Abstract
Aim
This study explored the prevalence of atopic and nonatopic asthma in 12‐year‐old children and whether they were associated with different risk factors. In particular, we wanted to analyse whether receiving antibiotics during the first week of life was associated with asthma at that age.
Methods
Data were obtained from a longitudinal cohort study of 5654 Swedish children born in 2003. The parents answered questionnaires from the age of six months until 12 years. The response rate at 12 years was 3637/4777 (76%).
Results
At 12 years, 6.4% reported current doctor‐diagnosed asthma. Treatment with antibiotics during the first week of life was associated with an increased risk of atopic asthma, with an adjusted odds ratio of 2.2 and 95% confidence interval of 1.2–4.2. Being born small for gestational age was associated with an increased risk of nonatopic asthma, with an adjusted odds ratio of 3.8 and 95% confidence interval of 1.1–13.7. Asthma that only occurred with colds was reported by 28%.
Conclusion
Antibiotic treatment during the first week of life was associated with an increased risk of atopic asthma at 12 years, suggesting an immune‐mediated effect. Being born small for gestational age increased the risk of nonatopic asthma.
Keywords: Antibiotics, Asthma, Atopy, Breastfeeding, Small for gestational age
Abbreviations
- aOR
Adjusted odds ratio
- OR
Odds ratio
Key notes.
Long‐term follow‐up studies of the association between early antibiotic treatment and asthma are scarce.
We now report that antibiotics given during the first week of life increased the risk of atopic asthma at the age of 12 years.
The association between antibiotics given during the first week of life and atopic asthma suggests that the effect is mediated via an influence on the immune system.
Introduction
Asthma is one of the most common chronic diseases in children and, following decades of increasing prevalence, it seems to have reached a high‐level plateau in western countries 1. School‐age asthma is considered to be a fairly homogeneous disease, with the majority of cases being related to allergic diseases, namely atopic asthma that is associated with allergic sensitisation, allergic rhinitis, food allergies or eczema 2, 3, 4, 5, 6. However, there are school children with nonatopic asthma, who do not experience allergic sensitisation or allergic comorbidity 3, 4, 5, 6. This suggests different underlying mechanisms for the development of asthma, including the possibility that atopic asthma is associated with immune modulation at a very early age 7.
The developing immune system interacts with, and is affected by, gastrointestinal microbiota 8 and a more diverse microbiota has been associated with lower risks of allergies and asthma 9. On the other hand, disturbed microbiota can affect the development of the immune system and thereby the risk of asthma and allergies. Antibiotic treatment is known to affect the gastrointestinal microbiota, and treatment of this kind during a vulnerable period of life could therefore have long‐term effects.
The most common indication for treatment with antibiotics for newborn infants admitted to the neonatal ward is suspected sepsis. Broad‐spectrum antibiotics are always used, and the first‐line treatment for infants aged 72 hours or less is benzylpenicillin combined with an aminoglycoside 10.
An increased risk of asthma following early antibiotic treatment in children has been reported, but studies have discussed the possible impact of confounding by both post‐natal vulnerability and indication 9, 11, 12, 13. It has been suggested that exposure to antibiotics before the onset of wheeze and the adjustment for post‐natal vulnerability, such as gestational age, delivery mode and heredity, could clarify the association.
In addition, other factors present in early life, such as breastfeeding and the introduction of food, have been reported to affect the risk of future asthma. For example, breastfeeding is known to reduce the risk of early life infections 14 and we know that early viral infections are related to infant wheeze and asthma. However, it is unclear how long the effects of breastfeeding are seen and long‐term follow‐up studies are needed to establish this.
The aim of this study was to examine the prevalence of, and risk factors for, asthma at 12 years of age and to examine the associations with atopic asthma and nonatopic asthma. In particular, we wanted to analyse whether the long‐term effects of antibiotics during the first week of life could be seen at the age of 12 years.
