Abstract
Background
Group 2 innate lymphoid cells (ILC2s) were closely associated with asthma. However, there were no perspective studies about the effects of glucocorticoid on ILC2s in asthma patients. Our objective was to perform a perspective study and evaluate the ILC2 activity after glucocorticoid therapy in asthma patients.
Methods
The asthma and asthma with allergic rhinitis patients were treated with glucocorticoid for 3 months. The circulating ILC2 levels were evaluated. The effects of glucocorticoid on ILC2s and possible signalling pathways were investigated in vitro.
Results
The patients were well‐controlled, and the high ILC2 levels were significantly decreased at 1 and 3 months after treatment. Peripheral blood monocytes from allergic patients produced dramatic IL‐5, IL‐13 and IL‐9 in response to IL‐25, IL‐33 plus IL‐2, and glucocorticoid significantly decreased their levels. Moreover, ILC2s were identified to be the predominant source of IL‐5, IL‐13 and IL‐9, and glucocorticoid treatment was able to reverse their high levels. STAT3, STAT5, STAT6, JAK3 and MEK signalling pathways were proved to be involved in regulating ILC2 activity under the glucocorticoid treatment.
Conclusion
The data suggested that glucocorticoid administration could be effective in treating asthma by regulating ILC2s via MEK/JAK‐STAT signalling pathways. This provides a new understanding of glucocorticoid application in regard to allergic diseases.
Keywords: asthma patient, glucocorticoid, group 2 innate lymphoid cells, inhibition, STAT signalling pathway
1. INTRODUCTION
Allergic airway inflammatory diseases, including asthma and allergic rhinitis (AR), represent a serious global health problem in many counties.1, 2 Type 2‐associated cytokines are essential for driving the pathology of bronchial asthma and AR. It is traditionally believed that CD4+ type 2 helper T (Th2) cells are the principal drivers of type 2 inflammatory conditions.3, 4, 5 However, recent studies have suggested that group 2 innate lymphoid cells (ILC2s) seemed to play a more critical role in allergic diseases in innate immune responses.6, 7, 8 ILC2s produced dramatic amounts of IL‐5, IL‐13, some IL‐4 and IL‐9 in response to the Th2 cell‐stimulating cytokines IL‐25, IL‐33 and thymic stromal lymphopoietin (TSLP) produced by epithelial cells.9, 10, 11
It was identified that ILC2s mediated airway hyper‐reactivity or asthmatic inflammation induced by influenza,7 protease,12, 13 ryegrass14 or mite15 in adaptive immunodeficiency mice. IL‐25 and IL‐33 mediating airway hyperactivity mostly depended on ILC2s but not the adaptive T cells in mouse.15, 16, 17 In addition, ILC2s were also found to be increased in several allergic immune diseases in human such as atopic dermatitis,18, 19 active eosinophilic esophagitis20 and chronic rhinosinusitis with nasal polyps or eosinophilia.21, 22 Importantly, high ILC2 levels were found in asthma patients23 and persistent airway eosinophilia,24 or rhinovirus‐induced asthma exacerbations25 and AR patients.26, 27, 28
Only a few studies have reported the effects of medicine treatments on ILC2 levels. ILC2 levels in the lung were decreased after corticosteroid treatment in mouse allergic airway inflammation.29 Patients with nasal polyps treated with steroids had significantly reduced ILC2s in nasal polyp tissue as compared to those in patients who did not receive steroid treatment.24 Corticosteroids are the most effective treatment to reduce the airway inflammation and symptoms in asthma and AR patients.1, 30 The predominant role of ILC2s in asthma pathology indicates the strong possibility of corticosteroid on ILC2 function. Until now only one cross‐sectional study has shown that there were high frequencies of IL‐13+ ILC2s in uncontrolled and partly controlled asthma patients than in those in the well‐controlled group.31 Therefore, studies are needed to investigate the effects of corticosteroid therapy on ILC2 levels and their activity in asthma and/or AR patients. Additionally, the signalling pathways involved in the effects of corticosteroid treatment on ILC2s are still unclear.
The aim of this study was to perform a prospective study to evaluate the levels and the function of ILC2s, as well as the relationship with the pulmonary function after glucocorticoid therapy in asthma and asthma with AR patients. In vitro studies were used to investigate the possible signalling pathways involved in the effects of glucocorticoid on ILC2 in allergic airway diseases.
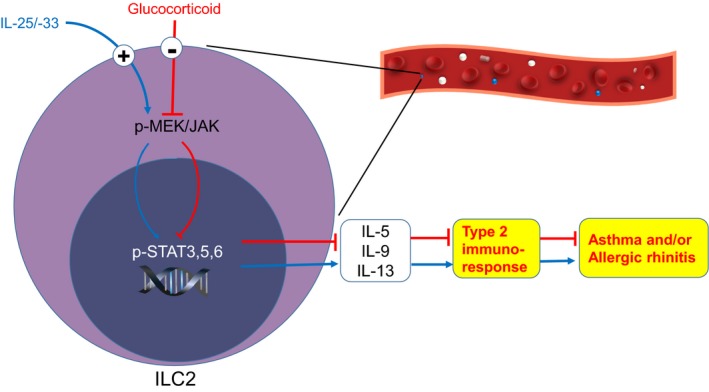
High circulating ILC2s were found in asthma and asthma with allergic rhinitis patients, and significantly decreased after treatment of glucocorticoid.
High levels of IL‐5, IL‐13 and IL‐9 in response to epithelium‐derived cytokines were mostly produced by the increased ILC2s from asthma patients.
Glucocorticoid treatment is able to reverse the high levels of IL‐5 and IL‐13 produced by ILC2s via STAT3, STAT5 and STAT6 signalling pathways.
2. METHODS
Detailed description of all experimental procedures is provided in the Data S1.
2.1. Study subjects
The study was approved by the Ethics Committee of The First Affiliated Hospital, Sun Yat‐sen University. Written informed consents were obtained from a total of 83 subjects (age ≥18 years and ≤65 years), including 32 asthma patients (15 for follow‐up, 7 for glucocorticoid experiments in vitro, 6 for testosterone and prostaglandin [PG] E2 experiments in vitro, and 4 for cell sorting), 27 asthma with AR patients (15 for follow‐up, 6 for glucocorticoid experiments in vitro and 6 for testosterone and PGE2 experiments in vitro) and 24 healthy controls (13 for follow‐up, 5 for glucocorticoid experiments in vitro and 6 for testosterone and PGE2 experiments in vitro). The inclusion and exclusion criterion are presented in Data S1.
