Abstract
Background
Modifying attentional processes with attentional bias modification (ABM) might be a relevant add‐on to treatment in addiction. This study investigated whether influencing cortical plasticity with transcranial direct current stimulation (tDCS) could increase training effects. tDCS could also help alcohol‐dependent patients to overcome craving and reduce relapse, independent of training. These approaches were combined to investigate effects in the treatment of alcoholism.
Methods
Ninety‐eight patients (analytical sample = 83) were randomly assigned to 4 groups in a 2‐by‐2 factorial design. Patients received 4 sessions of ABM (control or real training) combined with 2 mA tDCS (active: 20 minutes or sham: 30 seconds) over the left dorsolateral prefrontal cortex. Alcohol bias and craving were assessed, and treatment outcome was measured as relapse after 1 year.
Results
Attentional bias scores indicated that during the training only the group with active tDCS and real ABM displayed an overall avoidance bias (p < 0.05). From pre‐ to postassessment, there were no main or interaction effects of tDCS and ABM on the bias scores, craving, or relapse (p > 0.2). However, effects on relapse after active tDCS were in the expected direction.
Conclusions
There was no evidence of a beneficial effect of tDCS or ABM or the combination. Whether the absence of effect was due to issues with the outcome measurements (e.g., lack of craving, high dropout, and unreliable measurements) or aspects of the intervention should be further investigated.
Keywords: Transcranial Direct Current Stimulation, Cognitive Bias Modification, Addiction, Alcohol
For someone with an alcohol use disorder (AUD), the image of alcohol can be a salient stimulus that captures attention and activates an emotional response (Franken, 2003). Automatically triggered responses to alcohol are potentially relevant for various stages of AUD and have been studied broadly (for a review, see Wiers et al., 2013). Cognitive bias modification (CBM) paradigms are a family of novel interventions that aim to directly address these processes. In these paradigms, relatively automatic responses are retrained into more beneficial reactions with computerized training. Several CBM studies (mostly with another variety of CBM: approach bias retraining) have shown positive findings in alcohol‐dependent populations (Eberl et al., 2013; Manning et al., 2016; Rinck et al., in press; Schoenmakers et al., 2010; Wiers et al., 2011). This clinical study focused on attentional bias modification (ABM), in which participants are trained to no longer focus attention on alcohol‐related stimuli.
An attentional bias is often measured with a visual probe task (VPT) in which targets can follow nonalcohol or alcohol‐related stimuli. A relatively fast response time when a target is presented on the location where the alcohol‐related stimuli had been indicates an attentional bias. In a training version, targets usually appear at the location of nonalcohol stimuli, training attention away from the alcohol. Most ABM studies included smokers or hazardous drinkers, as of yet only 3 ABM studies have been published with an alcohol‐dependent sample (for review, see Wiers et al., 2018). At the start of this study, a first small study ABM was found to reduce attentional bias and time to relapse (Schoenmakers et al., 2010). In a second small study, AUD patients with high levels of comorbid social anxiety performed ABM both for alcohol and for social anxiety, and no effects were found (Clerkin et al., 2016). A recent large study (N > 1,400) examined the effects of ABM (real/sham) and a different form of CBM (approach bias modification) and found specific effects on biases and a significantly reduced relapse rate for both types of training a year after treatment discharge (Rinck et al., in press).
There is currently debate on the effectiveness of CBM and ABM methods (Christiansen et al., 2015; Cox et al., 2014; Cristea et al., 2016; Macleod, 2012; Wiers et al., 2018), also with regard to anxiety research, a field where ABM has been tested more extensively (Beard, 2011; Clarke et al., 2014b; Cristea et al., 2015; Emmelkamp, 2012; Mogoase et al., 2014). Regarding anxiety, MacLeod and Grafton (2016) have demonstrated that beneficial clinical results have consistently been found in anxiety studies when ABM successfully changed the bias, and no effects were found when ABM did not result in a change in bias. It is therefore relevant to further investigate ABM in a clinical sample and examine whether these bias changing effects can be improved. In the field of addiction, a meta‐analysis combined proof‐of‐principle studies in students not motivated to change with clinical randomized controlled trials (RCTs) and “cast serious doubts on the clinical utility of CBM interventions for addiction problems” (Cristea et al., 2016, p. 2). However, once the apples (proof‐of‐principle experimental studies) and oranges (RCTs in clinical samples) were separated, the picture became clear; with small short‐lasting effects of CBM on immediate outcome measures (e.g., a taste test) in students in case the bias was successfully manipulated (as in anxiety), and consistent small but clinically meaningful add‐on effect of CBM to regular treatment in RCTs in clinical samples, with effect sizes approximately the same size as medication (Wiers et al., 2018). Here, we tested whether the effects of ABM could be augmented by stimulating relevant neural networks.
An attentional bias toward alcohol can be explained by incentive sensitization, a neural process in which the neural activity underlying the incentive value of stimuli becomes more responsive to addictive substances after repeated use (Robinson and Berridge, 1993). From an evolutionary perspective, it makes sense that rewarding stimuli rapidly grab attention (Chelazzi et al., 2013; Theeuwes and Belopolsky, 2012). An attentional bias may play a role in the maintenance of addiction and may hinder recovery. The relation between attentional bias and dependence‐related processes such as relapse and craving is complex: Relapse predictions showed rather inconsistent findings (Christianen et al., 2015), and a meta‐analysis showed only a modest relation to craving (Field et al., 2009). This could also be due to the measurement issues; for example, craving is complex and difficult to measure (Breiner et al., 1999), and the assessment of attentional bias is often unreliable (Ataya et al., 2012). Neuroimaging studies show that subcortical brain regions, such as the amygdala, play a significant role in triggering attentional processes (Vuilleumier, 2005). The lateral prefrontal cortex is involved in controlling attention over emotional stimuli, and the lack of control over an attentional bias might be related to impairments in these regions (Arnsten and Rubia, 2012; Bishop, 2009; Browning et al., 2010).
