Abstract
Cardiac hypertrophy is a common response of cardiac myocytes to stress and a predictor of heart failure. While in vitro cell culture studies have identified numerous molecular mechanisms driving hypertrophy, it is unclear to what extent these mechanisms can be integrated into a consistent framework predictive of in vivo phenotypes. To address this question, we investigate the degree to which an in vitro-based, manually curated computational model of the hypertrophy signaling network is able to predict in vivo hypertrophy of 52 cardiac-specific transgenic mice. After minor revisions motivated by in vivo literature, the model concordantly predicts the qualitative responses of 78% of output species and 69% of signaling intermediates within the network model. Analysis of four double-transgenic mouse models reveals that the computational model robustly predicts hypertrophic responses in mice subjected to multiple, simultaneous perturbations. Thus the model provides a framework with which to mechanistically integrate data from multiple laboratories and experimental systems to predict molecular regulation of cardiac hypertrophy.
Keywords: cardiac hypertrophy, heart failure, transgenic mice, systems biology, computational modeling
1. Introduction
Heart failure is a common chronic phase of multiple cardiovascular diseases that afflicts more than 37 million people [1]. Treatment of underlying disease and prevention of progression to overt heart failure is critical, as treatment options for heart failure remain limited. Hypertrophy is often an early response of the cardiac myocytes to biochemical and mechanical stresses on the heart, and is a leading independent predictor of heart failure. The cardiac hypertrophy response at the cellular level is manifest as an increase in cardiomyocyte size. Hypertrophy is a complex multi-scale response that alters multiple aspects of cellular and organ physiology including growth, gene expression, excitation-contraction coupling, energetic and metabolic states, and intercellular and extracellular matrix interactions. The complexity of the response limits our ability to understand the mechanisms and identify effective treatment strategies that are specific to cardiac hypertrophy.
As a first step towards addressing these complexity challenges, we previously developed a largescale model of the hypertrophy intracellular signaling network incorporating 106 signaling molecules, transcription factors, and cellular processes (collectively referred to as species) [2]. This model was based on, and experimentally validated against, in vitro data from primary neonatal rat ventricular cardiomyocytes. While cultured myocytes allow for detailed study of individual molecular processes contributing to hypertrophy, this is accomplished in a 2-dimensional, immature and incomplete system. Mature cardiomyocytes in vivo are highly differentiated and subject to 3-dimensional, cell-cell and cell-extracellular matrix interactions, synchronous contractions and functional loading [3]. Mouse models have allowed for analysis of in vivo effects of gene perturbations, yet these models are subject to their own limitations and the context of the perturbations must be considered when interpreting results [3,4]. While individual cases of concordance or discordance between in vitro and in vivo hypertrophy have been noted [3,5], the extent to which knowledge of cardiomyocyte hypertrophy gained in vitro is predictive of in vivo responses remains unclear. A more systematic analysis should permit a more integrative understanding of the molecular networks driving cardiac myocyte hypertrophy as well as highlight specific knowledge gaps or limitations in these experimental systems.
In this manuscript, we investigate the degree to which our in vitro-based computational model of the hypertrophy signaling network is able to predict and explain in vivo hypertrophy of cardiac specific transgenic mice. Transgenic overexpression of 34 genes was simulated using network model and results were compared to experimental data from 52 mouse models. We found that the original network model qualitatively predicts in vivo cell size and hypertrophy gene expression responses with 72% concordance with experimental data. After minor revisions motivated by in vivo literature, the model concordance improves to 78% and predicts with 69% concordance the qualitative responses of signaling intermediates within the network model. Analysis of four double-transgenic mouse models reveals that the computational model is able to robustly predict tissue hypertrophy responses in mice subjected to multiple, simultaneous perturbations.
2. Materials and Methods
The computational model of the cardiac myocyte hypertrophy signaling network was developed using neonatal rat ventricular myocyte data from experimental literature [2]. It uses logic-based normalized Hill ordinary differential equations (ODEs) to predict activity of each species. The system of ODEs is generated from the Network Model Reactions database (Database S1) using Netflux software implemented in MATLAB. Netflux software is available at the Saucerman laboratory web page (http://bme.virginia.edu/saucerman/), with code deposited at https://github.com/saucermanlab/Netflux. The database is an Excel file containing the logic based activating and inhibitory reaction information and default parameters. Default weight for all of the input species was set 0.06 to reflect a low level of basal stimuli under normal in vivo conditions. This weight was chosen because it produced a baseline activity of all output species to be approximately halfmaximal, allowing a suitable range to detect either an increase or a decrease when modeling species overexpression. Overexpression was modeled for each species by setting its activity level at 0.5. Overexpression for GSK3β, which at baseline has a level of 0.95, was modeled at a level of 1.0. Overexpression levels of each species in the mouse models was often not reported, but when reported highly variable, making any attempts to model overexpression more specifically for the individual experimental results impossible for the full collection of mouse models. To simulate in vivo cardiac hypertrophy, an additional reaction was added from “cell area” to “heart weight/body weight “(HW/BW), as this output measure is frequently reported for cardiac transgenic mice.
Cardiac myocyte specific transgenic mice which overexpress species in the computational model were identified in the literature. Mouse models were identified through review articles about myocardial hypertrophy and by PubMed and Google Scholar searches for transgenic mice expressing species included in the computational model. Mouse models were included if they were generated using a myocardial specific promoter, and expressed a gene encoding either an input or intermediate species from the network model in either a constitutive or inducible manner. Wild-type and activating mutations of the genes were included. All 52 of the transgenic mouse models identified are tabulated in a supplemental table (Table S2). Details include the genetic source material with any additional modifications, the gene promoter used to achieve cardiac myocyte specificity, and the literature reference. All experimental data for outputs in the computational model were collected for in vivo computational model validation. To allow for uniform comparisons, output data was extracted using ternary logic as either increased, unchanged, or decreased compared to the wild-type (not overexpressed) condition according to the statistics reported in the original reference for the experimental data. When experimental data was available from multiple sources or conditions, data was collapsed into a single value by an increase or decrease at any time point or experimental condition within a model taking precedence over an unchanged value, and in cases where there were multiple overexpressing models of different isoforms of a species the isoform with the more canonical cardiac role taking precedence. Database S3 demonstrates compilation of output data and examples of data collapse for the species akt, CaMK, and PKC. These species are chosen for illustration as they had the most numerous mouse models published. Mouse Output Data (Database S4), the compilation of literature reported output data, was processed using MATLAB code for automated comparison with the Netflux system of ODEs generated from the Network Model Reactions. Model predictions were classified qualitatively as increased or decreased if the change from the baseline condition was greater than 0.01(1%). This threshold was selected because the range of the model is from 0 to 1, and the 0.001 threshold used in the in vitro model [2] seemed too sensitive for in vivo modeling. Discrepancies between model predictions and Mouse Output Data were used to perform a focused search of in vivo cardiac literature related to mechanism in those portions of the model. New or altered mechanisms identified from this literature review were then used to generate a Revised Network Model Reactions database (Database S5) and generate a revised system of ODEs, again with Netflux. Subsequent to model revision, agreement between the computational model and Mouse Output Data and Mouse Intermediate Data (Database S6) was determined using automated comparison.
3. Results
3.1. Computational network model predictions and validation of transgenic overexpressing mouse models for cardiac hypertrophy
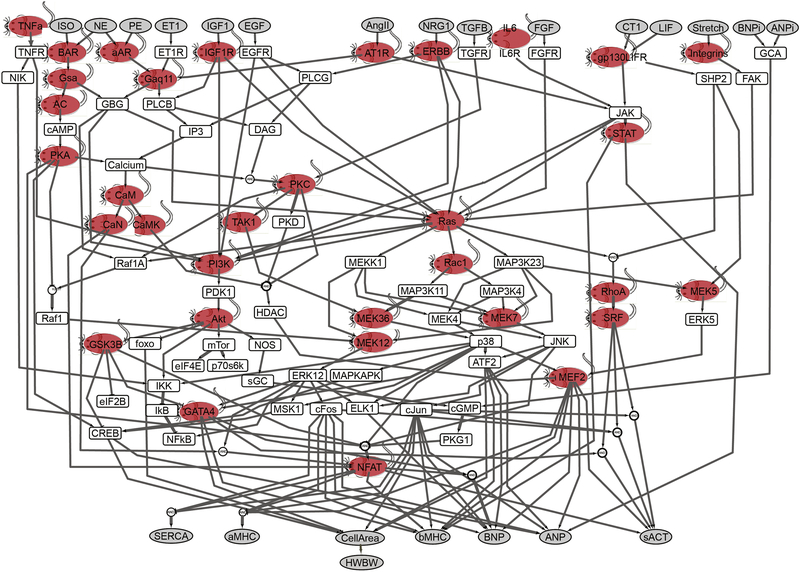
Fifty-two transgenic mouse models were identified with cardiac-specific overexpression of 34 of the species in the computational model. Figure 1 shows the positioning of these 34 species within the context of the hypertrophy signaling network model. Of the 99 species in the computational model (excluding outputs), we modeled mice overexpressing 2 of the 16 extracellular signaling inputs, 8 of the 14 cell surface receptors, and 24 of 68 intracellular signaling species. All of these transgenic mouse models identified are tabulated in a supplemental table (S2). The genetic source material is human in 22 and murine in 13 of the transgenes. Wild-type genes were used in 26 of the transgenes and constitutively activating mutations or subunits were used in 22 of the transgenes. The Jeff Robbins’ laboratory derived alpha-myosin heavy chain (α-MHC) promoter [6,7] was used to confer myocardial specificity in 37 of the transgenes, and it was used as part of an inducible expression system in an additional 6 models.