2.2. Research design and patient treatment
The detailed research design and patient treatment are described in Figure 1 and Data S1.
Figure 1.
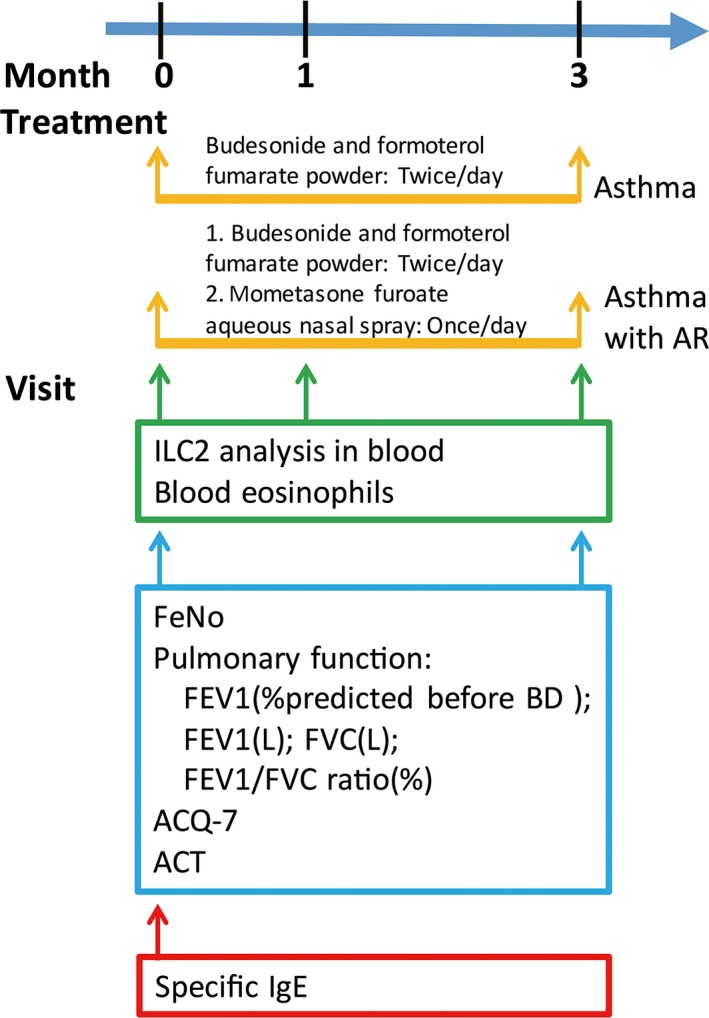
The detailed study design of this study. The patients were evaluated for pulmonary function, FeNO, routine blood test, ILC2 levels and specific IgE at the first visit (0 month), and were treated with glucocorticoid. They were followed up for routine blood test and ILC2 evaluation after 1 month. Three months later, they were evaluated for pulmonary function, FeNO, and routine blood test and ILC2 evaluation again. ACQ‐7, seven‐item asthma control questionnaire; ACT, asthma control test; AR, allergic rhinitis; FeNO, fractional exhaled nitric oxide; FEV 1, forced expiratory volume in 1 second [Colour figure can be viewed at http://wileyonlinelibrary.com]
2.3. Peripheral blood collection and flow cytometry analysis for ILC2s
Peripheral blood from the patients was collected for ILC2 examination using flow cytometry. The plasma was used for the determination of IL‐13, IL‐5 and IL‐9.
2.4. ILC2 sorting
Briefly, peripheral blood mononuclear cells (PBMCs) were stained with FITC‐Lin+ antibodies and FITC‐FceR1, and then, the Lin− cells were negatively isolated. The isolated Lin− cells were further stained with PE‐CRTH2 and PE‐Cy7‐CD127 and then sorted into 3 populations.
2.5. The stimulation and treatment of PBMCs, Lin‐ cells and ILC2s
A detailed method is provided in Data S1.
2.6. Western blot
Proteins from sorted ILC2s were separated by SDS‐PAGE and transferred to polyvinylidene difluoride membranes. The membrane was blotted with 10% nonfat milk, probed with the primary antibodies, incubated with secondary antibodies and then visualized by Enhanced Chemiluminescence Plus.
2.7. Statistical analyses
SPSS10.0 software was used for data analysis. The data were presented as mean ± SEM. Statistical analyses were performed using paired or unpaired nonparametric tests (Mann‐Whitney test, Kruskal‐Wallis test, Dunn's multiple comparison test and Spearman rank correlation). P value less than .05 was considered statistically significant.
3. RESULTS
3.1. Enhanced ILC2 levels in the patient peripheral blood
The detailed research design and patient treatment are described in Figure 1. Firstly, we evaluated the ILC2 levels in blood. Human ILC2s were defined as Lin−FceR1−CRTH2+CD127+ cells (Figure 2). We observed that there were the clear dot plot clusters of ILC2s in asthma and asthma with AR patients but not in healthy controls (Figure 2A). ILC2 frequencies were significantly increased in asthma patients (P < .05) and asthma with AR patients (P < .01) compared with that in healthy controls (Figure 2B).
Figure 2.
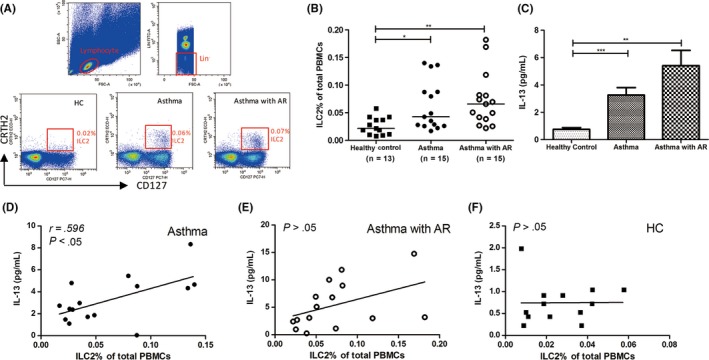
High ILC2 and IL‐13 levels in asthma and asthma with AR patients. (A) ILC2s were identified as Lin−FceR1− CRTH2+ CD127+ lymphocytes. ILC2 (B) and IL‐13 (C) levels in asthma patients (n = 15), asthma with rhinitis patients (n = 15) and healthy controls (n = 13). IL‐13 levels in the plasma were correlated with ILC2 levels in asthma patients (D) but not asthma with AR patients (E) or healthy control (F). *P < .05, **P < .01, ***P < .001. AR, allergic rhinitis
There were higher levels of IL‐13 in the plasma from asthma patients (P < .001) and asthma with AR patients (P < .01) compared with that from healthy controls (Figure 2C). A significant positive correlation was found between the ILC2 percentages and IL‐13 levels in asthma patients (P < .05; Figure 2D) but not in asthma with AR patients (Figure 2E) and healthy controls (Figure 2F). No IL‐5 was detected in the plasma from all subjects. No significant difference for the levels of IL‐4 and IL‐9 was found between the patients and healthy controls (Figure S1). These findings suggest that ILC2 frequencies were enhanced in both asthma patients and asthma with AR patients.