Clarke and colleagues (2014a) found that sending a small electrical current through the left dorsolateral prefrontal cortex (DLPFC) with transcranial direct current stimulation (tDCS) could help modulate attentional bias in highly anxious participants. Anodal stimulation, which is believed to increase excitability under the electrode (whereas cathodal decreases excitability), increased bias acquisition. A similar study found no effect on reaction time measures of attentional bias, but did find that anodal stimulation combined with ABM reduced fixation on angry faces (Heeren et al., 2015). Although the exact underlying mechanisms of tDCS are still under investigation, anodal tDCS is used to increase efficiency and plasticity in the underlying cortical area (Medeiros et al., 2012; Nitsche and Paulus, 2000; Rahman et al., 2013), increase cognitive performance (Hill et al., 2016; Nitsche and Paulus, 2000, 2011), and enhance effects of cognitive training (Elmasry et al., 2015). Adding tDCS to the ABM training might increase the effect of modifying the alcohol bias and thus provide a valuable addition to alcohol dependence treatment. Stimulation of the DLPFC (either left or right) without any simultaneous task or training has previously been used to reduce craving (meta‐analysis: Jansen et al., 2013); furthermore, there are initial results indicating that it can be used to reduce relapse (Klauss et al., 2014). A previous study combining tDCS with an alcohol approach bias retraining also showed a trend‐level effect on relapse, although it did not lead to a stronger bias at posttest (den Uyl et al., 2017). Modulating approach bias with tDCS in a heavy drinking population was also not successful (den Uyl et al., 2016). In this study, we want to investigate whether tDCS can have an effect on ABM training and craving in a clinical setting with alcohol‐dependent inpatients.
We tested whether 4 sessions of ABM (control vs. real) training combined with 2 mA anodal DLPFC tDCS (sham vs. active) had beneficial effects on behavior and clinical measures in alcohol‐dependent inpatients. We hypothesize that those patients who received tDCS stimulation while doing ABM training would develop stronger avoidance for alcohol‐related stimuli and demonstrate a reduction in craving. We also expected, similar to den Uyl and colleagues 2017, where we found a trend‐level effect of tDCS on relapse, that tDCS would benefit relapse prevention and that this effect may be more pronounced in the ABM training group.
Materials and Methods
Participants
Inpatients were recruited from the Salus Clinic in Lindow, Germany, where inpatient treatment takes an average of 3 months. Patients were included in the study from December 2014 to June 2015. Participants were allowed to participate in the study if none of the tDCS exclusion criteria applied (epilepsy, multiple sclerosis or other neurological illness, previous brain injury/infection, metal in the brain, pacemaker, pregnancy, claustrophobia, recent fainting/panic attack, frequent headaches or dizziness, eczema, or other skin conditions). Based on previous studies and feasibility, we aimed for a sample of 100 participants (Klauss et al., 2014). Ninety‐eight patients were included in the study (Fig. 1), 13 dropped out during testing (10 no reason/no further motivation, 1 due to craving, 1 due to relapse, and 1 due to an inclusion error), and 2 were later excluded (1 was analphabetic and 1 had a primary diagnosis of gambling addiction). The final analytical sample consisted of 83 patients (23 women and 60 men), with an average age of 48.6 years (Table 1). All patients gave written informed consent, and the study was approved by the Ethical Committee of the German Pension Fund and the University of Chemnitz. The trial was registered in the Dutch Clinical Trial Registry (NTR5016).
Figure 1.
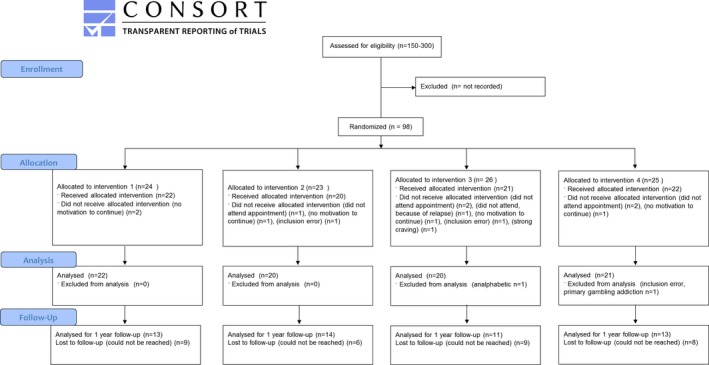
Flow diagram according to CONSORT 2010. Intervention 1 consists of control attentional bias modification (ABM) combined with sham transcranial direct current stimulation (tDCS), intervention 2 consists of control ABM combined with active tDCS, intervention 3 consists of real ABM combined with sham tDCS, and intervention 4 consists of real ABM combined with active tDCS.
Table 1.
Demographic Variables
| 1. Control ABM + sham tDCS | 2. Control ABM + active tDCS | 3. Real ABM + sham tDCS | 4. Real ABM + active tDCS | Total | p | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | M | SE | ||
| Gender (F/M) | 5/17 | 4/16 | 5/15 | 7/14 | 21/62 | 0.78 | |||||
| Smoker (Y/N) | 13/9 | 16/4 | 17/3 | 14/7 | 60/23 | 0.22 | |||||
| Age (years) | 48.23 | 1.93 | 48.65 | 1.63 | 49.20 | 1.98 | 48.38 | 2.06 | 48.60 | 0.94 | 0.99 |
| Duration of alcohol problems (years) | 18.15 | 2.28 | 15.37 | 2.31 | 16.80 | 2.89 | 19.00 | 2.44 | 17.37 | 1.23 | 0.75 |
| Alcohol problems (AUDIT score) | 27.27 | 1.66 | 23.70 | 1.52 | 24.40 | 1.47 | 26.00 | 1.50 | 25.40 | 0.77 | 0.36 |
| Number of detoxifications | 4.00 | 1.29 | 1.80 | 0.77 | 2.80 | 0.79 | 3.71 | 1.12 | 3.11 | 0.52 | 0.43 |
| Duration of treatment (days) | 80.59 | 2.53 | 80.30 | 3.78 | 75.10 | 4.30 | 73.43 | 3.70 | 77.39 | 1.80 | 0.38 |
| Start experiment (days) | 10.68 | 1.05 | 11.30 | 1.22 | 10.00 | 1.11 | 12.62 | 1.49 | 11.16 | 0.61 | 0.49 |
| Depression (BDI score) | 14.27 | 2.67 | 12.57 | 2.41 | 11.10 | 2.96 | 12.52 | 2.33 | 12.66 | 1.28 | 0.86 |
| Mental burden (GSI SCL‐90‐R score) | 58.18 | 3.44 | 58.27 | 3.05 | 57.40 | 3.20 | 58.33 | 3.21 | 58.05 | 1.59 | 1.00 |
| Craving baseline (PACS score) | 3.59 | 0.68 | 2.95 | 0.64 | 5.45 | 1.26 | 4.38 | 1.08 | 4.08 | 0.48 | 0.30 |
AUDIT, Alcohol Use Disorders Identification Test; ABM, attentional bias modification; BDI, Beck Depression Inventory; GSI, global severity index; SCL‐90‐R, Symptom Checklist‐90—Revised; PACS, Penn Alcohol Craving Scale; tDCS, transcranial direct current stimulation.