Figure 1. Transgenic mouse models developed to overexpress many of the species in the cardiac hypertrophy signaling network model.
The model consists of 106 species and 192 reactions. Model inputs and outputs are shown in gray. This model focused on neonatal rat ventricular myocytes for initial implementation using a normalized-Hill differential equation approach [2]. Fifty-two transgenic mouse models representing 34 of the species were identified in the literature for this analysis (red mice, see supplemental Table S5). ISO, isoproterenol; NE, norepinephrine; ET1, endothelin-1; IGF1, insulin-like growth factor 1; AngII, angiotensin II; NGR1, neuregulin 1; IL6, interleukin 6; FGF, fibroblast growth factor; CT1, cardiotropin 1; BNP, brain natriuretic peptide; ANP, atrial natriuretic peptide; SERCA, sarcoplasmic reticulum ATPase; α-MHC, α- myosin heavy chain; sACT, skeletal α-actin; CREB, cAMP-responsive element-binding protein; EGFR, EGF receptor; FGFR, FGF receptor; IP3, inositol 1,4,5-trisphosphate; β-AR, β-adrenergic receptor; α-AR, α-adrenergic receptor; NOS, nitric oxide synthase; NIK, NFκB-inducing kinase; AC, adenylyl cyclase; DAG, diacylglycerol; PLC, phospholipase C; FAK, focal adhesion kinase; SRF, serum response factor; GCA, guanylyl cyclase subtype A.
We first examined to what extent the original computational network model could predict in vivo changes in fetal gene expression and basic hypertrophic phenotypes. We found that experimental data was often reported qualitatively, or had quantitative values that varied greatly between mouse models or outputs. Database S3 illustrates the experimental variability for the three species for which the most in vivo data is available. Akt overexpression (Database S3, sheet 1) was evaluated in 6 different mouse lines over various time points from 2 to 18 weeks. The output cell area was evaluated in 7 of 9 conditions, but was only quantified in 3 of these conditions, and these 3 measurements were done by 2 different methods. HWBW was evaluated in all 9 conditions, and showed a fold change from control that ranged from 1.4 to 2.7. The only other outputs measured were βMHC and ANP gene expression measured in 1 study. Similar variability for CaMK and PKC are illustrated in sheets 2 and 3 of Database S3. Therefore to allow for uniform comparisons, output data was extracted using ternary logic.
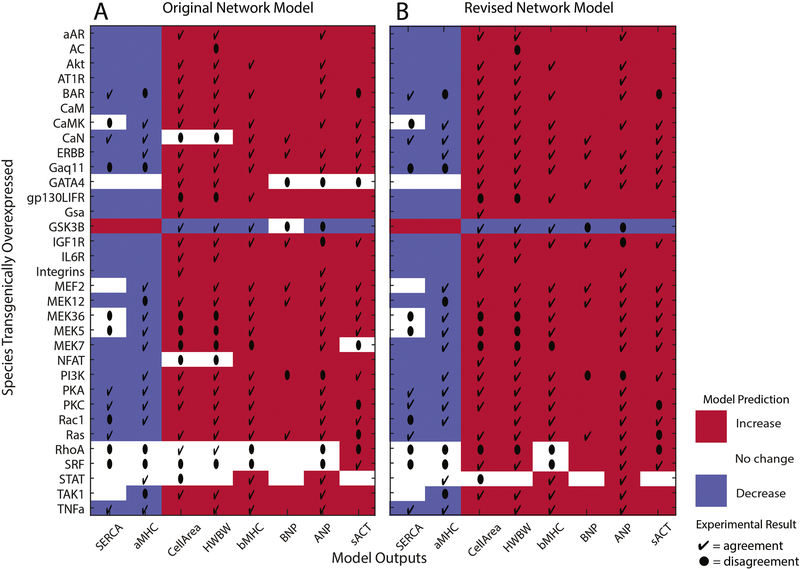
Qualitative agreement between Mouse Output Data (S4) and the computational model predictions is 72% overall (Figure 2A). We tested the robustness of this finding to the threshold selected for determining a qualitative increase or decrease from baseline in the model predictions. Thresholds of 0.001, 0.01, 0.1, 0.2 and 0.5 resulted in qualitative agreement with in vivo data of 74%, 72%, 70%, 67% and 51%, respectively. The qualitative agreement is similar up to a 20% threshold.
Figure 2. Cardiac hypertrophy model accurately predicts majority of in vivo overexpression output species data.
Model predictions for the output species (x-axis) with individual species overexpression (y-axis) are color coded for A) the original and B) the revised cardiac hypertrophy signaling network. Red indicates increase, white indicates no change, and blue indicates decrease. In vivo experimental data in agreement with the model predictions are indicated by a check (4) and data that disagree are indicated by a dot (l). There is 72% agreement with the original model, which increases to 78% after model revision.
Cell area and HW/BW were the most frequently investigated features of hypertrophy in the mouse models. βMHC and ANP were the most frequently assayed fetal genes. Other upregulated fetal genes and the downregulated genes were investigated less consistently in these transgenic mice. For the hypertrophic phenotypes (cell area and HW/BW) the agreement is 72% (21/29) and 73% (22/30), respectively. For the upregulated genes in the fetal gene program agreement is 88% (21/24) for beta-myosin heavy chain (β-MCH), 77% (20/26) for A natriuretic peptide (ANP), 67% (6/9) for B natriuretic peptide (BNP), and 67% (12/18) for skeletal actin (sACT). For the downregulated genes, agreement between model predictions and experimental data is 68% (13/19) for α-MHC, and 46% (6/13) for sarcoendoplasmic reticulum calcium transport ATPase (SERCA). As shown in Figure 2A, the original network model in general predicts an overall concordance between hypertrophy phenotypes and upregulation of all 4 fetal genes and downregulation of αMHC and SERCA. While this concordance is not always maintained in the transgenic models [8–13], there is a consistent trend. Attention to this pattern in the hypertrophy response helped guide subsequent revision of the computational model.
The lack of full concordance in the hypertrophy responses along with a high degree of disagreement between the predictions and experimental data for the GATA4, NFAT, and SRF transgenics led us to perform more focused literature searches. We justified several modifications to the computational model from additional in vitro and in vivo data. Modifications are of 3 types, discussed in detail below.
Four new links (red links in Figure S7) are included from transcription factors to outputs that were not part of the neonatal rat ventricular cardiomyocyte data from which the original model was developed but are supported by the transgenic mouse data. In the in vivo transgenic models, NFAT and SRF contribute to hypertrophy as measured both by an increase in cell area and HW/BW [8,14,15]. In the computational model cell area directly drives HW/BW, so addition of the links to cell area result in agreement for both output measures and also agreement of cell area and HW/BW for the calcineurin transgenic mouse. SRF upregulates expression of the peptides ANP and BNP in vivo, therefore these links were added to the computational model [14,16]. Addition of these links increases overall agreement from 72% to 76%.
The link from IL6R to JAK is modified to signal through gp130LIFR rather than directly to JAK (blue link in Figure S7) to more accurately represent this signaling pathway [17,18]. This update did not alter agreement between predicted and experimental data
Eight new OR reaction links (purple links in Figure S7) are included that directly link transcription factors with outputs for relationships that were initially modeled as logic-based AND links. Including the OR links to model the interactions between transcription factors and output species that were already part of AND links from in vitro interaction data was motivated by in vivo data indicating that transgenic overexpression of SRF, NFAT, and GATA4 are each sufficient to induce the corresponding output species mRNA [14,19]. Addition of these links increased overall agreement from 72% in the original in vitro model to 74%. Dropping the AND logic links from the computational model did not change the agreement between predictions and experimental data. We decided to keep both the AND and the OR logic links in the computational model because of the in vitro data supporting the cooperative interactions between the transcription factors [8,20–23]. Cooperative transcription factor interactions supporting cardiac hypertrophy are complex and not fully characterized, and retaining these links may allow for refinement of the model in the future as the transcription factor interactions are better defined. Inclusion of all the model modifications results in 78% agreement between mouse transgenic data and the computational model for output species (Figure 2B).