3.2. Glucocorticoid treatment led to better control in patients
Baseline characteristics of the subjects were listed in Table 1. The 1‐ and 3‐month follow‐ups were completed by 13 and 10 asthma patients, and 15 and 9 asthma with AR patients, respectively (Table 2). Five asthma patients and 6 asthma with AR patients were missed for a follow‐up examination at 3 months. However, there was no difference in age, gender or clinical characteristics between the patients in 3‐month follow‐up examinations, and then, all patients included at 0 months were included for statistical analysis.
Table 1.
Baseline characteristics of the participants’ demographics, pulmonary function, sIgE and blood eosinophils involved in this study
| Characteristic | HC | Asthma | Asthma with AR | P value |
|---|---|---|---|---|
| No. of patients | 13 | 15 | 15 | |
| Age (y) | 35.2 ± 2.2 | 35.6 ± 3.4 | 35.5 ± 3.6 | .99 |
| Gender, female/male | 9/4 | 11/4 | 11/4 | .97 |
| Ever smoked (%) | 0 | 0 | 6.7 | .33 |
| Specific IgE positive/subjects tested (%) | 0 | 20 | 100 | .07 |
| Blood eosinophils (%) | 1.58 ± 0.27 | 4.98 ± 1.01 | 6.77 ± 1.24 | <.05b, <.001c |
| FEV1 (%predicted before BD) | 107.6 ± 1.76 | 85.27 ± 6.23 | 78.59 ± 5.58 | <.01b, <.001c |
| FEV1/FVC ratio (%) | 93.32 ± 1.82 | 76.62 ± 3.78 | 71.39 ± 3.02 | <.01b, <.001c |
| FeNO (ppb) | 16.15 ± 1.99 | 44.40 ± 8.18 | 92.86 ± 11.96 | <.01a , b, <.001c |
| ACQ‐7 | – | 1.57 ± 0.25 | 1.53 ± 0.23 | .91 |
| ACT | – | 16.67 ± 0.99 | 16.07 ± 1.13 | .69 |
ACQ‐7, seven‐item asthma control questionnaire; ACT, asthma control test; AR, allergic rhinitis; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; HC, healthy control; sIgE, specific immunoglobulin E.
Asthma with AR vs asthma
HC vs asthma
HC vs asthma with AR.
Table 2.
Comparison of the subjects’ demographics, pulmonary function and blood eosinophils between first‐visit patients and patients after 3‐month glucocorticoid treatment
| Characteristic | Asthma | P value | Asthma with AR | P value | ||
|---|---|---|---|---|---|---|
| First visit | 3 month | First visit | 3 month | |||
| No. of patients | 10 | 9 | ||||
| Age (y) | 38 ± 4.72 | 32.33 ± 4.98 | .37 | |||
| Sex, female/male | 8/2 | 6/3 | .56 | |||
| Blood eosinophils (%) | 6.17 ± 1.27 | 6.68 ± 1.80 | .82 | 6.26 ± 1.50 | 2.67 ± 1.00 | .08 |
| FEV1 (%predicted before BD) | 84.37 ± 7.75 | 97.02 ± 5.87 | .21 | 72.90 ± 7.27 | 98.81 ± 3.21 | .005 |
| FEV1 (L) | 2.21 ± 0.21 | 2.53 ± 0.14 | .22 | 2.08 ± 0.22 | 2.87 ± 0.21 | .02 |
| FVC (L) | 2.99 ± 0.16 | 3.13 ± 0.19 | .23 | 2.99 ± 0.21 | 3.57 ± 0.28 | .11 |
| FEV1/FVC ratio (%) | 76.07 ± 5.42 | 77.55 ± 4.04 | .23 | 70.28 ± 4.01 | 82.96 ± 3.89 | .04 |
| FeNO (ppb) | 47.40 ± 10.17 | 33.50 ± 6.54 | .29 | 97.38 ± 17.54 | 34.63 ± 10.22 | .008 |
| ACQ‐7 | 1.76 ± 0.31 | 0.51 ± 0.21 | .006 | 1.62 ± 0.31 | 0.51 ± 0.24 | .01 |
| ACT | 16.30 ± 1.42 | 23.40 ± 0.72 | .004 | 15.89 ± 1.62 | 22.67 ± 0.98 | .04 |
ACQ‐7, seven‐item asthma control questionnaire; ACT, asthma control test; AR, allergic rhinitis; FeNO, fractional exhaled nitric oxide; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity.
Pulmonary functions were significantly improved in both patient groups including increasing predicted forced expiratory volume in 1 second (FEV1), FEV1/forced vital capacity (FVC) ratio, decreasing fractional exhaled nitric oxide (FeNO) levels, lower seven‐item Asthma Control Questionnaire (ACQ‐7) and higher Asthma Control Test (ACT) values (Table 2). Taken together, glucocorticoid treatment leads to the better control of asthma and asthma with AR patients.
3.3. Glucocorticoid treatment decreased the ILC2 level and function in asthma and asthma with AR patients
After 1‐month glucocorticoid treatment, the ILC2 percentages were significantly decreased in asthma patients (P < .05, n = 13) and asthma with AR patients (P < .01, n = 15) compared with those of the first visit (Figure S2, Figure 3A). The ILC2 percentages were further significantly decreased at 3 months in asthma (n = 10) and asthma with AR patients (n = 9) (Figure 3A, P < .01 or P < .05). The ILC2 levels in both asthma patients and asthma with AR patients after 3‐month treatment were similar to that in healthy controls (Figure 3B, P > .05).
Figure 3.