Methods
Participants
Data were obtained from a prospective, longitudinal cohort study of children born in western Sweden in 2003. We randomly selected 50% of the birth cohort (n = 8176) and invited them to participate and 5654 families agreed. After written informed consent, the parents answered postal questionnaires when their child was six months and one, four, eight and 12 years of age. Details regarding the questionnaires and earlier response rates have previously been published 15, 16, 17. At 12 years of age, questionnaires were distributed to 4777 of the 5654 families who entered the study, as we excluded those that had indicated that they no longer wished to participate. The response rate at the age of 12 years was 3637/4777 (76%) of the questionnaires distributed. This was equal to 64% of the families who entered the study and 90% of the families who responded at eight years of age.
Information on pregnancy and post‐natal factors were collected at six months of age. Supplementary information about pregnancy and delivery was obtained from the Swedish Medical Birth Register.
Information on admission to a neonatal ward during the first week of life and treatment with antibiotics during this period was obtained from the six‐month questionnaire. Information on the duration of breastfeeding and the introduction of different foods, including fish, was collected at 12 months of age. Prolonged breastfeeding was defined as any breastfeeding for four months or more. The early introduction of fish and eggs was defined as introducing the food before nine months of age. Having a cat or dog at home was defined as having a pet in the home for more than six months during the first year of life. Dampness at home was defined as reporting damage in the home due to dampness when the child was six months old. Specific information on these questions has previously been published 15.
At 12 years of age, questions were asked on current health, airway symptoms, eczema, rhinitis, food allergies, allergic sensitisation and environment.
Definitions
Current asthma at 12 years of age was defined as reported doctor‐diagnosed asthma and either current treatment or symptoms. It was based on the answers to the following questions:
Has your child been diagnosed with asthma by a physician?
Has your child received medication for asthma during the last 12 months
Has your child had problems with, or symptoms of, wheezing during the last 12 months?
Atopic asthma at 12 years was defined as current asthma, as stated above, plus reported allergic sensitisation and, or, current doctor‐diagnosed allergic rhinitis, food allergies or eczema (a reported diagnosis of allergic rhinitis, food allergy or eczema and either treatment and/or symptoms of the disease during the last 12 months). The questions regarding allergic diagnoses, treatment and symptoms are summarised in Table S1.
Nonatopic asthma at 12 years was defined as current asthma, as stated above, and not having any reported allergic sensitisation or current doctor‐diagnosed rhinitis, food allergy or eczema.
As allergic sensitisation was reported by the child's parents, we also analysed current asthma with and without current allergic rhinitis at the age of 12 years, to verify our analyses on atopic and nonatopic asthma.
In Figure 1, the children aged one and four years are labelled as having asthma if their parents had reported recurrent wheeze, namely at least three episodes of wheeze during the last year, as many children do not receive an asthma diagnosis at such an early age. At the age of eight, we used the same definition of asthma as at the age of 12 years, as stated above.
Figure 1.
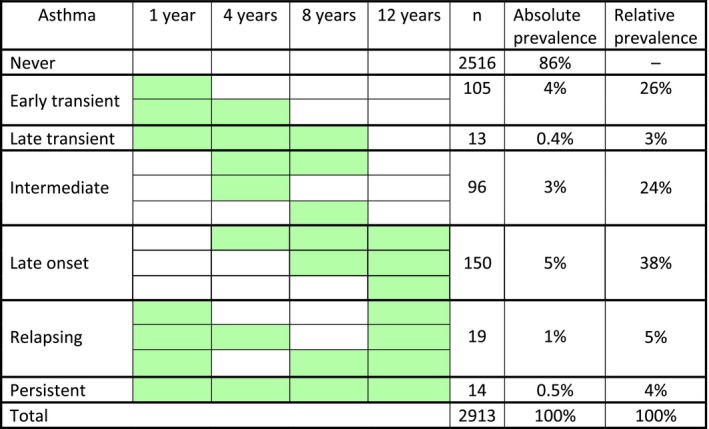
The course of asthma from infancy to age 12 years. The figure is based on the 2913/3637 participants for whom we had information on asthma in all questionnaires. The absolute prevalence is population based, i.e., of 2913 children. The relative prevalence is based on children with ever asthma, i.e., of 397 children. Green colour denotes asthma during the 12 months preceding the follow‐up.
