Abstract
Multiple lines of evidence suggest the RNA modification N 6‐methyladonsine (m6A), which is installed in the nucleus cotranscriptionally and, thereafter, serves as a reversible chemical imprint that influences several steps of mRNA metabolism. This includes but is not limited to RNA folding, splicing, stability, transport and translation. In this Review we focus on the current view of the nuclear installation of m6A as well as the molecular players involved, the so called m6A writers. We also explore the effector proteins, or m6A readers, that decode the imprint in different cellular contexts and compartments, and ultimately, the way the modification influences the lifecycle of an RNA molecule. The wide evolutionary conservation of m6A and its critical role in physiology and disease warrants further studies into this burgeoning and exciting field.
Keywords: cotranscriptional regulation, m6A, RNA modifications
Abbreviations
AML, acute myeloid leukaemia
CPT, camptothecin
DMS, dimethylsulphate
mESC, mouse embryonic stem cell
MIRES, m6A‐induced ribosome engagement site
Eukaryotic gene expression is regulated at the transcriptional and post‐transcriptional levels. Nascent transcripts are subject to additional levels of regulation resulting in their processing and/or decay. RNA maturation events often involve chemical modifications that expand the basic A, U, C and G nucleosides. As of 2017, 163 distinct RNA post‐transcriptional modifications have been reported and this number continues to grow 1. Among the best studied modifications in eukaryotes are the 5′ 7‐methylguanylate cap and the 3′ poly(A) tail of mRNA which have important regulatory functions in RNA export, translation and stability.
Our understanding of internal RNA modifications and their functions is unfortunately not as advanced. In eukaryotes, m6A is the most abundant internal modification in mRNA and has been studied for well over four decades. In mammalian cells 0.1–0.4% of adenosines are m6A modified 2, 3. A current estimation of modified sites per transcript is 1–3 m6As in mammalian cells 4, 5 and 1.4‐2.0 m6As in Arabidopsis 6. These approximations likely underrepresent the actual amount of m6A modified sites as these tend to cluster together and are therefore not detectable by meRIP‐seq. m6A is highly conserved and has important physiological functions in different organisms including yeast, plants, flies and mammals. Interestingly Schizosaccharomyces pombe and Caenorhabditis elegans have evolved without this molecular pathway, as many of the core components are absent in these organisms 7, 8.
Technological breakthroughs have recently allowed the genome‐wide mapping of m6A 4, 5, 9, 10 and revealed a degenerate consensus motif, DRACH (where D = A, G or U; R = A or G; H = A, C or U), which is consistent with earlier studies 11, 12. Yet, low complexity of this motif implies the involvement of other sequence or structural motifs in guiding the methylation machinery to target RNAs. Another key recent finding was the discovery that the m6A modification is a reversible imprint (not to be confused with genomic imprinting where a gene is expressed in a parent of origin‐specific manner), with the characterization of the demethylase fat mass and obesity‐associated (FTO) 13, 14 and a second m6A demethylase alkB homologue 5 (ALKBH5) 15. A reversible modification on RNA opened the exciting possibility of dynamic regulation and simultaneously sparked a lively debate over the extent of this dynamism 16, 17, 18.
In this review we will focus on consolidating the recent advances made towards understanding the molecular players involved in m6A deposition. The list of m6A functions continues to expand within different cellular contexts, cell types and organisms. In fact, m6A has been linked to essentially every single process within the RNA life cycle. Here we attempt to summarize the best‐described functions. We briefly discuss the structural effects of m6A as well as the specific protein binders and the way they can influence the fate of a particular RNA species.
m6A writers
Core enzymatic components
The proteins that install m6A can be thought of as RNA writers, just as enzymes that chemically modify N‐terminal tails of core histones are referred to as chromatin writers. Identified in humans, the methyltransferase (MTase) that catalyses m6A is methyltransferase like 3 (METTL3, also called MT‐A70) 19. A second, highly related protein, methyltransferase METTL14 was later characterized 20, 21, 22. It forms a stable heterodimer with METTL3 and is essential for m6A deposition in vivo. Intriguingly, this work found that physical association between METTL3 and METTL14 has a synergistic effect, resulting in higher activity of METTL3 alone. Recent structural studies have further examined the interactions and molecular activities of METTL3 and METTL14 23, 24, 25, revealing that only METTL3 contains catalytic activity, whereas METTL14 has a degenerate active site that is unable to accommodate donor and acceptor substrates in the context of a heterodimer, and is probably inactive 25. Instead METTL14 serves as an RNA‐binding platform, which enhances METTL3 enzymatic activity by binding substrate RNA and by positioning the methyl group for transfer to adenosine, explaining the synergistic effect observed in vitro.
Expanding list of in vivo regulators of the core MTase complex
While there is detailed structural data of the subunits that compose a minimal catalytic core of the complex, studies in several organisms have shown that additional factors are essential for in vivo methylation of mRNA 22, 26, 27, 28, 29, 30. Prominent examples are Wilms tumour 1‐associated protein (WTAP) and Vir‐like m6A methyltransferase associated (VIRMA), as their ablation has drastic effects on global mRNA m6A levels. WTAP is proposed to ensure the stability and localization of the MTase heterodimer to nuclear speckles 22, 28. VIRMA (Vir) was first characterized as having a role in sex determination in Drosophila 31 but its molecular function remains to be determined in the context of m6A. In Drosophila, the RNA binding protein (RBP) Nito promotes m6A incorporation and also has a role in the sex determination and dosage compensation pathways 28. Mammals have a Nito homologue, RNA Binding Motif 15 (RBM15) and a paralogue RBM15B, which are both suggested to regulate m6A 27. Mapping their binding sites using individual‐nucleotide resolution UV cross‐linking and immunoprecipitation (iCLIP) showed similar binding patterns 27. It is thought that RBM15 and RBM15B recruit the MTase complex to its target transcripts via direct binding to U‐rich sequences on mRNA. In humans this function is important to control X chromosome inactivation through the m6A modification of XIST RNA, which is essential for its transcriptional silencing activity. The E3 ubiquitin‐ligase that binds to E‐cadherin, Hakai, was recently found to interact with other subunits of the m6A methyltransferase complex in plants 30. Down regulation of Hakai in plants led to a reduced level of m6A, but its direct role within the MTase complex remains to be elucidated. The latest described factor involved in m6A deposition is Zc3h13 32, 33, 34. Zc3h13 is essential for the localization of the MTase complex in mammalian cells and sex determination in flies. In mouse embryonic stem cell (mESC), Zc3h13 is necessary for self‐renewal. Importantly, Zc3h13 bridges the interaction of Wtap and Rbm15 in both organisms. Consistent with an earlier study examining the WTAP interactome in the context of splicing and cell cycle regulation 35, the above mentioned proteins (WTAP, VIRMA, RBM15, HAKAI and ZC3H13) form a stable complex now referred to as MACOM (methylation associated complex, Fig. 1) 34. MACOM weakly interacts with the Mettll3/14 dimer 20, 34 but is nevertheless indispensible for the correct targeting of m6A to mRNA.
Figure 1.
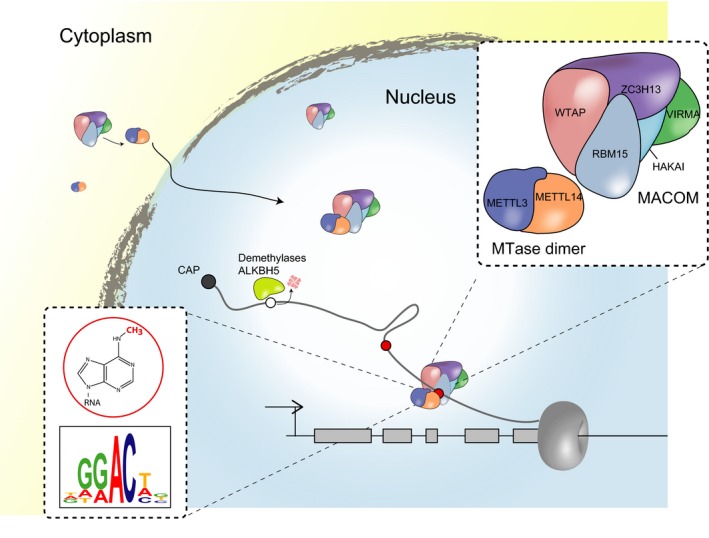
Nuclear installation of m6A. In the most updated model, m6A installation is coupled to transcription and is catalysed by an MTase dimer (METTL3 and METTL14) where METTL3 is the active catalytic subunit. The consensus motif methylated by METTL3 is DRACH (where D = A, G or U; R = A or G; H = A, C or U). A complex termed MACOM composed of WTAP, VIRILIZER, HAKAI, RBM15 and ZC3H13 is essential for in vivo deposition of m6A. MACOM is most likely essential in localizing the MTase to the nucleus and to its RNA targets. Nuclear proteins which have demethylase activity have been characterized including ALKBH5 and FTO.