Overview of the mean (M) and standard deviation (SD) of the baseline scores for all demographic variables per group. The p‐values represent outcomes of an ANOVA with group as between‐subject variable.
Design and Intervention
Design
We used a 2‐by‐2 double‐blind factorial design (control vs. real ABM and sham vs. active tDCS). Patients were randomly assigned to 1 of the 4 experimental groups. The tDCS device (NeuroConn DC‐stimulator Plus; neuroCare Group GmbH, Ilmenau, Germany) had a function incorporated that could apply sham or active stimulation with predetermined codes. In the script for the ABM training, a function was also incorporated to determine control or real ABM with predetermined codes. The codes that determined the placebo or real training variety for each group were covertly randomized within an Excel list with the rand function. The training consisted of 4 sessions of ABM training (control or real) during which patients received either sham or active tDCS.
Transcranial Direct Current Stimulation
The 2 mA stimulation was given through saline‐soaked sponges that contained the electrodes, which were attached to the head with rubber straps. The 35 cm2 anodal electrode was positioned over the F3 (10 to 20 electroencephalogram [EEG]) location (used for left DLPFC), and the 100 cm2 cathodal electrode was positioned over the F4. A 100 cm2 cathodal electrode was used to approximate unilateral stimulation (Boggio et al., 2008; den Uyl et al., 2017). To reduce shunting, we aimed for a 8 cm gab between electrodes (by slightly adjusting the F4 electrode). When giving active stimulation, the device was turned on for 20 minutes, and for sham stimulation, the active stimulation was automatically turned off after 30 seconds (while the display was still on). A fade‐in period of 30 seconds and a fade‐out period of 10 seconds were used.
Attentional Bias Modification
In this task, participants were required to respond to probes (arrows pointing up or down), which appeared on the location of 1 of the preceding pictorial stimuli. The trial started with a fixation cross (with a variable interstimulus interval (ISI) between 500 and 1,000 ms), followed by 2 pictures on the left and right of the screen presented for 500 ms. These 2 pictures were followed by an arrow on 1 of the 2 locations. For upward arrows, participants were required to press the G of the keyboard, for downward arrows the B, and the probe remained on the screen until a response was given. The attentional bias task was made similar to Zvielli and colleagues (2014) and also included trials on which the target was absent and surprise trials. The 2 stimuli were either alcohol and nonalcohol beverages, or in some cases 2 nonalcohol beverages (absent target), or 2 objects (surprise trial). In the training version, the probe appeared after the nonalcohol stimulus in 9 of 12 trials, and the probe appeared after the alcohol stimulus in 1 of 12 trials (contingency probe after nonalcohol vs. probe after alcohol: 90 to 10%); in the control version of the training, the contingency was kept equivalent (5/12 probe after alcohol and 5/12 probe after nonalcohol). In 1 of 12 trials, the 2 stimuli were both nonalcohol, and in another 1 of 12 of the trials, the 2 stimuli were both objects. The trials with objects were surprise trials, to increase the semantic processing of the pictures, and required a different response (pressing the space bar; Zvielli et al., 2014). For the trials with alcohol and nonalcohol stimuli, 16 alcohol and 16 nonalcohol stimuli were used, and 16 different nonalcohol images were used for the absent target trials. The pictures were matched for color (e.g., vodka with water) and composition (e.g., the presence of a glass/person). Local beverages were used, and the images were shot according to the protocol from Pronk and colleagues (2015). Each training session consisted of 468 trials, which took approximately 15 to 20 minutes (depending on the reaction time of the participant). When the training was completed before the 20 minutes of tDCS, the participant remained seated until the stimulation time was also finished.
Outcome Measures
Alcohol VPT
The design of the VPT was similar to the ABM training, only with equal amounts of alcohol, nonalcohol, and absent target trials (36 trials each), and it included 12 surprise trials. The first session was preceded by 20 practice trials to familiarize participants with the task. We calculated the original attentional bias score by subtracting average reaction times (only for accurate trials, and outliers >3 SD were deleted) for the congruent (alcohol target) trials from the incongruent (nonalcohol target) trials. Reliability scores were calculated by taking the split‐half (first half vs. second half) and the test–retest (preassessment vs. postassessment) correlations.
Implicit Association Task
Transfer in approach and avoidance associations was measured with a 7‐block Implicit Association Task (IAT; Ostafin and Palfai, 2006). Words were presented in the center of the screen and were categorized into a category shown on the left or right bottom of the screen (with k or d of the keyboard). Target categories were alcohol (e.g., beer) or nonalcohol (e.g., cola), and attribute categories were approach (e.g., grab) or avoid (e.g., push away). Each attribute categories were shown together in 1 block to measure the speed to categorize alcohol with approach (and nonalcohol with avoid) and vice versa. The blocks were organized into target category practice (block 1, 24 trials), attribute category practice (block 2, 24 trials), combined approach‐alcohol (practice block 3, 24 trials, test block 4, 48 trials), reversed target category practice (block 4, 24 trials), and combined avoid‐alcohol (practice block 3, 24 trials; test block 4, 48 trials). The reaction times for the congruent block (approach‐alcohol) were subtracted from the incongruent block (avoid‐alcohol) to form an alcohol bias score. As order effects can be asymmetrical, all subjects received the same order to increase predictive validity (Perugini et al., 2010).
Craving
Craving during the previous week was measured with the Penn Alcohol Craving Scale (PACS) (Flannery et al., 1999), which included 5 questions (with a 6‐point scale) on severity and frequency of craving in the previous week.
Relapse
We investigated relapse with standard follow‐ups that were gathered by the clinic. One year after discharge, patients were contacted by the clinic via letter, and in case of no response via phone. Clinicians who were blind to the study conditions used 3 different scores in accordance with the German Addiction Society: continued abstinence, improvement (where a lapse may have occurred, but the last month a patient had been abstinent again), and 1 for relapse without improvement. In our analyses, we used a binary scoring with improvement and abstinence together as a success, equivalent to previous research (den Uyl et al., 2017).
