Abstract
Background
Hospitalization for low‐risk pulmonary embolism (PE) is common, expensive, and of questionable benefit.
Objective
The objective was to determine if low‐risk PE patients discharged from the emergency department (ED) on rivaroxaban require fewer hospital days compared to standard of care (SOC).
Methods
Multicenter, open‐label randomized trial in low‐risk PE defined by Hestia criteria. Adult subjects were randomized to early ED discharge on rivaroxaban or SOC. Primary outcome was total number of initial hospital hours, plus hours of hospitalization for bleeding or venous thromboembolism (VTE), 30 days after randomization. A 90‐day composite safety endpoint was defined as major bleeding, clinically relevant nonmajor bleeding, and mortality.
Results
Of 114 randomized subjects, 51 were early discharge and 63 were SOC. Of 112 (98.2%) receiving at least one dose of study drug, 99 (86.8%) completed the study. Initial hospital LOS was 4.8 hours versus 33.6 hours, with a mean difference of –28.8 hours (95% confidence interval [CI] = –42.55 to –15.12 hours) for early discharge versus SOC, respectively. At 90 days, mean total hospital days (for any reason) were less for early discharge than SOC, 19.2 hours versus 43.2 hours, with a mean difference of 26.4 hours (95% CI = –46.97 to –3.34 hours). At 90 days, there were no bleeding events, recurrent VTE, or deaths. The composite safety endpoint was similar in both groups, with a difference in proportions of 0.005 (95% CI = –0.18 to 0.19). Total costs were $1,496 for early discharge and $4,234 for SOC, with a median difference of $2,496 (95% CI = –$2,999 to –$2,151).
Conclusions
Low‐risk ED PE patients receiving early discharge on rivaroxaban have similar outcomes to SOC, but fewer total hospital days and lower costs over 30 days.
Of the approximately 900,000 annual venous thromboembolism (VTE) events occurring in the United States,1 it is estimated that more than 250,000 are diagnosed with pulmonary embolus in the emergency department (ED).2 In a U.S. National Hospital Ambulatory Medical Care Survey analysis, during 2006 to 2010, >90% of ED patients diagnosed with pulmonary embolism (PE) were hospitalized.3 Since the average PE hospitalization costs approximately $14,000,1 this represents >$2 billion in annual expenditures. The necessity of routine PE admission is unclear. Although mortality rates of PE with shock exceed 30%,4 the 30‐day mortality rate of low‐risk PE (LRPE) patients is less than 1%.5 One study of LRPE patients, defined by a simplified PE severity index score = 0, found an average patient cost saving of >$6,000 for discharged versus hospitalized patients.5
Importance
Avoidance of hospitalization may not only reduce costs, it is associated with fewer adverse clinical outcomes. In LRPE patients, longer hospitalization is associated with as much as an 880% greater risk (1.5% vs 13.3%; 95% confidence interval [CI]] = 3.77%–19.94%) of hospital‐acquired conditions.6, 7 Although few prospective U.S. studies have discharged LRPE patients, the American College of Chest Physicians supports early discharge of these patients with adequate home cirumstances.8
In 2012, rivaroxaban (an oral factor Xa inhibitor) was approved for the treatment of PE. Because anticoagulation onset occurs within 2 hours of oral administration, rivaroxaban could obviate the historical requirement for bridging therapy with a parenteral anticoagulant agent. As this strategy questions the necessity for hospitalization to simply take an oral medication, we performed the first U.S. study randomizing ED LRPE patients to hospital discharge on an oral factor Xa inhibitor (rivaroxaban) versus standard‐of‐care (SOC) therapy.
Goals of This Investigation
The goal of this investigation was to determine if LRPE patients discharged from the ED on rivaroxaban require less time in the hospital and lower costs compared to SOC.