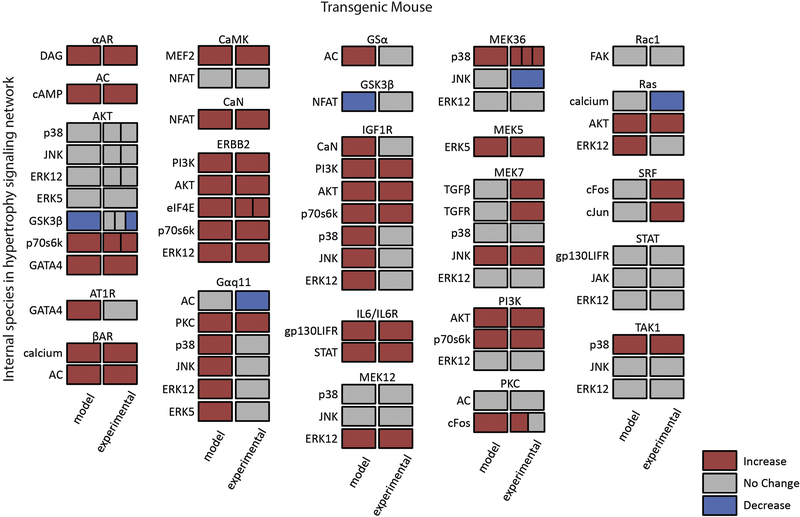
As an independent validation of the computational models’ applicability to in vivo hypertrophy, we examined the extent to which the revised model could predict internal species (proteins that are not inputs or outputs) measured in these transgenic mouse models. Agreement between mouse transgenic data and the revised computational model for internal species is 69% (Figure 3, and Database S6). This good overall level of agreement is consistent with the model predictions for in vivo hypertrophic outputs. Areas of agreement indicate internal species whose role can be accounted for based on the prior knowledge and assumptions on which the network model was based. Cases in which model predictions and experimental data disagree highlight knowledge gaps, where the mechanisms underlying cardiac hypertrophy are not fully understood. These gaps indicate areas where additional experiments and model revision may be needed.
Figure 3. Cardiac hypertrophy model accurately predicts majority of in vivo overexpression internal species data.
Model predictions and experimental data are depicted side-by-side; red indicates increase, gray indicates no change, and blue indicates decrease. Species overexpressed is indicated above and the species for which the response is predicted or measured is indicated on the left. The divisions in the experimental blocks indicate species for which there are multiple transgenic models, and the data for each model are included.
A notable discrepancy is the model prediction that transgenic overexpression of Gαq11 or IGF1R activates p38, JNK, and ERK1/2, which is not reported experimentally [9,24]. As these MAPK pathways are downstream effectors of many hypertrophic stimuli, this discrepancy may indicate that crosstalk or other compensatory mechanisms may need to be investigated or that the often transient activation of MAPK species [25] was not detected at the time points evaluated in the mouse models. Another gap is the model’s inability to predict that MEK7 overexpression induces activation of upstream TGFβ and TGFR species, suggesting a potentially important autocrine/paracrine loop that may need to be incorporated into future models. Since these experimental data were from cardiac tissue homogenate [13], further study will be required to determine the underlying mechanisms and whether feedback from MEK7 to TGFβ signaling involves only cardiomyocytes or other cell types. The disagreements between the model and the GSK3β and SRF transgenic mice illustrate that transcription factor interactions are incompletely delineated, both in the model and experimentally [21,26].
3.2. New predictions at the network level
Experimental studies are able to interrogate limited proteins, transcripts, or physiologic responses with respect to hypertrophy signaling. To gain a more comprehensive view of how the 34 overexpressed species affect hypertrophy signaling, the network-wide responses to transgenic overexpression were simulated and visualized (Figure S8). Figure S8 illustrates the change in species activity from baseline conditions for the entire network (y axis) for each transgenic overexpressing mouse (x axis).
In order to identify groups of transgenic mouse models that induce similar global network responses, K-means clustering was performed on the columns of Figure S8. This analysis revealed 8 groups of mouse models (Table 1). These groups are color coded onto the full network schematic in Figure S9A. The smaller groupings consist of species that are in close proximity within the network. The larger groupings are comprised of species with similar network locations: the TG1 group contains cell surface receptors and closely related species that activate multiple signaling pathways, the TG4 group contains both receptors and upstream signaling hubs, and the TG6 group contains species more distal in the signaling pathways and downstream transcription factors. We did not find any consistency between the groupings by network response similarity and the clinical phenotypes, type of cardiomyopathy and timing and type of morbidity and mortality, for the transgenic overexpressing mice (data not shown).
Table 1. Transgenic overexpressing mice clustering.
Groups identified by k-means clustering of mouse models by similarity of global network responses to transgenic overexpression of a single network species.
| Group | Transgenic mouse models |
|---|---|
| TG1 | αAR, IGF1R, AT1R, ERBB, Gαq/11 |
| TG2 | βAR, Gsα |
| TG3 | AC, PKA |
| TG4 | IL6/IL6R, gp130LIFR, Integrins, PKC, Ras |
| TG5 | GSK3β |
| TG6 | MEK12, MEK7, MEK5, RhoA, SRF, GATA4, MEF2, NFAT, STAT, Calmodulin, calcineurin, CaMK |
| TG7 | Rac1, MEK36, TAK1, |
| TG8 | TNFα, PI3K, Akt |
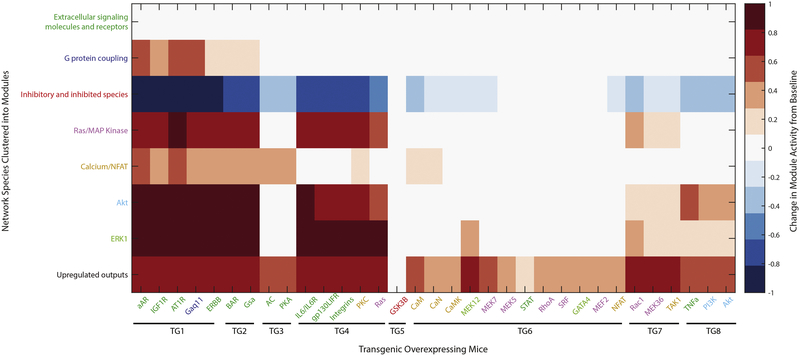
In order to identify signaling pathways and modules that are similarly responsive across these transgenic mouse models, K-means clustering was performed on the rows of Figure S8. This analysis enables us to condense the 107 input, intermediate and output species of the hypertrophy signaling model into 8 functional modules (Table 2). These modules are color coded onto the full network schematic in Figure S9B.
Table 2. Functional modules for hypertrophy signaling network.
Functional species modules identified by k-means clustering of the predicted activity change in each species activity from transgenic overexpression of 33 individual species.
| Module | Members |
|---|---|
| Extracellular signaling molecules and receptors | TNFα, TNFR, NIK, ISO, βAR, Gsα, AC, cAMP, PKA, NE, PE, αAR, ET1, ET1R, IGF1, IGF1R, EGF, EGFR, PLCγ, AngII, AT1R, NRG1, ERBB, TGFβ, TGFR, IL6, IL6R, FGF, FGFR, CT1, LIF, gp130LIFR, JAK, STAT, SHP2, stretch, Integrins, FAK, BNPi, ANPi, GCA |
| G protein coupling | Gαq/11, Gβγ, PLCβ, IP3, DAG |
| Inhibitory and inhibited species | HDAC, GSK3β, foxo, IκB, SERCA, αMHC |
| Ras/MAP Kinase | Ras, Raf1A, MEKK1, Rac1, MAP3K11, MAP3K4, MAP3K23, MEK36, MEK4, MEK7, p38, MAPKAPK, JNK, ATF2, ELK1, RhoA, SRF, MEK5, ERK5, MEF2 |
| Calcium/NFAT | Calcium, calmodulin, calcineurin, CaMK, PKC, TAK1, PKD,,NFAT |
| Akt | PI3K, PDK1, Akt, mTor, eIF4E, IKK, p70s6k, eIF2B, CREB, NOS, sGC, cGMP, PKG1 |
| ERK1 | Raf1, NFκB, MEK12, ERK12, MSK1, GATA4, cFos, cJun |
| Upregulated outputs | Cell area, heart weight/body weight, βMHC, BNP, ANP, sACT |
The largest functional module contains 41 of the 107 species in the hypertrophy signaling network. It is designated “Extracellular signaling molecules and receptors” (dark green in Figure S9B) because all 17 of the model inputs and all 14 of the cell surface receptors for these inputs along with 10 species that are deeper in the network were clustered into this module. The second largest module is the Ras/MAP kinase module which contains 20 species located centrally in the network (purple in Figure S9B). This module includes the small signaling molecule Ras and most, but not all, of the MAP kinase signaling pathway. The cluster entitled “Inhibitory and inhibited-species” contains species that are least proximate to each other in the network; these are grouped together because their activity is inhibited by their inputs.