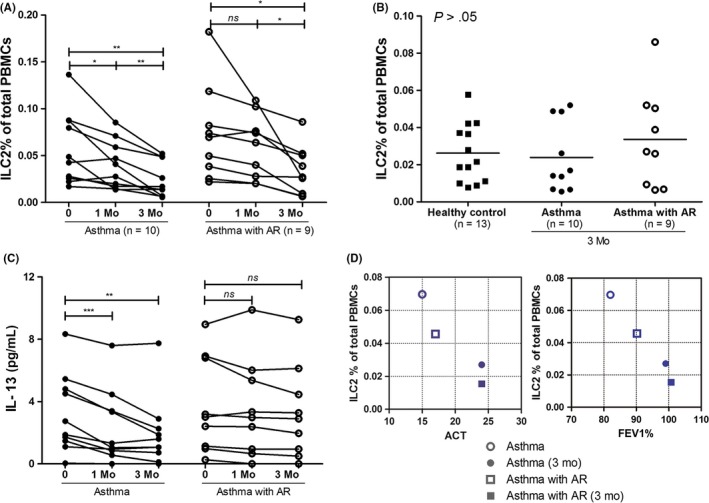
The treatment of glucocorticoids significantly decreases ILC2 levels in asthma and asthma with AR patients. (A) ILC2 levels in PBMCs of asthma patients, asthma with AR patients at first visit, 1 and 3 months follow‐up time. (B) ILC2 levels in PBMCs of asthma patients, asthma with AR patients compared to healthy controls after 3‐month treatment. (C) IL‐13 levels in the plasma of the patients after the treatment. (D) The relationship between ILC2 levels and FEV1% or ACT after the treatment. The coordinative points represent the mean values of FEV 1 (X) or ACT (X) and ILC2s (Y) for the same group of patients. *P < .05, **P < .01, ***P < .001. ACT, asthma control test; AR, allergic rhinitis; FEV 1, forced expiratory volume in 1 second; HC, healthy control; Mo, month. [Colour figure can be viewed at http://wileyonlinelibrary.com]
IL‐13 levels in the patient's plasma were also significantly decreased after the treatment (P < .001), and there was a significant difference of IL‐13 levels in asthma patients but not asthma with AR patients between 1‐ or 3‐month treatments and 0‐month (P < .01, Figure 3C).
After 3‐month treatment, the patients showed the decreased ILC2 frequency but increased FEV1 and ACT levels. The increased predicted FEV1 and ACT had relationships with the decreased ILC2 percentages in both asthma and asthma with AR patient groups after the glucocorticoid treatment (Figure 3D), which indicates that the patient recovery was accompanied by the decreased ILC2 levels after glucocorticoid treatment.
3.4. The glucocorticoid effects on Th2 cytokine production in PBMCs from the patients in response to epithelial cytokines
Without stimulation of IL‐25, IL‐33 plus IL‐2, the PBMCs from both groups of patients exhibited higher levels of IL‐5 (P < .01), IL‐13 (P < .01) but not IL‐9 compared with those from the healthy control group (Figure 4A‐C). Under the stimulation, there were dramatic increases of IL‐5 and IL‐13 levels in cultured PBMCs from both patients and healthy groups, and with higher IL‐5 in the patients (Figure 4A,B). We found increased IL‐9 levels in both types of patients but not control subjects in response to the epithelial cytokines (Figure 4C). The responses of IL‐9 to IL‐25/‐33/‐2 were much lower compared to those of IL‐5 and IL‐13. No significant difference was found for IL‐4 levels after the treatment of IL‐25, IL‐33 plus IL‐2 by PBMCs for 3 groups of subjects (Figure S3). Moreover, budesonide administration dramatically decreased the levels of IL‐5 (from 47 296 to 533 pg/mL, P < .01) and IL‐13 (from 4599 to 113 pg/mL, P < .05) (Figure 4D) in asthma patients under both situations of with and without stimulation. High IL‐9 was also decreased with budesonide treatment (Figure 4E, P < .01). We further identified that budesonide administration significantly decreased the levels of IL‐13+ILC2s (Figure 4F) after the stimulation for PBMCs from asthma patients. However, we did not observe any difference of the ILC2 percentages with the stimulation or the budesonide treatment using flow cytometry (data not shown). These results suggest that glucocorticoid may mostly affect the ILC2 function but not their frequencies.
Figure 4.
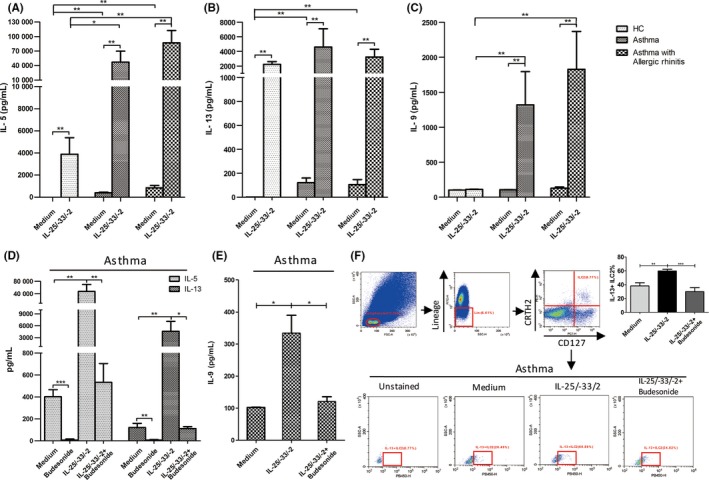
Budesonide significantly decreases the large quantity of IL‐5, IL‐13 and IL‐9 produced by PBMCs from asthma and asthma with AR patients in response to IL‐25 and IL‐33. PBMCs from asthma, asthma with AR patients or healthy controls were treated with budesonide under the stimulation of IL‐2, IL‐25 and IL‐33 for 5 days. IL‐5 (A), IL‐13 (B) and IL‐9 (C) levels measured using ELISA. IL‐5, IL‐13 (D) and IL‐9 (E) levels with budesonide treatment were determined with ELISA. (F) IL‐13+ ILC2s levels determined with flow cytometry from asthma patients. The ILC2 cells were gated in Lin− CRTH2+ CD127+ cells. *P < .05, **P < .01, ***P < .001
Testosterone was reported to negatively regulate ILC2 proliferation and cytokine expression as well as ILC2‐mediated allergic airway inflammation.32 PGE2 suppresses IL‐5 and IL‐13 production in human tonsillar ILC2s.33 We examined the effects of testosterone and PGE2 on ILC2 function (Figure S4). Testosterone and PGE2 partly and significantly decreased the levels of IL‐5 and IL‐13 by PBMCs for patients in response to IL‐25/‐33/‐2 (P < .05). The levels of IL‐9 were decreased with the treatment of testosterone and PGE2 in asthma patients (P < .05) but not the other 2 groups. Additionally, the levels of IL‐5 and IL‐13 were decreased for healthy controls with the treatment of testosterone and PGE2 (Figure S4). It suggests that testosterone and PGE2 have some ability of inhibiting ILC2s to produce of IL‐5, IL‐13 and IL‐9, but weaker than glucocorticoid.