Methods
Study Design and Setting
MERCURY PE (MulticEnter trial of Rivaroxaban for early disCharge of pUlmonaRY embolism from the Emergency Department, NCT02584660) was a randomized, open‐label, parallel‐group, multicenter study, initially planned to be conducted at 57 U.S. sites. All sites had institutional review board approval. This study is reported consistent with CONSORT standards and its methodology has been previously published.9
Selection of Participants
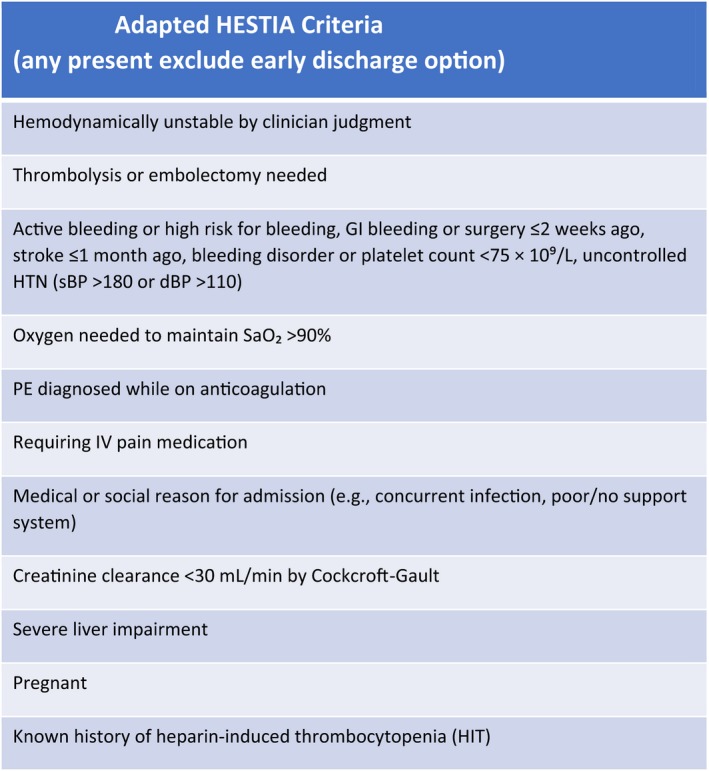
Adult patients presenting to the ED with objectively confirmed, LRPE were eligible for enrollment. LRPE was defined by the absence of any Hestia criteria, adapted for emergency medicine by removing 24‐hour requirements10, 11 (see Table 1). Patients were excluded for a troponin level above the institutional upper reference level, contraindications to anticoagulation, or by investigator determination of barriers to treatment or follow‐up. Although the Hestia criteria exclude hemodynamically unstable patients, instability is not defined and was determined per the physician's judgment. Study logs were maintained to provide description of excluded patients.
Table 1.
Adapted HESTIA Criteria (Any Present Exclude Early Discharge Option)
| Hemodynamically unstable by clinician judgment |
| Thrombolysis or embolectomy needed |
| Active bleeding or high risk for bleeding, GI bleeding or surgery ≤2 weeks ago, stroke ≤1 month ago, bleeding disorder or platelet count < 75 × 109/L, uncontrolled HTN (sBP > 180 or dBP > 110) |
| Oxygen needed to maintain SaO2 > 90% |
| PE diagnosed while on anticoagulation |
| Requiring IV pain medication |
| Medical or social reason for admission (e.g., concurrent infection, poor/no support system) |
| Creatinine clearance < 30 mL/min by Cockcroft‐Gault |
| Severe liver impairment |
| Pregnant |
| Known history of heparin‐induced thrombocytopenia |
GI = gastrointestinal; HTN = hypertension; PE = pulmonary embolism.
Interventions
After obtaining written informed consent, patients were randomly assigned in a 1:1 ratio to ED discharge on open‐label rivaroxaban or standard care (as determined by the attending physician) by an interactive Web system within 12 hours of diagnosis. Patients randomized to early discharge on rivaroxaban were discharged within 24 hours of ED triage and were instructed to take rivaroxaban with food, 15 mg twice daily for 21 days and then 20 mg once daily to study completion. SOC patients were treated per local protocol, which could include hospitalization and any Food and Drug Administration (FDA)‐approved anticoagulant strategy, including rivaroxaban. If receiving warfarin, the target international normalized ratio (INR) was 2.0 to 3.0, with testing per local protocol.
Measurements
Patients, nursing staff, physicians, and local investigators were aware of group assignment. Principal investigators and outcome adjudicators were masked to group assignment. Data collection was by trained research staff using standardized e‐forms.