The course of asthma from infancy to the age of 12, as illustrated in Figure 1, was based on 2913/3637 participants whose parents provided information on asthma in all the questionnaires. Early transient asthma was defined as asthma at one year, but not later than four years. Late transient asthma was defined as asthma at one, four and eight years, but not at 12 years of age. Intermediate asthma was defined as only having asthma at the age of four and, or, eight. Late‐onset asthma was defined as asthma at 12 years of age, which had been reported since four or eight years of age, but was not reported at one year of age. Relapsing asthma was defined as asthma at one and 12 years, but not persistently at four and eight years of age. Persistent asthma was defined as asthma at all ages.
Statistical methods
In the statistical analysis, contingency tables were produced using the chi‐square test and binary logistic regression. Odds ratios (OR) were estimated with 95% confidence intervals (95% CI). Crude ORs are indicated as ORs and adjusted ORs as aORs.
Potential risk factors for current asthma at the age of 12 years are summarised in Table S2.
Risk factors with p value of less than 0.1 in the univariate analysis were analysed in the multivariate model. A cut‐off of 0.1 was chosen so that potential confounders would be included. The multivariate model also controlled for maternal smoking during pregnancy, being born at less than 37 weeks of gestation and any breastfeeding for four months or more, as these factors have been reported to affect the risk of childhood asthma. In addition, the parental levels of education were included as a marker of socio‐economic status. Adjustments were made for all factors simultaneously in one multivariate model. Missing values were coded as missing.
Current asthma at 12 years of age was used as the primary outcome. In addition, the multivariate analyses were performed for atopic and nonatopic asthma, respectively.
SPSS Statistics version 23.0 (IBM Corp., Armonk, NY, USA) was used for the statistical analyses.
Ethical approval
The study was approved by the ethics committee at the University of Gothenburg.
Results
Representativeness of the study sample
We analysed the differences between children whose parents completed questionnaires at 12 years of age with those who did not take part, but did provide data when their child was six months and 12 months. Low parental educational level and maternal smoking during pregnancy were more common among nonresponders than responders. There was also a lower prevalence of atopic heredity and breastfeeding among the nonresponders. No significant differences were seen regarding wheeze, reported doctor‐diagnosed eczema or food allergies. The results from the dropout analysis are shown in Table S3.
Prevalence
Current asthma was reported by 6.4% (233/3637) and, of these, 65% had atopic asthma and 35% had nonatopic asthma. Of the children with current asthma, 62% reported three or more wheezing episodes during the last year. Any wheeze during the last year was reported by 8.4% (303/3621). Using medication for asthma during the last year was reported by 8.7% (312/3599). Numbers vary because of varying responses received on each question.
Current allergic rhinitis was reported in 52% (121 of 231) of the children with current asthma and in 78% (81 of 103) of the children with atopic asthma.
Any breastfeeding, defined as breastfeeding for four months or more, was 78% (149 of 190) in the total current asthma group 81% (99 of 122) in the atopic asthma group and 74% (50 of 68) in the nonatopic asthma group. Of those who participated in the 12‐year follow‐up, 4.2% (152 of 3602) had received antibiotics at the neonatal ward during the first week of life.
Course of asthma
The course of asthma from age one to 12 years is shown in Figure 1. The cumulative prevalence of asthma was 14% (397 of 2913), with regard to reporting asthma at some time point from one to 12 years of age. Almost 30% (n = 118) of the 397 children with asthma had transient asthma, while approximately 38% (n = 150) had late‐onset asthma. About 24% (n = 96) of the asthmatics had intermediate asthma, while very few, 4% (n = 14), had persistent asthma.
Univariate and multivariate analyses
The univariate analysis for current asthma at 12 years of age is shown in Table S4.