The factors essential for the deposition of m6A continue to be characterized, emphasizing that our understanding of this intricate pathway is incomplete. Additional players involved in m6A deposition could yet be identified. Supporting this idea, global m6A levels vary in different tissues, developmental stages and cell states 4, 5, as do the levels of writers and erasers 36. It is possible that cell‐type‐specific modulators could regulate the Mettl3/14 complex, altering its activity, specificity or localization. Alternative MTase's with different specificities could also install m6A in a cell‐type‐specific manner. For example several studies have described Mettl16 as having m6A activity modifying coding and noncoding RNAs 37, 38, 39.
Spatiotemporal installation of m6A
Evidence for m6A on pre‐mRNA
Early exploratory studies of m6A supported a model where installation occurs early in the life of RNA. Pioneering reports examining adenovirus type 2 RNA methylation suggested early RNA processing and m6A installation are coupled, with methylation occurring soon after transcription 40. Detection of m6A in pre‐mRNA of an endogenous mammalian gene supported a similar model for mammalian RNAs, where methylation occurs either cotranscriptionally, or very soon after transcription 41. Furthermore, Bokar and colleagues demonstrated that the methylation activity resides in the nuclear fraction of HeLa cell extracts 19.
A breakthrough in our general understanding of the m6A landscape came with the advent of genome‐wide m6A profiling technology using antibody‐based approaches coupled to massive parallel sequencing 4, 5. These comprehensive works revealed the widespread distribution of the modification. In mouse and human cells approximately 10 thousand peaks could be identified within coding and noncoding RNAs. In addition, the distribution and global abundance of m6A were shown to vary depending on cell type, developmental stage and environmental stimulus. These studies revealed evolutionary conservation of prominent features of m6A distribution, including methylation at a degenerate consensus motif, enrichment in 3′UTRs and a sharp peak close to stop codons. Subsequent methodological refinements including ultraviolet light cross‐linking and site‐specific antibody induced mutagenesis improved the resolution to the nucleotide level, and revealed m6A on small nucleolar RNAs (snoRNAs) 9, 10.
An important additional observation from the genome‐wide m6A profiling studies was the identification of enrichment peaks within introns, despite the starting RNA material not being enriched for unspliced pre‐mRNAs. This finding is again supportive of RNA methylation happening at early stages of RNA processing. To directly quantify m6A within growing nascent pre‐mRNA chains, one study used a method to enrich for chromatin‐associated pre‐mRNA (CA‐RNA) using stringent purification conditions 42. This study then compared m6A using a cross‐linking and immunoprecipitation approach of chromatin‐associated, nuclear, and cytoplasmic RNA fractions. Surprisingly, pre‐mRNA enrichment did not show a massive increase in the number of m6A modified introns in CA‐RNA relative to nuclear or cytoplasmic fractions, suggesting the methylation machinery has a preference for exonic sequences (the sequence of events regarding methylation and splicing and interplay between these processes is further discussed below). Additionally, the content of m6A in cytoplasmic mRNA was largely indistinguishable from that in the newly synthesized CA‐RNA or nucleoplasmic mRNA. This suggests m6A is mostly deposited when RNA is still associated with chromatin. The authors further interpreted these observations as indicative of m6A levels remaining unchanged in the three cellular compartments studied and by extension proposed that quantitatively little methylation or demethylation occurs on cytoplasmic mRNA. This view is at odds with the idea of m6A being a dynamic modification and is currently a matter of debate 16, 17, 18.
Using an alternative approach to isolate nascent RNA transcripts through BrU labelling, a recent report profiling m6A found the mark to reside primarily in intronic sequences 43. Perhaps the discrepancy between abundance of m6A in introns compared to previous work is due to differing efficiencies in capturing unspliced pre‐mRNA. In the same study, a comparison between CA‐RNA and 15 min BrU‐labelled RNA shows the latter to be more substantially unspliced. Supportive of intronic deposition is our observation that Mettl3 ChIP‐seq data sets very often localize the protein within intronic regions albeit, high confidence peaks are often broad and span several features, likely due to the cross‐linking strategy used 44. Additionally, METTL3/14 and WTAP PAR‐CLIP profiling experiments often colocalized the writers within introns (~ 29–34%) 20. All together, these findings are consistent with a model of early cotranscriptional deposition of the m6A mark as RNA is newly transcribed.
Another example of early nuclear RNA processing events coupled to m6A deposition is the microRNA (miRNA) biogenesis pathway. Reports linking miRNA maturation to m6A identified an enrichment for the modification on primary miRNA sequences in nuclear RNA 45, 46. Recently, our own work has shown that Mettl3 and Dgcr8, the RNA binding cofactor of Drosha necessary for pri‐miRNA cleavage, often colocalize throughout the genome at miRNA encoding loci, as well as other RNA species including coding, long noncoding RNAs (lncRNAs) and snoRNAs 44. Early deposition of m6A is intriguing because it allows the machinery that recognizes and acts upon the modification to influence the processing and fate of RNAs subsequently, as will be discussed further.
Nuclear localization of the m6A installation machinery
Identification of the proteins required for m6A installation enabled a subcellular interrogation of this process. Using microscopy, the METTL3/METTL14 dimer and associated factor WTAP were localized to nuclear speckles 20, 22, 47. Interestingly, WTAP knockdown in human cells resulted in loss of endogenous METTL3/METTL14 nuclear localization and not vice versa. This experiment suggested WTAP has a critical role in guiding the catalytic dimer to its RNA targets (Fig. 1). Other subunits of MACOM, the WTAP containing complex 34, have also been shown to have a role in localizing the Mettl3/14 dimer. Loss of function of Zc3h13 (discussed below), for example results in Mettl3 and Mett14 re‐localizing to the cytoplasm, as revealed by imaging and cell fractionation experiments in mESCs 48. Given that the disruption of Zc3h13 and other MACOM components severely reduces m6A levels on mRNA, it is tempting to conclude that localizing the MTase dimer to the correct nuclear compartment is indispensable to install the mark.
The use of chromatin immunoprecipitation (ChIP) experiments to localize m6A writers revealed important insights into the localization of the machinery with respect to the genome. ChIP‐sequencing of METTL proteins in mammalian cells demonstrate their proximity to chromatin 44, 49. The profiles of METTL3 binding are variable in these reports, perhaps due to differences in organisms, cell states (mESC vs. human acute myeloid leukaemia [AML] cells) and cross‐linking strategies. Whereas Mettl3 binding in mESCs showed a strong bias towards the 3′ end of target genes, in accordance with m6A enrichment at the 3′UTRs and stop codons, in human AML cells METTL3 bound predominantly to transcription start sites (TSS) and to far fewer targets. Interestingly, METTL3 binding often did not correlate with METTL14 targets in AML cells, raising the provocative idea that they might have independent roles on chromatin in human AML cells. Notably, the role of m6A deposition at the TSS in the malignant cells was suggested to promote translation of target mRNAs essential to maintain AML growth. A similar model had been suggested for 5′UTR methylation of heat shock genes promoting cap‐independent translation upon heat stress (discussed below) 50, 51. Supporting the notion of an environmentally responsive m6A writing machinery, Mettl3 binding radically changes upon heat shock in mESCs, selectively re‐localizing to heat shock response genes 44.
Although the predominant view is that m6A is installed in the nuclear compartment, METTL3 itself has been described to associate with ribosomes and promote translation in human cancer cells 52. This study suggested the machinery could localize outside of the nucleus and associate with specific mRNAs. It was also proposed that METTL3 influences translation, independent of its catalytic activity and of its association with METTL14 and WTAP. These observations would be consistent with the idea that stimuli such as cellular stress or oncogenic transformation can alter the localization of the m6A writers, prompting them to modify novel mRNA targets. These can fall within noncanonical genic locations, such as the 5′UTR of mRNAs, thereby altering translation dynamics. In addition, it is possible that the methylation machinery can even shuttle to the cytoplasm to de novo methylate mRNAs.