Outcomes
The primary efficacy outcome was the total amount of time spent in the hospital, expressed in hours for venous thromboembolic or bleeding events, in the 30 days after randomization. The primary safety outcome was major bleeding within 90 days. Patient safety was monitored by an independent data safety monitoring board. As participants could not be blinded to their own hospitalization, to reduce observer bias clinical and safety endpoints were adjudicated by a panel blinded to treatment allocation. The study protocol was approved by all participating institutional review boards.
For outcome definitions, hospital time included all the time a patient was admitted to an inpatient service or hospital observation unit. Readmissions were identified based on record review and patient self‐report by telephone contact at 30 and 90 days. Readmissions for reasons unrelated to VTE were excluded. Adverse events were reviewed by trained adjudicators blinded to study assignment.
Patient satisfaction with inpatient and outpatient care was rated using 5‐ and 3‐point Likert scales, respectively, with higher scores indicating greater satisfaction. Satisfaction was further evaluated with the Anti‐Clot Treatment Score, which uses two subscales of burdens (12 items) and benefits (three items), both measured on a 5‐point Likert scale, with higher scores indicating greater satisfaction.
Prespecified secondary efficacy endpoints included 90‐day rates of new/recurrent VTE, VTE‐related death, unplanned hospital or physician office visits for VTE or bleeding, total length of initial and subsequent hospitalizations for any reason, and patient‐reported satisfaction with the site of care assessed on Day 7; satisfaction with anticlot treatment assessed on Days 14, 30, and 90; and the total costs of care. A prespecified secondary safety endpoint was clinically relevant nonmajor bleeding, based on the International Society on Thrombosis and Haemostasis (ISTH) definitions.12
Standard U.S. FDA outcome definitions13 were utilized and included the following: 1) adverse event (AE), any untoward medical occurrence associated with the use of a drug in humans whether or not considered drug related, or 2) serious adverse event (SAE), any experience or reaction of any untoward medical occurrence that at any dose results in death, is life‐threatening, requires inpatient hospitalization or prolongation of existing hospitalization, or results in persistent or significant disability/incapacity or a congenital anomaly/birth defect. Treatment‐emergent adverse events are defined as adverse events with onset or worsening on or after date of first dose of study treatment up to and including 2 days after the last dose date
We evaluated total treatment costs for early discharge on rivaroxaban and SOC cohorts during 30 days following randomization for all patients in the intention to treat analysis. This consisted of direct medical care costs (2016 U.S. dollars), including the qualifying index PE encounter, all subsequent encounters related to any ISTH major or clinically relevant nonmajor bleeding, adjudicated recurrent PE or new/recurrent DVT, and the costs of anticoagulation and related monitoring. Since PE treatment costs are higher on the first 1 to 2 days of an inpatient or ED visit, we valued the cost of the initial encounter on an hourly basis using unit costs derived from a previous economic analysis of PE.14 The ED and outpatient physician office visits, procedures, and tests (including INR testing) were assigned costs based on current procedural terminology coding and the Medicare fee schedule.15 All anticoagulants were assigned costs based upon mean Red Book wholesale prices. When necessary, costs were inflated to 2016 U.S. dollars using the Consumer Price Index for Medical Care.16
Data Analysis
Analyses were conducted by intention‐to‐treat basis, regardless of anticoagulant used. Baseline characteristics (demographic and clinical data) are summarized with appropriate statistics. The primary clinical endpoint was calculated for each cohort and is presented as a two‐sided 95% CI for the mean difference of length of hospital stay, dichotomized by treatment. Descriptive statistics are provided for other endpoints. Cost data are reported as medians and interquartile ranges (IQRs). The nonparametric Hodges‐Lehman independent‐samples test was used to estimate the difference in median cost between early discharge on rivaroxaban and SOC and is reported with 95% CIs
Sample Size and Power Calculation
A large‐sample CI approach was used to determine the sample size required for estimating the difference in mean length of stay for VTE or bleeding related events during the first 30 days after randomization between groups. Using a standard deviation of 24 and 74.4 hours, for outpatient and inpatient groups, respectively,17 an average number of hours of hospitalization after discharge of <48, and the percentage of patients with VTE‐related rehospitalization as <5%, we projected a total of 150 subjects per group would provide a two‐sided 95% CI with about a 12‐hour margin of error. Margin of error was defined as the quantity from the observed difference in means to the endpoint of the CI. This provided an 82% power to detect a 24‐hour difference, or 99% power to detect a 48‐hour difference, in total hospital hours. Because recurrent VTE rates are very low in LRPE patients,17 we did not power for this parameter.
