Abstract
Background: Preliminary evidence suggests that diet manipulation may influence motor and nonmotor symptoms in PD, but conflict exists regarding the ideal fat to carbohydrate ratio.
Objectives: We designed a pilot randomized, controlled trial to compare the plausibility, safety, and efficacy of a low‐fat, high‐carbohydrate diet versus a ketogenic diet in a hospital clinic of PD patients.
Methods: We developed a protocol to support PD patients in a diet study and randomly assigned patients to a low‐fat or ketogenic diet. Primary outcomes were within‐ and between‐group changes in MDS‐UPDRS Parts 1 to 4 over 8 weeks.
Results: We randomized 47 patients, of which 44 commenced the diets and 38 completed the study (86% completion rate for patients commencing the diets). The ketogenic diet group maintained physiological ketosis. Both groups significantly decreased their MDS‐UPDRS scores, but the ketogenic group decreased more in Part 1 (−4.58 ± 2.17 points, representing a 41% improvement in baseline Part 1 scores) compared to the low‐fat group (−0.99 ± 3.63 points, representing an 11% improvement) (P < 0.001), with the largest between‐group decreases observed for urinary problems, pain and other sensations, fatigue, daytime sleepiness, and cognitive impairment. There were no between‐group differences in the magnitude of decrease for Parts 2 to 4. The most common adverse effects were excessive hunger in the low‐fat group and intermittent exacerbation of the PD tremor and/or rigidity in the ketogenic group.
Conclusions: It is plausible and safe for PD patients to maintain a low‐fat or ketogenic diet for 8 weeks. Both diet groups significantly improved in motor and nonmotor symptoms; however, the ketogenic group showed greater improvements in nonmotor symptoms. © 2018 The Authors. Movement Disorders published by Wiley Periodicals, Inc. on behalf of International Parkinson and Movement Disorder Society.
Keywords: Parkinson's disease, low‐fat diet, ketogenic diet, MDS‐UPDRS
Many new therapies for Parkinson's disease (PD) have emerged in recent decades, yet levodopa remains the primary treatment for motor symptoms. However, l‐dopa is associated with the development of motor fluctuations and dyskinesias,1 and it does not adequately control many nonmotor symptoms,2 which are often under‐recognized yet ultimately more disabling than motor symptoms.3, 4, 5, 6 In this context, there is growing interest in the largely unexplored, patient‐empowering approach of diet manipulation.7
Preliminary evidence suggests that diet manipulation may influence motor and nonmotor symptoms in PD, yet conflict exists regarding the ideal fat to carbohydrate ratio.8 On one hand, a low‐fat, high‐carbohydrate diet may ease the passage of the dopamine precursor, tyrosine, into cerebrospinal fluid and/or trigger an insulin‐induced rise in brain dopamine.9, 10 Moreover, an increase in dietary fiber might enhance fermentation of neuroactive short‐chain fatty acids in the gut, which could, theoretically, affect gut motility in PD.11, 12 On the other hand, defects in respiratory chain complex I activity have been demonstrated in the substantia nigra (SN) and frontal cortex of people with PD13, 14; it has been suggested that the ketones produced by a high‐fat, low‐carbohydrate “ketogenic” diet may be able to circumvent this defect through a complex II–dependent mechanism, thereby enhancing mitochondrial oxidative phosphorylation in the brain.15 In addition, such a diet may also enhance central and peripheral neuron energy metabolism by stimulating mitochondrial biogenesis.16 To date, only one study has examined the effects of a ketogenic diet in PD.17 The 5 patients improved their motor scores, but in addition to the small sample size, the study was limited by a short duration of 4 weeks, the consumption of only 8% protein, which likely enhanced l‐dopa bioavailability, and the lack of a control group such that a placebo effect may have contributed to the improved scores.
On this background, we designed this study to examine a general neurology hospital clinic of PD patients with regard to the plausibility and safety of maintaining a low‐fat or ketogenic diet for 8 weeks, whether either diet group significantly improved in motor and/or nonmotor symptoms, and whether one group showed greater improvements compared to the other.