Transcriptional mode influences m6A deposition
Given that METTL3/METTL14 deposition of m6A is an early RNA processing event, it is tempting to speculate that it might be directly linked to the act of transcription. Protein–protein interaction studies do not point towards direct associations of m6A writers with the core transcriptional machinery under normal physiological conditions 26, 34, 35. Recently, however, it was found that downstream members of the TGFβ pathway, the SMAD2/3 transcriptional effectors, directly interact with METTL3/METTL14 and WTAP in human pluripotent stem cells 53. In this study, SMAD2/3 proteins were shown to promote the recruitment of m6A writers to TGFβ target mRNAs that are essential for pluripotency, thereby destabilizing them and allowing the rapid transition out of pluripotency upon differentiation. Importantly, this study implies that transcriptional modulators can influence the target specificity of m6A writers and warrants additional interrogation of yet to be identified regulatory binding partners of the METTL3/METTL14 and MACOM complexes.
A study investigating the relationship between transcription and translation efficiency proposes these two processes are coupled and that the former regulates the latter 54. In addition, the authors propose that communication between these compartmentalized processes is mediated by m6A on mRNAs. Mainly through pharmacological manipulation (low level camptothecin (CPT) treatment, which slows down RNA polymerase II [RNAPII] 55), the authors show that slowly transcribed genes are more likely to be m6A modified. Furthermore, METTL3 coprecipitated with RNAPII upon CPT treatment. These results suggest augmented cotranscriptional m6A modification if RNAPII elongation is impeded. Although this is an attractive idea, more evidence is needed to support such a model. Future studies should address how the m6A machinery is coupled to transcription. For example METTL3 has been shown to be post‐translationally modified, phosphorylated and SUMOylated at several sites 33, 47. Such modification could impact catalytic activity, as has been shown when METTL3 is SUMOylated, as well as binding to interaction partners.
Intrinsic effects of m6A
m6A alters RNA secondary structure
The most direct effects of the m6A modification on structure and base pairing of RNA are subtle. Perhaps this partially explains why it has been challenging to assign functions to the modification in vivo. The methyl group at the N6 position of adenosine does not alter Watson–Crick A•U base pairing 56, but nevertheless affects RNA structure by stabilizing unpaired bases and destabilizing duplexes. In solution, the 6‐methyl group on an adenine base is known to exist in two conformations anti and syn, with the latter being energetically favoured 57. NMR studies revealed that m6A•U base pairing requires flipping and trapping of the methylamino group into an energetically unfavourable spring‐loaded anti orientation 58. In this model m6A acts as a compressed spring that is locked into place by its paired context 58 resulting in a net destabilization of the duplex. In unpaired positions, m6A stacks better than an unmodified base, thereby stabilizing stretches of single‐stranded RNA.
Global in vivo secondary structure profiling to examine the impact of m6A
Recent methodological advances permit the examination of RNA secondary structure at the genome‐wide level and to single‐nucleotide resolution in vivo (comprehensively reviewed in Ref. 59). One strategy employs an alkylating reagent [dimethylsulphate (DMS)] that reacts with unpaired adenosines and cytidines and blocks reverse transcription. Coupled to massive parallel sequencing, prematurely terminated cDNA can be used as a proxy for DMS modification and unpaired RNA 60, 61. Another chemical approach termed SHAPE (selective 2′‐hydroxyl acylation analysed by primer extension) uses chemicals that attack the sugar‐phosphate backbone and also block reverse transcription 62. Using an adaptation of SHAPE (icSHAPE), which allows in vivo RNA structure determination for all four bases, the influence of m6A on RNA structure was examined 63. In mESCs comparison of icSHAPE signals at m6A‐modified vs. unmodified sites revealed stronger icSHAPE reactivity (suggesting unpaired RNA) at positions surrounding the modified A. Moreover, in Mettl3 KO mESCs canonical motif sites that lost m6A also widely lost icSHAPE signal, suggesting a gain in pairing and secondary structure 63. These findings are consistent with structural studies showing that m6A has a destabilizing effect on paired bases. Future work including other genome‐wide secondary structure probing strategies such as DMS‐sequencing will be important to further characterizing the role of m6A in regulating RNA secondary structure in vivo.
m6A decoders
YTH proteins as m6A readers
Following the conceptual framework of m6A writers selectively decorating RNAs, ‘reader’ proteins can recognize, decode and influence RNA fate (Fig. 2). The best‐described m6A readers are the YTH domain family of proteins, initially identified by RNA affinity chromatography coupled to mass spectrometry using m6A modified RNA as bait 4. The YTH domain has been identified by sequence comparison in 174 different proteins expressed in eukaryotes 64. Vertebrate YTH proteins can be classified into three categories: YTHDF (YTH domain‐containing family proteins) family, YTHDC1 (YTH domain‐containing protein 1, also called DC1) and YTHDC2 (YTH domain‐containing protein2, also called DC2). Humans have three YTHDF family members, and a single copy of YTHDC1 and YTHDC2 (for an excellent review refer to 65). Structural studies have shown that the methyl moiety of m6A is selectively recognized by an aromatic cage that is formed by two tryptophans and a leucine 66, 67, 68 or three tryptophans 69. The selective binding of YTH proteins to methylated RNA was further confirmed by gel shift assays where the YTH domain preferentially binds methylated RNA oligonucleotides and in vitro pull‐down assays confirming enhanced binding to methylated vs. nonmethylated RNA 4, 21, 67, 70. Transcriptome‐wide binding studies of endogenous YTH proteins using CLIP methods demonstrated that most YTH proteins bind to the m6A mammalian motif (DRACH) in RNA with a nearly complete overlap for the YTHDF members 27, 70, 71. YTHDC1 is localized to a specific nuclear compartment, the YT body, where it was first proposed to regulate splicing 72. YTHDC1 binds to nuclear RNAs including XIST, which is highly m6A modified. As discussed above, m6A is required for X chromosome gene silencing 27 and ablating METTL3 or RBM15 impairs this process. Strikingly, artificial tethering of YTHDC1 to XIST rescues XIST‐mediated silencing upon loss of m6A. YTHDF1–3 are cytoplasmic proteins that have been proposed to have independent roles in regulating mRNA stability and translation (discussed below) 70, 71, 73, despite the fact that YTHDF proteins are highly structurally related and share high amino acid identity. Whether these proteins are functionally fully redundant or indeed have specialized functions remains to be further investigated.
Figure 2.
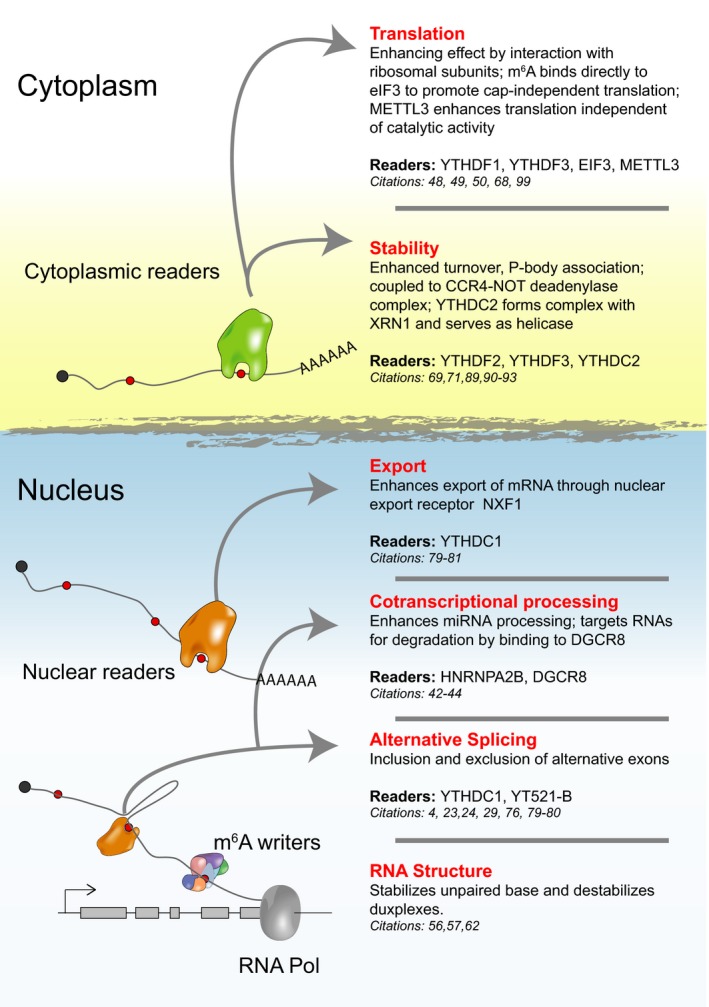
Functional implications of m6A modified RNA. Since transcription, m6A modified RNAs are subject to effects altering their structure, splicing, processing, nuclear export, stability and translation at distinct cellular compartments. Some of these effects are mediated by reader proteins including YTH family proteins that contain a dedicated motif to recognize the methyl moiety as well as others such as HNRNPA2B and EIF3.