Results
Characteristics of Study Subjects
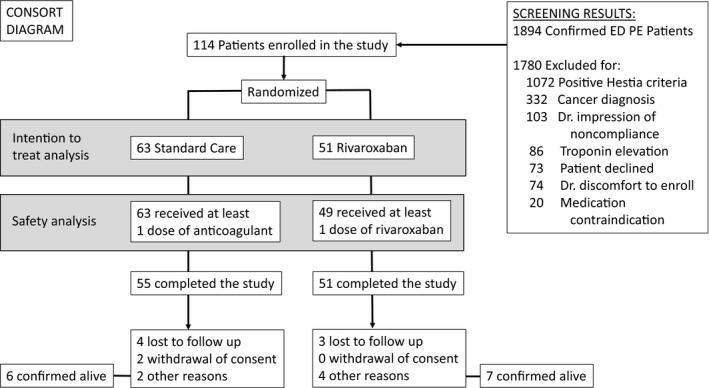
The flow of screened and randomized patients is presented in Figure 1. Because of an unanticipated funding decrease, only 35 hospitals participated, each enrolling a median (IQR) of 3 (1–5) patients. A total of 114 subjects were randomized and 99 (86.8%) completed the study. Of 15 (13.2%) discontinuations, seven (13.7%) were on rivaroxaban, and eight (12.7%) received SOC. The most frequent reasons for early discontinuation were lost to follow‐up (see Figure 1) and adverse events (n = 4, 7.8%), all on rivaroxaban (see Table 2). Demographic characteristics were similar between treatment groups (see Table 3). The median (range) duration of treatment was 90 (2–109) days; early discharge on rivaroxaban, 91 (3–109) days; and SOC, 89 (2–105) days. Overall, 60 patients (53.6%) received treatment for ≥90 days: early discharge on rivaroxaban, 33 (67.3%); and SOC, 27 (42.9%).
Figure 1.
CONSORT diagram.
Table 2.
Summary of AEs
| Rivaroxaban (n = 49) | SOC (n = 63) | Total (n = 112) | Chi‐square p‐value | |
|---|---|---|---|---|
| AE | 29 (59.2) | 25 (39.7) | 54 (48.2) | 0.0405 |
| TE‐AE | 28 (57.1) | 24 (38.1) | 52 (46.4) | 0.0450 |
| Chest pain | 6 (12.2) | 3 (4.8) | 9 (8) | 0.1484 |
| Dyspnea | 1 (2) | 7 (11) | 8 (7.1) | 0.0645 |
| Headache | 2 (4.1) | 3 (4.8) | 5 (4.5) | 0.8627 |
| SAE | 5 (10.2) | 7 (11.1) | 12 (10.7) | 0.8776 |
| TE‐SAE | 5 (10.2) | 7 (11.1) | 12 (10.7) | 0.8776 |
| AE leading to study drug discontinuation | 2 (4.1) | 4 (6.3) | 6 (5.4) | 0.5970 |
| Drug‐related TE‐AE leading to drug discontinuation | 1 (2) | 2 (3.2) | 3 (2.7) | 0.7124 |
| SAE leading to hospitalization | 5 (10.2) | 7 (11.1) | 12 (10.7) | 0.8776 |
| Deaths | 0 | 0 | 0 |
Data are reported as n (%). Percentages are calculated with the number of subjects in each treatment group as the denominators. Drug‐related adverse event is defined as a relationship to the study drug is possible, probable, or very likely.
AE = adverse event; SAE = serious adverse event; TE = treatment emergent.
Table 3.