Comparison of the signaling modules and groups of transgenic mice permits an integrative view of how transgenic overexpression induces distinct signaling programs (Figure 4). For the extracellular signaling module the average change in species activity for each transgenic mouse is not different from the baseline activation state of the network (top row, Figure 4). This reflects the upstream location of these species and the 0.8 to 1 ratio of receptors to inputs in the network. Fourteen transgenic mice exist for species in this module, but when the mouse models are clustered perpendicularly by global network responses these 14 transgenics represent 6 of the 8 mouse groups (Figure S9A compared to S9B). Eight transgenic mice exist for species in the Ras/MAPK module which cluster into 3 of the mouse groups.
Figure 4. Network activation by modular relationships of species and mice.
The K-means clustering algorithm in MATLAB allowed condensations of the 107 network model species into 8 functional modules (y-axis) and clustering of the 34 species for which transgenic overexpressing mice exist into 8 groups (x-axis) with the most similar network responses. The predicted average response of each module is shown for overexpression of an individual species.
Ras and NFAT are the species in the network with the most combined input/output reactions (n=14 for each), and were identified as hubs in the original in vitro signaling network model. Ras has the most input reactions at 8, followed by PI3K at 7. The species directly upstream from the global network outputs are transcription factors; these include ATF2, CREB, foxo, GATA4, cFos, cJun, ELK1, NFAT, MEF2, SRF, and STAT. Their responses for species overexpression represent 6 of the 8 functional modules, but the transgenic mice that overexpress 5 of these transcription factors all cluster into a single mouse group.
Network responses are more related to upstream/downstream location in the network, both when analyzed by mouse model and global network response predictions. Additionally, predicted network output responses are quite uniform, illustrated by color in Figure 2, for the transgenic overexpressing mouse models. Model development from in vitro cellular hypertrophy data, the high level of crosstalk between signaling pathways, the majority of relationships being activating, and a lack of feedback mechanisms are some features of the current computational model that contribute to these predictions.
3.3. Double transgenic predictions using in vivo hypertrophy signaling network
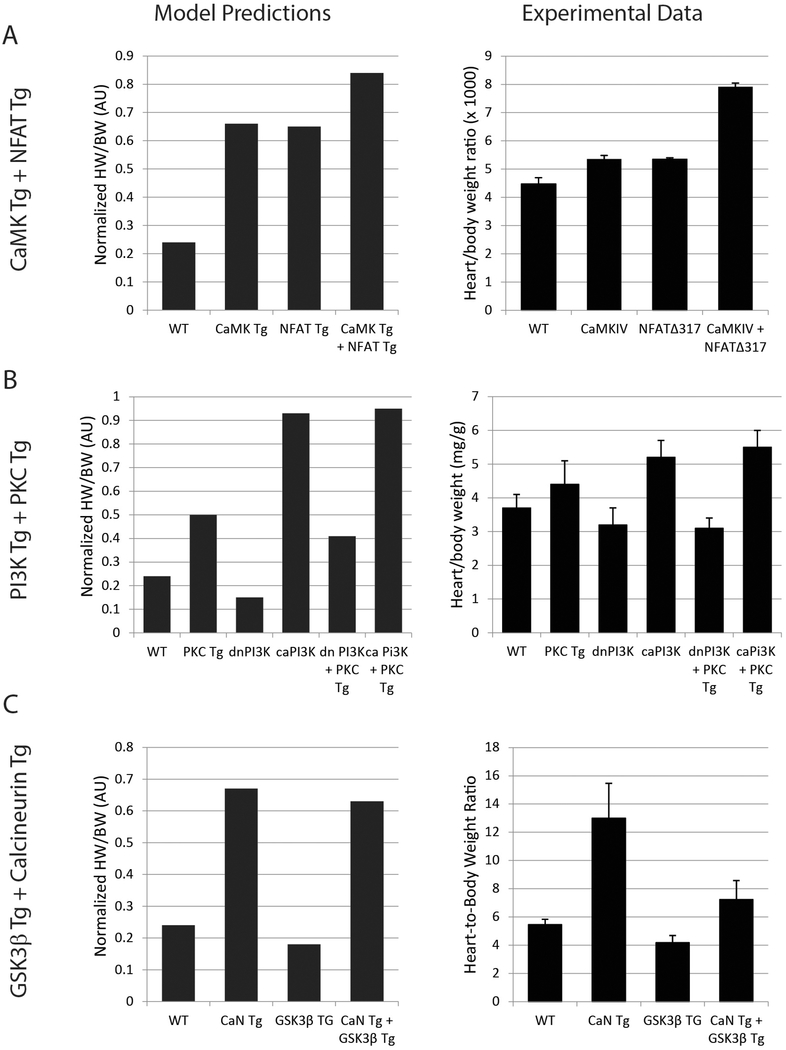
Analysis of transgenic mouse models that overexpress multiple distinct genes may provide a more stringent validation of the model’s predictive abilities and allow for identification of cooperative or compensatory hypertrophic mechanisms. Therefore we analyzed 4 different double transgenic mouse models from the literature where both transgenic genes are in the computational model. We focused this analysis primarily on heart weight/body weight (HW/BW) as this is an integrative measure of in vivo cardiac hypertrophy.
First, we performed simulations representing a double transgenic mouse that overexpresses the Ca2+/calmodulin-dependent protein kinase IV (CaMK) and a constitutively activated NFAT3 [15]. Overexpression was simulated as in the above single-gene analyses, with uniform initial input values, setting the overexpressed gene(s) at 0.5 activation, and prediction of normalized HW/BW on a range from 0 to 1. The model predicted increased HW/BW for individual overexpression of CaMK and NFAT, as well as further hypertrophy with combined overexpression of CaMK and NFAT (Figure 5A). All of these predictions are in good qualitative agreement with the experimentally measured hypertrophy for these individual and double transgenic mice [15]. This mutualistic hypertrophic response reflects the parallel pathways leading to hypertrophy activated by NFAT transcription factors and by CaMK through the MEF2 transcription factors [8,15,27].
Figure 5. Cardiac hypertrophy model accurately predicts in vivo hypertrophy responses in single and double transgenic mice.
The in vivo hypertrophy response is indicated by the heart to body weight ratio. In each panel, network model predictions are shown on the left and experimental data from the literature are replotted on the right. A) Individual and double transgenic overexpression of calcium/calmodulin-dependent protein kinase VI (CaMK) and constitutively activated NFAT3, with experimental data from [15]. B) Individual and double transgenic expression of constitutively activated or dominant negative phosphoinosotide 3-kinase (PI3K) and protein kinase C β2, with experimental data from [28]. C) Individual and double transgenic overexpression of activated calcineurin (CaN) and activated glycogen synthase-3β, with experimental data from [29].
The next pairings of doubly transgenic mice that we computationally modeled combined phosphoinositide 3-kinase (PI3K) and protein kinase C (PKC), which are predominantly involved in physiologic and pathologic hypertrophy signaling, respectively (Figure 5B) [28]. In these mouse experiments overexpression of either constitutively- activated (ca) or dominant-negative (dn) PI3K transgenes were combined with PKCβ2 transgene overexpression. Using default parameters, the model predicted HW/BW responses to individual PKC, dnPI3K or caPI3K overexpression that were qualitatively consistent with experimental data. While this model also correctly predicted that combined caPI3K and PKC overexpression would induce cardiac hypertrophy, the model did not correctly predict the experimentally observed reduced hypertrophy with combination of dnPI3K and PKC overexpression (Figure S10A). Analysis revealed that this inaccuracy may be explained by the differing baseline activities of these kinases in the model and the extent of overexpression that was simulated. With the default parameters there is a mismatch between the PI3K and PKC effects which was further investigated by tuning the modeled level of PKC overexpression. With the default overexpression of PKC at 0.5 activation (Figure S10A), PI3K is collaterally activated to 0.6 on the 0 to 1 activation scale. PKC was tuned to an overexpression level of 0.05, a 5 fold increase from the baseline activation of 0.013, which results in a minimal PI3K collateral activation, 0.17 to 0.15 at baseline, and a qualitatively appropriate HW/BW increase, from 0.24 to 0.5. After tuning of the PKC activation level for overexpression, additional tuning of PI3K expression was not required to produce qualitative similarity between the computational model predictions and experimental data for both HW/BW (Figure 5B) and cell area (Figure S10B). This experimental study additionally measured protein levels of PKC and phospho-AKT which also was well predicted by our computational model with the single exception of the PKC protein level in the PKC/caPI3K double transgenic (Figure S10C).