Expanding the m6A reader list
In addition to the YTH domain proteins, other m6A readers have been described. The initial m6A RNA pull‐down assays also identified the RRM domain protein ELAVL1 4. A more recent systematic mass‐spectrometry‐based report screened for m6A interactors in various cell types and sequence contexts 74. Prominent proteins described in this work, as expected, included YTH family proteins and the ALKBH5 demethylase. Novel factors included FMR1 and its paralogues FXR1 and FXR2 as sequence‐context‐dependent m6A readers. Interestingly, some proteins were repelled specifically by the presence of the methyl moiety on RNA including G3BP1 in various cell types and mRNA sequence contexts.
m6A‐dependent regulation of splicing
A role for m6A in the process of pre‐mRNA splicing has been apparent since the beginning of the 1980s when this modification was found at position 43 of the U6 small nuclear RNA (U6 snRNA, an abundant RNA critical for splicing) 75. Until very recently the MTase that catalyses m6A on U6 snRNA was unknown, although it was clear that the enzyme was distinct from the MTase that methylated mRNA in HeLa nuclear extracts (later identified as Mettl3) 76. The elusive MTase, METTL16, that methylates U6 snRNA was eventually identified 38. Interestingly, METTL16 has evolved additional roles in vertebrates including the crucial homeostasis maintenance of methyl donor S‐adenosylmethionine (SAM). In humans the SAM synthetase MAT2A generates SAM from methionine and ATP. There are two MAT2A RNA isoforms, a cytoplasmic mRNA and a nuclear retained‐intron isoform (MAT2A‐RI), which is subject to decay 77. In conditions of low cellular SAM, METTL16 m6A methylates MAT2A pre‐mRNA on a conserved hairpin and promotes splicing of the MAT2A retained intron. The balance in isoforms then shifts to the cytoplasmic transcripts, which promotes SAM synthesis. Of note, a recent report suggests that m6A regulation of MAT2A may also be mediated through mRNA degradation by recognition of the reader protein YTHDC1 39.
The role of Mettl3‐mediated m6A in splicing has been best characterized in Drosophila 28, 29, 78. In particular, m6A is necessary for correct splicing and to achieve maximal Sex lethal (Sxl) expression, the master regulator of sex determination. Sxl then suppresses dosage compensation in females by inhibiting the translation of Male‐specific lethal‐2 (Msl‐2), which is required for the upregulation of transcription from the single male X chromosome 8. The functional role of m6A in splicing of Sxl was addressed in three studies and all coincided on sex determination phenotypes with varying penetrance depending on the m6A component ablated. Furthermore, the Drosophila YTH family member m6A reader, YT521‐B recently renamed Ythdc1, is necessary for correct exon exclusion and to achieve maximum Sxl expression in females 28, 29. Finally, a splicing defect in Sxl and a sex determination phenotype is also apparent in flies with impaired expression of the MACOM component Zc3h13/Flacc 34. In addition to m6A installation, MACOM components have additional important roles. In flies it was found through mRNA profiling experiments that MACOM components showed a much stronger perturbation upon knock down when compared to the MTAses 34. In human cells, a study has shown that WTAP expression is cell cycle regulated, and that it controls the stability of cyclin A2 mRNA 79. Therefore, we propose that future studies should identify mutations disrupting the interaction between MACOM and the MTAse duplex to cleanly dissect m6A independent functions.
In humans the nuclear reader protein YTHDC1 has a role in the inclusion of alternative exons 80. YTHDC1 interacts with pre‐mRNA splicing factors, including splicing enhancer‐binding SR proteins (Fig. 2). Mechanistically, m6A present in alternative exons induces their inclusion by recruiting YTHDC1, which in turn recruits the splicing factor Serine and arginine‐rich splicing factor 3 (SRSF3) 80. YTHDC1 also inhibits pre‐mRNA binding of the exon‐skipping factor SRSF10 by competitively binding to SRSF3 and occupying SRSF10 RNA landing sites. In conclusion, although m6A has been clearly linked to pre‐mRNA splicing, more work will be required for a full mechanistic understanding.
Nuclear export of methylated mRNA
Another key aspect that has been linked to m6A modification is nuclear export. For example RNA viruses exploit the endogenous methylation machinery to modify their mRNAs. Zika and HIV‐1 are extensively m6A methylated and exploit the modification to enhance nuclear export and other processing steps during replication 81, 82. In noninfected human cells, the m6A‐binding protein YTHDC1 mediates export of methylated mRNA from the nucleus to the cytoplasm 83. As described above, YTHDC1 interacts with SRSF3, which can also interact with the nuclear export receptor NXF1 (Fig. 2). Inhibition of YTHDC1 does not affect global m6A levels in mRNA, but instead results in accumulation of the mark in nuclear mRNA and in depletion from the cytoplasmic pool. This finding was supported by tethering YTHDC1 to a synthetic mRNA, which facilitated its export and concomitantly increased cytoplasmic abundance and translation. Expanding on the reader‐tethering assay, future studies on the role of m6A in RNA transport should include single‐molecule imaging techniques. These types of experiments will be crucial to yield quantitative information on the rate at which transport is enhanced.
Turnover of methylated RNA
One of the best‐described functions for m6A so far is in controlling RNA stability. In this respect, the modification is remarkably versatile in employing distinct mechanisms and molecular partners for turnover, depending on target RNA and cellular compartment (Fig. 2). Some have proposed that modulating RNA turnover is the main function of m6A, at least in some cellular contexts such as human cancer cells and mESCs, 16, 17 and that its role in pre‐mRNA splicing is minor.
The physiological importance of m6A‐mediated regulation of RNA stability has been nicely exemplified in the context of mESCs, where Mettl3 is necessary to terminate naïve pluripotency 84. Mettl3 KO mESCs fail to terminate their naïve state and instead undergo aberrant and restricted lineage priming at the post‐implantation stage, eventually resulting in embryonic lethality. Thus, by directly reducing mRNA stability of key naïve pluripotency transcripts, m6A controls the transition from naïve to primed pluripotency.
Mechanisms of m6A‐mediated RNA decay
From nucleus to cytoplasm, the earliest example of m6A‐mediated RNA turnover occurs cotranscriptionally 44. As previously mentioned, the miRNA biogenesis machinery can bind and degrade non‐miRNA targets in the nucleus in an m6A‐dependent manner 85, 86, 87, 88, 89, 90, 91. In this context, m6A presumably confers specificity and facilitates the recognition of RNA structures by DGCR8. The molecular mechanism by which m6A aids DGCR8/DROSHA to recognize and cleave its targets has been addressed and is in part mediated by the reader protein HNRNPA2B 46. This cotranscriptional regulation mechanism is responsive to the environment as the players involved can rapidly re‐localize to essential genes. This is the case for Hsp70 during heat shock where there is massive accumulation of Mettl3 and Dgcr8 44. Future studies should examine the response of these players to other environmental stimuli or conditions that globally alter the transcriptome.
An important mechanism of m6A‐mediated RNA decay functioning in the cytoplasm employs the reader protein YTHDF2 70. In this study RNA half‐life profiling revealed a pronounced increase in stability in YTHDF2 knockdown cells compared to control samples. The effect correlated well with YTHDF2 binding, where RNAs with more binding displayed increased stabilization. Importantly, YTHDF2 colocalizes with three markers (DCP1a, GW182 and DDX6) of processing bodies (P bodies) in the cytoplasm 70. The highly related m6A reader protein YTHDF3 has been reported to synergize with YTHDF1 and YTHDF2 by potentiating their binding to target RNAs thereby promoting their effects on translation and decay, respectively 73. A separate study found that m6A‐modified RNAs exhibit accelerated deadenylation mediated by the CCR4–NOT deadenylase complex 92. The deadenylation is mediated through the direct recruitment of the CCR4–NOT complex by YTHDF2. Another reader protein that was recently shown to serve as an intermediary between m6A and the RNA degradation machinery is YTHDC2. This cytoplasmic reader and RNA helicase is essential for meiosis in mice 93, 94, 95, 96. Data suggesting that Ythdc2 mediate m6A‐dependent RNA degradation includes the observation that m6A decorated transcripts are upregulated in Ythdc2 knockout testes compared to wild‐type controls 93, 94. Biochemical characterization of Ythdc2 binding partners identified the exoribonuclease Xrn1 as the top associated factor in mouse testis protein extracts in addition to Meioc, a highly conserved meiosis‐specific protein. In sum, the usage of m6A reader proteins to target a particular subset of transcripts for expedited degradation is a conserved strategy employed in diverse cell types and executed by varying molecular partners.