3.5. ILC2s were predominantly responsible for the robust production of IL‐5, IL‐13 and IL‐9, and glucocorticoid treatment
PBMCs from asthma patients were sorted to 4 subgroups using flow cytometry: ILC2s (Group 1), Lin+ PBMCs (Group 2), Lin− CD127+ CRTH2− cells (Group 3) and Lin− CD127 −CRTH2− cells (Group 4) as shown in Figure 5A. ILC2s were identified to exhibit typical morphology of general lymphocytes and became bigger under IL‐25 and IL‐33 plus IL‐2 stimulation (Figure 5B). After 3 days of stimulation with IL‐25, IL‐33 plus IL‐2, the IL‐5 levels in ILC2s (Group 1) (97% in PBMCs) were climbed up to 317 577 pg/mL and were about 50‐folds to those (6548 pg/mL) in Lin+ PBMCs (Group 2) (Figure 5C). There were also greater IL‐13 and IL‐9 levels in ILC2 group compared with those in Lin+ PBMCs. It suggests that ILC2s were predominantly responsible for the robust production of IL‐5, IL‐13 and even IL‐9. The stimulated high levels of IL‐5, IL‐13 and IL‐9 in the sorted ILC2s from asthma patients were dramatically reversed with the treatment of budesonide (Figure 5D).
Figure 5.

ILC2s are predominantly responsible for the robust production of IL‐5, IL‐13 and IL‐9, and the decrease in glucocorticoid on them. Four subsets of ILC2s (group 1), Lin+ cells (group 2), Lin− CRTH2− CD127− cells (group 3), Lin− CRTH2− CD127+ cells (group 4) from PBMCs of asthma patients were sorted using FACSAria flow cytometer. (A) The 4 subsets showed under FACSCalibur flow cytometer. (B) Haematoxylin‐eosin staining for general lymphocytes, the sorted ILC2s from asthma patients, or ILC2s stimulated by IL‐25, IL‐33 plus IL‐2. Scale bar = 10 μm. IL‐5, IL‐13 and IL‐9 levels in the sorted 4 subsets (C) or in the sorted ILC2s (D) with budesonide treatment from asthma patients after stimulation. *P < .05, **P < .01 compared to ILC2 group in (c)
Using flow cytometry, we found that glucocorticoid receptor (GR) was expressed in ILC2s, and there was no change after the stimulation of epithelial cytokines for 5 days (Figure S5).
3.6. p‐STAT3, p‐STAT5 and p‐STAT6 were involved in the production of IL‐5 and IL‐13 in ILC2 cells and the effects of glucocorticoid treatment
The Lin− cells which were sorted from the buffy coats from healthy volunteers were used for the evaluation of the phosphorylation of STAT3, STAT5 and STAT6 at the different time points. The phosphorylation of STAT3, STAT5 and STAT6 in Lin− cells under stimulation markedly increased after 6 hours and reached the maximum levels in 12 hours (Figure 6A). Next, we used the isolated ILC2s to examine the effects of budesonide on the above signalling pathways. Budesonide administration almost completely reversed the levels of p‐STAT3, p‐STAT5 and p‐STAT6 back to normal in the activated ILC2s (Figure 6B). Budesonide also decreased the high levels of total STAT3, STAT5 and STAT6 (Figure S6). Both S3I‐201 (STAT3 inhibitor) and IQDMA (STAT5 inhibitor) significantly reversed the high levels of IL‐5 and IL‐13, and AS1517499 (STAT6 inhibitor) reversed the high IL‐5 but not IL‐13 levels in ILC2s (Figure 6C). We also investigated the possible upstream signalling pathways. We found that budesonide significantly inhibited the levels of p‐JAK3 (Figure 6B) and total JAK3 (Figure S6), upstream regulator of STAT3, STAT5 and STAT6 in the ILC2s. Moreover, JAK3 inhibitor JANEX‐1 significantly inhibited the levels of p‐STAT3, p‐STAT5 and p‐STAT6 (Figure 6B), and reversed the high levels of IL‐5 and IL‐13 (Figure 6C). Mitogen‐activated protein kinase kinase (MEK) phosphorylates and activates extracellular signal‐regulated kinases (ERKs), and further phosphorylates and activates STAT3.34 We found that budesonide significantly decreased p‐MEK1/2 to a certain degree (Figure 6B) but not total MEK1/2 (Figure S6) under the stimulation. Moreover, MEK inhibitor, U0126, decreased the high levels of p‐STAT3, p‐STAT5 and p‐STAT6, and partly decreased p‐JAK3 levels. We also observed the reversion of MEK inhibitor on the production of IL‐5 and IL‐13 (Figure 6C). These findings suggest that budesonide inhibits ILC2 function in IL‐5 and IL‐13 production via MEK/JAK‐STAT signalling pathways.
Figure 6.