Questionnaires
Alcohol Use Disorders Identification Test
The Alcohol Use Disorders Identification Test (AUDIT) was used to measure hazardous alcohol use (Dybek et al., 2006; Saunders et al., 1993). The score can be between 0 and 40 (sum of 10 questions), with higher scores representing heavier use/problems in the last year.
Beck Depression Inventory
The Beck Depression Inventory (BDI) contained 21 questions to measure depressive feelings in the past week with answer options ranging from 0 to 3 points (Beck and Steer, 1993; Hautzinger et al., 1994).
Symptom Checklist‐90—Revised
The Symptom Checklist‐90—Revised (SCL‐90‐R) contained 90 questions (answers from 0 to 4) and measured physical and psychological impairment of a person in the past week (Derogatis, 1983; Franke, 1995). We used the average score (global severity index) as a general indication.
Adverse Effects tDCS Questionnaire
Side effects of the stimulation were assessed with a questionnaire that checked 10 possible side effects (itching, tingling, burning, scalp pain, neck pain, headache, dizziness, sleepiness, trouble concentrating, and nausea). Answers could be given from 0 (not present) to 3 (strongly present). We also included 2 questions on the estimated strength and the uncomfortableness of the stimulation, on a 10‐point scale.
Procedure
When entering the clinic, alcohol‐dependent patients were invited to an information session on the experiment, those willing and able (inclusion criteria were checked with the physician) made appointments to participate in the study. During the first appointment, patients were randomly assigned to 1 of the 4 groups and gave written informed consent. During the pre‐ or postassessment sessions, patients filled out the PACS (at the beginning) and picture ratings (at the end) and performed several experimental tasks (in this order: alcohol VPT, alcohol memory task, IAT, self‐ordered pointing task) and a cue‐reactivity task with physiological measurements.1 The first training session was started directly after the preassessment. The 4 training sessions were performed within 1 week. The postassessment was made at least 1 day and maximum 7 days after the last training session (with 1 exception, due to illness 1 patient had the postassessment 10 days after the last training session).
Data Analysis
Baseline group differences in demographics and questionnaires (AUDIT, SCL‐90‐R, BDI) are entered in a multivariate analysis of variance (MANOVA), and ANOVA comparisons for each variable with the group as between‐subject variable are reported. The PACS scores were analyzed with nonparametric tests. An effect of time was investigated with the Wilcoxon signed rank test, and the Kruskal–Wallis test was used on difference scores for group effects. The continuous data (attentional bias and approach associations) were analyzed with a repeated‐measures ANOVA with time as within‐subject factor, and the group variables ABM (control vs. real) and tDCS (sham vs. active) were entered as between‐subject factors. For each variable in the continuous data, participants with scores higher than 3 times the standard deviation were considered outliers and were adjusted to the highest score + 1 (no more than 3 participants were adjusted for each variable). For the analysis on relapse, we performed a logistic regression with complete case (CC) and multiple imputation (MI) analysis. We used all demographic variables from Table 1 and the outcome measures from Table 2 as predictors. We performed 40 imputations (as we had 40% missing data; Bodner, 2008) with SPSS Version 20.0 (IBM Corp., Armonk, NY). In step 1 of the regression, we used the same predictors as similar studies (den Uyl et al., 2017). In step 2, we entered the group variables ABM (coded as −1, 1), tDCS (coded as −1, 1), and the interaction (ABM × tDCS) as dummy variables. To obtain a pooled result in the MI analysis of the second step, we used the median p‐value, which gives a good estimate of the significance of a categorical variable (personal communication with I. Eekhout).
Table 2.
Intervention Outcomes. Results on Outcome Measurements, Craving, Alcohol Bias Measures, and Relapse. (A) The Mean and Standard Error is Given for the Pre‐ and Postassessment, and p‐Values Represent Outcomes of the ANOVA Interaction Time × ABM × tDCS (or Nonparametric Test on Difference Score for PACS). (B) For Relapse Rates, the Multiple Imputation Estimates Are Given with Complete Case Results Between Parentheses
| 1. Control ABM + sham tDCS | 2. Control ABM + active tDCS | 3. Real ABM + sham tDCS | 4. Real ABM + active tDCS | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| M | SE | M | SE | M | SE | M | SE | ||
| A. Outcome measurements | |||||||||
| Craving (PACS) | |||||||||
| Preassessment | 3.6 | 0.9 | 3.0 | 1.0 | 5.5 | 1.0 | 4.4 | 0.9 | 0.20 |
| Postassessment | 3.3 | 0.8 | 2.7 | 0.8 | 4.0 | 0.8 | 3.6 | 0.8 | |
| Attentional bias | |||||||||
| Preassessment | −2.1 | 6.9 | −6.2 | 6.2 | −14.3 | 6.6 | −5.7 | 11.1 | 0.46 |
| Postassessment | 3.5 | 5.9 | 3.0 | 6.9 | 2.7 | 7.2 | 0.6 | 6.0 | |
| IAT | |||||||||
| Preassessment | −71.6 | 79.4 | −91.8 | 83.2 | 18.9 | 55.5 | −62.9 | 61.3 | 0.63 |
| Postassessment | −58.7 | 67.5 | −68.3 | 60.7 | 10.3 | 60.7 | −11.6 | 75.7 | |
| B. Outcome measurements | Relapse | Success | Relapse | Success | Relapse | Success | Relapse | Success | |
| Relapse after 1 year | 9.0 (5) | 13.0 (8) | 5.7 (3) | 14.3 (11) | 9.1 (5) | 10.9 (6) | 6.8 (4) | 14.2 (9) | 0.56 (0.61) |
ABM, attentional bias modification; PACS, Penn Alcohol Craving Scale; tDCS, transcranial direct current stimulation.
The p‐values represent outcomes of a chi‐square test.
Results
All patients tolerated the stimulation well, and there were no differences in reported side effects between sham stimulation and active stimulation (see Appendix S1). There was no difference in how often people thought they had received real stimulation if they were in sham or active tDCS group, χ2(4) = 4.5, p = 0.35. There were no significant differences between any of the baseline variables (Table 1).