The final double transgenic mouse that we compared to our computational signaling model was the combination of activated calcineurin (CaN) and activated glycogen synthase-3β (GSK3β) transgenes, species that are pro- and anti-hypertrophic, respectively. In this case, the default parameters for reactions, baseline activation and overexpression of the species resulted in good qualitative agreement between the computational model predictions and experimental data for HW/BW and cell area (Figures 5C and S11A) [29]. Model prediction agreement with data could not be improved with tuning because the CaN results were already good and there was no range available for tuning the GSK3β effect; baseline activation was at 0.95 and transgenic activation is limited to maximum of 1.0. Our computational model predicts responses of similar direction and magnitude for cell and tissue hypertrophy (cell area and HW/BW) and for the fetal genes (βMHC, ANP, BNP, sACT) reactivated in hypertrophic responses, consistent with the majority of both in vitro and in vivo literature. Interestingly, experimentally for this combination of CaN and GSK3β transgenic overexpression, ANP and BNP expression were mutually enhanced in a manner decoupled with hypertrophy and βMHC expression (Figure S11B). This unexplained decoupling among fetal genes indicates a knowledge gap regarding crosstalk of feedback in some conditions.
4. Discussion
We examined the ability of a cardiac myocyte hypertrophy signaling network model, based on in vitro mechanistic studies, to predict biochemical and hypertrophic responses of transgenic mice. Although the network model includes 99 species, each species is treated as a single unit and there is no distinction with respect to selective modulation of individual isoforms. We have included much “crosstalk” between various signaling cascades, but have not yet incorporated feedback loops or micro-RNA regulation. Dynamic activation of input signals and feedback loops are likely necessary to fully capture the complexities of in vivo cardiac hypertrophy and its progression to heart failure [30]. Despite these limitations, we found this network model predicts in vivo responses of cellular and organ hypertrophy and cardiac gene expression with 78% concordance. Further, the model provides a mechanistic framework with which to identify whether mispredictions are due to mechanisms that are not yet represented in the model or differences between in vitro and in vivo experimental systems.
The computational modeling approach used here facilitates comparison and integration of knowledge acquired from in vitro primary neonatal cardiac myocytes and in vivo whole organism models. Our computational model of the cardiomyocyte hypertrophy signaling network was initially created using cultured neonatal rat cardiomyocyte data [2]. The highly controlled setting of primary cell culture systems allows detailed dissection of signaling and regulatory interactions that drive hypertrophy. However, a cardiac myocyte within a heart is subject to a rich context that includes multiple other cell types, a complex 3-dimensional extracellular matrix, synchronous electrical depolarizations, cyclic functional loading and stretching, and the paracrine and endocrine signaling milieu of the whole organism. While progress is being made in the development of in vitro models that better represent complex cardiac microenvironments [31,32], in vivo models remain the benchmark. Temporal, tissue-specific gene modification and genome editing technologies available for mice allow for astounding precision in manipulating cardiovascular genetics; however they also make careful planning and controlling of experiments imperative [3,4,33,34]. We chose to use transgenic overexpression of species in the hypertrophy signaling network for this in vivo analysis of our computational network because the hypertrophy response generally involves increased signaling, gene expression and protein responses. Investigation of knockdown or knockout mouse models with respect to hypertrophy would require modeling additional stimuli in order to have a baseline level of hypertrophy to evaluate responses; thus modeling overexpression provides a more straightforward approach. Limitations of transgenic overexpressing mouse models include insufficient statistical power due to small numbers of animals in compared groups and a lack of reproducibility between studies utilizing different transgenic lines. The gene expression and protein levels interrogated, methods for characterizing phenotypes, time points examined, and duration of survival for different transgenic mice can be quite disparate (Database S3). Despite qualitatively similar model outputs of cell area and HW/BW, the variability in the clinical phenotypes resulting from the four AKT transgenic lines illustrates well these limitations [35–38]. Given the variability in published studies, the current model does not directly account for potential contributions of genetic background and gender to hypertrophic responses. While caution is advised in interpretation of in vivo results that are generated by complete knockouts or non-physiologic overexpression [39], the integration of multiple overexpressing models with the framework of our mathematical model manually curated from in vitro studies offers a new level of support for in vivo overexpression models.
We report concordance between our computational model and in vivo data based on qualitative similarity. While both experimental measurements and model predictions are quantitative, continuous variables, two main factors prevent direct quantitative comparison. First, the hypertrophic outputs all have distinct experimental units with different dynamic ranges, which complicate a uniform application of thresholds for gradations. Second and more importantly, there is extensive variability in the change of these hypertrophic outputs across experimental studies. Database S3 illustrates this experimental variability. We concluded that given the extent of this variability and incomplete data across mouse models, a qualitative comparison using ternary logic, increased, unchanged, or decreased compared to the control conditions and statistical analysis employed within the individual studies, was the appropriate metric for the in vivo experimental data. This comparison utilized the originally reported statistical significance testing to maintain objectivity and consistency with the experimental study design despite substantially different quantitative values and statistical methods used across studies. This model is our first step in efforts to synthesize a significant number of experimental studies that, while fundamentally similar, are quite varied with respect to the details of experimental design.
In contrast because the computational model has a single baseline condition, comparisons between model predictions can be made more directly. We initially selected a threshold of 1% for defining a change as significant for the model. The model was found to perform robustly up to a 20% change threshold. This suggests that changes in hypertrophy outputs predicted by the computational model are generally of sufficient magnitude to reflect changes that are generated by experimental manipulations. Protein overexpression ranging from 5- to 400-fold (Database S3) were used in the mouse models of cardiac hypertrophy. However, experimentally generated perturbations in the function of hypertrophy signaling species are likely exaggerated in comparison to the in vivo stressors that lead to hypertrophy in clinical situations. Thus while the original and the modified computational models perform well in predicting hypertrophy for experimental perturbations, additional refinements will likely be required for our model to predict clinical hypertrophy signaling responses. The stepwise approach of integrating detailed in vitro and in vivo experimental data before attempting to make predictions concerning clinical hypertrophy is necessary because of the complexity of the hypertrophy signaling network.
Our analysis reveals a broad representation across species in the network as compared to the transgenic model groupings (Figure S9). This reflects the complexity of the hypertrophy response, which makes this type of systems-based analysis necessary to allow for meaningful insights to inform new therapeutic options. Cases in which model predictions and experimental data disagree (Figure 2) highlight knowledge gaps, where the mechanisms underlying cardiac hypertrophy are not fully understood. These gaps indicate areas where additional experiments and model revision may be needed.
In order to simulate in vivo cardiac hypertrophy in our computational model, we added a direct reaction from “cell area” to “heart weight/body weight”. While other factors also contribute to HW/BW such as cardiac fibrosis and cardiomyocyte proliferation, the current computational hypertrophy signaling model was not designed to predict those additional in vivo phenotypic responses or the temporal progression of cardiac dysfunction. The cardiac phenotypes of mouse models are often interpreted as cardiomyopathies such as dilated, restrictive, or hypertrophic, but the evaluation and interpretation of these clinical conditions is in no way standardized for mice. These will be a challenges for future iterations of the model. At present, the model is most applicable to adult mice at relatively early stages of cardiac remodeling, where apoptosis, fibrosis and proliferation responses are more limited. Mis-predictions between “cell area” and “HW/BW” will help identify signaling pathways that also impact the additional stress responses as we work to further extend our modeling.