Patients and Methods
Overview
This was a single‐phase, parallel‐group (1:1 randomization) study conducted at Waikato Hospital in Hamilton, New Zealand. Waikato Hospital is a tertiary hospital serving the Waikato and Bay of Plenty regions, representing a combined population of 750,000 people. The study was approved by local Ethics and Maori Consultation Research Review Committees.
Screening
The study sought volunteers through advertisements in local and national PD newsletters in late 2016, followed by presentations at Parkinson's New Zealand meetings in Hamilton, Rotorua, and Tauranga in early 2017.
Prospective patients underwent a 2‐hour screening visit in April or May 2017 with the lead investigator, nutrition specialist, and PD nurse specialist. The visit entailed (1) a presentation of the study and diet plans, (2) a demographic, medical, and social history, (3) Montreal Cognitive Assessment (MoCA), (4) data collection for body mass index (BMI) and recommended calorie intake calculations, and (5) written informed consent.
Eligible patients were aged 40 to 75 years with a diagnosis fulfilling the UK Parkinson's Disease Society Brain Bank criteria, MoCA score > 20, BMI > 18.5, and were able and willing to follow either diet plan. Exclusion criteria were inability to speak or understand English, H & Y stages 0 or 5, or a medical, psychiatric, or substance abuse condition that in the opinion of the investigators would make it difficult to complete the study.
Patients were instructed to eat their usual, nonmodified diets from the screening visit to the start of the diet intervention and to take their l‐dopa at least 1 hour before or after any meal from the screening visit to the end of the intervention.
Procedure
Patients engaged in the 8‐week diet intervention from June 26 to August 18, 2017. Four 1‐hour clinical visits were scheduled: two baseline visits over the 2 consecutive weeks immediately preceding the diet intervention (while on usual diet), followed by visits in weeks 4 and 8 after commencing the diet intervention.
Patients were instructed to take their medications at the same time before each visit, which involved an International Parkinson and Movement Disorder Society UPDRS (MDS‐UPDRS) assessment by a diet‐blinded neurologist certified by the MDS.18 It was strictly forbidden for either patient or neurologist to discuss any aspect of diet during the assessments. Patients were always assessed by the same neurologist, on the same weekday, at the same time of day. Each visit also included body weight measurements and blood tests for glycosylated hemoglobin (HbA1C), triglycerides, high‐density lipoprotein (HDL), low‐density lipoprotein (LDL), total cholesterol, urate, and C‐reactive protein (CRP).
At the first baseline clinical visit, patients received a blood glucose and ketone (beta‐hydroxybutyrate) monitor (Freestyle Neo; Abbott Diabetes Care, Whitney, UK) and finger prick training.19 After the first baseline visit but before the second visit, patients were randomized (1:1 allocation) to a low‐fat or ketogenic diet plan using an online randomization generator.20 To avoid selection bias, all patients were randomized simultaneously, stratified by recommended calorie intake (<2,000, 2,000‐2,500, and >2,500 kcal per day, block size of two). Randomization was generated by the lead investigator, witnessed by the PD nurse specialist, and signed and dated by both. Patients received their randomized plan at the second baseline visit and commenced the plan at the start of the following week, returning for repeat clinical visits in weeks 4 and 8 after commencing the plan.
Both 4‐week diet plans included weekly shopping lists containing ingredients readily available at local supermarkets, daily set menus with space to tick the completion of each meal as well as record daily (bedtime) blood glucose and ketone levels, and simple recipes (for diet plan details, see the Supplementary Appendix). The low‐fat plan provided 1,750 kcal per day composed of 42 g of fat (10 g saturated), 75 g of protein, 246 g net carbohydrate, and 33 g of fiber, and for those with higher energy needs, ad libitum “calorie‐booster” recipes each providing on average 500 extra kcal composed of 4 g of fat (1 g saturated), 6 g of protein, 102 g net carbohydrate, and 13 g of fiber. The ketogenic plan provided 1,750 kcal per day composed of 152 g of fat (67 g saturated), 75 g of protein, 16 g net carbohydrate, and 11 g of fiber, with each calorie‐booster recipe providing on average 500 extra kcal composed of 50 g of fat (22 g saturated), 6 g of protein, 5 g net carbohydrate, and 4 g of fiber.