The results of the multivariate analyses of risk factors and protective factors for current asthma, atopic asthma and nonatopic asthma are shown in Table 1. We found that treatment with antibiotics during the first week of life was an independent risk factor for atopic asthma, but not for nonatopic asthma at the age of 12 years, with an aOR of 2.2 (1.2–4.2) versus 1.4 (0.5–3.4). Breastfeeding for four months or more reduced the risk to an aOR of 0.5 (0.3–0.95), while being born small for gestational age increased the risk to an aOR of 3.8 (1.1–13.7) for nonatopic asthma.
Table 1.
Multivariate analyses of risk factors and protective factors for current asthma (n = 233), atopic asthma (n = 151) and nonatopic asthma (n = 82) at 12 years of age
| Factors | Current asthma | Atopic asthma | Nonatopic asthma |
|---|---|---|---|
| aOR (95% CI) | aOR (95% CI) | aOR (95% CI) | |
| Parental asthma | 2.6 (1.9–3.7) | 2.5 (1.7–3.6) | 3.0 (1.7–5.0) |
| Parental rhinitis | 1.1 (0.8–1.6) | 1.6 (1.1–2.3) | 0.7 (0.4–1.1) |
| Parental eczema | 1.2 (0.9–1.6) | 1.0 (0.7–1.4) | 1.7 (1.1–2.8) |
| Male gender | 1.3 (0.99–1.8) | 1.6 (1.1–2.3) | 0.9 (0.6–1.4) |
| Gestational age <37 weeks | 1.0 (0.5–1.8) | 0.8 (0.4–1.9) | 1.3 (0.6–3.2) |
| Maternal medication during pregnancy | 1.2 (0.9–1.6) | 1.1 (0.8–1.6) | 1.4 (0.9–2.3) |
| Small for gestational age | 2.6 (1.1–5.9) | 2.3 (0.9–6.3) | 3.8 (1.1–13.7) |
| Antibiotics first week | 1.9 (1.1–3.2) | 2.2 (1.2–4.2) | 1.4 (0.5–3.4) |
| Doctor‐diagnosed food allergy first year | 2.2 (1.3–3.7) | 3.0 (1.8–5.1) | 0.6 (0.1–2.5) |
| Eczema during the first year | 2.1 (1.5–2.8) | 2.7 (1.9–4.0) | 1.1 (0.6–2.0) |
| Recurrent wheeze first year | 3.3 (2.1–5.0) | 2.4 (1.4–4.4) | 5.1 (2.8–9.5) |
| Introduction of egg before nine months of age | 0.9 (0.7–1.4) | 1.1 (0.7–1.7) | 0.8 (0.4–1.5) |
| Introduction of fish before nine months of age | 0.8 (0.5–1.1) | 0.7 (0.4–1.1) | 0.9 (0.5–1.8) |
| Fish once a month or more in infancy | 1.1 (0.6–1.9) | 1.0 (0.5–1.9) | 1.4 (0.5–3.7) |
| Damp mould in the home at 6 months of age | 0.3 (0.1–0.9) | 0.4 (0.1–1.2) | 0.3 (0.03–2.1) |
| Cat at home during infancy | 0.8 (0.6–1.1) | 0.9 (0.6–1.3) | 0.7 (0.4–1.2) |
| Breastfeeding for four months or more | 1.2 (0.8–1.7) | 1.1 (0.7–1.9) | 0.5 (0.3–0.95) |
| Smoking during pregnancy | 0.6 (0.3–1.2) | 1.1 (0.5–2.1) | — |
| Parental educational level | 0.9 (0.7–1.2) | 0.7 (0.5–1.1) | 1.3 (0.8–2.1) |
Adjusted odds ratios and 95% confidence interval, aOR (95% CI). Adjustments were made for all factors simultaneously. Independently significant aORs, p < 0.05, are in bold.
Having a history of atopic disease during the first year of life, namely a food allergy or eczema, predicted current asthma as well as atopic asthma at 12 years of age (Table 1).