Another way in which m6A regulates RNA stability, independent of direct reader proteins, is through its effect on secondary structure (discussed above). One example is the RBP HuR which binds to U‐rich regions in the 3′‐UTR of transcripts 97 and can block miRNA guided Argonaute complexes, thus preventing degradation 98. mESC transcripts encoding developmental regulators are highly m6A decorated 21, 84. One study proposed that HuR binding is impaired in m6A modified transcripts allowing miRNA‐mediated repression 21. Accordingly, Mettl3 knockdown results in decreased Ago2 binding to a known methylated transcript and its concomitant stabilization.
m6A effect on translation
A growing body of work supports the hypothesis that m6A also serves as means to communicate nuclear transcriptional information to the downstream translation machinery 54, 71 (Fig. 2). In yeast and human cells, when examining the distribution of m6A modified mRNAs there was a noticeable enrichment in ribosome associated fractions. Conversely, nonribosome‐associated fractions were depleted for m6A 71, 99. A study using single‐molecule methods to probe the effect of m6A on mRNA showed changes in translational dynamics 100. Although m6A base pairs with uridine during decoding, methylated codons have slower translation‐elongation dynamics, leading the authors to propose that the presence of an m6A within a codon slows down cognate‐tRNA decoding by acting as a barrier to tRNA accommodation 100.
On the other hand, a comprehensive examination of the functional role of the m6A reader YTHDF1 showed that it enhances the translation efficiency of m6A modified RNAs 71, through direct interactions with initiation factors and ribosomal subunits. High‐throughput sequencing experiments uncovered a positive correlation between the ribosome association of YTHDF1 bound mRNAs and the number of YTHDF1‐binding sites on the target mRNAs. Furthermore, tethering experiments revealed 72% increased translation efficiency for YTHDF1‐tethered transcripts 71. Whether such effects on translation are restricted to the subset of transcripts bound by YTHDF1 remains an open question. Notably, analysis of translation efficiency in WT vs. Mettl3 KO ESCs by ribosome profiling revealed only a minor yet significant increase in translation efficiency in KO cells, on both methylated and unmethylated transcripts 84.
m6A as a mediator of cap‐independent translation during stress
Although the majority of m6A shows a 3′ bias and is close to the stop codon, substantial methylation is detectable in 5′ UTRs. Antibody‐based m6A profiling experiments have been shown to also detect a related RNA modification, N6,2′‐O‐dimethladenosine (m6Am). This modification has the unique property that it is present only when the first cap‐adjacent nucleotide is an adenosine and is thought to enhance stability of mRNAs 101. m6A is not detected at the first position of mRNA and can therefore be readily distinguished from m6Am by position in single‐nucleotide resolution approaches 102. It has been proposed that mRNAs containing m6A in their 5′ UTR can be translated in a cap‐independent manner 50, 51. In cap‐independent translation, mRNAs do not require eIF4E and are translated under normal resting conditions, as well as under environmental stress, viral infection, or other disease conditions 103. m6A in the 5′ UTR can bind eukaryotic initiation factor 3 (eIF3) and thus act as an m6A‐induced ribosome engagement site (MIRES), which promotes translation of mRNA independent of the presence of a cap structure. Interestingly, cells subjected to heat stress exhibit increased m6A methylation (and not m6Am) within the 5′UTR of newly transcribed mRNAs, selectively on stress‐inducible mRNAs. The 5′ accumulation was observed also when cells were exposed to UV radiation, supporting a generalized mechanism for other forms of stress 50. The accumulation of 5′UTR methylation was proposed to be mediated through nuclear translocation of YTHDF2, where it protects methylated adenosines from eraser FTO 51. Recently, however, a report suggested that in vivo, FTO demethylates m6Am as its preferred substrate. It remains to be tested if the nuclear translocation and shielding effect of YTHDF2 prevents demethylation by ALKBH5.
Conclusions and Perspectives
The field of RNA modifications has recently garnered a great deal of attention. Among the most prominent modifications, m6A stands out as multi‐purpose signal on RNA. Deposited early during transcription by multiprotein writer complexes that are responsive to cell context and environmental cues, m6A functions in key steps of mRNA metabolism. Intrinsic properties of RNA are affected by the modification, including base‐pairing stability and secondary structure. The repertoire of proteins that can interact with a given RNA is also different once it is modified. Reader proteins affect early RNA processing events including splicing, poly‐A usage and cotranscriptional cleavage 10. In the cytoplasm, m6A drastically influences mRNA turnover. Cytoplasmic YTH proteins mediate association to the decay machinery. It is also evident that m6A can affect translation dynamics, in some cases potentiated through direct recruitment of translation initiation factors.
Despite significant progress in recent years, important basic questions regarding m6A biology remain elusive. For example a better understanding of the rules that define the methylation machinery's specificity is urgently needed. It remains unclear which determinants make an mRNA a good substrate for methylation. Although the catalytic core of the methylation machinery and its obligate cofactors have been characterized with some detail, it is likely that m6A biogenesis is a carefully orchestrated process that requires multiple additional factors and cell‐specific regulators. We therefore anticipate that additional players involved in m6A deposition will be discovered and characterized in the future.
Besides the advancement of our mechanistic understanding of m6A biology, its physiological roles are also becoming increasingly evident in multiple organisms and cell types (description of which unfortunately is beyond the scope of this review). It has also become abundantly clear that, as with many fundamental cell biological processes, m6A can be hijacked in several forms of cancer. A growing body of work suggests that m6A is implicated in glioblastoma 104, 105, 106. There is also a clear link of m6A to leukaemia. For example METTL3 inhibition in human myeloid leukaemia cell lines induces differentiation, apoptosis and delays leukaemia in transplantation experiments 49, 107. Some forms of acute megakaryoblastic leukaemias are mediated by a chromosomal translocation of RBM15 with the MAL gene, where the fusion protein drives the development of haematological malignancy 108. It is thus tempting to speculate that a RBM15 fusion protein could re‐direct the m6A machinery to new targets, thereby tilting the transcriptome towards malignancy. Hence, manipulation of the m6A pathway as a novel approach towards cancer therapy might be not too far‐fetched.
Acknowledgements
We are grateful to Alex Tuck and Sarah Carl for comments on the manuscript. Work performed in MB's laboratory is supported by the Novartis Research Foundation and the Swiss National Science Foundation NCCR RNA & Disease (grant no. 141735). We apologize to colleagues whose work we were not able to cite due to space constraints.