Demographics, Past Medical History, and Treatment
| Total (N = 114) | Rivaroxaban (n = 51) | SOC (n = 63) | Rivaroxaban vs. SOC (95% CI) | |
|---|---|---|---|---|
| Characteristics | ||||
| Female | 59 (51.8) | 27 (52.9) | 32 (50.8) | –0.1629 to 0.2059 |
| White | 77 (67.5) | 29 (56.9) | 48 (76.2) | –0.3651 to –0.0214 |
| African American | 34 (29.8) | 20 (39.2) | 14 (22.2) | 0.0011 to 0.3387 |
| Age (years) | 48.26 (±15.5) | 49.14 (±13.3) | 47.56 (±17.2) | –4.2323 to 7.3957 |
| 18 to 65 | 100 (±87.7) | 48 (±94.1) | 52 (±82.5) | 0.0019 to 0.2296 |
| 66 to <76 | 9 (±7.9) | 3 (±5.9) | 6 (±9.5) | –0.1335 to 0.0607 |
| >76 | 5 (±4.4) | 0 | 5 (±7.9) | –4.2323 to 7.3957 |
| BMI | 31.12 (±8.2) | 31.41 (±8.7) | 30.87 (±7.9) | –2.5557 to 3.6441 |
| Past medical history | ||||
| PE | 16 (14) | 8 (15.7) | 8 (12.7) | –0.0994 to 0.1592 |
| DVT | 6 (5.3) | 2 (3.9) | 4 (6.3) | –0.1047 to 0.0561 |
| MI | 0 | 0 | 0 | |
| AF | 4 (3.5) | 4 (7.8) | 0 | 0.0046 to 0.1522 |
| CHF | 1 (0.9) | 1 (2) | 0 | –0.0184 to 0.0577 |
| Cancer | 7 (6.1) | 3 (5.9) | 4 (6.3) | –0.0930 to 0.0836 |
| Diabetes | 9 (7.9) | 6 (11.8) | 3 (4.8) | –0.0329 to 0.1729 |
| ED medication | ||||
| Aspirin | 3 (2.7) | 1 (2) | 2 (3.2) | –0.0700 to 0.0473 |
| Heparin | 29 (25.9) | 12 (24.5) | 17 (27) | –0.1878 to 0.1379 |
| Low‐molecular‐weight heparin | 6 (5.4) | 3 (6.1) | 3 (4.8) | –0.0717 to 0.0989 |
Data are reported as n (%) or mean (±SD).
AF = atrial fibrillation; BMI = body mass index; CHF = congestive heart failure; DVT = deep vein thrombosis; MI = myocardial infarction; PE = pulmonary embolism; SOC = standard of care.
As the SOC arm was required to be per local practice patterns, our results reflect standard U.S. practice. We found > 75% of LRPE patients ultimately received some type of direct‐acting oral anticoagulant (DOAC; see Table 4). However, nearly 75% of SOC patients were initially treated with some type of parenteral heparin (49.2% low molecular weight, 25.4% unfractionated).
Table 4.
Anticoagulant Medications in Use for the Longest Duration After Randomization in the SOC Group, Safety Analysis (n = 63)
| Apixaban | 16 (25.4) |
| Dabigatran | 1 (1.6) |
| Unfractionated heparin | 2 (3.2) |
| Low‐molecular‐weight heparin | 2 (3.2) |
| Rivaroxaban | 32 (50.8) |
| Warfarin | 10 (15.9) |
Data are reported as n (%).
SOC = standard of care.
Main Results
Primary Endpoint
The mean (±SD) duration of initial and subsequent hospitalizations for bleeding and/or VTE events within 30 days of randomization was shorter with early discharge on rivaroxaban than SOC: 4.8 (±16.8) hours versus 33.6 (±48.0) hours (p < 0.0001), respectively. The mean difference (95% CI) of LOS between treatment groups was 28.8 hours (–41.5 to –16.2).
Secondary Endpoints
There was no recurrence of VTE, or VTE‐related death, within 7, 14, 30, or 90 days from randomization in any group, and there were no differences in the bleeding‐related hospitalizations or physician visits within 90 days from randomization, 2 (3.9%) versus SOC, 4 (6.3%); 95% CI = –0.024 (–0.206 to 0.160). The mean (±SD) length of initial and subsequent hospitalizations for any reason within 90 days from randomization was shorter for early discharge on rivaroxaban than SOC, 19.2 (52.8) hours vs. SOC, 43.2 (64.8) hours (p = 0.024), respectively. The difference (95% CI) of LOS in the ED and hospital between groups was –26.4 (–47.0 to –3.3) hours.
