Summary
Aim
Previous studies and national assessments indicate an undertreatment of mineralocorticoid receptor antagonists (MRA) in heart failure with reduced ejection fraction (HFrEF). This study aimed to investigate why MRA is not used to full extent.
Methods
A complete community‐based heart failure population was studied. Several variables were collected, and medical records were scrutinized to identify reasons for not prescribing MRA.
Results
Of 2029 patients, 812 had EF ≤40%. Five hundred and fifty‐three patients (68%) tried MRA at some point but 184 of these (33%) discontinued therapy. There were 259 patients that never tried MRA with 177 with a listed explanation or contraindication. Eighty‐two patients, 10% of the total HFrEF population, had no clear contraindications. They were older and had less HF hospitalizations compared to patients on MRA (P < 0.05) and 32% did not have any follow‐up at the cardiology clinic. Contraindications to MRA were renal dysfunction (93 patients), hypotension (28 patients), and hyperkalemia (25 patients). Only six patients had hyperkalemia without renal dysfunction. Of the patients with renal dysfunction, 66 (72%) had eGFR >30 mL/min.
Conclusions
The reasons why MRA are underutilized were mainly because of contraindications. However, the data suggest that physicians are overly cautious about moderately reduced kidney function. There seems to be a 10%‐18% avoidable undertreatment with MRA, especially for elderly patients that are admitted to the hospital for other reasons than heart failure. This suggests that patients with heart failure would benefit from routine follow‐up at a cardiology clinic.
Keywords: heart failure, heart failure with reduced ejection fraction, mineralocorticoid receptor antagonist, undertreatment
1. INTRODUCTION
Heart failure is a disease with high mortality and morbidity, but major advances have been made with the treatment during the last decades. The treatment of heart failure has developed with several medicines and devices that have proven benefit for both a majority of patients with heart failure with reduced ejection fraction (HFrEF) as well as therapies that benefit subgroups of patients. With so many treatment options for such a large patient population which is often old, frail, and have significant comorbidities, heart failure treatment has become more and more complex. With every new add‐on therapy, the incremental absolute benefit decreases for the individual patient but the reward for patients who lack the most basic therapies are large. Perhaps there is a higher group benefit to make sure the guidelines are implemented to as many as possible. Registry studies have shown that many patients with heart failure do not receive all the proper treatment that they should.1, 2, 3
The cornerstones in treating HFrEF are angiotensin‐converting enzyme (ACE) inhibitors or angiotensin‐receptor blockers (ARBs) and beta blockers but also mineralocorticoid receptor antagonists (MRAs).4 ACE inhibitors only temporary suppress the production of aldosterone, a phenomenon called “aldosterone escape.”5, 6, 7 An over activation of mineralocorticoid receptor by aldosterone leads to increased sodium retention and potassium loss but also causes reduced myocardial perfusion, myocardial interstitial fibrosis, increased peripheral vascular resistance, and baroreceptor dysfunction in patients with chronic heart failure.8, 9, 10, 11 This explains why MRA not only lowers the risk of further hypervolemia and hypertension through blocking excessive sodium retention but are also cardio protective.8, 12, 13 Landmark studies have shown that MRA treatment reduces mortality and morbidity in patients with HFrEF13, 14, 15 and European guidelines recommend MRA to all patients that despite standard therapy remain symptomatic and have reduced ejection fraction.4, 16
MRA treatment should be used for eligible patients as it both makes pathophysiological sense and has proven benefit. However, several registry studies and national assessments indicate that heart failure patients are prescribed ACE inhibitors and beta blockers to a high extent, but it seems that MRA treatment is underutilized.1, 2, 3, 17 There are valid explanations to not use MRA in some patients. One reason is the common side effects of MRA such as renal dysfunction, hypotension, and hyperkalemia which restrict the use in some patients. Furthermore, the major contraindications which is potassium levels >5 mmol/L, eGFR <30 mL/min/1.73 m2, serum creatinine >221 μmol/L, and use of potassium‐sparing diuretics also limits use of MRAs in the overall heart failure population.13, 14, 15 It is largely unknown to which degree patients do not receive treatment explained by valid contraindications and side effects and to which degree it is an avoidable undertreatment.
The overall aim of the study was to examine why MRAs are not used to a full extent in eligible heart failure patients. Which are the major obstacles to ensuring that the patients get proper treatment?
