Abstract
Background
The pulsed‐dye laser has been used to treat facial redness and rosacea for decades. Recent advances in dye laser technology enable 50% higher output energies supporting 50% larger treatment areas, and beam‐diameters up to 15 mm with clinically‐relevant fluences. In this study, we investigate this novel pulsed‐dye laser using a 15 mm diameter beam for treatment of rosacea.
Methods
Twenty subjects with erythemato‐telangiectatic rosacea were enrolled in the study. A total of 4 monthly treatments were administered, first treating linear vessels with a 3 × 10 mm elliptical beam, then diffuse redness with a 15‐mm diameter circular beam. Blinded assessment of digital, cross‐polarized photographs taken 2 months following the last treatment was performed using an 11‐point clearance scale.
Results
Nineteen subjects completed the study. Blinded reviewers correctly identified baseline photos in 55 out of the total of 57 images (96.5%). The blinded reviewers scored 17 of the 19 subjects with an improvement greater than 40%, and 11 of the 19 subjects greater than 50%. The average improvement was 53.9%. Side effects were limited to mild edema, mild to moderate erythema, and mild to moderate bruising.
Conclusion
This study demonstrates that a newly designed pulsed‐dye laser having a novel 15‐mm diameter treatment beam improves the appearance of rosacea with a favorable safety profile. Lasers Surg. Med. 50:808–812, 2018. © 2018 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: rosacea, laser, treatment, pulsed dye laser, 610‐645‐5551 p, 610‐645‐5151 f
INTRODUCTION
Rosacea results from chronic sun‐exposure and is more commonly seen in lightly pigmented individuals who are genetically susceptible to develop diffuse redness, telangiectasias, and/or papules and pustules in response to chronic sun‐exposure. Numerous stimuli cause these extra blood vessels that occur due to chronic sun‐exposure to temporarily dilate, including: hot, cold, exercise, sunlight, coffee, alcoholic beverages, and many other common aspects of normal life 1, 2, 3, 4. The main culprit in causing rosacea is sun‐exposure as evidenced by the predominance of photodamage, including facial redness and telangiectasias, on the side of the face adjacent to the car window for drivers and passengers, which varies by country 5, 6. This would implicate UVA as having a significant impact on developing rosacea, because window glass blocks virtually all UVB. Rosacea is characterized by facial redness, spider veins, flushing in response to numerous stimuli, and papules and/or pustules. Subjects may also present with facial stinging and burning, ocular symptoms, and hypertrophy of nasal sebaceous glands, termed rhinophyma 2, 4. Rosacea prevalence ranges widely from 1% to 22% depending upon the population and the means of surveying various populations, with one study demonstrating a prevalence of 9.6% in a diverse population, and 16% in a more focused study of Caucasian women 7. Another study directly examining people for rosacea, and not depending upon historical data, found 10% of those examined had rosacea in one community, with women having a higher prevalence (14%) than men (5%) 8. Whatever the true numbers, rosacea is a very common disorder, caused in very large part by chronic sun‐exposure.
Vascular‐targeting lasers are a mainstay for treating superficial skin vessels, which are the root‐cause of the flushing and blushing that characterize rosacea. In this study, we investigate the safety and effectiveness of treating rosacea using a novel pulsed dye laser (PDL). The improved efficiency of this new PDL enables delivery of higher fluences, and/or much larger beam diameters than were previously available, as well as longer dye life than currently‐available PDLs.
MATERIALS AND METHODS
Subjects
This IRB‐approved study enrolled twenty subjects diagnosed with rosacea, with 19 subjects completing the study, and one subject discontinuing due to moving away from the area where the study was being performed. The age of the subjects ranged from 36 to 69 years with a mean age of 52 ± 10 (mean ± sd). Three subjects were male and 16 were female. Fifteen subjects had Fitzpatrick skin type II, three had skin type III, and one had skin type IV. Subjects who had previous laser treatments or deep chemical peels, a history of keloid formation, or a history of vitiligo were excluded from the study.