Attentional Bias
The attentional bias score showed poor split‐half correlations (r = −0.07) and a poor test–retest correlation (r = 0.15). The bias score at baseline did not correlate with alcohol problems (r = −0.04) or craving (r = −0.06). The accuracy on the ABM task in the preassessment was very high (mean: 96.7%, SD = 3.7), except for 2 participants who had only 50% accuracy rates in the first session and were therefore deleted from the analysis. In the ANOVA on the bias scores with time (pre‐ and postassessment) and experimental group (control/real ABM and sham/active tDCS), there were no significant interactions (p > 0.46, Table 2). There was a main effect of time, F(1, 77) = 4.03, p = 0.048, η 2 = 0.05, the overall bias went from a slight avoidance (1‐sample t‐test: M = −8.03, p = 0.06) to more neutral at postassessment (M = 0.54, p = 0.59).
We also analyzed the attentional bias during training. An ANOVA with time (4 sessions) was run for the different attentional bias scores (due to infrequent alcohol‐target trials, this could also be done for the real ABM training). The bias score showed no significant within‐subject main effects or interactions with group (p > 0.15); however, a significant between‐subject effect was found for ABM, F(1, 79) = 4.87, p = 0.03, η 2 = 0.06, and for the interaction between tDCS and ABM, F(1, 79) = 4.17, p = 0.04, η 2 = 0.05. Bonferroni‐controlled comparisons indicated that on average (4 training sessions combined) there was only a negative alcohol bias (faster when the target was behind the nonalcohol target), for those in the group that received both real ABM and active tDCS compared to the other groups (group 4 vs. group 1, p = 0.046, vs. group 2, p = 0.02, vs. group 3, p = 0.08; Fig. 2).
Figure 2.
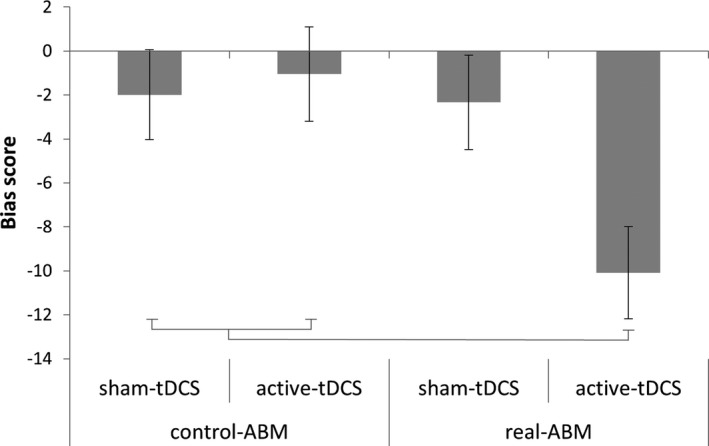
Bias scores during training. As there is only a significant interaction of attentional bias modification (ABM) × transcranial direct current stimulation (tDCS) (without an interaction with time), average bias scores over all 4 training sessions are given per group. Error bars represent standard error of the mean. Group 4 that received real ABM and active tDCS differs significantly from groups 1 and 2; there is a trend‐level difference with group 3 (p = 0.08).
Implicit Alcohol Approach Association
The IAT approach association score showed good split‐half correlations (r = 0.83) and good test–retest correlations (r = 0.72). The baseline score did not correlate with alcohol problems (r = −0.09) or craving (r = 0.14). An ANOVA on the bias scores with time (preassessment and postassessment), tDCS, and ABM did not give any significant main or interaction effects (p > 0.44, Table 2). The overall bias was slightly negative at baseline, in the direction of an avoidance association, but this was not significant (M = −52.5, SE = 35.2, p = 0.14).
Craving
Craving was extremely low; that is, 72% of patients scored 5 (seldom craving) or lower at preassessment. The Wilcoxon signed rank test showed that craving reduced from preassessment (M = 4.08, SE = 0.48) to postassessment (M = 3.39, SE = 0.41, z = −3.194, p < 0.01). The Kruskal–Wallis tests on difference scores showed no differences between the groups (p > 0.20).
Relapse After 1 Year
Follow‐up data were obtained from 61.4% of the patients. The chi‐square analysis without predictors was not significant (Table 2). There was also no main effect of ABM (CC: χ2(1) = 0.35, p = 0.55, φ = 0.08, MI: χ2(1) = 0.29, p = 0.59, φ = 0.06) or tDCS (CC: χ2(1) = 1.42. p = 0.23, φ = 0.17, MI: χ2(1) = 0.38. p = 0.24, φ = 0.13). However, there was the least amount of relapse in patients from the groups that received active tDCS (group 2: 21%, group 4: 31%), compared to sham tDCS (group 1: 38% and group 3: 45%; Table 2). The logistic regression also did not yield any significant effects (step 2, CC: χ2(3) = 2.53, p = 0.47, MI: χ2(3) = 2.10, p = 0.55). There were no significant predictors of relapse and no main effects of tDCS and ABM (Table 3).
Table 3.
Logistic Regression Results with Multiple Imputation Data for 1‐Year Relapse Data
| Variable | 1‐year relapse | |||
|---|---|---|---|---|
| B | SE | p | ||
| Step 1 | Gender | 0.110 | 0.755 | 0.885 |
| Duration alcohol problems | −0.026 | 0.032 | 0.429 | |
| Number of detoxifications | 0.020 | 0.066 | 0.765 | |
| Alcohol problems (AUDIT) | 0.030 | 0.048 | 0.537 | |
| Duration of treatment (days) | −0.008 | 0.018 | 0.673 | |
| Depression (BDI) | 0.020 | 0.039 | 0.610 | |
| SCL‐90‐R | −0.009 | 0.033 | 0.777 | |
| Step 2 | ABM | 0.114 | 0.297 | 0.701 |
| tDCS | −0.286 | 0.297 | 0.336 | |
| ABM × tDCS | −0.035 | 0.303 | 0.907 | |
AUDIT, Alcohol Use Disorders Identification Test; ABM, attentional bias modification; BDI, Beck Depression Inventory; SCL‐90—R, Symptom Checklist‐90—Revised; PACS, Penn Alcohol Craving Scale; tDCS, transcranial direct current stimulation.
Discussion
In this experiment, we investigated the potential beneficial effects of anodal tDCS over the DLPFC, while performing an alcohol ABM training, by studying the effects on craving, alcohol bias, and relapse. We found some evidence of enhanced learning during the training with tDCS; there was a stronger avoidance bias during training in the combined group. However, from pre‐ to postassessment, no beneficial effects of tDCS on changing attentional bias were found on the attentional bias and the implicit association scores. There were also no effects of tDCS and ABM on relapse.