2. METHODS
2.1. Study population and data collection
All patients, hospitalized, and out clinic patients, that were diagnosed with heart failure, both main and side diagnosis (ICD codes I50.X, I42.0, I42.6, I42.7, I42.9, I11.0, I13.0, I13.2) at the Heart Centre or Department of Internal Medicine between January 2010 and March 2016, and were residents within the catchment area of the Umeå University Hospital, Sweden, were identified in the hospital's medical records. Both incident and prevalent patients were included. Once the patients had been identified, we manually collected data from the medical records regarding drug therapy, laboratory data, clinical, echocardiogram, and electrocardiography parameters. Baseline data were collected at the time of diagnosis, and follow‐up data were collected by the journal entry closest to the end of data collection period (September 2016). Patients diagnosed before 2010 had their baseline data collected by the journal entry closest to January 2010. All patients who were alive at the end of the collection period and had an ejection fraction (EF) of 40% or less at either the baseline collection or at the end of the collection period were included. The text in the medical records was further scrutinized by the first author of the paper on all patients not receiving MRA to determine if the treatment had been considered. If the treating physician had mentioned MRA, those text segments were pasted into a data file where we later could categorize the reasons for not treating the patients.
2.2. Statistical analysis
For baseline and follow‐up data, continuous variables are presented as means with standard deviations and analyzed with Student's t test. Continuous variables without normal distribution are presented as medians with interquartile range and analyzed with Mann‐Whitney U test. Categorical variables are presented with frequencies and percentage and analyzed with Pearson's chi‐squared test. A P value less than 0.05 was considered statistical significant, and we performed all analyses in SPSS version 24.
3. RESULTS
There were 3636 patients that received a heart failure diagnosis at Umeå University Hospital between January 2010 and March 2016, of which 2029 were alive at the end of March 2016. In total, 1607 patients died before March 2016, whereof 626 had an EF ≤40% and out of these, 186 had been treated with MRA. Of the 2029 living patients, 812 had EF ≤40%, either at index or latest echocardiography. Baseline characteristics are listed in Table 1. The median duration of heart failure was 45 months.
Table 1.
Baseline characteristics
| Characteristics | Patients with EF ≤40% (N = 812) |
|---|---|
| Sex—n (%) | |
| Male | 563 (69) |
| Female | 249 (31) |
| Age—years, mean (SD) | 75 (12) |
| Systolic blood pressure—mm Hg, mean (SD) | 126 (19) |
| Diastolic blood pressure—mm Hg, mean (SD) | 74 (11) |
| Ejection fraction—%, mean (SD) | 33 (7) |
| Heart rate—beats/min, mean (SD) | 75 (15) |
| Body mass index—kg/m2, mean (SD) | 27 (5) |
| Laboratory values | |
| NT‐proBNP—pg/mL, median (IQR) | 1224 (432‐2899) |
| Potassium—mmol/L, mean (SD) | 4.4 (1.5) |
| Serum creatinine—μmol/La, mean (SD) | 108 (60) |
| Creatinine clearance—mL/min, mean (SD) | 68 (33) |
| Creatinine clearance—n (%) | |
| ≥90 mL/min | 182 (22) |
| 60‐89 mL/min | 235 (29) |
| 30‐59 mL/min | 305 (38) |
| 15‐29 mL/min | 76 (9) |
| <15 mL/min | 6 (1) |
| Medical history—n (%) | |
| Atrial fibrillation | 387 (48) |
| Diabetes | 185 (23) |
| Hypertension | 554 (68) |
| Coronary diseaseb | 386 (48) |
| Medications and devices—n (%) | |
| ACE inhibitor | 383 (47) |
| ARB | 353 (43) |
| Beta‐blocker | 730 (90) |
| Diuretics | 497 (61) |
| Digitalis | 95 (12) |
| Implantable cardioverter‐defibrillatorc | 108 (13) |
| Cardiac resynchronization therapyc | 105 (13) |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; IQR, interquartile range; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide; SD, standard deviation.
To convert the values for creatinine to mg/dL, divide by 88.4.
Coronary artery disease defined as either previous myocardial infarction or documented stenosis of at least 50%.
Including patients with Cardiac Resynchronization Therapy Defibrillator (CRT‐D).