Regular support and education sessions were provided. The lead investigator and nutrition specialist sent global e‐mails to all patients every second day and filmed and posted 10‐minute videos on the study's website every weekend. Both diets were equally presented as potentially conferring health benefits, and both groups were consistently reminded to eat until satiated.
Primary and Secondary Outcomes
Primary outcomes were within‐ and between‐group changes in MDS‐UPDRS Parts 1 to 4 from the mean of the two baseline clinical visits to week 8 after commencing the diet intervention. Secondary outcomes were within‐ and between‐group changes in metabolic parameters, including weight, BMI, HbA1C, triglycerides, HDL, LDL, total cholesterol, urate, and CRP.
Statistical Analysis
Sample‐size calculations were based on previous studies showing the smallest clinically meaningful MDS‐UPDRS score improvement to be −2.64 points in Part 1, −3.05 points in Part 2, and −3.25 points in Part 321, 22; to our knowledge, a clinically meaningful change in Part 4 has not been determined. To obtain 80% power using a significance level of 0.05, we calculated that 32 patients (16 per diet group) would detect a Part 1 change of 2.64 ± 2.64 points and/or Part 2 change of 3.05 ± 3.05 points and/or Part 3 change of 3.25 ± 3.25 points. Given that previous studies involving ketogenic diets in adults show an average dropout rate of 40% to 50% over 3 to 12 months,23 we aimed to recruit 40 to 60 patients.
Given the finite sample size and pre‐study uncertainty as to whether data would be normally distributed or not, all outcomes were analyzed using nonparametric tests (Wilcoxon signed‐rank test for within‐group comparisons, Mann‐Whitney U test for between‐group comparisons). Statistical tests were two‐tailed and used a significance level of 5%. All data are presented as mean ± standard deviation unless stated otherwise.
We analyzed primary and secondary outcomes using data from all randomized patients, with missing data imputed using regression imputation. To evaluate the robustness of the primary outcome findings to various imputation conditions, we additionally performed sensitivity analyses on all primary outcomes using mean imputation, baseline observation carried forward, median imputation, and complete case analyses.
Results
Patient Flow and Baseline Characteristics
Details of patient flow, including all study exclusions and withdrawals, are shown in Figure 1. We randomized 47 patients, of which 44 commenced the diets and 38 completed the study (86% completion rate for patients commencing the diets); 6 patients withdrew for reasons unrelated to the diets and 3 patients withdrew as a result of diet‐related difficulties. Randomized patient baseline characteristics are shown in Table 1. There were no significant between‐group differences in any baseline characteristics.
Figure 1.
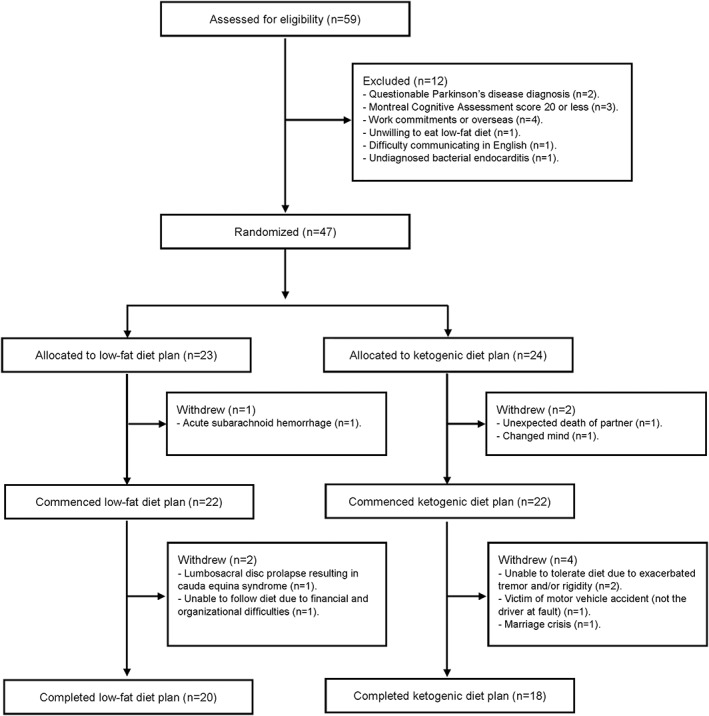
Patient flow.