In vitro and in vivo data support the concordance in our model between hypertrophy phenotype (cell area and HW/BW) and gene expression (bMHC, BNP,ANP, sACT) upregulation. Literature generally supports this concordance for pathologic hypertrophy, and in fact this concordance is used as one of the distinguishing features of pathologic as opposed to physiologic hypertrophy [40,41]. However, there are gaps in this distinction. For instance, our model predicts, and the experimental data supports, the concordant pattern for the PI3K and AKT species (Figure 2, Database S3), which are generally interpreted as physiologic hypertrophy signals. The clinical phenotypes of the transgenic AKT lines vary from physiologic hypertrophy to dilated cardiomyopathy [35–38], while the PI3K transgenic line has a physiologic hypertrophy phenotype [24,42]. Whether pathologic hypertrophy is distinct from or a progression of unresolved physiologic hypertrophy remains a question in the field of cardiovascular biology. With the use of single species transgenic overexpressing mice we cannot address this controversy using our computational model. However, additional transgenic models with multiple perturbations and further tuning of our model provide ways to enhance understanding of this issue.
SERCA2 gene expression in general decreases with hypertrophy. However, it changes dramatically with maturity [43] which may result in the higher rate of disagreement between model predictions and in vivo data than occurs for the other output species (Figure 2). SERCA mRNA levels are decreased consistently in failing human hearts regardless of etiology, including both dilated and hypertrophic cardiomyopathies [44]. The failure of the model to accurately predict the SRF and RhoA overexpression effects may be due to complex regulatory interactions involving thyroid hormone and miR280 that are not yet modeled [45]. Given the complexity of this system, it appears most appropriate for future detailed work.
While additional complexity in the signaling model may help to discriminate subtleties in the timing and gene expression changes and species specificity of the hypertrophy response, development of multi-scale models may be required to predict clinical phenotypes such as eccentric vs. concentric and physiologic vs. pathologic hypertrophy. Systems biology approaches to describing organ and organism level responses related to circulatory stressors will require integration of multiple phenotypic responses, such as hypertrophy signaling, apoptosis, fibrosis, and contractility, across varying time-scales using complex multi-scale modeling [30]. The ability of the hypertrophy signaling network model to predict in vivo hypertrophy of transgenic mouse models indicates that this computational model will be a strong basis for understanding multi-scale relationships between molecular regulation and organ level remodeling.
Supplementary Material
Highlights.
Hypertrophy network model is used to simulate transgenic overexpression of 34 genes.
In vitro based network model predicts 72% of 168 in vivo hypertrophic responses.
Model revision using in vivo data improves concordance to 78%.
Hypertrophic responses of 4 double transgenic mice are well predicted by the model.
This is a framework for integrating multi-source data to predict cardiac hypertrophy.
5. Acknowledgement
This study was supported by the National Institutes of Health (HL137100, HL137755, and HL105242), the National Science Foundation (1252854), and the University of Virginia.
Footnotes
Disclosures
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9 References
- [1].Ziaeian B, Fonarow GC, Epidemiology and aetiology of heart failure., Nat Rev Cardiol. 13 (2016) 368–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Ryall KA, Holland DO, Delaney KA, Kraeutler MJ, Parker AJ, Saucerman JJ, Network reconstruction and systems analysis of cardiac myocyte hypertrophy signaling., J. Biol. Chem 287 (2012) 42259–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Molkentin JD, Robbins J, With great power comes great responsibility: using mouse genetics to study cardiac hypertrophy and failure., J. Mol. Cell. Cardiol 46 (2009) 130–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cook SA, Clerk A, Sugden PH, Are transgenic mice the “alkahest” to understanding myocardial hypertrophy and failure?, J. Mol. Cell. Cardiol 46 (2009) 118–29. [DOI] [PubMed] [Google Scholar]
- [5].Liang Q, Molkentin JD, Redefining the roles of p38 and JNK signaling in cardiac hypertrophy: dichotomy between cultured myocytes and animal models., J. Mol. Cell. Cardiol 35 (2003) 1385–94. [DOI] [PubMed] [Google Scholar]
- [6].Gulick J, Subramaniam A, Neumann J, Robbins J, Isolation and characterization of the mouse cardiac myosin heavy chain genes., J. Biol. Chem 266 (1991) 9180–5. [PubMed] [Google Scholar]
- [7].Rindt H, Subramaniam A, Robbins J, An in vivo analysis of transcriptional elements in the mouse alpha-myosin heavy chain gene promoter., Transgenic Res. 4 (1995) 397–405. [DOI] [PubMed] [Google Scholar]
- [8].Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. , A calcineurin-dependent transcriptional pathway for cardiac hypertrophy., Cell. 93 (1998) 215–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].D’Angelo D, Sakata Y, Lorenz J, Boivin G, Walsh R, Liggett S, et al. , Transgenic Gαq overexpression induces cardiac contractile failure in mice, Proceedings of the National Academy of Sciences. 94 (1997) 8121–8126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liao P, Georgakopoulos D, Kovacs A, Zheng M, Lerner D, Pu H, et al. , The in vivo role of p38 MAP kinases in cardiac remodeling and restrictive cardiomyopathy., Proc. Natl. Acad. Sci. U.S.A 98 (2001) 12283–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Martindale JJ, Wall JA, Martinez-Longoria DM, Aryal P, Rockman HA, Guo Y, et al. , Overexpression of mitogen-activated protein kinase kinase 6 in the heart improves functional recovery from ischemia in vitro and protects against myocardial infarction in vivo., J. Biol. Chem 280 (2005) 669–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nicol RL, Frey N, Pearson G, Cobb M, Richardson J, Olson EN, Activated MEK5 induces serial assembly of sarcomeres and eccentric cardiac hypertrophy., EMBO J. 20 (2001) 2757–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Petrich B, Eloff B, Lerner D, Kovacs A, Saffitz J, Rosenbaum D, et al. , Targeted activation of cJun N-terminal kinase in vivo induces restrictive cardiomyopathy and conduction defects., The Journal of Biological Chemistry. (2004). [DOI] [PubMed] [Google Scholar]
- [14].Zhang X, Azhar G, Chai J, Sheridan P, Nagano K, Brown T, et al. , Cardiomyopathy in transgenic mice with cardiac-specific overexpression of serum response factor, American Journal of Physiology-Heart and Circulatory Physiology. 280 (2001) H1782–H1792. [DOI] [PubMed] [Google Scholar]
- [15].Passier R, Zeng H, Frey N, Naya FJ, Nicol RL, McKinsey TA, et al. , CaM kinase signaling induces cardiac hypertrophy and activates the MEF2 transcription factor in vivo., J. Clin. Invest 105 (2000) 1395–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Dasgupta T, Stillwagon SJ, Ladd AN, Gene expression analyses implicate an alternative splicing program in regulating contractile gene expression and serum response factor activity in mice., PLoS ONE. 8 (2013) e56590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Tone E, Kunisada K, Kumanogoh A, Negoro S, Funamoto M, Osugi T, et al. , gp130DEPENDENT SIGNALLING PATHWAY IS NOT ENHANCED IN gp130 TRANSGENIC HEART AFTER LIF STIMULATION, Cytokine. 12 (2000) 1512–1518. [DOI] [PubMed] [Google Scholar]
- [18].Hirota H, Yoshida K, Kishimoto T, Taga T, Continuous activation of gp130, a signal-transducing receptor component for interleukin 6-related cytokines, causes myocardial hypertrophy in mice., Proc. Natl. Acad. Sci. U.S.A 92 (1995) 4862–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liang Q, De Windt LJ, Witt SA, Kimball TR, Markham BE, Molkentin JD, The transcription factors GATA4 and GATA6 regulate cardiomyocyte hypertrophy in vitro and in vivo., J. Biol. Chem 276 (2001) 30245–53. [DOI] [PubMed] [Google Scholar]