Edited by Wilhelm Just
References
- 1. Boccaletto P, MacHnicka MA, Purta E, Pitkowski P, Baginski B, Wirecki TK, De Crécy‐Lagard V, Ross R, Limbach PA, Kotter A et al (2018) MODOMICS: a database of RNA modification pathways. 2017 update. Nucleic Acids Res 46, D303–D307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wei CM, Gershowitz A and Moss B (1975) Methylated nucleotides block 5′ terminus of HeLa cell messenger RNA. Cell 4, 379–386. [DOI] [PubMed] [Google Scholar]
- 3. Rottman F, Shatkin AJ and Perry RP (1974) Sequences containing methylated nucleotides at the 5′ termini of messenger RNAs: possible implications for processing. Cell 3, 197–199. [DOI] [PubMed] [Google Scholar]
- 4. Dominissini D, Moshitch‐moshkovitz S, Schwartz S, Ungar L, Osenberg S, Cesarkas K, Jacob‐hirsch J, Amariglio N, Kupiec M, Sorek R et al (2012) Topology of the human and mouse m6A RNA methylomes revealed by m6A‐seq. Nature 485, 201–206. [DOI] [PubMed] [Google Scholar]
- 5. Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE and Jaffrey SR (2012) Resource comprehensive analysis of mRNA methylation reveals enrichment in 3 0 UTRs and near stop codons. Cell 149, 1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wan Y, Tang K, Zhang D, Xie S, Zhu X, Wang Z and Lang Z (2015) Transcriptome‐wide high‐throughput deep m6A‐seq reveals unique differential m6A methylation patterns between three organs in Arabidopsis thaliana . Genome Biol 16, 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C, Zhu Y, Bao H, Jiang Y, Xu C, Wu J and Shi Y (2016) A novel RNA‐binding mode of the YTH domain reveals the mechanism for recognition of determinant of selective removal by Mmi1. Nucleic Acids Res 44, 969–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roignant JY and Soller M (2017) m6A in mRNA: an ancient mechanism for fine‐tuning gene expression. Trends Genet 33, 380–390. [DOI] [PubMed] [Google Scholar]
- 9. Linder B, Grozhik AV, Olarerin‐George AO, Meydan C, Mason CE and Jaffrey SR (2015) Single‐nucleotide‐resolution mapping of m6A and m6Am throughout the transcriptome. Nat Methods 12, 767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ke S, Alemu EA, Mertens C, Gantman EC, Fak JJ, Mele A, Haripal B, Zucker‐Scharff I, Moore MJ, Park CY et al (2015) A majority of m6A residues are in the last exons, allowing the potential for 3ʹ UTR regulation. Genes Dev 29, 2037–2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dimock K and Stoltzfus CM (1977) Sequence specificity of internal methylation in B77 avian sarcoma virus RNA subunits. Biochemistry 16, 471–478. [DOI] [PubMed] [Google Scholar]
- 12. Schibler U, Kelley DE and Perry RP (1977) Comparison of methylated sequences in messenger RNA and heterogeneous nuclear RNA from mouse L cells. J Mol Biol 115, 695–714. [DOI] [PubMed] [Google Scholar]
- 13. Jia G, Fu Y, Zhao X, Dai Q, Zheng G, Yang Y, Yi C, Lindahl T, Pan T, Yang YG et al (2011) N6‐Methyladenosine in nuclear RNA is a major substrate of the obesity‐associated FTO. Nat Chem Biol 7, 885–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bartosovic M, Molares HC, Gregorova P, Hrossova D, Kudla G and Vanacova S (2017) N6‐methyladenosine demethylase FTO targets pre‐mRNAs and regulates alternative splicing and 3′‐end processing. Nucleic Acids Res 45, 11356–11370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zheng G, Dahl JA, Niu Y, Fedorcsak P, Huang CM, Li CJ, Vågbø CB, Shi Y, Wang WL, Song SH et al (2013) ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol Cell 49, 18–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darnell RB, Ke S and Darnell JE (2017) Pre‐mRNA processing includes N6 methylation of adenosine residues that are retained in mRNA exons and the fallacy of “RNA epigenetics”. RNA 24(3), 262–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosa‐Mercado NA, Withers JB and Steitz JA (2017) Settling the m6A debate: methylation of mature mRNA is not dynamic but accelerates turnover. Genes Dev 31, 957–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao BS, Nachtergaele S, Roundtree IA and He C (2017) Our views of dynamic. RNA J 24, 268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bokar JA, Shambaugh ME, Polayes D, Matera AG and Rottman FM (1997) Purification and cDNA cloning of the AdoMet‐binding subunit of the human mRNA (N6‐adenosine)‐methyltransferase. RNA 3, 1233–1247. [PMC free article] [PubMed] [Google Scholar]
- 20. Liu J, Yue Y, Han D, Wang X, Fu Y, Zhang L and Jia G (2014) A METTL3 – METTL14 complex mediates mammalian nuclear RNA N6‐adenosine methylation. Nat Chem Biol 10, 93–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang Y, Li Y, Toth JI, Petroski MD, Zhang Z and Zhao JC (2014) N6‐methyladenosine modification destabilizes developmental regulators in embryonic stem cells. Nat Cell Biol 16, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ping XL, Sun BF, Wang L, Xiao W, Yang X, Wang WJ, Adhikari S, Shi Y, Lv Y, Chen YS et al (2014) Mamalian WTAP is a regulatory subunit of the RNA N6‐methyladenosine methyltransferasme. Cell Res 24, 177–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wang P, Doxtader KA and Nam Y (2016) Structural basis for cooperative function of Mettl3 and Mettl14 methyltransferases. Mol Cell 63, 306–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wang X, Feng J, Xue Y, Guan Z, Zhang D, Liu Z, Gong Z, Wang Q, Huang J, Tang C et al (2016) Structural basis of N6‐adenosine methylation by the METTL3‐METTL14 complex. Nature 534, 575–578. [DOI] [PubMed] [Google Scholar]
- 25. Śledź P and Jinek M (2016) Structural insights into the molecular mechanism of the m6A writer complex. eLife 5, e18434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schwartz S, Mumbach MR, Jovanovic M, Wang T, Maciag K, Bushkin GG, Mertins P, Ter‐Ovanesyan D, Habib N, Cacchiarelli D et al (2014) Perturbation of m6A writers reveals two distinct classes of mRNA methylation at internal and 5′ sites. Cell Rep 8, 284–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Patil DP, Chen C‐K, Pickering BF, Chow A, Jackson C, Guttman M and Jaffrey SR (2016) m6A RNA methylation promotes XIST‐mediated transcriptional repression. Nature 537, 369–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lence T, Akhtar J, Bayer M, Schmid K, Spindler L, Ho CH, Kreim N, Andrade‐Navarro MA, Poeck B, Helm M et al (2016) m6A modulates neuronal functions and sex determination in Drosophila. Nature 540, 242–247. [DOI] [PubMed] [Google Scholar]
- 29. Haussmann IU, Bodi Z, Sanchez‐Moran E, Mongan NP, Archer N, Fray RG and Soller M (2016) m6A potentiates Sxl alternative pre‐mRNA splicing for robust Drosophila sex determination. Nature 540, 301–304. [DOI] [PubMed] [Google Scholar]
- 30. Růžička K, Zhang M, Campilho A, Bodi Z, Kashif M, Saleh M, Eeckhout D, El‐Showk S, Li H, Zhong S et al (2017) Identification of factors required for m6A mRNA methylation in Arabidopsis reveals a role for the conserved E3 ubiquitin ligase HAKAI. New Phytol 215, 157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Niessen M, Schneiter R and Nothiger R (2001) Molecular identification of virilizer, a gene required for the expression of the sex‐determining gene sex‐lethal in Drosophila melanogaster . Genetics 157, 679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guo J, Tang H‐W, Li J, Perrimon N and Yan D (2018) Xio is a component of the Drosophila sex determination pathway and RNA N 6‐methyladenosine methyltransferase complex. Proc Natl Acad Sci U S A 115, 3674–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Du Y, Hou G, Zhang H, Dou J, He J, Guo Y, Li L, Chen R, Wang Y, Deng R et al (2018) SUMOylation of the m6A‐RNA methyltransferase METTL3 modulates its function. Nucleic Acids Res 04, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Knuckles P, Lence T, Haussmann IU, Jacob D, Kreim N, Carl SH, Masiello I, Hares T, Villaseñor R, Hess D et al (2018) Zc3h13/Flacc is required for adenosine methylation by bridging the mRNA‐binding factor Rbm15/Spenito to the m6A machinery component Wtap/Fl(2)d. Genes Dev 32, 415–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Horiuchi K, Kawamura T, Iwanari H, Ohashi R, Naito M, Kodama T and Hamakubo T (2013) Identification of Wilms' tumor 1‐associating protein complex and its role in alternative splicing and the cell cycle. J Biol Chem 288, 33292–33302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chang M, Lv H, Zhang W, Ma C, He X, Zhao S, Zhang Z‐W, Zeng Y‐X, Song S, Niu Y et al (2017) Region‐specific RNA m6A methylation represents a new layer of control in the gene regulatory network in the mouse brain. Open Biol 7, 170166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Warda AS, Kretschmer J, Hackert P, Lenz C, Urlaub H, Höbartner C, Sloan KE and Bohnsack MT (2017) Human METTL 16 is a N6‐methyladenosine (m6A) methyltransferase that targets pre‐mRNAs and various non‐coding RNAs. EMBO Rep 18, 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pendleton KE, Chen B, Liu K, Hunter OV, Xie Y, Tu BP and Conrad NK (2017) The U6 snRNA m6A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell 169, 824–835.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shima H, Matsumoto M, Ishigami Y, Ebina M, Muto A, Sato Y, Kumagai S, Ochiai K, Suzuki T and Igarashi K (2017) S‐Adenosylmethionine synthesis is regulated by selective N6‐adenosine methylation and mRNA degradation involving METTL16 and YTHDC1. Cell Rep 21, 3354–3363. [DOI] [PubMed] [Google Scholar]