Laser
The pulsed dye laser used in this study (V‐Beam Prima, Syneron‐Candela, Wayland, MA) was a re‐designed version of the earlier generation PDL (V‐Beam Perfecta, Syneron‐Candela) with a newly‐designed laser cavity, and incorporating a dynamic spray cooling device as did the earlier‐generation PDL. Modifications included a more efficient laser cavity design allowing up to a 12 J maximum energy, compared to 8 J delivered with the predecessor PDL, and a maximum 15 mm diameter treatment beam compared to 12 mm with the earlier PDL. This is a 56% increase in area for the largest available spot size. Available pulse durations ranged from 0.45 to 40 ms.
Laser Treatment
All subjects received full‐face treatments with the novel PDL (V‐Beam Prima, Syneron‐Candela). Of the 19 subjects, 17 received the maximum four laser treatments with a 1 month interval between treatments, while two subjects missed a single treatment and had just three treatments due to scheduling difficulties. All treatments were administered and visualized by the investigator using a cross‐ and parallel‐polarizing head lamp (v900 Syris Scientific, Gray, ME). Linear vessels were first treated using a 3 × 10 mm elliptical spot at a fluence of 15 J/cm2 and a 40 ms pulse duration. Then diffuse redness was treated over the entire face with a 15 mm diameter circular beam, a pulse‐duration of 3 ms, and increasing average fluences over the four treatments starting at 6.25 J/cm2 for the first treatment and averaging 6.97 J/cm2 for the final treatment (Table 1). Fluences were selected by the treating physician based on extensive experience with earlier generation PDLs, and observation of tissue responses during laser treatment. On average, approximately 200 pulses were required to cover the full face with the 15 mm diameter treatment beam (Table 1). The median number of pulses delivered per treatment with the 3 × 10 mm elliptical spot was 143 (range: 41–394 pulses) for the first treatment, and decreased to a median 65 pulses (range: 15–240) at the fourth treatment, due to clearance of some vessels during the treatment period (Table 1). The average number of pulses delivered with the 15 mm diameter treatment beam was 195 (range: 172–206) over the four treatments (Table 1). There was little variation in the number of pulses delivered with the 15 mm diameter treatment beam because the entire facial surface was covered during each treatment. Epidermal protection was afforded by administration of cryogen spray cooling with the integrated dynamic cooling device (DCD), using a 40 ms spray duration delivered 20 ms prior to the laser pulse.
Table 1.
Rosacea Treatment Fluences and Typical Pulses Delivered for the 3 × 10 mm Table 1
| Spot size | Parameter | Tx1 | Tx2 | Tx3 | Tx4 |
|---|---|---|---|---|---|
| 15 mm | Mean fluence ± Std Dev (J/cm2) | 6.25 | 6.59 ± 0.15 | 6.91 ± 0.15 | 6.97 ± 0.12 |
| Range | – | 6.25–6.75 | 6.5–7.0 | 6.5–7.0 | |
| Median number of pulses | 206 | 199 | 204 | 172 | |
| Range | 168–387 | 147–327 | 140–436 | 60–390 | |
| 3 × 10 | Mean fluence ± Std Dev (J/cm2) | 15 ± 0 | 15 ± 0 | 15 ± 0 | 15 ± 0 |
| Range | – | – | – | – | |
| Median number of pulses | 143 | 89 | 69 | 65 | |
| Range | 41–394 | 28–431 | 10–147 | 15–240 |
Rosacea treatment fluences and typical pulses delivered for the 3 × 10 mm elliptical and 15 mm diameter treatment beams. Pulse duration was 40 and 3 ms, respectively.
Blinded Evaluation of Digital Images
A commercial research‐grade digital facial photography system (Visia CR, Canfield Scientific Inc., Fairfield, NJ) was used for pre‐ and post‐treatment photographs. This system enables standardization of digital images by providing a photographic booth for the patients face, ensuring controlled lighting conditions. In addition, the positioning of the subject's faces was matched pre‐ and post‐treatment using a digital mode that enables visualization of the pre‐treatment image while superimposing the subject's face in real‐time. Cross‐polarized images were used to compare pre‐ and post‐treatment images, because cross‐polarization eliminates surface reflection, enabling unencumbered visualization of linear vessels, diffuse redness, and the red inflammatory papules that characterize rosacea. Each subject had a frontal photograph as well as left and right photographs taken at a 45° angle, for a total of three image pairs per subject per visit.