- [20].Belaguli NS, Sepulveda JL, Nigam V, Charron F, Nemer M, Schwartz RJ, Cardiac tissue enriched factors serum response factor and GATA-4 are mutual coregulators., Mol. Cell. Biol 20 (2000) 7550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Akazawa H, Komuro I, Roles of cardiac transcription factors in cardiac hypertrophy., Circ. Res 92 (2003) 1079–88. [DOI] [PubMed] [Google Scholar]
- [22].Jeong MY, Kinugawa K, Vinson C, Long CS, AFos dissociates cardiac myocyte hypertrophy and expression of the pathological gene program., Circulation. 111 (2005) 1645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Paradis P, MacLellan WR, Belaguli NS, Schwartz RJ, Schneider MD, Serum response factor mediates AP-1-dependent induction of the skeletal alpha-actin promoter in ventricular myocytes., J. Biol. Chem 271 (1996) 10827–33. [DOI] [PubMed] [Google Scholar]
- [24].McMullen JR, Shioi T, Huang W-YY, Zhang L, Tarnavski O, Bisping E, et al. , The insulin-like growth factor 1 receptor induces physiological heart growth via the phosphoinositide 3kinase(p110alpha) pathway., J. Biol. Chem 279 (2004) 4782–93. [DOI] [PubMed] [Google Scholar]
- [25].Rose BA, Force T, Wang Y, Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale., Physiol. Rev 90 (2010) 1507–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kohli S, Ahuja S, Rani V, Transcription factors in heart: promising therapeutic targets in cardiac hypertrophy., Curr Cardiol Rev. 7 (2011) 262–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lu J, McKinsey TA, Nicol RL, Olson EN, Signal-dependent activation of the MEF2 transcription factor by dissociation from histone deacetylases., Proc. Natl. Acad. Sci. U.S.A 97 (2000) 4070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rigor D, Bodyak N, Bae S, Choi J, Zhang L, Ter-Ovanesyan D, et al. , Phosphoinositide 3-kinase Akt signaling pathway interacts with protein kinase Cβ2 in the regulation of physiologic developmental hypertrophy and heart function, Am J Physiology - Hear Circulatory Physiology. 296 (2009) H566–H572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, et al. , Activated glycogen synthase-3 beta suppresses cardiac hypertrophy in vivo., Proc. Natl. Acad. Sci. U.S.A 99 (2002) 907–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Kang JH, Lee H-SS, Kang Y-WW, Cho K-HH, Systems biological approaches to the cardiac signaling network., Brief. Bioinformatics 17 (2016) 419–28. [DOI] [PubMed] [Google Scholar]
- [31].Kofron CM, Mende U, In vitro models of the cardiac microenvironment to study myocyte and non-myocyte crosstalk: bioinspired approaches beyond the polystyrene dish., J. Physiol. (Lond.) 595 (2017) 3891–3905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sadeghi AH, Shin SR, Deddens JC, Fratta G, Mandla S, Yazdi IK, et al. , Engineered 3D Cardiac Fibrotic Tissue to Study Fibrotic Remodeling., Adv Healthc Mater. 6 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Doetschman T, Georgieva T, Gene Editing With CRISPR/Cas9 RNA-Directed Nuclease., Circ. Res 120 (2017) 876–894. [DOI] [PubMed] [Google Scholar]
- [34].Doetschman T, Azhar M, Cardiac-specific inducible and conditional gene targeting in mice., Circ. Res 110 (2012) 1498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Matsui T, Li L, Wu JC, Cook SA, Nagoshi T, Picard MH, et al. , Phenotypic spectrum caused by transgenic overexpression of activated Akt in the heart., J. Biol. Chem 277 (2002) 22896–901. [DOI] [PubMed] [Google Scholar]
- [36].Condorelli G, Drusco A, Stassi G, Bellacosa A, Roncarati R, Iaccarino G, et al. , Akt induces enhanced myocardial contractility and cell size in vivo in transgenic mice., Proc. Natl. Acad. Sci. U.S.A 99 (2002) 12333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shioi T, McMullen JR, Kang PM, Douglas PS, Obata T, Franke TF, et al. , Akt/protein kinase B promotes organ growth in transgenic mice., Mol. Cell. Biol 22 (2002) 2799–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Shiojima I, Sato K, Izumiya Y, Schiekofer S, Ito M, Liao R, et al. , Disruption of coordinated cardiac hypertrophy and angiogenesis contributes to the transition to heart failure, Journal of Clinical Investigation. 115 (2005) 2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wang Y, Su B, Sah VP, Brown JH, Han J, Chien KR, Cardiac hypertrophy induced by mitogen-activated protein kinase kinase 7, a specific activator for c-Jun NH2-terminal kinase in ventricular muscle cells., J. Biol. Chem 273 (1998) 5423–6. [DOI] [PubMed] [Google Scholar]
- [40].Bernardo BC, Weeks KL, Pretorius L, McMullen JR, Molecular distinction between physiological and pathological cardiac hypertrophy: experimental findings and therapeutic strategies., Pharmacol. Ther 128 (2010) 191–227. [DOI] [PubMed] [Google Scholar]
- [41].Maillet M, van Berlo JH, Molkentin JD, Molecular basis of physiological heart growth: fundamental concepts and new players., Nat. Rev. Mol. Cell Biol 14 (2013) 38–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, et al. , The conserved phosphoinositide 3-kinase pathway determines heart size in mice., EMBO J. 19 (2000) 2537–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Periasamy M, Bhupathy P, Babu GJ, Regulation of sarcoplasmic reticulum Ca2+ ATPase pump expression and its relevance to cardiac muscle physiology and pathology., Cardiovasc. Res 77 (2008) 265–73. [DOI] [PubMed] [Google Scholar]
- [44].Arai Matsui, Periasamy, Sarcoplasmic reticulum gene expression in cardiac hypertrophy and heart failure., Circulation Research. 74 (1994) 555–564. [DOI] [PubMed] [Google Scholar]
- [45].van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN, Control of stressdependent cardiac growth and gene expression by a microRNA., Science. 316 (2007) 575–9. [DOI] [PubMed] [Google Scholar]
- [46].Cotecchia S, Exum S, Caron M, Lefkowitz R, Regions of the alpha 1-adrenergic receptor involved in coupling to phosphatidylinositol hydrolysis and enhanced sensitivity of biological function., Proceedings of the National Academy of Sciences. 87 (1990) 2896–2900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Cotecchia S, Schwinn DA, Randall RR, Lefkowitz RJ, Caron MG, Kobilka BK, Molecular cloning and expression of the cDNA for the hamster alpha 1-adrenergic receptor., Proc. Natl. Acad. Sci. U.S.A 85 (1988) 7159–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Milano CA, Dolber PC, Rockman HA, Bond RA, Venable ME, Allen LF, et al. , Myocardial expression of a constitutively active alpha 1B-adrenergic receptor in transgenic mice induces cardiac hypertrophy., Proc. Natl. Acad. Sci. U.S.A 91 (1994) 10109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gao MH, Lai NC, Roth DM, Zhou J, Zhu J, Anzai T, et al. , Adenylylcyclase increases responsiveness to catecholamine stimulation in transgenic mice., Circulation. 99 (1999) 1618–22. [DOI] [PubMed] [Google Scholar]
- [50].Yu Z, Redfern CS, Fishman GI, Conditional transgene expression in the heart., Circ. Res 79 (1996) 691–7. [DOI] [PubMed] [Google Scholar]
- [51].Hein L, Stevens ME, Barsh GS, Pratt RE, Kobilka BK, Dzau VJ, Overexpression of angiotensin AT1 receptor transgene in the mouse myocardium produces a lethal phenotype associated with myocyte hyperplasia and heart block., Proc. Natl. Acad. Sci. U.S.A 94 (1997) 6391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Paradis P, Dali-Youcef N, Paradis F, Thibault G, Nemer M, Overexpression of angiotensin II type I receptor in cardiomyocytes induces cardiac hypertrophy and remodeling, Proceedings of the National Academy of Sciences. 97 (2000) 931–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Engelhardt S, Hein L, Wiesmann F, Lohse MJ, Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice., Proc. Natl. Acad. Sci. U.S.A 96 (1999) 7059–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Engelhardt S, Hein L, Dyachenkow V, Kranias E, Isenberg G, Lohse M, Altered Calcium Handling Is Critically Involved in the Cardiotoxic Effects of Chronic β-Adrenergic Stimulation, Circulation. 109 (2004) 1154–1160. [DOI] [PubMed] [Google Scholar]
- [55].Bisognano J, Weinberger H, Bohlmeyer T, Pende A, Raynolds M, Sastravaha A, et al. , Myocardial-Directed Overexpression of the Human β1-Adrenergic Receptor in Transgenic Mice, Journal of Molecular and Cellular Cardiology. 32 (2000) 817830. [DOI] [PubMed] [Google Scholar]