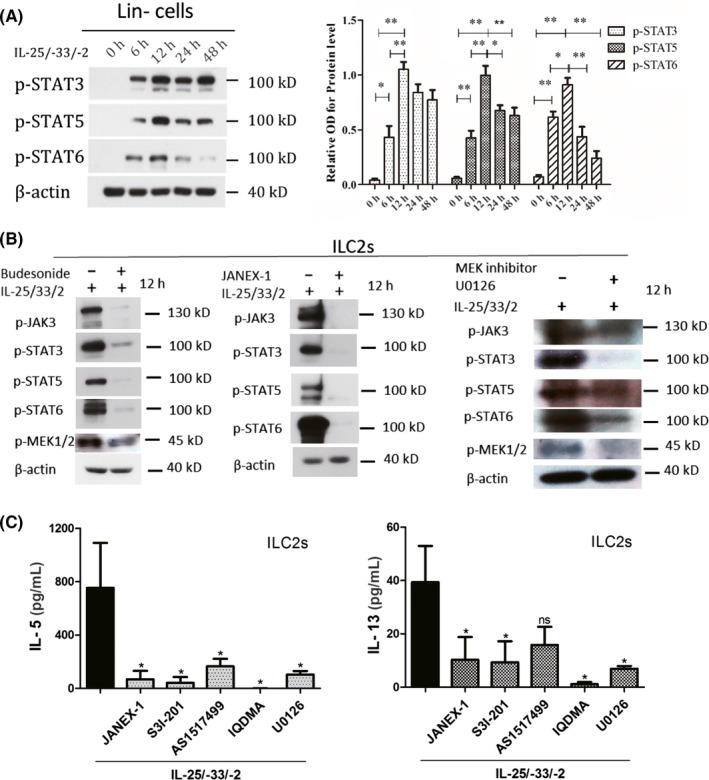
p‐STAT3, p‐STAT5 and p‐STAT6 are the main signalling pathways that are responsible for the production of IL‐5 and IL‐13 with the stimulation and the effects of glucocorticoid. (A) Western blot analysis of p‐STAT3, p‐STAT5 and p‐STAT6 levels in Lin− cells from the buffy coats from healthy volunteers in response to IL‐25 and IL‐33 plus IL‐2 at different time points. (B‐C) ILC2s from the buffy coats from healthy volunteers were sorted using flow cytometer. (B) p‐STAT3, p‐STAT5, p‐STAT6 and p‐MEK1/2 levels in sorted ILC2s with the treatments of IL‐25 and IL‐33 plus IL‐2, budesonide, JAK3 inhibitor (JANEX‐1) or MEK inhibitor (U0126). (C) IL‐5 and IL‐13 levels in ILC2s were measured under STAT3 inhibitor (S3I‐201), STAT5 inhibitor (IQDMA), STAT6 inhibitor (AS1517499), JAK3 inhibitor (JANEX‐1) and MEK inhibitor (U0126) treatments after the stimulation of IL‐25, IL‐33 plus IL‐2. *P < .05, **P < .01, ***P < .001 [Colour figure can be viewed at http://wileyonlinelibrary.com]
4. DISCUSSION
The high frequency and the predominant role of ILC2s in asthma provide a strong hypothesis that therapies for asthma should also consider the ILC2 levels and function. Until now only one study reported that dexamethasone suppresses IL‐33‐induced airway inflammation and lung natural help cells, a member of ILC2s in mice.19 For human patients, only one cross‐sectional report showed an increased frequency of IL13+ILC2s in uncontrolled and partly controlled asthma patients when compared to those in the well‐controlled group.31 In our study, we performed a prospective study to evaluate the ILC2 levels and function after glucocorticoid therapy in asthma patients. More importantly, many clinical indicators were evaluated. We found that the glucocorticoid therapy led to ideal control in asthma and asthma with AR patients. More importantly, for both types of patients, the glucocorticoid treatments significantly decreased the blood high frequencies of ILC2s, and ILC2 frequencies at 3 months after the treatment almost reached to normal levels. Additionally, the improvement of predicted FEV1 and ACT had relationships with the decreased ILC2 percentages after the glucocorticoid treatment. This finding also implies that the patient recovery was accompanied by the ILC2 decrease after glucocorticoid treatment. To our knowledge, it was the first prospective study to investigate the effects of glucocorticoid on ILC2 levels and their function in asthma patients.
We further confirmed the effects of glucocorticoid on ILC2 function in vitro. The PBMCs from the patients exhibited strong and almost 10‐ to 20‐fold increase in IL‐5, IL‐13 and IL‐9 levels in response to the epithelial cytokines compared with those of the healthy controls. The glucocorticoid treatment significantly reversed high levels of IL‐5, IL‐13 and IL‐9. More importantly, glucocorticoid treatment significantly decreased intracellular IL‐13 in ILC2s. Previous studies also identified that IL‐13+ILC2s but not IL‐5+ILC2s were sensitive in response to IL‐25, IL‐33.35 Our data suggest that the patients with allergic airway inflammation have stronger responses to the epithelial cytokine stimulation and glucocorticoid is able to inhibit the production of Th2 cytokines. We found that ILC2s became bigger after the stimulation of IL‐25, IL‐33 plus IL‐2, suggesting the activation of ILC2s. Additionally, we observed that ILC2s but not the Lin+ cells are the most dominant cells to produce IL‐5, IL‐13 and IL‐9. In vitro data further provided strong evidence to support our in vivo findings, by which glucocorticoid is able to regulate the function of ILC2s. It is consistent with previous study that dexamethasone inhibited type 2 cytokine expression by blood ILC2s in response to IL‐25 and IL‐33 but not IL‐7 and TSLP.30 Furthermore, they reported that unlike blood ILC2s, bronchoalveolar lavage (BAL) fluid ILC2s from asthmatic patients were resistant to dexamethasone.30 It suggests that glucocorticoid could behave different effects on ILC2 activity depending on different sources of ILC2s and/or in response to the different stimulators. Our findings provide solid evidence that ILC2s could be a novel target for the treatment of allergic airway inflammation.
Previous study has reported that ILC2s were increased in peripheral blood in asthma patients.20 Increased ILC2s were found in the blood and sputum in severe asthma patients compared to mild asthma.23 In our study, all of the patients were newly diagnosed with asthma or asthma with AR, and most of them were not severe. We identified the enhanced circulating ILC2s in asthma and asthma with AR patients. Previous studies reported higher frequencies of ILC2s in seasonal but not nonseasonal AR patients,26 and in house dust mite (HDM)—but not mugwort—AR patients.28 We recently reported that the levels of ILC2s were increased in the HDM‐AR patients.27 In this study, 20% the asthma patients and 100% asthma with AR patients were positive for specific IgE. Our findings have shown that asthma with AR patients had high levels of ILC2s but with no significant difference compared to asthma patients. Recent studies reported that allergen‐experienced ILC2s have the activity to acquire memory‐like properties, and respond more efficiently during secondary encounters with allergens.36, 37 Of course, the role of the antigens in the pathology of asthma with or without AR should be further addressed. Additionally, we found that there were higher levels of IL‐13 but not IL‐4 and IL‐9 in plasma in both types of patients, and there was a linear correlation between the ILC2 percentages and the IL‐13 levels in asthma patients. All data have provided strong evidence that asthma patients and asthma with AR patients have the increased frequencies of ILC2s and functional cytokine of IL‐13 in the circulation.
Previous study has shown that women had increased circulating ILC2 numbers compared to men with moderate‐to‐severe asthma.32 Testosterone decreased lung ILC2 numbers and IL‐5 and IL‐13 expression from ILC2s both in vitro and in vivo.32 In our study, we did not find significant difference for circulating ILC2 levels in the patients between women and men (data not shown). The inconsistent results may be because of many factors such as the severity of the diseases or different races. PGE2 was reported to suppress IL‐5 and IL‐13 production in human tonsillar ILC2s.33 We observed that the treatment of testosterone and PGE2 significantly decreased the high levels of IL‐5, IL‐13 and IL‐9 by the PBMCs from asthma patients in response to epithelial cytokines. However, the effects of both testosterone and PGE2 on the inhibition of Th2 cytokine production were much weaker compared to glucocorticoid.