Improvement of rosacea was determined by blinded comparison of randomized baseline and final, cross‐polarized images taken 2 months following the final treatment. Three blinded physician reviewers were first asked to identify the baseline image, and then asked to rate any improvement on an 11‐point scale ranging from 0 to 100% in 10% increments (0, 1–10%, 11–20% up to 91–100%). If a reviewer incorrectly identified a baseline image, the score was given a negative value (e.g., a 3 was reassigned as −3).
Side‐Effects
Side‐effects including: pain, post treatment erythema, edema, and purpura were evaluated throughout the study. The subjects self‐reported pain scores using a 0–10 scale (0 = No pain to 10 = severe pain). Post‐treatment erythema, edema, and purpura were evaluated by the treating physician using a 0–3 scale corresponding to no (0), mild (1), moderate (2), and severe (3) erythema, edema, or purpura. Longer‐term side effects such as hyperpigmentation, hypopigmentation, and scarring were evaluated by the treating physician at the final 2‐month follow‐up visit using the same three‐point scale.
RESULTS
Blinded Evaluation of Digital Photographs
Blinded evaluators correctly identified the baseline image from the 2‐month follow‐up image in 55 out of a total of 57 image pairs (19 subjects, 3 reviewers) for a success rate of 96.5% (Fig. 1). One reviewer correctly identified the baseline image in all images pairs, while the remaining two reviewers misidentified the baseline image in a single pair. The two image pairs that were misidentified were from different subjects, with two of three reviewers correctly identifying the baseline image in each of these two cases. Improvement scores averaged 53.9 ± 2.6% (mean ± sem), and ranged from 6.6–86.7%. Sixteen out of the 19 subjects (84.2%) had over a 40% improvement, and 11 out of the 19 subjects (57.9%) had a greater than 50% improvement.
Figure 1.
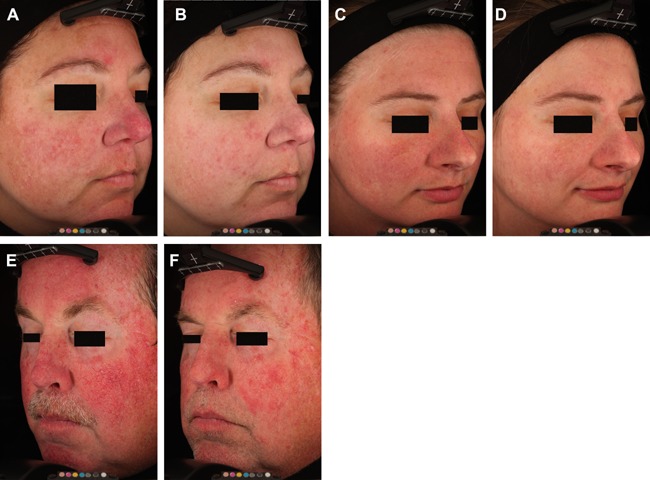
Baseline (A,C,E) and post‐treatment (B,D,F) cross‐polarized images. All follow‐up images were collected 2‐months following the last treatment. The subject in figures E and F had only three treatments.
Side Effects
Subjects reported an average pain score of 5.6 ± 1.8 out of a maximum of 10. Post‐treatment, most subjects reported mild edema, mild to moderate erythema, and mild to moderate purpura. For the first treatment four subjects had mild purpura, and for the second eight had mild and one had moderate purpura, while for the third eight had mild purpura and eight had moderate purpura, and for the final treatment nine had mild purpura and four had moderate purpura. Edema and erythema typically resolved within a couple days following treatment, while purpura usually resolved within a week. There were no incidents of blistering, pigmentary alterations, or scarring.