Table 2 demonstrates safety outcomes. No ISTH major bleeding events occurred, although two (1.8%) subjects reported ISTH clinically relevant nonmajor bleeding, one from each randomization group. One SOC subject had a minimal bleeding event. There were no deaths. The most frequent treatment‐emergent (TE)‐SAE was dyspnea, three (4.8%) in SOC; one was considered related to study drug. No TE‐SAE was considered related to rivaroxaban. There were three TE‐SAEs leading to study agent discontinuation: one (2.0%) on rivaroxaban (arthralgia) and two (3.2%) on SOC (one embolic pneumonia and one dyspnea). There were more TE‐AEs on rivaroxaban, of which most were mild or moderate in severity (see Table 2).
As to patient reported satisfaction, most, 66 of 107 (61.7%), were “very satisfied” with their care, regardless of assignment to early discharge on rivaroxaban (60.4%) or SOC (62.7%; p = 0.619) or its location as outpatient (rivaroxaban cohort, 62.5%) or inpatient (SOC cohort, 59.3%; p = 0.728). However, a greater percentage of early discharge on rivaroxaban patients preferred outpatient care, 24 of 48 (50.0%), compared to SOC, 28 of 59 (47.5%; p = 0.003).
When patient perspective of early discharge on rivaroxaban versus SOC treatment was evaluated using the Anti‐Clot Treatment Scale, more early discharge on rivaroxaban subjects reported it as “not at all” (64.4% vs. 54.4%) or “a little” (28.9% vs. 18.2%) burdensome versus SOC patients who reported it as “moderately” (14.5% vs. 4.4%), “quite a bit” (7.3% vs. 2.2%), and “extremely” (5.5% vs. 0%) burdensome, although no comparisons were significantly different (p = 0.099). Similarly, a higher proportion reported early discharge on rivaroxaban as “extremely beneficial” versus SOC (37.8% vs. 23.6%), but without differences between groups (p = 0.265).
Overall, early discharge on rivaroxaban was markedly less expensive than the SOC. Index visits and total costs were $2,638 (p < 0.001) and $2,496 (p < 0.001) less with rivaroxaban (see Table 5).
Table 5.
Total Costs of Rivaroxaban Versus SOC in LRPE Patients
| Rivaroxaban* (n = 51) | SOC* (n = 63) | Median Difference (95% CI) | p‐value | |
|---|---|---|---|---|
| Total costs | $1,496 ($1,410 to $1,641) | $4,234 ($3,191 to $5,827) | –$2,496 (–$2,999 to –$2,151) | <0.001 |
| Index stay costs | $704 ($618 to $849) | $3,461 ($2,534 to $5,553) | –$2,638 (–$3,288 to –$2,287) | <0.001 |
| Anticoagulation costs | $792 (NA) | $792 ($575 to $792) | NA | NA |
*Data are reported as median (IQR).
LRPE = low‐risk pulmonary embolism; SOC = standard of care.
Discussion
This is the first prospective randomized trial to evaluate ED discharge in LRPE patients in the United States. Our primary endpoint demonstrates that the prospective identification of LRPE patients in the ED is feasible, can result in much shorter hospitalization exposure, and markedly decreases costs without a negative impact on patient satisfaction and with no increase in VTE or bleeding events. While our small sample size limits safety statements, very large studies (n > 3,500) have previously and definitively established that there are very low rates of bleeding and recurrent VTE events, 18, 19 such that a redundant repetition would contribute little to the safety literature (except to be extremely costly). Ultimately, the results of MERCURY‐PE suggest a strategy of early discharge is feasible and demonstrates that the practice of admission for all PE patients should be questioned.
The advantage of potentially shorter inpatient hospitalization portends much greater benefits than simply cost reduction. The avoidance of hospitalization and shortening length of stay6, 7 may be associated with a lower risk of hospital acquired conditions (e.g., hospital‐acquired pneumonia, methicillin‐resistant Staphylococcus aureus infection).
Our data are timely, as significant changes are occurring in the management of anticoagulation. When the MERCURY‐PE protocol was first started, selected centers refused participation due to the perceived ethical, safety, and operational conflicts of discharging patients from the ED with a newly diagnosed pulmonary embolus. In fact, it was common at the participating study sites for enrollment to be met with initial trepidation. However, once implemented, the study protocol was rapidly adopted such that by the final enrollment period, SOC had frequently migrated to include early discharge on rivaroxaban in a number of the participating centers. This experience serves as a demonstration project on the feasibility of adoption of the MERCURY‐PE model to improve patient care.