In Figure 1, we show that 553 patients (68%) tried MRA at some point, 393 (71%) with spironolactone and 53 (10%) with eplerenone. Mean doses of spironolactone and eplerenone were 23 mg and 33 mg, respectively. Of those who were prescribed MRA, 184 (33%) had to discontinue therapy before end of March 2016.
Figure 1.
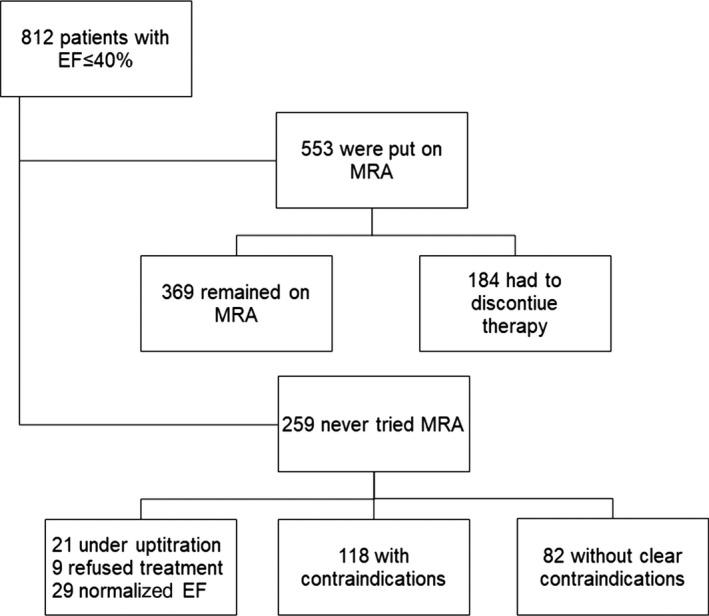
Flow chart of patient distribution
Out of the 812 patients with EF ≤40%, there were 259 patients (32%) that were never treated with MRA. Of these, 29 patients (11%) normalized their EF on ACE‐inhibitors/ARB and beta blockers alone, 9 patients (3%) refused additional treatment, and 21 patients (8%) were still under up titration of ACE inhibitor/ARB or beta blocker. Of the remaining 200 patients, 118 patients had contraindications listed in their medical records but there were an additional 82 patients without clear contraindications.
The contraindications to MRA treatment documented in the records comprised of renal dysfunction, hypotension/orthostatic hypotension, and hyperkalemia (Figure 2). The most common contraindication was renal dysfunction. Of the patients with hyperkalemia, only 6 patients did not have renal dysfunction as well. Of the patients where renal dysfunction was listed as a contraindication, mean e‐GFR was 42 mL/min and 26 patients (28%) had an e‐GFR <30 mL/min, which is the formal contraindication.
Figure 2.
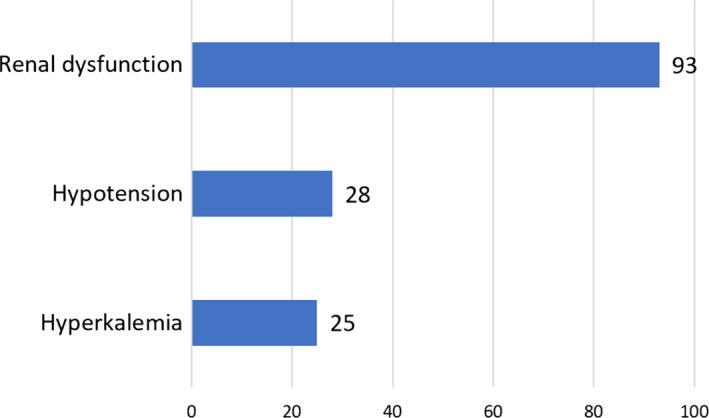
Contraindications to mineralocorticoid receptor antagonists
The patients who never were subject to treatment with MRA and without clear contraindications were compared to the patients who were on MRA at the latest follow‐up (Table 2). The MRA naïve patients were significantly older, had slightly higher systolic blood pressure and lower Body Mass Index (BMI), less patients had a Cardiac Resynchronization Therapy (CRT) and fewer patients had been hospitalized owing to heart failure. In addition, these patients had less treatment with ACE inhibitors/ARB and beta blockers and fewer patients who reached guideline recommended target doses. Examining the medical records of the patients without treatment but without clear contraindications, 26 of the 82 patients (32%) did not have any follow‐up at the cardiology clinic.
Table 2.