DISCUSSION
This study investigates the efficacy and safety of a newly‐designed, long pulse‐duration, higher‐energy, 595 nm PDL incorporating a DCD for epidermal protection for treating rosacea. This laser proved highly effective at improving rosacea including both the diffuse redness and telangiectasias, as evaluated by blinded physicians rating randomized digital images after an average of 4 monthly treatments. All side‐effects were expected and self‐limited including erythema, edema, and purpura. When treating linear telangiectasias, longer pulse‐durations (>10 ms) are generally required to deliver purpura‐free removal of linear vessels, as was used in current study with the elliptical spot. For removing diffuse redness, longer pulse‐durations are generally less effective than shorter ones; however, balancing the risk of purpura at pulse durations of 3 ms and under, as chosen in the current study, must be weighed against the decreased ability to remove diffuse redness and the risk of increased edema at longer pulse‐durations. Clinicians will have to choose for themselves whether to treat diffuse redness with longer pulse durations in the 6–10 ms range, or shorter pulse‐durations risking purpura.
Blinded evaluators rated an average improvement of over 50% in randomized pairs of digital, cross‐polarized images, taken with an imaging system that controls for external lighting and enables replication of head positioning with a video system that matches the pre‐ and post‐treatment head positions. Cross‐polarization removes any surface reflection from the skin, enhancing the appearance of sub‐surface features such as linear blood vessels, erythema, and pigmentation. Because cross‐polarized photography accentuates the appearance of telangiectasias and erythema, it enables the best assessment of laser‐effects on rosacea. Although this is not a true image of what an observer would see, any use of flash photography alters the appearance of the subject. Cross‐polarized images are best for assessing laser treatment effects on any sub‐surface pigment or vasculature.
Bernstein and Kligman studied the earlier generation of this laser (V‐Beam Perfecta, Syneron‐Candela) for treating rosacea 9. The laser in that study used an identical wavelength and pulse‐durations to the current study, with an identical treatment beam for treating linear telangiectasias (3 × 10 mm), but a different beam diameter for treating the entire face with a circular spot for diffuse redness (12 mm diameter treatment beam in the previous study vs. 15 mm in the current study). Treatment of linear vessels was with identical pulse‐durations and beam diameters, with the earlier study using slightly higher fluences than the current study, ranging from 17 to 19 J/cm2 in the earlier study and staying fixed at 15 J/cm2 in the current study. The fluences used for treatment of the entire face were virtually identical ranging from 6 to 7 J/cm2 in the earlier study 9, and 6.25–7 J/cm2 in the current study. The major difference between the two studies in terms of laser treatment parameters, was the 12 mm diameter treatment beam in the earlier study versus a 15 mm beam diameter used to treat the entire face in the current study. The larger 15 mm spot is 56% larger than the 12 mm‐diameter treatment beam. This not only translates into more rapid treatments, but also can impact the dynamics of laser‐tissue interactions increasing the subsurface fluence and the depth of penetration of laser energy into the skin.
Due to tissue scattering of light, the probability of light scattering to regions outside the collimated treatment beam is higher for smaller diameter beams compared to larger diameter beams. As a result, even though light scatters with similar distances independent of beam diameter, the probability of staying inside the collimated treatment column is higher for light scattered in a larger beam diameter. The net result is higher subsurface fluence for the larger beam diameter when measured at comparable depths 10, 11, 12. In addition, similar subsurface fluences will be found deeper in tissue for the larger diameter beam. For similar incident fluences, the safety profile is increased due to the higher subsurface fluences 10, 11.
Larger beam diameters should also lead to more complete coverage of a given area. In comparing the two studies, the current study had 96% of the images correctly identified by blinded observers, whereas in the previous study 80% were correctly identified. A different rating scale was used in the two studies so direct comparisons cannot be made; however, in terms of the percentage improvement of blinded digital images, the current study showed a 54% improvement, versus 39% in the previous study 9. Although treatment parameters were quite similar in the two studies, with the main variable being the larger treatment beam of the higher‐powered laser used in the current study; direct comparisons between the two studies are limited by the different rating scales used.