The only potentially relevant effect in the reaction time data was the difference during training in bias scores while receiving active ABM and tDCS. This fits the expectation that tDCS could enhance bias acquisition (Clarke et al., 2014a). This could be due to a modulation of attentional deployment or due to a more general learning effect. Those receiving tDCS may have been better at noticing and learning the stimulus–response contingency and therefore were able to speed reaction times to probes at the contingent location. However, the lack of effects on the postassessment bias raises the question whether the potential tDCS effects could still be maintained offline. More crucially, the absence of effects in the postassessment bias task could also be due to the difficulties in measuring attentional bias; the visual dot‐probe task is very unreliable. One can question the relevance of this finding given the low reliability of the task. However, tasks that give suboptimal correlation effect may still produce robust experimental effects (Hedge et al., 2018). The minor observed effect during training may have been due to the vast amount of trials; all sessions combined the training consisted of 1,872 trials, compared to 120 trials in the assessment. This may cause a small effect to become measurable in an unreliable task. Reaction times were relatively fast and accuracy was high so it was not a necessity to use the stimulus information. Patients were not explicitly told of the training goal; hence, there was no explicit motivation to focus attention on the nonalcoholic pictures. It is unlikely that patients were completely ignoring the contents of the stimuli, due to the inclusion of surprise trials; however, the content of the stimuli appears to have had little impact on their attention. This is congruent with others who have also recently stated that the visual dot‐probe task has serious limitations (van Bockstaele et al., 2016). There is a need to develop better paradigms for measuring attentional bias, for example, by including eye tracking or EEG (Kappenman et al., 2014).
We also did not find any tDCS or ABM effect on automatic approach associations toward alcohol with a (more reliable) implicit association test. This specific sample of patients already demonstrated a very negative attitude toward alcohol and already showed an indication of alcohol avoidance association before the intervention. In previous studies, approach associations were found at baseline (Eberl et al., 2013; Wiers et al., 2013) but not in Rinck and colleagues (in press). This could be the result of random variation (approach and avoidance biases both occur) or simply a lack of power (the other studies were larger). Nevertheless, in the absence of a specific detrimental bias, it is difficult to determine which automatic processes could be targeted with CBM. There was no effect of tDCS on craving, which was not entirely surprising, given that craving was very low, as shown in the previous studies in a clinical inpatient context (Schoenmakers et al., 2010; Wiers et al., 2011). Future studies could use more active craving manipulations than simple pictures of alcohol stimuli, for example, videos, or more context relevant pictures or situations (instead of the safe environment of the clinic). Another useful addition could be to include craving induction with imagery or stress manipulations (Sinha, 2007). In the current study, patients were already abstinent, and a previous study also found an effect of CBM during detoxification (Manning et al., 2016). It may be more useful to do an intervention while patients feel stronger automatic or subjective approach tendencies toward the alcohol.
tDCS was not found to influence automatic biases or craving, and no effect on relapse was found. However, the effects were going in the expected direction with 16% less relapse in active tDCS groups, which was of comparable magnitude to the effect found in the previous study (den Uyl et al., 2017) that showed a trend‐level effect with 19% less relapse. The absence of significant effects in the current study could be due to a large amount of dropout in the follow‐up. With other studies in mind (Boggio et al., 2008; Klauss et al., 2014), it is still possible that tDCS over the DLPFC can have a beneficial effect on addiction treatment, although more research is necessary to increase understanding of underlying neurocognitive mechanisms. If not due to an effect on automatic processes, stimulating the DLPFC repeatedly may, for example, have led to a general increase in neuroplasticity and better retention during the cognitive behavior therapies (Stagg and Nitsche, 2011). The most appropriate electrode montage is also a topic of debate, as different protocols have been used. Perhaps more optimal results could be obtained with right anodal DLPFC stimulation, differently sized electrodes, or different stimulation lengths (Jansen et al., 2013; Klauss et al., 2014).
Except for a minor effect during training, this study did not yield evidence that anodal tDCS over the DLPFC could enhance the effectiveness of alcohol ABM training in alcohol treatment. ABM training did not have any effects on bias or other clinical measures, which is likely due to problems in measuring the underlying bias and a low sample, as a larger recent study did find the effect on the attentional bias (Rinck et al., in press). tDCS also did not affect craving or relapse, which might be due to low reporting of craving and high dropout at follow‐up. As in previous studies, the dot‐probe task showed very low reliability (Ataya et al., 2012; van Bockstaele et al., 2016; Kappenman et al., 2014). Care should be taken that psychometric properties of tasks are reported and that more valid and more reliable clinical outcome measurements are used. This study does not demonstrate any benefit in the specific combination of tDCS and CBM; however, it does not exclude the potential use of tDCS and CBM. Given the amount of review articles CBM and tDCS inspired in the last years, these techniques are a hot topic of debate (e.g., Antal et al., 2015; Cristea et al., 2016; Horvath et al., 2014; Wiers, 2018; Wiers et al., 2018). The field of research would benefit from large experimental trials to provide further answers. Underlying mechanisms of ABM should be further explored to find potential targets for enhancement. In addition, tDCS is a feasible technique with little side effects that may be useful as an add‐on to treatment, but a more appropriate method of tDCS application should be further investigated.
Conflict of Interest
The authors report no conflict of interest.
Supporting information
Appendix S1. Supplementary materials on side‐effects, blinding, mood, and working memory.
Fig. A. Overview of reported side‐effects.
Fig. B. Stimulation type blinding.
Fig. C. Pre and post scores for working memory and mood.
Acknowledgments
The study is based on a chapter of the unpublished dissertation of T.E. den Uyl. This work was supported by N.W.O. (Dutch Science Foundation) Research Talent Grant [406‐11‐203] and a grant from the European Foundation for Alcohol Research (ERAB) [EA 1239].
Note
We included multiple craving measurements (Alcohol Urge Questionnaire and visual analog scales), which assessed momentary craving; however, due to low scores it did not give additional information and was excluded from the article. We assessed visual analog scales on mood at the beginning and end of experimental sessions. Brief results on the working memory task and mood questionnaires are included in the Supplementary materials. Analysis on physiological findings will be reported elsewhere.