- 40. Chen‐Kiang S, Nevins JR and Darnell JE (1979) N‐6‐methyl‐adenosine in adenovirus type 2 nuclear RNA is conserved in the formation of messenger RNA. J Mol Biol 135, 733–752. [DOI] [PubMed] [Google Scholar]
- 41. Carroll SM, Narayan P and Rottman FM (1990) N6‐methyladenosine residues in an intron‐specific region of prolactin pre‐mRNA. Mol Cell Biol 10, 4456–4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ke S, Pandya‐jones A, Saito Y, Fak JJ, Vågbø CB, Geula S, Hanna JH, Black DL, Darnell JE Jr and Darnell RB (2017) m6A mRNA modifications are deposited in nascent pre‐mRNA and are not required for splicing but do specify cytoplasmic turnover. Genes Dev 31, 990–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Louloupi A, Ntini E, Conrad T and Orom UA (2018) Transient N‐6‐methyladensosine transcriptome sequencing reveals a regulatory role of m6A in splicing efficiency. bioRxiv 242966 [PREPRINT]. [DOI] [PubMed] [Google Scholar]
- 44. Knuckles P, Carl SH, Musheev M, Niehrs C, Wenger A and Bühler M (2017) RNA fate determination through cotranscriptional adenosine methylation and microprocessor binding. Nat Struct Mol Biol 24, 561–569. [DOI] [PubMed] [Google Scholar]
- 45. Alarcón CR, Lee H, Goodarzi H, Halberg N and Tavazoie SF (2015) N6‐methyladenosine marks primary microRNAs for processing. Nature 519, 482–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alarcón CR, Goodarzi H, Lee H, Liu X, Tavazoie S and Tavazoie SF (2015) HNRNPA2B1 is a mediator of m6A‐dependent nuclear RNA processing events. Cell 162, 1299–1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Schöller E, Weichmann F, Treiber T, Ringle S, Flatley A, Feederle R, Bruckmann A and Meister G (2018) Interactions, localization and phosphorylation of the m6A generating METTL3‐ METTL14‐WTAP complex. RNA 24, 499–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wen J, Lv R, Ma H, Shen H, He C, Wang J, Jiao F, Liu H, Yang P, Tan L et al (2018) Zc3h13 regulates nuclear RNA m6A methylation and mouse embryonic stem cell self‐renewal. Mol Cell 69, 1028–1038.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barbieri I, Tzelepis K, Pandolfini L, Shi J, Millán‐Zambrano G, Robson SC, Aspris D, Migliori V, Bannister AJ, Han N et al (2017) Promoter‐bound METTL3 maintains myeloid leukaemia by m6A‐dependent translation control. Nature 552, 126–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, Pestova TV, Qian SB and Jaffrey SR (2015) 5′ UTR m6A promotes cap‐independent translation. Cell 163, 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR and Qian SB (2015) Dynamic m6A mRNA methylation directs translational control of heat shock response. Nature 526, 591–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lin S, Choe J, Du P, Triboulet R, Gregory Correspondence RI and Gregory RI (2016) The m6A methyltransferase METTL3 promotes translation in human cancer cells a methyltransferase METTL3 promotes translation in human cancer cells. Mol Cell 62, 335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bertero A, Brown S, Madrigal P, Osnato A, Ortmann D, Yiangou L, Kadiwala J, Hubner NC, de Los Mozos IR, Sadee C et al (2018) The SMAD2/3 interactome reveals that TGFβ controls m6A mRNA methylation in pluripotency. Nature 555, 256–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Slobodin B, Han R, Calderone V, Vrielink JAFO, Loayza‐puch F, Elkon R, Agami R and Methylation N (2017) Transcription impacts the efficiency of mRNA translation via co‐transcriptional N6‐adenosine article transcription impacts the efficiency of mRNA translation via co‐transcriptional. Cell 169, 326–337.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dujardin G, Lafaille C, de la Mata M, Marasco LE, Muñoz MJ, Le Jossic‐Corcos C, Corcos L and Kornblihtt AR (2014) How slow RNA polymerase II elongation favors alternative exon skipping. Mol Cell 54, 683–690. [DOI] [PubMed] [Google Scholar]
- 56. Kierzek E and Kierzek R (2003) The thermodynamic stability of RNA duplexes and hairpins containing N6‐alkyladenosines and 2‐methylthio‐N6‐alkyladenosines. Nucleic Acids Res 31, 4472–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Engel JD and von Hippel PH (1974) Effects of methylation on the stability of nucleic acid conformations: studies at the monomer level. Biochemistry 13, 4143–4158. [DOI] [PubMed] [Google Scholar]
- 58. Roost C, Lynch SR, Batista PJ, Qu K, Chang HY and Kool ET (2015) Structure and thermodynamics of N6‐methyladenosine in RNA: a spring‐loaded base modification. J Am Chem Soc 137, 2107–2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Bevilacqua PC, Ritchey LE, Su Z and Assmann SM (2016) Genome‐wide analysis of RNA secondary structure. Annu Rev Genet 50, 235–266. [DOI] [PubMed] [Google Scholar]
- 60. Ding Y, Tang Y, Kwok CK, Zhang Y, Bevilacqua PC and Assmann SM (2014) In vivo genome‐wide profiling of RNA secondary structure reveals novel regulatory features. Nature 505, 696–700. [DOI] [PubMed] [Google Scholar]
- 61. Rouskin S, Zubradt M, Washietl S, Kellis M and Weissman JS (2014) Genome‐wide probing of RNA structure reveals active unfolding of mRNA structures in vivo. Nature 505, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Watts JM, Dang KK, Gorelick RJ, Leonard CW, Bess JW, Swanstrom R, Burch CL and Weeks KM (2009) Architecture and secondary structure of an entire HIV‐1 RNA genome. Nature 460, 711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Spitale RC, Flynn RA, Zhang QC, Crisalli P, Lee B, Jung J, Kuchelmeister HY, Batista PJ, Torre EA, Kool ET et al (2015) Structural imprints in vivo decode RNA regulatory mechanisms. Nature 519, 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zhang Z, Theler D, Kaminska KH, Hiller M, Grange PDe, Pudimat R, Rafalska I, Heinrich B, Bujnicki JM and Stamm S (2010) The YTH domain is a novel RNA binding domain. J Biol Chem 285, 14701–14710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Patil DP, Pickering BF and Jaffrey SR (2017) Reading m6A in the transcriptome: m6A‐binding proteins. Trends Cell Biol 28, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Xu C, Wang X, Liu K, Roundtree IA, Tempel W, Li Y, Lu Z, He C and Min J (2014) Structural basis for selective binding of m6A RNA by the YTHDC1 YTH domain. Nat Chem Biol 10, 927–929. [DOI] [PubMed] [Google Scholar]
- 67. Xu C, Liu K, Ahmed H, Loppnau P, Schapira M and Min J (2015) Structural basis for the discriminative recognition of N6‐Methyladenosine RNA by the human YT521‐B homology domain family of proteins. J Biol Chem 290, 24902–24913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Theler D, Dominguez C, Blatter M, Boudet J and Allain FHT (2014) Solution structure of the YTH domain in complex with N6‐methyladenosine RNA: a reader of methylated RNA. Nucleic Acids Res 42, 13911–13919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Li F, Zhao D, Wu J and Shi Y (2014) Structure of the YTH domain of human YTHDF2 in complex with an m6A mononucleotide reveals an aromatic cage for m6A recognition. Cell Res 24, 1490–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Wang X, Lu Z, Gomez A, Hon GC, Yue Y, Han D, Fu Y, Parisien M, Dai Q, Jia G et al (2013) N6‐methyladenosine‐dependent regulation of messenger RNA stability. Nature 505, 117–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang X, Zhao BS, Roundtree IA, Lu Z, Han D, Ma H, Weng X, Chen K, Shi H and He C (2015) N6‐methyladenosine modulates messenger RNA translation efficiency. Cell 161, 1388–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Rafalska I, Zhang Z, Benderska N, Wolff H, Hartmann AM, Brack‐Werner R and Stamm S (2004) The intranuclear localization and function of YT521‐B is regulated by tyrosine phosphorylation. Hum Mol Genet 13, 1535–1549. [DOI] [PubMed] [Google Scholar]