In addition, multivariate analyses were carried out for current asthma with and without current allergic rhinitis at 12 years of age. The results were in line with the findings for atopic and nonatopic asthma. Treatment with antibiotics during the first week of life also increased the risk of current asthma with allergic rhinitis to an aOR of 2.6 (1.3–5.2). Being born small for gestational age increased the risk of current asthma without allergic rhinitis to an aOR of 3.6 (1.2–10.8). However, breastfeeding for four months or more did not significantly reduce the risk of current asthma without allergic rhinitis, with an aOR of 1.1 (0.6–2.1).
Triggers for asthma
Triggers for asthma were reported in 232 of 233 children with current asthma at 12 years of age. Exercise was reported as a trigger factor in 59% (137 of 232) of the subjects with current asthma, with colds in 59% (n = 136), pollen in 27% (n = 62), furry animals in 22% (n = 50) and mites in 10% (n = 24). Asthma that only occurred with colds was reported by 28% (n = 64).
Discussion
The main result of this prospective birth cohort study was that we found an increased risk of atopic asthma at 12 years in children who received antibiotics during the first week in life, suggesting an immune‐mediated effect. Being born small for gestational age increased the risk of nonatopic asthma. In addition, we found that asthma triggered by colds was not just restricted to preschool children, as is often suggested, but was reported by a considerable percentage of the parents of 12‐year‐olds with asthma.
Long‐term follow‐up studies of the association between early antibiotic treatment and asthma, such as our study, are scarce. In addition, most research groups have studied the effects of antibiotic treatment during the first year of life and not of antibiotic treatment during the first week of life, as we did. Focusing on the first week of life enabled us to avoid reverse causation by treatment of early wheezing episodes with antibiotics and to avoid confounding by indication. To minimise confounding by post‐natal vulnerability, we adjusted for parental asthma and allergy and being born small for gestational age, as well as for preterm birth and Caesarean section. The correlation between early antibiotic treatment and asthma was in line with our previous findings from the same cohort of children at the ages of one, four and eight years 15, 16, 17.
When we considered atopic and nonatopic asthma separately, we found that treatment with antibiotics during the first week of life was an independent risk factor for atopic asthma. However, we found no significant effect on nonatopic asthma. This was also true for current asthma with and without current allergic rhinitis. Antibiotics can affect the intestinal flora and, in this way, affect the maturation of the immune system and disrupt the development of immunological tolerance 9. It has been reported that the immune response in the newborn child is characterised by a dominant T‐helper cell type 2 response and that maturation towards a T‐helper cell type 1 response is stimulated by microbial exposure. This could potentially reduce the risk of allergic diseases 8. Our finding that antibiotic treatment in the first week of life had an effect on atopic, but not nonatopic asthma, was in line with the hypothesis that immune‐mediated mechanisms were involved.
On the other hand, being born small for gestational age was found to affect the risk of nonatopic asthma. This means that factors present at a very early age, perhaps in utero, appeared to be associated with the development of asthma in our cohort. Our finding that being born small for gestational age increased the risk of nonatopic asthma was supported by a meta‐analysis of 25 000 children 18. It has been suggested that reduced lung function in children born small for gestational age could explain this association 19 and a lower forced expiratory volume in one‐second than expected has been shown in children born small for gestational age 18.
Breastfeeding appeared to reduce the risk of nonatopic asthma at 12 years of age, but the association was not confirmed when we compared asthma with and without current allergic rhinitis. We saw an effect of breastfeeding on asthma in the same cohort at 12 months of age 15, but not at age four or eight years of age 16, 17. Moreover, other studies have found that breastfeeding reduced the risk of viral wheeze in infants and during the first years of life 20, possibly by reducing the risk of infections in early life 14. This does not explain any long‐term effect and the effects of breastfeeding on asthma later in life remain uncertain. A meta‐analysis published in 2014, which was based on 117 studies, confirmed that prolonged breastfeeding reduced the risk of asthma up to the age of two years, but the authors called for more prospective longitudinal studies on older children 21.