Reflective of the changing anticoagulant landscape, the majority of patients enrolled in MERCURY‐PE received treatment with a DOAC, most commonly rivaroxaban (51%) or apixaban (25%). While both of these agents have a rapid onset, 75% of all patients still were initially treated with unfractionated or low‐molecular‐weight heparin. The use of a heparinoid agent, despite no bridging therapy requirement with DOACs, may reflect historical patterns of early treatment in suspected PE or may represent concern for selecting a reversible agent (heparin can be reversed, factor Xa inhibitors do not currently have an available antidote) in the initial management period.
Finally, we noted a higher frequency of TE‐AEs with rivaroxaban. This seem to be a composite of several subjective complaints, which in the final analysis did not result in study drug termination or an association with AEs. While the precise causality of these TE‐AEs cannot be determined and are not reflected in very large investigations of rivaroxaban, it is possible that our finding represents a nociceptive bias (where subjects report a higher frequency of events in randomized controlled trials compared to observational registries, as they are specifically asked about these events randomized controlled trials).
Limitations
Our study has several limitations. First our sample size, originally powered to enroll 300 patients, was decreased due to slower than anticipated enrollment and unanticipated funding limitations. Nevertheless, it should be noted that our primary endpoint was met, which speaks to the large effect size attainable from immediate ED discharge. Our unexpectedly large effect size is most likely the result of practice differences in the rates of ED PE discharge in the United States compared to European studies used for its prediction.
Additional limitations include the fact that ED physicians could exclude patients based on a subjective evaluation of hemodynamic stability and their impression of the patient's ability to adhere to the protocol. While some exclusion bias may have occurred, this does not detract from the pragmatic decision strategies required of contemporary ED practice, and the use of the Hestia criteria provides relatively objective data supported guidance for candidate selection.
Further, because a patient would know their admission status, they could not be blinded to their assigned cohort. Thus the use of an endpoint committee, blinded to the intervention, mitigated but did not eliminate this potential bias.
Some may consider the fact that 75% of our control group were discharged on a NOAC as a limitation and that randomization alone may have contributed to our between group differences. We would point out that this reflects current ED practice patterns of hospitalization on an intravenous anticoagulant, then followed by discharge on a NOAC. It should be clear from this analysis that, in selected patients, ED admission for a limited period of intravenous anticoagulation provides little benefit over immediate ED discharge.
Finally, this was a pragmatic trial, and as such we did not dictate SOC, especially when intravenous therapy could result. This strategy was chosen because SOC is not well defined (as demonstrated by our control group), and our dictating therapy would have required a degree of arbitrary decision making (we would have to pick one of several reasonable pathways). Although allowing the physician to define SOC (thus rivaroxaban could be included in the standard care arm) may have contributed to bias to the null, we feel that this actually strengthens our ultimately positive results.
Conclusions
Compared to standard of care, early ED discharge of low‐risk pulmonary embolus patients on rivaroxaban results in markedly lower costs and shorter duration of initial and subsequent hospitalizations without an increase in serious adverse events or patient dissatisfaction.
Academic Emergency Medicine 2018;25:995–1003.
This research was presented as an abstract at the American College of Emergency Physicians Research Forum, Washington, DC, Oct 29, 2017.
Funding for this research was provided by Janssen Pharmaceuticals, Raritan, NJ.
Consulting for commercial interests, including advisory board work—WFP, CC, DD, and AS have received funding personally from Janssen for consulting. WFP, and CC have received funding personally from Bayer, AG. JK has received grant funding from Janssen for a separate study. Payment for writing independent of grant funding—No author received payment from for writing any part of this manuscript. Employment—PW and JX are employed by Janssen, which manufactures rivaroxaban. Institutional Grant Receipt—WFP, CC, DD, SF, CKa, CKe, JM, and AS's institution has received funding from Janssen for this investigator‐initiated research. Miscellaneous—DD has been a member of the SAEM board of directors.
Author Contributions: WFP—study concept and design, acquisition of the data, analysis and interpretation of the data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding. CC—acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and statistical expertise. DD—study concept and design, acquisition of the data, and critical revision of the manuscript for important intellectual content. SK—acquisition of the data and critical revision of the manuscript for important intellectual content. CKa—study concept and design, analysis and interpretation of the data, and critical revision of the manuscript for important intellectual content. CKe—acquisition of the data and critical revision of the manuscript for important intellectual content. JK—study concept and design and critical revision of the manuscript for important intellectual content. JM—acquisition of the data and critical revision of the manuscript for important intellectual content. PW—study concept and design, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding. JX—analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, and statistical expertise. AS—study concept and design, acquisition of the data, analysis and interpretation of the data, critical revision of the manuscript for important intellectual content, statistical expertise, and acquisition of funding.