Patients with MRA and without clear contraindications compared to patients on MRA
| Characteristics | Patients without MRA treatment and no clear contraindication (N = 82) | Patients on MRA treatment at follow up (N = 369) | P value |
|---|---|---|---|
| Female sex—n (%) | 32 (39) | 104 (28) | 0.05 |
| Age—years, mean (SD) | 77.2 (12.6) | 72.1 (11.2) | <0.001 |
| Systolic blood pressure—mm Hg, mean (SD) | 130 (19) | 124 (18) | 0.01 |
| Ejection fraction—%, mean (SD) | 35 (6) | 32 (7) | <0.001 |
| Heart rate—beats/min, mean (SD) | 75 (17) | 73 (15) | 0.33 |
| Body mass index—kg/m2, mean (SD) | 26 (3.7) | 28 (5.4) | <0.001 |
| Laboratory values | |||
| NT‐proBNP—pg/mL, median (IQR) | 986 (331‐1650) | 970 (334‐2226) | 0.89 |
| Creatinine clearance—mL/min, mean (SD) | 69 (26) | 74 (34) | 0.11 |
| Hemoglobin—mg/L, mean (SD) | 132 (17) | 136 (16) | 0.049 |
| Sodium—mmol/L, mean (SD) | 140 (2.3) | 139 (2.6) | 0.01 |
| Potassium—mmol/L, mean (SD) | 4.2 (0.4) | 4.4 (0.4) | <0.001 |
| Previous hospitalization for heart failure—n (%) | 21 (26) | 221 (60) | <0.001 |
| Medical history—n (%) | |||
| Atrial fibrillation | 34 (42) | 170 (46) | 0.42 |
| Diabetes | 14 (17) | 83 (23) | 0.28 |
| Hypertension | 54 (66) | 242 (66) | 0.96 |
| Coronary diseasea | 38 (46) | 177 (48) | 0.79 |
| Medications and devices—n (%) | |||
| ACE inhibitor or ARB | 73 (89) | 351 (95) | 0.04 |
| Beta‐blocker | 68 (83) | 354 (96) | <0.001 |
| Diuretics | 36 (44) | 240 (65) | <0.001 |
| Digitalis | 8 (10) | 50 (14) | 0.35 |
| Implantable cardioverter‐defibrillatora | 2 (2) | 73 (20) | <0.001 |
| Cardiac resynchronization therapya | 4 (5) | 59 (16) | 0.01 |
| Proportion of target dose—mean (SD) | |||
| ACE inhibitor or ARB | 60 (31) | 77 (30) | <0.001 |
| Beta‐blocker | 49 (30) | 67 (32) | <0.001 |
ACE, angiotensin converting enzyme; ARB, angiotensin receptor blocker; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; NT‐proBNP, N‐terminal pro–B‐type natriuretic peptide.
Statistical significance level P < 0.05.
Coronary artery disease defined as either previous myocardial infarction or documented stenosis of at least 50%.
Including patients with Cardiac Resynchronization Therapy Defibrillator (CRT‐D).
4. DISCUSSION
The majority of patients with clear indication for MRA either received treatment or had a contraindication to treatment corresponding to around 90% of all eligible patients. Overall, a third of the population had never tried MRA which is in line with the latest national evaluation and heart failure registry studies.1, 3, 17 A majority of these patients had contraindications listed in their medical records, but it is striking that such a large proportion of patients had an eGFR over 30 mL/min but where the treating physician still chose to not initiate therapy. Patients with borderline eGFR require more surveillance and controls owing to higher risk of hyperkalemia on MRA which may imply that the frailest patients are denied treatment because of inadequate means of follow‐up. Hyperkalemia as contraindication was not nearly as common as renal dysfunction. A part of the explanation could be that it is the combination of minor hyperkalemia and moderate kidney dysfunction that prevents the treating physicians to prescribe MRA, which is unfortunate as data indicate survival benefit even with minor hyperkalemia.18 This suggests that a major part of the undertreatment of MRA could be avoided if the attending physician had better knowledge about the side effects of MRA in combination with better routines of follow‐up for patient with borderline eGFR. Today the intervals of check‐ups can vary which may explain why treating physicians are reluctant to risk side effects and possible harm. Potassium binders that control potassium levels and that are tolerated by patients have not been available but with the emergence of such treatments,19, 20 there may be additional potential to increase MRA use.