References
- Antal A, Keeser D, Priori A, Padberg F, Nitsche MA (2015) Conceptual and procedural shortcomings of the systematic review “Evidence that transcranial direct current stimulation (tDCS) generates little‐to‐no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review” by Horvath and co‐workers. Brain Stimul 8:846–849. [DOI] [PubMed] [Google Scholar]
- Arnsten AFT, Rubia K (2012) Neurobiological circuits regulating attention, cognitive control, motivation, and emotion: disruptions in neurodevelopmental psychiatric disorders. J Am Acad Child Adolesc Psychiatry 51:356–367. [DOI] [PubMed] [Google Scholar]
- Ataya AF, Adams S, Mullings E, Cooper RM, Attwood AS, Munafò MR (2012) Internal reliability of measures of substance‐related cognitive bias. Drug Alcohol Depend 121:148–151. [DOI] [PubMed] [Google Scholar]
- Beard C (2011) Cognitive bias modification for anxiety: current evidence and future directions. Expert Rev Neurother 11:299–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA (1993) Manual for the Beck Depression Inventory Psychological Corporation, San Antonio, TX. [Google Scholar]
- Bishop SJ (2009) Trait anxiety and impoverished prefrontal control of attention. Nat Neurosci 12:92–98. [DOI] [PubMed] [Google Scholar]
- van Bockstaele B, Salemink E, Bögels SM, Wiers RW (2016) Limited generalisation of changes in attentional bias following attentional bias modification with the visual probe task. Cogn Emot 31:369–376. [DOI] [PubMed] [Google Scholar]
- Bodner TE (2008) What improves with increased missing data imputations? Struct Eq Model Multi J 15:651–675. [Google Scholar]
- Boggio PS, Sultani N, Fecteau S, Merabet L, Mecca T, Pascual‐Leone A, Basaglia A, Fregni FF (2008) Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double‐blind, sham‐controlled study. Drug Alcohol Depend 92:55–60. [DOI] [PubMed] [Google Scholar]
- Breiner M, Stritzke WGK, Lang AR (1999) Approaching avoidance. A step essential to the understanding of craving. Alcohol Res Health 23:197–206. [PMC free article] [PubMed] [Google Scholar]
- Browning M, Holmes EA, Murphy SE, Goodwin GM, Harmer J (2010) Lateral prefrontal cortex mediates the cognitive modification of attentional bias. Biol Psychiatry 67:919–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelazzi L, Perlato A, Santandrea E, Della Libera C (2013) Rewards teach visual selective attention. Vision Res 85:58–62. [DOI] [PubMed] [Google Scholar]
- Christiansen P, Schoenmakers TM, Field M (2015) Less than meets the eye: reappraising the clinical relevance of attentional bias in addiction. Addict Behav 44:43–50. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Browning M, Hammond G, Notebaert L, Macleod C (2014a) The causal role of the dorsolateral prefrontal cortex in the modification of attentional bias: evidence from transcranial direct current stimulation. Biol Psychiatry 76:946–952. [DOI] [PubMed] [Google Scholar]
- Clarke PJF, Notebaert L, MacLeod C (2014b) Absence of evidence or evidence of absence: reflecting on therapeutic implementations of attentional bias modification. BMC Psychiatry 14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clerkin EM, Magee JC, Wells TT, Beard C, Barnett NP (2016) Randomized controlled trial of attention bias modification in a racially diverse, socially anxious, alcohol dependent sample. Behav Res Ther 87:58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox WM, Fadardi JS, Intriligator JM, Klinger E (2014) Attentional bias modification for addictive behaviors: clinical implications. CNS Spectr 19:215–224. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P (2015) Efficacy of cognitive bias modification interventions in anxiety and depression: meta‐analysis. Br J Psychiatry 206:7–16. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P (2016) The efficiency of cognitive bias modification interventions for addictions: a meta‐analysis. PLoS One 18:1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis LR (1983) SCL‐90‐R Administration, Scoring, & Procedures. Manual II Clinical Psychometric Research, Towson, MD. [Google Scholar]
- Dybek I, Bischof G, Grothues J, Reinhardt S, Meyer C, Hapke U, Rumpf HJ (2006) The reliability and validity of the alcohol use disorders identification test (AUDIT) in a German general practice population sample. J Stud Alcohol 67:473–481. [DOI] [PubMed] [Google Scholar]
- Eberl C, Wiers RW, Pawelczack S, Rinck M, Becker ES, Lindenmeyer J (2013) Approach bias modification in alcohol dependence: do clinical effects replicate and for whom does it work best? Dev Cogn Neurosci 4:38–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmasry J, Loo C, Martin D (2015) A systematic review of transcranial electrical stimulation combined with cognitive training. Restor Neurol Neurosci 33:263–278. [DOI] [PubMed] [Google Scholar]
- Emmelkamp PMG (2012) Attention bias modification: the Emperor's new suit? BMC Med 10:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M, Munafo MR, Franken IHA (2009) A meta‐analytic investigation of the relationship between attentional bias and subjective craving in substance abuse. Psychol Bull 135:589–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM (1999) Psychometric properties of the Penn Alcohol Craving Scale. Alcohol Clin Exp Res 23:1289–1295. [PubMed] [Google Scholar]
- Franke GH (1995) SCL‐90‐R Die Symptom—Checkliste vonDerogatis—Deutsche Version: Manual Hogrefe, Göttingen. [Google Scholar]
- Franken IHA (2003) Drug craving and addiction: integrating psychological and neuropsychopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry 27:563–579. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer MHW, Keller F (1994) Beck‐Depressions‐Inventar (BDI). Bearbeitung der deutschen Ausgabe. Testhandbuch Huber, Bern, Göttingen, Toronto, Seattle. [Google Scholar]
- Hedge C, Powell G, Sumner P (2018) The reliability paradox: why robust cognitive tasks do not produce reliable individual differences. Behav Res Methods 50:1166–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeren A, Baeken C, Vanderhasselt M‐A, Philippot P, de Raedt R (2015) Impact of anodal and cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex during attention bias modification: an eye‐tracking study. PLoS One 10:e0124182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AT, Fitzgerald PB, Hoy KE (2016) Effects of anodal transcranial direct current stimulation on working memory: a systematic review and meta‐analysis of findings from healthy and neuropsychiatric populations. Brain Stimul 9:197–208. [DOI] [PubMed] [Google Scholar]