References
- 1. Centers for Disease Control and Prevention . Venous Thromboembolism (Blood Clots); Data & Statistics. 2015. Available at: http://www.cdc.gov/ncbddd/dvt/data.html. Accessed Dec 14, 2015.
- 2. Schissler AJ, Rozenshtein A, Schluger NW, Einstein AJ. National trends in emergency room diagnosis of pulmonary embolism, 2001‐2010: a cross‐sectional study. Respir Res 2015;16:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Singer AJ, Thode HC, Peacock WF. Admission rates for emergency department patients with venous thromboembolism and estimation of the proportion of low risk pulmonary embolism patients: a US perspective. Clin Exp Emerg Med 2016;3:126–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol 1997;30:1165–71. [DOI] [PubMed] [Google Scholar]
- 5. Beam DM, Kahler ZP, Kline JA. Immediate discharge and home treatment with rivaroxaban of low‐risk venous thromboembolism diagnosed in two U.S. emergency departments: a one‐year preplanned analysis. Acad Emerg Med 2015;22:788–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandilov A. Analysis Report: Estimating the Incremental Costs of Hospital‐acquired Conditions (HACs). Centers for Medicare and Medicaid Services. Available at: https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/HospitalAcqCond/index.html. Accessed May 20, 2016.
- 7. Wang L, Baser O, Wells P, et al. Benefit of early discharge among patients with low‐risk pulmonary embolism. PLoS One 2017;12:e0185022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest 2016;149:315–52. [DOI] [PubMed] [Google Scholar]
- 9. Singer AJ, Xiang J, Kabrhel C, et al. Multicenter Trial of Rivaroxaban for Early Discharge of Pulmonary Embolism From the Emergency Department (MERCURY PE): rationale and design. Acad Emerg Med 2016;23:1280–6. [DOI] [PubMed] [Google Scholar]
- 10. Zondag W, Hiddinga BI, Crobach MJ, et al. Hestia criteria can discriminate high‐ from low‐risk patients with pulmonary embolism. Eur Respir J 2013;41:588–92. [DOI] [PubMed] [Google Scholar]
- 11. Zondag W, Vingerhoets LM, Durian MF, et al. Hestia criteria can safely select patients with pulmonary embolism for outpatient treatment irrespective of right ventricular function. J Thromb Haemost 2013;11:686–92. [DOI] [PubMed] [Google Scholar]
- 12. Schulman S, Kearon C. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non‐surgical patients. J Thromb Haemost 2005;3:692–4. [DOI] [PubMed] [Google Scholar]
- 13. CFR – Code of Federal Regulations Title 21. 21CFR312.32. Available at: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=312.32. Accessed Mar 23, 2018.
- 14. Dasta JF, Pilon D, Mody SH, et al. Daily hospitalization costs in patients with deep vein thrombosis or pulmonary embolism treated with anticoagulant therapy. Thromb Res 2015;135:303–10. [DOI] [PubMed] [Google Scholar]
- 15. Tumeh JW, Moore SG, Shapiro R, Flowers CR. Practical approach for using Medicare data to estimate costs for cost‐effectiveness analysis. Expert Rev Pharmacoecon Outcomes Res 2005;5:153–62. [DOI] [PubMed] [Google Scholar]
- 16. U.S. Bureau of Labor Statistics . Consumer Price Indexes (CPI). Available at: https://www.bls.gov/cpi/. Accessed Aug 2, 2017.
- 17. Aujesky D, Roy PM, Verschuren F, et al. Outpatient versus inpatient treatment for patients with acute pulmonary embolism: an international, open‐label, randomised, non‐inferiority trial. Lancet 2011;378:41–8. [DOI] [PubMed] [Google Scholar]
- 18. EINSTEIN Investigators , Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med 2010; 363:2499–510. [DOI] [PubMed] [Google Scholar]
- 19. Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med 2011;365:883–91. [DOI] [PubMed] [Google Scholar]