An asthma diagnosis was reported at some time point from birth to 12 years of age for 14% of the children in our cohort, which was in line with the finding of 16% in the Swedish BAMSE study (translates as Children, Allergy, Milieu, Stockholm, Epidemiology) 22. Of the children with asthma during childhood in our study, 30% had transient symptoms, 38% had late onset and only 8% had persistent or relapsing symptoms. However, as many as 24% of the children with asthma during childhood had symptoms on and off at four and eight years of age. We find this interesting, as the results do not fit the traditional pattern of early transient wheeze, persistent asthma or late‐onset asthma. In addition, it should be noted that some of the parents of children with transient asthma did not report that they were symptom free until eight or even 12 years.
In our study, 65% of the asthmatic children had atopic asthma and 35% had nonatopic asthma, which was well in line with the Swedish BAMSE study just mentioned, in which 67% of children with asthma had rhinitis or eczema at the age of 12 years 22. Other studies from the north and west of Europe have reported that atopic and nonatopic asthma are equally common 3, 4, but much lower ratios for atopic asthma have been found among asthmatic children in nonaffluent countries 5. The reason for this has not been fully understood, but a difference in immune regulation caused by a difference in microbial and infectious exposures has been proposed.
The definition of current atopic asthma was chosen based on the well‐known association with allergic comorbidity and allergic sensitisation. For example, the association between atopic asthma and allergic rhinitis has been well established. In a Finnish postbronchiolitis follow‐up study of children aged five to seven years, current asthma was associated with prolonged rhinitis and allergic sensitisation skin prick test positivity 23.
We are aware that parent‐reported allergic sensitisation is not a strong parameter. Therefore, we want to point out that the definition of current atopic asthma included reported allergic sensitisation and, or, current doctor‐diagnosed rhinitis, food allergies or eczema. Furthermore, we want to emphasise the main result that antibiotics given during the first week of life increased the risk of atopic asthma at the age of 12 years and remained for asthma with current allergic rhinitis.
Our finding that 28% of the 12‐year‐old asthmatics only had asthma with a cold is notable and was higher than we expected. However, the percentage was in line with British findings 6. In a large population‐based cohort of children from Leicestershire, UK, Jurca et al. 6 found that the relative prevalence of episodic viral wheeze in the children aged 10–13 was 40%. That means that four in 10 children with wheeze only wheezed during respiratory infections.
Strengths and limitations
The strengths of this prospective follow‐up study included the large size of the birth cohort, access to perinatal data and the high response rate at 12 years of age.
Questionnaire‐based studies are accompanied by limitations relating to the validity and interpretation of the answers. To avoid this, we have used validated, well‐known questions that had previously been used in the ISAAC study (International Study of Asthma and Allergies in Childhood) 24 and the Swedish BAMSE study 25. As our cohort was not clinically tested, all the answers were based on parental reports. This could have resulted in some bias in the sample, but this is difficult to avoid in studies based on questionnaires. For example, questionnaire‐based data on the results of allergy tests should be interpreted with caution, as parents may not remember or remember incorrectly.
At inclusion, the material was largely representative of the population 15, but the loss of participants during follow‐up might have affected the results. Study participants suffering from allergic diseases may be more likely to continue participating. However, although atopic heredity was lower among nonparticipants, early wheeze, eczema and food allergies were not significantly lower. As is often seen in questionnaire studies, responders were somewhat more health conscious and well educated than nonresponders. Other basic characteristics were similar in the study participants and nonparticipants.
Conclusion
Antibiotic treatment during the first week of life increased the risk of atopic asthma at the age of 12 years. This suggests an immune‐mediated effect. Being born small for gestational age increased the risk nonatopic asthma. As many as 28% of the 12‐year‐olds only reported asthma when they had colds.
Funding
The study was supported by the Swedish Government under the ALF Agreement between the Government and the County Councils relating to the economic support of medical research, the Research Foundation of the Swedish Asthma and Allergy Association, the Gothenburg Masonic Order Orphanage Foundation and the Health & Medical Care Committee of the Regional Executive Board, Västra Götaland Region, Sweden.