There were several reports about the signalling pathways involved in ILC2s in asthma. STAT6 deficient will lead to impaired IL‐4 and IL‐13 receptor expressions as well as proliferation of ILC2s in mouse.38 Activation of GR inhibits expression of mRNA for STAT5 and JAK3.39 STAT5 is the key molecule in TSLP‐induced corticosteroid resistance in mouse airway inflammation model.29 IL‐7 and TSLP abrogated the inhibition of dexamethasone on type 2 cytokine production by blood ILC2s and induced steroid resistance of ILC2s in a MEK‐ and STAT5‐dependent manner.30 The steroid resistant of bronchoalveolar lavage fluid (BALF) ILC2s from asthma patients was reversed by inhibitors of MEK and STAT5.30 In that study, the PBMCs or BALF cells but not sorted ILC2s were treated with the steroid. However, it is necessary to use ILC2s to investigate the signalling pathways involved in with the treatments of steroid. Using sorted human ILC2s, we identified that the levels of p‐STAT3, p‐STAT5 and p‐STAT6 and their upstream p‐JAK3, p‐MEK1/2, were significantly increased with the stimulation of IL‐25, IL‐33 plus IL‐2, and the glucocorticoid administration decreased their levels. Moreover, the inhibitors of STAT3, STAT5, STAT6 and JAK3 phosphorylation significantly decreased the high IL‐5 and IL‐13 levels after the stimulation. Importantly, JAK3 inhibitor significantly decreased the levels of p‐STAT3, p‐STAT5 and p‐STAT6 in activated ILC2s, suggesting that JAK3 is upstream signalling pathways for STATs. We further identified that MEK inhibitor strongly decreased the levels of p‐STAT3, p‐STAT5 and p‐STAT6 in activated ILC2s, partly decreased p‐JAK3 levels, and reversed the high levels of IL‐5 and IL‐13. It suggests that MEK/JAK‐STAT signalling pathways were involved in the production of Th2 cytokines by ILC2s and the effects of glucocorticoid on ILC2 function.
We acknowledged the limitation that given the low relative abundance in PBMCs, the relative contribution of ILC2s to the pathogenesis of allergic diseases did not be studied. ILC2s in human bronchoalveolar lavage fluid were not taken into consideration due to the ethical limitation. Further study should be carried out to address glucocorticoid‐resistant asthma and ILC2s.
In summary, using a prospective study we identified that increased ILC2s in asthma and asthma with AR patients were inhibited by glucocorticoid treatment. It was confirmed that most IL‐5, IL‐13 and IL‐9 in response to epithelium‐derived cytokines were produced by ILC2s. Glucocorticoid treatment was able to reverse the high levels of IL‐5, IL‐13 and IL‐9 produced by ILC2s via STAT3, STAT5, STAT6, JAK3 and MEK signalling pathways. Taken together, our data suggested that glucocorticoid administration could be effective in treating allergic airway inflammation by regulating ILC2s. This will provide a new understanding of glucocorticoid application in regard to allergic diseases.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
Q.N.Y., Y.B.G., X.L., C.L.L., W.P.T., X.L.F., Z.L.Q., D.C., W.P.W. and S.G.Z. helped in collection and/or assembly of data; Q.N.Y. and X.L.F. contributed to data analysis and manuscript writing; Q.L.F. helped in concept and design, data analysis, manuscript writing and final approval of the manuscript.
Supporting information
ACKNOWLEDGMENT
This study was supported by grants from NSFC for Excellent Young Scholars (81322012 to Prof. QL Fu), NSFC (81373174, 81471832, 81470674, 81671882, 81671611, 81770984 and 81770024), the key grant from the Science and Technology Foundation of Guangdong Province of China (2015B020225001) and the Natural Science Foundation of Guangdong Province (2014A030313051, 2016A030308017, 2017A030313105).
Yu QN, Guo YB, Li X, et al. ILC2 frequency and activity are inhibited by glucocorticoid treatment via STAT pathway in patients with asthma. Allergy. 2018;73:1860–1870. 10.1111/all.13438
Q. N. Yu, Y. B. Guo and X. Li contributed equally to this manuscript.
REFERENCES
- 1. Brozek JL, Bousquet J, Baena‐Cagnani CE, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines: 2010 revision. J Allergy Clin Immunol. 2010;126:466‐476. [DOI] [PubMed] [Google Scholar]
- 2. Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fort MM, Cheung J, Yen D, et al. IL‐25 induces IL‐4, IL‐5, and IL‐13 and Th2‐associated pathologies in vivo. Immunity. 2001;15:985‐995. [DOI] [PubMed] [Google Scholar]
- 4. Paul WE, Zhu JF. How are T(H)2‐type immune responses initiated and amplified? Nat Rev Immunol. 2010;10:225‐235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Webb DC, McKenzie ANJ, Koskinen AML, Yang M, Mattes J, Foster PS. Integrated signals between IL‐13, IL‐4, and IL‐5 regulate airways hyperreactivity. J Immunol. 2000;165:108‐113. [DOI] [PubMed] [Google Scholar]
- 6. Halim TY, Steer CA, Matha L, et al. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell‐mediated allergic lung inflammation. Immunity. 2014;40:425‐435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chang YJ, Kim HY, Albacker LA, et al. Innate lymphoid cells mediate influenza‐induced airway hyper‐reactivity independently of adaptive immunity. Nat Immunol. 2011;12:631‐638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Drake LY, Iijima K, Kita H. Group 2 innate lymphoid cells and CD4 + T cells cooperate to mediate type 2 immune response in mice. Allergy. 2014;69:1300‐1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barlow JL, Peel S, Fox J, et al. IL‐33 is more potent than IL‐25 in provoking IL‐13‐producing nuocytes (type 2 innate lymphoid cells) and airway contraction. J Allergy Clin Immunol. 2013;132:933‐941. [DOI] [PubMed] [Google Scholar]
- 10. Salmond RJ, Mirchandani AS, Besnard AG, Bain CC, Thomson NC, Liew FY. IL‐33 induces innate lymphoid cell‐mediated airway inflammation by activating mammalian target of rapamycin. J Allergy Clin Immunol. 2012;130:1159‐1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hung LY, Lewkowich IP, Dawson LA, et al. IL‐33 drives biphasic IL‐13 production for noncanonical Type 2 immunity against hookworms. Proc Natl Acad Sci U S A. 2013;110:282‐287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell‐type cytokines in protease allergen‐induced airway inflammation. Immunity. 2012;36:451‐463. [DOI] [PubMed] [Google Scholar]