In an article from the Swedish Heart Failure Registry with over 11 000 patients21 they also conclude that MRA use is decreased in patients with moderately impaired renal function and further strengthen our findings. Our study adds data on how many patients are not chosen for treatment at all and the finding that patients who are hospitalized for other reasons than heart failure seem to be overlooked shows why massive data from registries must be accompanied by data from whole communities. With both our data and the data from the Swedish Heart Failure Registry concluding that there seems to be an undertreatment in patients with moderately reduced kidney function, we need to work harder to remove barriers for MRA use. Several barriers have been identified on patient‐, clinician‐, and system‐based levels22 and could be a good starting point to improve treatment.
Even removing the patients with clear or relative contraindication, the underutilization was 10% (82 of 812) of the HFrEF population. These patients were older, had slightly higher systolic blood pressure, lower BMI, and were less likely to receive a CRT. The most striking difference, however, was that these patients had to a much lesser extent been hospitalized with a main diagnosis of heart failure. With the high proportion of patients that had to discontinue MRA therapy, it is possible that many of the patients that never received treatment would not have tolerated treatment in the first place as they were older than those who tolerated treatment. Because of this it is difficult to say how many more patients would have benefitted from MRA treatment. On the other hand, it is evident that these patients many times were overlooked as they were hospitalized for other reasons than heart failure and almost a third of these patients did not receive follow‐up at a cardiology clinic. Perhaps these patients had less active heart failure and, as a result, went unnoticed. To ensure better treatment for every patient, we need better tools of identifying patients in need for treatment. Further, the patients who did not receive treatment with MRA despite lack of clear contraindications had lower doses of basic heart failure therapy which could be a partial explanation to why these patients did not receive treatment with MRA. It is unclear how many had attempted up titration and failed.
In addressing incorrect treatment, one should also take into account overutilization. As MRA have side effects that should not be taken lightly, patients with heart failure and midrange EF (40%‐50%) or heart failure with preserved EF should not routinely be prescribed MRA. This is, however, difficult to determine as some patients improve their EF after treatment and MRAs are also prescribed as antihypertensive therapy. Overutilization has not been investigated in this article. Additionally, local practices vary on how to initiate treatment. To follow guidelines correctly, one should perform an echocardiography and do a careful clinical evaluation after up titration of ACE inhibitors/ARB before commencing MRA treatment. However, at our center, patients sometimes are put on MRA before a new echocardiography is performed. It is possible that this practice may have led us to overestimate the number of patients eligible for MRA. Taking the time aspect into consideration, it is also likely that a longer duration of heart failure may increase the odds of exposure for MRA treatment. We have tried to ameliorate this by accounting for the patients who were still under up titration. Still, the earlier patients who are eligible for treatment are initiated, the better.
If the patient experiences side effects, such as hypotension, hyperkalemia or decreased kidney function on ACE inhibitor/ARB, guidelines are lacking whether higher doses of ACE inhibitor/ARB should be preferred over introducing MRA. MRA lower blood pressure but the low doses used in heart failure studies do not substantially decrease blood pressure. Hypotension was still listed as a common reason to not use MRA. In MRA studies, not all patients received maximum doses of ACE inhibitor/ARB but there was still a survival benefit in the MRA arm.23 This may indicate that lower doses of two different drug classes should be preferred over maximum dose of just one class.
In comparing these real‐world data to clinical studies, we can conclude that our heart failure population differs from the population in the latest major landmark trial, the PARADIGM‐HF study.24 In a previous work, we have shown that only 25% of patients in our HFrEF population would have fulfilled the strict inclusion criteria in the PARADIGM‐HF study.25 With the higher doses of ACE‐inhibitors and ARBs in the PARADIGM‐HF study, perhaps the reasoning of lower doses of several different drug classes applies to sacubitril–valsartan as well as MRAs.
We also showed that the proportion of women with reduced EF is less than a third than the overall population. The proportion of women without contraindications who were not initiated on treatment is higher than the proportion on women being on treatment. Although these results fail to reach statistical significance, this merits further investigation.
Another question of interest is what proportion of patients could be expected to tolerate MRA treatment in an unselected heart failure population—how high should we realistically aim for? In our HFrEF population, 45% of the patients were prescribed and remained on MRA treatment. Taking into account the 10% without MRA treatment and no clear contraindications, together with some of the patients listed in the contraindication group but only had moderate renal impairment, we estimate that about 60% would tolerate MRA treatment in long term. As previous studies have shown that only 33%‐40% of heart failure patients are prescribed MRA treatment,1, 3, 17 this leaves room for improvement in the management of the heart failure patient.