Conflicts of interests
The authors have no conflicts of interests to declare.
Supporting information
Table S1 Questions on current allergic symptoms and diagnoses at age 12 years.
Table S2 Variables considered in the univariate analyses as potential risk factors or protective factors for current doctor‐diagnosed asthma at 12 years.
Table S3 Responders at 12 years (n = 3637) versus non‐responders at age 12 years, i.e. children who answered the questionnaire at six and/or 12 months of age but not at 12 years (n = 2017).
Table S4 Risk factors and protective factors with a significance level of p < 0.1 in the univariate analysis for current doctor‐diagnosed asthma at 12 years of age.
References
- 1. Hicke‐Roberts A, Åberg N, Wennergren G, Hesselmar B. Allergic rhinoconjunctivitis continued to increase in Sweden up to 2007, but asthma and eczema levelled off from 1991. Acta Paediatr 2017; 106: 75–80. [DOI] [PubMed] [Google Scholar]
- 2. Sly PD, Boner AL, Björkstén B, Bush A, Custovic A, Eigenmann PA, et al. Early identification of atopy in the prediction of persistent asthma in children. Lancet 2008; 372: 1100–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kurukulaaratchy RJ, Fenn M, Matthews S, Arshad SH. Characterisation of atopic and non‐atopic wheeze in 10 year old children. Thorax 2004; 59: 563–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Janson C, Kalm‐Stephens P, Foucard T, Alving K, Nordvall SL. Risk factors associated with allergic and non‐allergic asthma in adolescents. Clin Respir J 2007; 1: 16–22. [DOI] [PubMed] [Google Scholar]
- 5. Moncayo AL, Vaca M, Oviedo G, Erazo S, Quinzo I, Fiaccone RL, et al. Risk factors for atopic and non‐atopic asthma in a rural area of Ecuador. Thorax 2010; 65: 409–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jurca M, Pescatore AM, Goutaki M, Spycher BD, Beardsmore CS, Kuehni CE. Age‐related changes in childhood wheezing characteristics: a whole population study. Pediatr Pulmonol 2017; 52: 1250–9. [DOI] [PubMed] [Google Scholar]
- 7. Gensollen T, Iyer SS, Kasper DL, Blumberg RS. How colonization by microbiota in early life shapes the immune system. Science 2016; 352: 539–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schaub B, Liu J, Schleich I, Hoppler S, Sattler C, von Mutius E. Impairment of T helper and T regulatory cell responses at birth. Allergy 2008; 63: 1438–47. [DOI] [PubMed] [Google Scholar]
- 9. Noverr MC, Huffnagle GB. The microflora hypothesis of allergic disease. Clin Exp Allergy 2005; 35: 1511–20. [DOI] [PubMed] [Google Scholar]
- 10. Svenska Neonatalföreningen [Swedish Neonal Society]. Neonatal sepsis – ny behandlingsrekommendation [Neonatal sepsis – new treatment recommendation]. [cited 2018 Jan 30]. Available from URL: http://neo.barnlakarforeningen.se/wp-content/uploads/sites/14/2014/03/infektioner_2013.pdf.