- 13. Reed CE, Kita H. The role of protease activation of inflammation in allergic respiratory diseases. J Allergy Clin Immunol. 2004;114:997‐1008. [DOI] [PubMed] [Google Scholar]
- 14. Kim HK, Lund S, Baum R, Rosenthal P, Khorram N, Doherty TA. Innate type 2 response to Alternaria extract enhances ryegrass‐induced lung inflammation. Int Arch Allergy Immunol. 2014;163:92‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein Wolterink RG, Kleinjan A, van Nimwegen M, et al. Pulmonary innate lymphoid cells are major producers of IL‐5 and IL‐13 in murine models of allergic asthma. Eur J Immunol. 2012;42:1106‐1116. [DOI] [PubMed] [Google Scholar]
- 16. Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H. IL‐33‐responsive lineage‐ CD25 + CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs. J Immunol. 2012;188:1503‐1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim HY, Chang YJ, Subramanian S, et al. Innate lymphoid cells responding to IL‐33 mediate airway hyperreactivity independently of adaptive immunity. J Allergy Clin Immunol. 2012;129:216‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Salimi M, Barlow JL, Saunders SP, et al. A role for IL‐25 and IL‐33‐driven type‐2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013;210:2939‐2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim BS, Siracusa MC, Saenz SA, et al. TSLP elicits IL‐33‐independent innate lymphoid cell responses to promote skin inflammation. Sci Transl Med. 2013;5:170ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bartemes KR, Kephart GM, Fox SJ, Kita H. Enhanced innate type 2 immune response in peripheral blood from patients with asthma. J Allergy Clin Immunol. 2014;134:671‐678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Miljkovic D, Bassiouni A, Cooksley C, et al. Association between group 2 innate lymphoid cells enrichment, nasal polyps and allergy in chronic rhinosinusitis. Allergy. 2014;69:1154‐1161. [DOI] [PubMed] [Google Scholar]
- 22. Ho J, Bailey M, Zaunders J, et al. Group 2 innate lymphoid cells (ILC2s) are increased in chronic rhinosinusitis with nasal polyps or eosinophilia. Clin Exp Allergy. 2015;45:394‐403. [DOI] [PubMed] [Google Scholar]
- 23. Smith SG, Chen R, Kjarsgaard M, et al. Increased numbers of activated group 2 innate lymphoid cells in the airways of patients with severe asthma and persistent airway eosinophilia. J Allergy Clin Immunol. 2016;137:75‐86. [DOI] [PubMed] [Google Scholar]
- 24. Walford HH, Lund SJ, Baum RE, et al. Increased ILC2s in the eosinophilic nasal polyp endotype are associated with corticosteroid responsiveness. Clin Immunol. 2014;155:126‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jackson DJ, Makrinioti H, Rana BM, et al. IL‐33‐dependent type 2 inflammation during rhinovirus‐induced asthma exacerbations in vivo. Am J Respir Crit Care Med. 2014;190:1373‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lao‐Araya M, Steveling E, Scadding GW, Durham SR, Shamji MH. Seasonal increases in peripheral innate lymphoid type 2 cells are inhibited by subcutaneous grass pollen immunotherapy. J Allergy Clin Immunol. 2014;134:1193‐1195. [DOI] [PubMed] [Google Scholar]
- 27. Zhong H, Fan XL, Yu QN, et al. Increased innate type 2 immune response in house dust mite‐allergic patients with allergic rhinitis. Clin Immunol. 2017;183:293‐299. [DOI] [PubMed] [Google Scholar]
- 28. Fan DC, Wang XD, Wang M, et al. Allergen‐dependent differences in ILC2s frequencies in patients with allergic rhinitis. Allergy Asthma Immunol Res. 2016;8:216‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kabata H, Moro K, Fukunaga K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun. 2013;4:2675. [DOI] [PubMed] [Google Scholar]
- 30. Liu S, Verma M, Michalec L, et al. Steroid resistance of airway type 2 innate lymphoid cells from patients with severe asthma: the role of thymic stromal lymphopoietin. J Allergy Clin Immunol. 2018;141:257‐268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jia Y, Fang X, Zhu X, et al. IL‐13 + type 2 innate lymphoid cells correlate with asthma control status and treatment response. Am J Respir Cell Mol Biol. 2016;55:675‐683. [DOI] [PubMed] [Google Scholar]
- 32. Cephus JY, Stier MT, Fuseini H, et al. Testosterone attenuates group 2 innate lymphoid cell‐mediated airway inflammation. Cell Rep. 2017;21:2487‐2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Maric J, Ravindran A, Mazzurana L, et al. Prostaglandin E2 suppresses human group 2 innate lymphoid cell function. J Allergy Clin Immunol. 2018;141:1761‐1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Schonwasser DC, Marais RM, Marshall CJ, Parker PJ. Activation of the mitogen‐activated protein kinase/extracellular signal‐regulated kinase pathway by conventional, novel, and atypical protein kinase C isotypes. Mol Cell Biol. 1998;18:790‐798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cavagnero K, Doherty TA. Cytokine and Lipid mediator regulation of group 2 innate lymphoid cells (ILC2s) in human allergic airway disease. J Cytokine Biol. 2017;2:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinez‐Gonzalez I, Matha L, Steer CA, Ghaedi M, Poon GF, Takei F. Allergen‐experienced group 2 innate lymphoid cells acquire memory‐like properties and enhance allergic lung inflammation. Immunity. 2016;45:198‐208. [DOI] [PubMed] [Google Scholar]
- 37. Martinez‐Gonzalez I, Matha L, Steer CA, Takei F. Immunological memory of group 2 innate lymphoid cells. Trends Immunol. 2017;38:423‐431. [DOI] [PubMed] [Google Scholar]
- 38. Lund S, Walford HH, Doherty TA. Type 2 innate lymphoid cells in allergic disease. Curr Immunol Rev. 2013;9:214‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bianchi M, Meng C, Ivashkiv LB. Inhibition of IL‐2‐induced Jak‐STAT signaling by glucocorticoids. Proc Natl Acad Sci U S A. 2000;97:9573‐9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