4.1. Strengths and limitations
One limitation is the single‐center study design and local traditions may differ between geographic areas. On the other hand, the strength is that the study population is an almost complete community‐based heart failure population with no exclusions and our data on HFrEF patients are in line with national assessments, which indicates that the results are valid. As no interviews have been performed with treating physicians, we can only speculate on the real reasons why moderate kidney dysfunction seems to prevent MRA usage in some cases. We have not analyzed reasons for discontinuing treatment with MRA, which needs to be elucidated further.
5. CONCLUSIONS
Most patients in our population with heart failure and a clear indication for MRA receive treatment but more than every third patient either had to discontinue therapy or have contraindications for MRA therapy. The data indicate that the treating physicians are overly cautious about adverse effects with MRA in patients with moderately reduced kidney function and minor hyperkalemia. We propose education on the risk–benefit ratio of moderate renal dysfunction and minor hyperkalemia vs the risks of not receiving complete heart failure treatment. We believe that we could reach a higher degree of MRA usage with better and regular monitoring of patients or using modern potassium binders that are tolerable by patients and give a predictable response. Additionally, there is a need for knowledge on whether to prioritize MRA over higher doses of ACE inhibitors/ARB. There seems to be a 10%‐18% avoidable undertreatment with MRA, especially for elderly patients that are admitted to hospital for other reasons than heart failure and patients with moderate renal dysfunction. This suggests that patients with heart failure would benefit from routine follow‐up at a cardiology clinic.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
AUTHOR CONTRIBUTIONS
All authors were involved in the study design, data collection, data analysis/interpretation, and critical revision of the manuscript. AJ and KL drafted the manuscript. All authors read and approved the final manuscript before submission and publication.
ETHICS
The Regional Ethical Review Board in Umeå, Sweden has approved this study (registration number 2015/419‐31).
ACKNOWLEDGMENTS
The authors thank all the personnel who obtained data for the database.
Jonsson A, Norberg H, Bergdahl E, Lindmark K. Obstacles to mineralocorticoid receptor antagonists in a community‐based heart failure population. Cardiovasc Ther. 2018;36:e12459 10.1111/1755-5922.12459
Funding information
This work was supported by the Heart Foundation of Northern Sweden.
REFERENCES
- 1. Socialstyrelsen . Nationella riktlinjer för hjärtsjukvård – Stöd för styrning och ledning. Stockholm: Socialstyrelsen; 2015. [Google Scholar]
- 2. Socialstyrelsen . Nationella riktlinjer – Utvärdering 2015 – Hjärtsjukvård – Indikatorer och underlag för bedömning. Stockholm: Socialstyrelsen; 2015. [Google Scholar]
- 3. Thorvaldsen T, Benson L, Dahlstrom U, Edner M, Lund LH. Use of evidence‐based therapy and survival in heart failure in Sweden 2003‐2012. Eur J Heart Fail. 2016;18:503‐511. [DOI] [PubMed] [Google Scholar]
- 4. Ponikowski P, Voors AA, Anker SD, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129‐2200. [DOI] [PubMed] [Google Scholar]
- 5. Biollaz J, Brunner HR, Gavras I, Waeber B, Gavras H. Antihypertensive therapy with MK 421: Angiotensin II–renin relationships to evaluate efficacy of converting enzyme blockade. J Cardiovasc Pharmacol. 1982;4:966‐972. [PubMed] [Google Scholar]
- 6. Borghi C, Boschi S, Ambrosioni E, Melandri G, Branzi A, Magnani B. Evidence of a partial escape of renin‐angiotensin‐aldosterone blockade in patients with acute myocardial infarction treated with ACE inhibitors. J Clin Pharmacol. 1993;33:40‐45. [DOI] [PubMed] [Google Scholar]
- 7. Weir RA, Tsorlalis IK, Steedman T, et al. Aldosterone and cortisol predict medium‐term left ventricular remodelling following myocardial infarction. Eur J Heart Fail. 2011;13:1305‐1313. [DOI] [PubMed] [Google Scholar]