- [56].Liggett SB, Tepe NM, Lorenz JN, Canning AM, Jantz TD, Mitarai S, et al. , Early and delayed consequences of beta(2)-adrenergic receptor overexpression in mouse hearts: critical role for expression level., Circulation. 101 (2000) 1707–14. [DOI] [PubMed] [Google Scholar]
- [57].Epstein PN, Overbeek PA, Means AR, Calmodulin-induced early-onset diabetes in transgenic mice., Cell. 58 (1989) 1067–73. [DOI] [PubMed] [Google Scholar]
- [58].Field LJ, Atrial natriuretic factor-SV40 T antigen transgenes produce tumors and cardiac arrhythmias in mice., Science. 239 (1988) 1029–33. [DOI] [PubMed] [Google Scholar]
- [59].Gruver CL, DeMayo F, Goldstein MA, Means AR, Targeted developmental overexpression of calmodulin induces proliferative and hypertrophic growth of cardiomyocytes in transgenic mice., Endocrinology. 133 (1993) 376–88. [DOI] [PubMed] [Google Scholar]
- [60].Zhang T, Johnson E, Gu Y, Morissette M, Sah V, Gigena M, et al. , The Cardiac-specific Nuclear δB Isoform of Ca2+/Calmodulin-dependent Protein Kinase II Induces Hypertrophy and Dilated Cardiomyopathy Associated with Increased Protein Phosphatase 2A Activity, Journal of Biological Chemistry. 277 (2002) 1261–1267. [DOI] [PubMed] [Google Scholar]
- [61].Zhang T, Maier L, Dalton N, Miyamoto S, Ross J, Bers D, et al. , The δC Isoform of CaMKII Is Activated in Cardiac Hypertrophy and Induces Dilated Cardiomyopathy and Heart Failure, Circulation Research. 92 (2003) 912–919. [DOI] [PubMed] [Google Scholar]
- [62].O’Keefe SJ, Tamura J, Kincaid RL, Tocci MJ, O’Neill EA, FK-506- and CsA-sensitive activation of the interleukin-2 promoter by calcineurin., Nature. 357 (1992) 692–4. [DOI] [PubMed] [Google Scholar]
- [63].Penuel E, Akita R, Sliwkowski M, Identification of a Region within the ErbB2/HER2 Intracellular Domain That Is Necessary for Ligand-independent Association, Journal of Biological Chemistry. 277 (2002) 28468–28473. [DOI] [PubMed] [Google Scholar]
- [64].Sysa-Shah P, Xu Y, Guo X, Belmonte F, Kang B, Bedja D, et al. , Cardiac-Specific OverExpression of Epidermal Growth Factor Receptor 2 (ErbB2) Induces Pro-Survival Pathways and Hypertrophic Cardiomyopathy in Mice, PLoS ONE. 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].D’Uva G, Aharonov A, Lauriola M, Kain D, Yahalom-Ronen Y, Carvalho S, et al. , ERBB2 triggers mammalian heart regeneration by promoting cardiomyocyte dedifferentiation and proliferation., Nat. Cell Biol 17 (2015) 627–38. [DOI] [PubMed] [Google Scholar]
- [66].Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, et al. , Overexpression of Gs alpha protein in the hearts of transgenic mice., J. Clin. Invest 95 (1995) 1676–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Iwase M, Bishop SP, Uechi M, Vatner DE, Shannon RP, Kudej RK, et al. , Adverse effects of chronic endogenous sympathetic drive induced by cardiac GS alpha overexpression., Circ. Res 78 (1996) 517–24. [DOI] [PubMed] [Google Scholar]
- [68].Iwase M, Uechi M, Vatner DE, Asai K, Shannon RP, Kudej RK, et al. , Cardiomyopathy induced by cardiac Gs alpha overexpression., Am. J. Physiol 272 (1997) H585–9. [DOI] [PubMed] [Google Scholar]
- [69].Valencik ML, McDonald JA, Cardiac expression of a gain-of-function alpha(5)-integrin results in perinatal lethality., Am. J. Physiol. Heart Circ. Physiol 280 (2001) H361–7. [DOI] [PubMed] [Google Scholar]
- [70].Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD, Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice., J. Biol. Chem 281 (2006) 9152–62. [DOI] [PubMed] [Google Scholar]
- [71].Kim Y, Phan D, van Rooij E, Wang D-ZZ, McAnally J, Qi X, et al. , The MEF2D transcription factor mediates stress-dependent cardiac remodeling in mice., J. Clin. Invest 118 (2008) 124–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Cowley S, Paterson H, Kemp P, Marshall CJ, Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells., Cell. 77 (1994) 841–52. [DOI] [PubMed] [Google Scholar]
- [73].Bueno OF, De Windt LJ, Tymitz KM, Witt SA, Kimball TR, Klevitsky R, et al. , The MEK1ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice., EMBO J. 19 (2000) 6341–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT, Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase., Science. 268 (1995) 100–2. [DOI] [PubMed] [Google Scholar]
- [75].Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, et al. , Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a., Circ. Res 89 (2001) 997–1004. [DOI] [PubMed] [Google Scholar]
- [76].Stebbins EG, Mochly-Rosen D, Binding specificity for RACK1 resides in the V5 region of beta II protein kinase C., The Journal of Biological Chemistry. 276 (2001) 29644–50. [DOI] [PubMed] [Google Scholar]
- [77].Braz JC, Gregory K, Pathak A, Zhao W, Sahin B, Klevitsky R, et al. , PKC-alpha regulates cardiac contractility and propensity toward heart failure., Nat. Med 10 (2004) 248–54. [DOI] [PubMed] [Google Scholar]
- [78].Hahn H, Marreez Y, Odley A, Sterbling A, Yussman M, Hilty C, et al. , Protein Kinase Cα Negatively Regulates Systolic and Diastolic Function in Pathological Hypertrophy, Circulation Research. 93 (2003) 1111–1119. [DOI] [PubMed] [Google Scholar]
- [79].Bowman JC, Steinberg SF, Jiang T, Geenen DL, Fishman GI, Buttrick PM, Expression of protein kinase C beta in the heart causes hypertrophy in adult mice and sudden death in neonates., J. Clin. Invest 100 (1997) 2189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Wakasaki H, Koya D, Schoen FJ, Jirousek MR, Ways DK, Hoit BD, et al. , Targeted overexpression of protein kinase C beta2 isoform in myocardium causes cardiomyopathy., Proc. Natl. Acad. Sci. U.S.A 94 (1997) 9320–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Takeishi Y, Ping P, Bolli R, Kirkpatrick DL, Hoit BD, Walsh RA, Transgenic overexpression of constitutively active protein kinase C epsilon causes concentric cardiac hypertrophy., Circ. Res 86 (2000) 1218–23. [DOI] [PubMed] [Google Scholar]
- [82].Diekmann D, Brill S, Garrett MD, Totty N, Hsuan J, Monfries C, et al. , Bcr encodes a GTPaseactivating protein for p21rac., Nature. 351 (1991) 400–2. [DOI] [PubMed] [Google Scholar]
- [83].Sussman MA, Welch S, Walker A, Klevitsky R, Hewett TE, Price RL, et al. , Altered focal adhesion regulation correlates with cardiomyopathy in mice expressing constitutively active rac1., J. Clin. Invest 105 (2000) 875–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Hunter JJ, Tanaka N, Rockman HA, Ross J, Chien KR, Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice., J. Biol. Chem 270 (1995) 23173–8. [DOI] [PubMed] [Google Scholar]
- [85].Gottshall KR, Hunter JJ, Tanaka N, Dalton N, Becker KD, Ross J, et al. , Ras-dependent pathways induce obstructive hypertrophy in echo-selected transgenic mice., Proc. Natl. Acad. Sci. U.S.A 94 (1997) 4710–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Zheng M, Dilly K, Dos Santos Cruz J, Li M, Gu Y, Ursitti JA, et al. , Sarcoplasmic reticulum calcium defect in Ras-induced hypertrophic cardiomyopathy heart., Am. J. Physiol. Heart Circ. Physiol 286 (2004) H424–33. [DOI] [PubMed] [Google Scholar]
- [87].Sah V, Minamisawa S, Tam S, Wu T, Dorn G, Ross J, et al. , Cardiac-specific overexpression of RhoA results in sinus and atrioventricular nodal dysfunction and contractile failure, Journal of Clinical Investigation. 103 (1999) 1627–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Kunisada K, Negoro S, Tone E, Funamoto M, Osugi T, Yamada S, et al. , Signal transducer and activator of transcription 3 in the heart transduces not only a hypertrophic signal but a protective signal against doxorubicin-induced cardiomyopathy, Proceedings of the National Academy of Sciences. 97 (2000) 315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, et al. , TAK1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice., Nat. Med 6 (2000) 556–63. [DOI] [PubMed] [Google Scholar]
- [90].Kubota T, McTiernan CF, Frye CS, Slawson SE, Lemster BH, Koretsky AP, et al. , Dilated cardiomyopathy in transgenic mice with cardiac-specific overexpression of tumor necrosis factor-alpha., Circ. Res 81 (1997) 627–35. [DOI] [PubMed] [Google Scholar]
- [91].Kubota T, Bounoutas GS, Miyagishima M, Kadokami T, Sanders VJ, Bruton C, et al. , Soluble tumor necrosis factor receptor abrogates myocardial inflammation but not hypertrophy in cytokine-induced cardiomyopathy., Circulation. 101 (2000) 2518–25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.