- 11. Wickens K, Ingham T, Epton M, Pattemore P, Town I, Fishwick D, et al. The association of early life exposure to antibiotics and the development of asthma, eczema and atopy in a birth cohort: confounding or causality? Clin Exp Allergy 2008; 38: 1318–24. [DOI] [PubMed] [Google Scholar]
- 12. Marra F, Marra CA, Richardson K, Lynd LD, Kozyrskyj A, Patrick DM, et al. Antibiotic use in children is associated with increased risk of asthma. Pediatrics 2009; 123: 1003–10. [DOI] [PubMed] [Google Scholar]
- 13. Sobko T, Schiött J, Ehlin A, Lundberg J, Montgomery S, Norman M. Neonatal sepsis, antibiotic therapy and later risk of asthma and allergy. Paediatr Perinat Epidemiol 2010; 24: 88–92. [DOI] [PubMed] [Google Scholar]
- 14. Ballard O, Morrow AL. Human milk composition: nutrients and bioactive factors. Pediatr Clin N Am 2013; 60: 49–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Alm B, Erdes L, Möllborg P, Pettersson R, Norvenius SG, Åberg N, et al. Neonatal antibiotic treatment is a risk factor for early wheezing. Pediatrics 2008; 121: 697–702. [DOI] [PubMed] [Google Scholar]
- 16. Goksör E, Alm B, Thengilsdottir H, Pettersson R, Åberg N, Wennergren G. Preschool wheeze – impact of early fish introduction and neonatal antibiotics. Acta Paediatr 2011; 100: 1561–6. [DOI] [PubMed] [Google Scholar]
- 17. Goksör E, Alm B, Pettersson R, Möllborg P, Erdes L, Åberg N, et al. Early fish introduction and neonatal antibiotics affect the risk of asthma into school age. Pediatr Allergy Immunol 2013; 24: 339–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. den Dekker HT, Sonnenschein‐van der Voort AM, de Jongste JC, Anessi‐Maesano I, Arshad SH, Barros H, et al. Early growth characteristics and the risk of reduced lung function and asthma: a meta‐analysis of 25,000 children. J Allergy Clin Immunol 2016; 137: 1026–35. [DOI] [PubMed] [Google Scholar]
- 19. Tedner SG, Örtqvist AK, Almqvist C. Fetal growth and risk of childhood asthma and allergic disease. Clin Exp Allergy 2012; 42: 1430–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dekker HT, Sonnenschein‐van der Voort AM, Jaddoe VW, Reiss IK, de Jongste JC, Duijts L. Breastfeeding and asthma outcomes at the age of 6 years: the Generation R Study. Pediatr Allergy Immunol 2016; 27: 486–92. [DOI] [PubMed] [Google Scholar]
- 21. Dogaru CM, Nyffenegger D, Pescatore AM, Spycher BD, Kuehni CE. Breastfeeding and childhood asthma: systematic review and meta‐analysis. Am J Epidemiol 2014; 179: 1153–67. [DOI] [PubMed] [Google Scholar]
- 22. Ballardini N, Kull I, Lind T, Hallner E, Almqvist C, Östblom E, et al. Development and comorbidity of eczema, asthma and rhinitis to age 12: data from the BAMSE birth cohort. Allergy 2012; 67: 537–44. [DOI] [PubMed] [Google Scholar]
- 23. Lauhkonen E, Koponen P, Nuolivirta K, Helminen M, Paassilta M, Toikka J, et al. Following up infant bronchiolitis patients provided new evidence for and against the united airway disease hypothesis. Acta Paediatr 2016; 105: 1355–60. [DOI] [PubMed] [Google Scholar]
- 24. Asher MI, Keil U, Anderson HR, Beasley R, Crane J, Martinez F, et al. International Study of Asthma and Allergies in Childhood (ISAAC): rationale and methods. Eur Respir J 1995; 8: 483–91. [DOI] [PubMed] [Google Scholar]
- 25. Wickman M, Kull I, Pershagen G, Nordvall SL. The BAMSE project: presentation of a prospective longitudinal birth cohort study. Pediatr Allergy Immunol 2002; 13(Suppl 15): 11–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Questions on current allergic symptoms and diagnoses at age 12 years.
Table S2 Variables considered in the univariate analyses as potential risk factors or protective factors for current doctor‐diagnosed asthma at 12 years.
Table S3 Responders at 12 years (n = 3637) versus non‐responders at age 12 years, i.e. children who answered the questionnaire at six and/or 12 months of age but not at 12 years (n = 2017).
Table S4 Risk factors and protective factors with a significance level of p < 0.1 in the univariate analysis for current doctor‐diagnosed asthma at 12 years of age.