The laser used in the current study (V‐Beam Prima, Syneron‐Candela) delivers 50% more power than its predecessor (V‐Beam Perfecta, Syneron‐Candela), and has an entirely new laser design enabling dramatically longer dye life, when operating at the maximum fluences available with the previous‐generation device. Ultimate dye life in normal clinical use will depend upon the fluence/beam‐diameter combinations available with the newer production laser, and how each individual clinician uses the device. This long‐awaited re‐design of the PDL, a mainstay of cutaneous laser therapy for over a quarter‐century, has enabled dramatically increased longevity of dye‐kits which should translate into even greater reliability in the field. The significantly larger diameter treatment beams with higher available fluences will reduce treatment times and should impact clinical results in a positive way.
ACKNOWLEDGMENTS
Dr. Bernstein is a consultant for, got equipment discounts and funding for this study, and holds equity in Syneron‐Candela. Dr. Schomacker, Dr. Paranjape, and Mr. Jones are employees of Syneron‐Candela at the time this work was done. Research funding for this project and equipment loan were provided by Syneron‐Candela.
Conflicts of Interest Disclosures: Eric F. Bernstein reports grants from Syneron‐Candela to support the study; consulting fees from Syneron‐Candela, equity from Syneron‐Candela, outside the submitted work. Kevin Schomacker, Amit Paranjape, and Christopher J. Jones are employees of Syneron‐Candela.
REFERENCES
- 1. Two AM, Wu W, Gallo RL, Hata TR. Rosacea: part I. Introduction, categorization, histology, pathogenesis, and risk factors. J Am Acad Dermatol 2015; 72(5):749–758. [DOI] [PubMed] [Google Scholar]
- 2. Wilkin J, Dahl M, Detmar M, et al. Standard classification of rosacea: report of the national rosacea society expert committee on the classification and staging of rosacea. J Am Acad Dermatol 2002; 46(4):584–587. [DOI] [PubMed] [Google Scholar]
- 3. Yamasaki K, Gallo RL. The molecular pathology of rosacea. J Dermatologic science 2009; 55(2):77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Powell FC. Clinical practice. rosacea. New Eng J Medicine 2005; 352(8):793–803. [DOI] [PubMed] [Google Scholar]
- 5. Parisi AV, Wong JC. Quantitative evaluation of the personal erythemal ultraviolet exposure in a car. Photodermatol Photoimmunol Photomed 1998; 14(1):12–16. [DOI] [PubMed] [Google Scholar]
- 6. Kimlin MG, Parisi AV. Ultraviolet radiation penetrating vehicle glass: a field based comparative study. Physics Med Biol 1999; 44(4):917–926. [DOI] [PubMed] [Google Scholar]
- 7.Rosacea now estimated to affect at least 16 million Americans. Rosacea Review 2010; http://www.rosacea.org/rr/2010/winter/article_1.php, Accessed November 26, 2017.
- 8. Berg M, Liden S. An epidemiological study of rosacea. Acta Derm Venereol 1989; 69(5):419–423. [PubMed] [Google Scholar]
- 9. Bernstein EF, Kligman A. Rosacea treatment using the new‐generation, high‐energy, 595nm, long pulse‐duration pulsed‐dye laser. Lasers Surg Med 2008; 40(4):233–239. [DOI] [PubMed] [Google Scholar]
- 10. Keijzer M, Pickering JW, van Gemert MJC. Laser beam diameter for port wine stain treatment. Lasers Surg Med 1991; 11:601–605. [DOI] [PubMed] [Google Scholar]
- 11. Ross EV, Childs J. Role of beam spot size in heating targets at depth. J Drugs Dermatol 2015; 14:1437–1442. [PubMed] [Google Scholar]
- 12. Ash C, Dubee M, Donne K, Bashford T. Effect of wavelength and beam width on penetration in light‐tissue interaction using computational methods. Lasers Med Sci 2017; 32:1909–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]