- Horvath JC, Forte JD, Carter O (2014) Evidence that transcranial direct current stimulation (tDCS) generates little‐to‐no reliable neurophysiologic effect beyond MEP amplitude modulation in healthy human subjects: a systematic review. Neuropsychologia 66:213–236. [DOI] [PubMed] [Google Scholar]
- Jansen JM, Daams JG, Koeter MWJ, Veltman DJ, van den Brink W, Goudriaan AE (2013) Effects of non‐invasive neurostimulation on craving: a meta‐analysis. Neurosci Biobehav Rev 37:2472–2480. [DOI] [PubMed] [Google Scholar]
- Kappenman ES, Farrens JL, Luck SJ, Proudfit GH (2014) Behavioral and ERP measures of attentional bias to threat in the dot‐probe task: poor reliability and lack of correlation with anxiety. Front Psychol 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klauss J, Penido Pinheiro LC, Silva Merlo BL, Correia Santos GDA, Fregni F, Nitsche MA, Miyuki Nakamura‐Palacios E (2014) A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 17:1793–1803. [DOI] [PubMed] [Google Scholar]
- Macleod C (2012) Cognitive bias modification procedures in the management of mental disorders. Curr Opin Psychiatry 25:114–120. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Grafton B (2016) Anxiety‐linked attentional bias and its modification: illustrating the importance of distinguishing processes and procedures in experimental psychopathology research. Behav Res Ther 86:68–86. [DOI] [PubMed] [Google Scholar]
- Manning V, Staiger PK, Hall K, Garfield JBB, Flaks G, Hughes LK, Lum JAG, Lubman DI, Verdejo‐Garcia A (2016) Cognitive bias modification training during inpatient alcohol detoxification reduces early relapse: a randomized controlled trial. Alcohol Clin Exp Res 40:2011–2019. [DOI] [PubMed] [Google Scholar]
- Medeiros LF, de Souza ICC, Vidor LP, de Souza A, Deitos A, Volz MS, Fregni F, Caumo W, Torres ILS (2012) Neurobiological effects of transcranial direct current stimulation: a review. Front Psychiatry 3:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogoase C, David D, Koster EHW (2014) Clinical efficacy of attentional bias modification procedures: an updated meta‐analysis. J Clin Psychol 70:1133–1157. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W (2000) Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 527:633–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W (2011) Transcranial direct current stimulation—update 2011. Restor Neurol Neurosci 29:463–492. [DOI] [PubMed] [Google Scholar]
- Ostafin BD, Palfai TP (2006) Compelled to consume: the Implicit Association Test and automatic alcohol motivation. Psychol Addict Behav 20:322–327. [DOI] [PubMed] [Google Scholar]
- Perugini M, Richetin J, Zogmaister C (2010) Prediction of behavior, in Handbook of Implicit Social Cognition: Measurement, Theory, and Applications (Gawronski B, Payne BK. eds), pp 255–278. Guilford Press, New York, NY. [Google Scholar]
- Pronk T, van Deursen DS, Beraha EM, Larsen H, Wiers RW (2015) Validation of the Amsterdam beverage picture set: a controlled picture set for cognitive bias measurement and modification paradigms. Alcohol Clin Exp Res 39:2047–2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M (2013) Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 591(Pt 10):2563–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinck M, Wiers RW, Becker ES, Lindenmeyer J (in press) Relapse prevention in abstinent alcoholics by cognitive bias modification, clinical effects of combining approach bias modification and attention bias modification. J Consult Clin Psychol. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC (1993) The neural basis of drug craving: an incentive‐sensitization theory of addiction. Brain Res Brain Res Rev 18:247–291. [DOI] [PubMed] [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M (1993) Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption–II. Addiction 88:791–804. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IFM, Goertz AG, Van Kerkhof DHAT, Wiers RW (2010) Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug Alcohol Depend 109:30–36. [DOI] [PubMed] [Google Scholar]
- Sinha R (2007) The role of stress in addiction relapse. Curr Psychiatry Rep 9:388–395. [DOI] [PubMed] [Google Scholar]
- Stagg CJ, Nitsche MA (2011) Physiological basis of transcranial direct current stimulation. Neuroscientist 17:37–53. [DOI] [PubMed] [Google Scholar]
- Theeuwes J, Belopolsky AV (2012) Reward grabs the eye: oculomotor capture by rewarding stimuli. Vision Res 74:80–85. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Rinck M, Lindenmeyer J, Wiers RW (2017) A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict Biol 22:1632–1640. [DOI] [PubMed] [Google Scholar]
- den Uyl TE, Gladwin TE, Wiers RW (2016) Electrophysiological and behavioral effects of combined transcranial direct current stimulation and alcohol approach bias retraining in hazardous drinkers. Alcohol Clin Exp Res 40:1–10. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P (2005) How brains beware: neural mechanisms of emotional attention. Trends Cogn Sci 9:585–594. [DOI] [PubMed] [Google Scholar]
- Wiers RW (2018) Cognitive training in addiction: does it have clinical potential? Biol Psychiatry Cogn Neurosci Neuroimaging 3:101–102. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Boffo M, Field MJ (2018) What's in a trial? On the importance of distinguishing between experimental lab‐studies and randomized controlled trials; the case of cognitive bias modification and alcohol use disorders. J Stud Alcohol Drugs 79:333–343. [PubMed] [Google Scholar]
- Wiers RW, Eberl C, Rinck M, Becker ES, Lindenmeyer J (2011) Retraining automatic action tendencies changes alcoholic patients’ approach bias for alcohol and improves treatment outcome. Psychol Sci 22:490–496. [DOI] [PubMed] [Google Scholar]
- Wiers RW, Gladwin TE, Hofmann W, Salemink E, Ridderinkhof KR (2013) Cognitive bias modification and cognitive control training in addiction and related psychopathology: mechanisms, clinical perspectives, and ways forward. Clin Psychol Sci 1:192–212. [Google Scholar]
- Zvielli A, Bernstein A, Koster EHW (2014) Temporal dynamics of attentional bias. Clin Psychol Sci 3:1–17. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Supplementary materials on side‐effects, blinding, mood, and working memory.
Fig. A. Overview of reported side‐effects.
Fig. B. Stimulation type blinding.
Fig. C. Pre and post scores for working memory and mood.