- 8. Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium. Fibrosis and renin‐angiotensin‐aldosterone system. Circulation. 1991;83:1849‐1865. [DOI] [PubMed] [Google Scholar]
- 9. Bauersachs J, Heck M, Fraccarollo D, et al. Addition of spironolactone to angiotensin‐converting enzyme inhibition in heart failure improves endothelial vasomotor dysfunction: Role of vascular superoxide anion formation and endothelial nitric oxide synthase expression. J Am Coll Cardiol. 2002;39:351‐358. [DOI] [PubMed] [Google Scholar]
- 10. Suzuki G, Morita H, Mishima T, et al. Effects of long‐term monotherapy with eplerenone, a novel aldosterone blocker, on progression of left ventricular dysfunction and remodeling in dogs with heart failure. Circulation. 2002;106:2967‐2972. [DOI] [PubMed] [Google Scholar]
- 11. Yee KM, Struthers AD. Aldosterone blunts the baroreflex response in man. Clin Sci. 1998;95:687‐692. [DOI] [PubMed] [Google Scholar]
- 12. Struthers AD. The clinical implications of aldosterone escape in congestive heart failure. Eur J Heart Fail. 2004;6:539‐545. [DOI] [PubMed] [Google Scholar]
- 13. Pitt B, Remme W, Zannad F, et al. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med. 2003;348:1309‐1321. [DOI] [PubMed] [Google Scholar]
- 14. Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341:709‐717. [DOI] [PubMed] [Google Scholar]
- 15. Zannad F, McMurray JJ, Krum H, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11‐21. [DOI] [PubMed] [Google Scholar]
- 16. Socialstyrelsen . Nationella riktlinjer för hjärtsjukvård – Vetenskapligt underlag – Bilaga. Stockholm: Socialstyrelsen; 2015. [Google Scholar]
- 17. Maggioni AP, Dahlstrom U, Filippatos G, et al. EURObservational Research Programme: Regional differences and 1‐year follow‐up results of the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail. 2013;15:808‐817. [DOI] [PubMed] [Google Scholar]
- 18. Rossignol P, Dobre D, McMurray JJ, et al. Incidence, determinants, and prognostic significance of hyperkalemia and worsening renal function in patients with heart failure receiving the mineralocorticoid receptor antagonist eplerenone or placebo in addition to optimal medical therapy: Results from the Eplerenone in Mild Patients Hospitalization and Survival Study in Heart Failure (EMPHASIS‐HF). Circ Heart Fail. 2014;7:51‐58. [DOI] [PubMed] [Google Scholar]
- 19. Pitt B, Anker SD, Bushinsky DA, Kitzman DW, Zannad F, Huang IZ. Evaluation of the efficacy and safety of RLY5016, a polymeric potassium binder, in a double‐blind, placebo‐controlled study in patients with chronic heart failure (the PEARL‐HF) trial. Eur Heart J. 2011;32:820‐828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosiborod M, Rasmussen HS, Lavin P, et al. Effect of sodium zirconium cyclosilicate on potassium lowering for 28 days among outpatients with hyperkalemia: The HARMONIZE randomized clinical trial. JAMA. 2014;312:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 21. Savarese G, Carrero JJ, Pitt B, et al. Factors associated with underuse of mineralocorticoid receptor antagonists in heart failure with reduced ejection fraction: An analysis of 11 215 patients from the Swedish Heart Failure Registry. Eur J Heart Fail. 2018; 10.1002/ejhf.1182. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22. Dev S, Hoffman TK, Kavalieratos D, et al. Barriers to adoption of mineralocorticoid receptor antagonists in patients with heart failure: A mixed‐methods study. J Am Heart Assoc. 2016;5:e002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krum H, Shi H, Pitt B, et al. Clinical benefit of eplerenone in patients with mild symptoms of systolic heart failure already receiving optimal best practice background drug therapy: Analysis of the EMPHASIS‐HF study. Circ Heart Fail. 2013;6:711‐718. [DOI] [PubMed] [Google Scholar]
- 24. McMurray JJ, Packer M, Desai AS, et al. Angiotensin‐neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993‐1004. [DOI] [PubMed] [Google Scholar]
- 25. Norberg H, Bergdahl E, Lindmark K. Eligibility of sacubitril‐valsartan in a real‐world heart failure population: A community‐based single‐centre study. ESC Heart Failure. 2018;5:337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]