- 73. Shi H, Wang X, Lu Z, Zhao BS, Ma H, Hsu PJ and Liu C (2017) YTHDF3 facilitates translation and decay of N6‐methyladenosine‐modified RNA. Cell Res 27, 315–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Edupuganti RR, Geiger S, Lindeboom RGH, Shi H, Hsu PJ, Lu Z, Wang SY, Baltissen MPA, Jansen PWTC, Rossa M et al (2017) N6‐methyladenosine (m6A) recruits and repels proteins to regulate mRNA homeostasis. Nat Struct Mol Biol 24, 870–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Epstein P, Reddy R, Henning D and Busch H (1980) The nucleotide sequence of nuclear U6 (4.7 S) RNA. J Biol Chem 255, 8901–8906. [PubMed] [Google Scholar]
- 76. Shimba S, Bokar JA, Rottman F and Reddy R (1995) Accurate and efficient N‐6‐adenosine methylation in spliceosomal U6 small nucelar RNA by HeLa cell extract in vitro. Nucleic Acids Res 23, 2421–2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Bresson SM, Hunter OV, Hunter AC and Conrad NK (2015) Canonical Poly(A) polymerase activity promotes the decay of a wide variety of mammalian nuclear RNAs. PLoS Genet 11, e1005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kan L, Grozhik A V, Vedanayagam J, Patil DP, Pang N, Lim KS, Huang YC, Joseph B, Lin CJ, Despic V et al (2017) The m6A pathway facilitates sex determination in Drosophila. Nat Commun 8, 15737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Horiuchi K, Umetani M, Minami T, Okayama H, Takada S, Yamamoto M, Aburatani H, Reid PC, Housman DE, Hamakubo T et al (2006) Wilms' tumor 1‐associating protein regulates G2/M transition through stabilization of cyclin A2 mRNA. Proc Natl Acad Sci U S A 103, 17278–17283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Xiao W, Adhikari S, Dahal U, Chen Y, Hao Y, Sun B‐F, Sun H, Li A, Ping X‐L, Lai W‐Y et al (2016) Nuclear m6A reader YTHDC1 regulates mRNA splicing. Mol Cell 61, 507–519. [DOI] [PubMed] [Google Scholar]
- 81. Lichinchi G, Zhao BS, Wu Y, Lu Z, Qin Y, He C and Rana TM (2016) Dynamics of human and viral RNA methylation during Zika virus infection. Cell Host Microbe 20, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lichinchi G, Gao S, Saletore Y, Gonzalez GM, Bansal V, Wang Y, Mason CE and Rana TM (2016) Dynamics of the human and viral m(6)A RNA methylomes during HIV‐1 infection of T cells. Nat Microbiol 1, 16011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Roundtree IA, Luo G, Zhang Z, Wang X, Zhou T, Cui Y, Sha J, Huang X, Guerrero L, Xie P et al (2017) YTHDC1 mediates nuclear export of N6‐methyladenosine methylated mRNAs. eLife 6, 1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Geula S, Moshitch‐Moshkovitz S, Dominissini D, Mansour AA, Kol N, Salmon‐Divon M, Hershkovitz V, Peer E, Mor N, Manor YS et al (2015) m6A mRNA methylation facilitates resolution of naive pluripotency toward differentiation. Science 347, 1002–1006. [DOI] [PubMed] [Google Scholar]
- 85. Han J, Pedersen JS, Kwon SC, Belair CD, Kim YK, Yeom KH, Yang WY, Haussler D, Blelloch R and Kim VN (2009) Posttranscriptional crossregulation between Drosha and DGCR8. Cell 136, 75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Macias S, Plass M, Stajuda A, Michlewski G, Eyras E and Cáceres JF (2012) DGCR8 HITS‐CLIP reveals novel functions for the microprocessor. Nat Struct Mol Biol 19, 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Heras SR, Macias S, Plass M, Fernandez N, Cano D, Eyras E, Garcia‐Perez JL and Cáceres JF (2013) The Microprocessor controls the activity of mammalian retrotransposons. Nat Struct Mol Biol 20, 1173–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Knuckles P, Vogt MA, Lugert S, Milo M, Chong MMW, Hautbergue GM, Wilson SA, Littman DR and Taylor V (2012) Drosha regulates neurogenesis by controlling Neurogenin 2 expression independent of microRNAs. Nat Neurosci 15, 962–969. [DOI] [PubMed] [Google Scholar]
- 89. Johanson TM, Keown AA, Cmero M, Yeo JHC, Kumar A, Lew AM, Zhan Y and Chong MMW (2015) Drosha controls dendritic cell development by cleaving messenger RNAs encoding inhibitors of myelopoiesis. Nat Immunol 16, 1134–1141. [DOI] [PubMed] [Google Scholar]
- 90. Rolando C, Erni A, Grison A, Beattie R, Engler A, Gokhale PJ, Milo M, Wegleiter T, Jessberger S and Taylor V (2016) Multipotency of adult hippocampal NSCs in vivo is restricted by Drosha/NFIB. Cell Stem Cell 19, 653–662. [DOI] [PubMed] [Google Scholar]
- 91. Macias S, Cordiner RA, Gautier P, Plass M and Cáceres JF (2015) DGCR8 acts as an adaptor for the exosome complex to degrade double‐stranded structured RNAs. Mol Cell 60, 873–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Du H, Zhao Y, He J, Zhang Y, Xi H, Liu M, Ma J and Wu L (2016) YTHDF2 destabilizes m6A‐containing RNA through direct recruitment of the CCR4‐NOT deadenylase complex. Nat Commun 7, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hsu PJ, Zhu Y, Ma H, Guo Y, Shi X, Liu Y, Qi M, Lu Z, Shi H, Wang J et al (2017) Ythdc2 is an N6‐methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res 27, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wojtas MN, Pandey RR, Mendel M, Homolka D, Sachidanandam R and Pillai RS (2017) Regulation of m6A transcripts by the 3ʹ→5ʹ RNA helicase YTHDC2 is essential for a successful meiotic program in the mammalian germline. Mol Cell 68, 374–387.e12. [DOI] [PubMed] [Google Scholar]
- 95. Bailey AS, Batista PJ, Gold RS, Grace Chen Y, de Rooij DG, Chang HY and Fuller MT (2017) The conserved RNA helicase YTHDC2 regulates the transition from proliferation to differentiation in the germline. eLife 6, e26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Jain D, Puno MR, Meydan C, Lailler N, Mason CE, Lima CD, Anderson KV and Keeney S (2018) Ketu mutant mice uncover an essential meiotic function for the ancient RNA helicase YTHDC2. eLife 7, e30919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Lebedeva S, Jens M, Theil K, Schwanhäusser B, Selbach M, Landthaler M and Rajewsky N (2011) Transcriptome‐wide analysis of regulatory interactions of the RNA‐binding protein HuR. Mol Cell 43, 340–352. [DOI] [PubMed] [Google Scholar]
- 98. Kundu P, Fabian MR, Sonenberg N, Bhattacharyya SN and Filipowicz W (2012) HuR protein attenuates miRNA‐mediated repression by promoting miRISC dissociation from the target RNA. Nucleic Acids Res 40, 5088–5100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Bodi Z, Bottley A, Archer N, May ST and Fray RG (2015) Yeast m6A methylated mRNAs are enriched on translating ribosomes during meiosis, and under rapamycin treatment. PLoS One 10, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Choi J, Ieong KW, Demirci H, Chen J, Petrov A, Prabhakar A, O'Leary SE, Dominissini D, Rechavi G, Soltis SM et al (2016) N6‐methyladenosine in mRNA disrupts tRNA selection and translation‐elongation dynamics. Nat Struct Mol Biol 23, 110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mauer J, Luo X, Blanjoie A, Jiao X, Grozhik AV, Patil DP, Linder B, Pickering BF, Vasseur J‐J, Chen Q et al (2016) Reversible methylation of m6Am in the 5′ cap controls mRNA stability. Nature 541, 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schibler U and Perry RP (1977) The 5′‐termini of heterogeneous nuclear RNA: a comparison among molecules of different sizes and ages. Nucleic Acids Res 4, 4133–4150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Stoneley M and Willis AE (2004) Cellular internal ribosome entry segments: structures, trans‐acting factors and regulation of gene expression. Oncogene 23, 3200–3207. [DOI] [PubMed] [Google Scholar]
- 104. Cui Q, Shi H, Ye P, Li L, Qu Q, Sun G, Sun G, Lu Z, Huang Y, Yang CG et al (2017) m6A RNA methylation regulates the self‐renewal and tumorigenesis of glioblastoma stem cells. Cell Rep 18, 2622–2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Zhang S, Zhao BS, Zhou A, Lin K, Zheng S, Lu Z, Chen Y, Sulman EP, Xie K, Bögler O et al (2017) m6A demethylase ALKBH5 maintains tumorigenicity of glioblastoma stem‐like cells by sustaining FOXM1 expression and cell proliferation program. Cancer Cell 31, 591–606.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Visvanathan A, Patil V, Arora A, Hegde AS, Arivazhagan A, Santosh V and Somasundaram K (2017) Essential role of METTL3‐mediated m6A modification in glioma stem‐like cells maintenance and radioresistance. Oncogene 37, 522–533. [DOI] [PubMed] [Google Scholar]
- 107. Vu LP, Pickering BF, Cheng Y, Zaccara S, Nguyen D, Minuesa G, Chou T, Chow A, Saletore Y, MacKay M et al (2017) The N6‐methyladenosine (m6A)‐forming enzyme METTL3 controls myeloid differentiation of normal hematopoietic and leukemia cells. Nat Med 23, 1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Jaffrey SR and Kharas MG (2017) Emerging links between m6A and misregulated mRNA methylation in cancer. Genome Med 9, 2. [DOI] [PMC free article] [PubMed] [Google Scholar]