Table 1.
Randomized patient baseline characteristics
| Low‐fat group (n = 23) | Ketogenic group (n = 24) | |
|---|---|---|
| Age (years) | 61.48 ± 7.12 | 64.29 ± 6.69 |
| Sex (male) | 14 (61%) | 17 (71%) |
| Ethnicity | ||
| European | 22 (96%) | 22 (92%) |
| Maori | 0 | 2 (8%) |
| Asian | 1 (4%) | 0 |
| Functional ability | ||
| Spouse at home | 20 (87%) | 21 (88%) |
| Screening MoCA | 25.4 ± 2.0 | 25.3 ± 2.5 |
| H & Y | 1.78 ± 0.82 (range, 1‐4) | 2.13 ± 0.76 (range, 1‐4) |
| MDS‐UPDRS | ||
| Part 1 (nonmotor daily living experiences) | 8.96 ± 4.34 | 11.15 ± 4.15 |
| Part 2 (motor daily living experiences) | 11.13 ± 5.59 | 12.75 ± 5.30 |
| Part 3 (motor examination) | 34.93 ± 13.40 | 36.48 ± 13.29 |
| Part 4 (motor complications) | 4.33 ± 4.44 | 4.90 ± 3.95 |
| PD meds | ||
| None | 4 (17%) | 1 (4%) |
| Monoamine oxidase B inhibitor | 5 (22%) | 4 (17%) |
| Dopamine agonist | 5 (22%) | 10 (42%) |
| l‐dopa | 18 (78%) | 22 (92%) |
| Catechol‐O‐methyl transferase inhibitor | 2 (9%) | 4 (17%) |
| Amantadine | 4 (17%) | 3 (13%) |
| Apomorphine | 0 | 1 (4%) |
| Comorbidities | ||
| Type 1 diabetes | 0 | 1 (4%) |
| Type 2 diabetes | 1 (4%) | 0 |
| Cholecystectomy | 1 (4%) | 1 (4%) |
| Past renal stones | 1 (4%) | 3 (13%) |
| Past gout | 2 (9%) | 1 (4%) |
| Physical profile | ||
| Weight (kg) | 78.13 ± 19.45 | 83.71 ± 19.38 |
| BMI | 26.96 ± 6.35 | 27.77 ± 5.29 |
| Recommended calorie intake (kcal per day) | 2,236 ± 430 | 2,242 ± 417 |
| Blood profile | ||
| HbA1C (mmol/mol) | 34.67 (5.32%) ± 5.22 | 36.15 (5.46%) ± 8.60 |
| Triglycerides (mmol/L) | 1.71 ± 0.88 | 1.81 ± 1.18 |
| HDL (mmol/L) | 1.51 ± 0.47 | 1.50 ± 0.44 |
| LDL (mmol/L) | 2.74 ± 0.82 | 2.73 ± 1.06 |
| Total cholesterol (mmol/L) | 5.03 ± 1.00 | 5.03 ± 1.05 |
| Urate (mmol/L) | 0.30 ± 0.08 | 0.30 ± 0.08 |
| CRP (mmol/L) | 2.95 ± 2.99 | 1.95 ± 1.59 |
Except for % variables, values are presented as mean ± standard deviation.
Baseline values were obtained by averaging the two baseline clinical visits; 1 patient withdrew post‐randomization, several hours before their scheduled second baseline visit, so the first visit was used as the baseline value.
Blood Glucose and Ketone Levels
Patient‐monitored blood glucose and ketone levels are shown in Figure 2. Over the 8‐week diet intervention, the two diet groups significantly differed in mean weekly bedtime blood glucose (low‐fat group: 6.28 ± 0.73 mmol/L vs. ketogenic group: 5.70 ± 1.20 mmol/L; P = 0.001) and ketone (0.16 ± 0.05 mmol/L vs. 1.15 ± 0.59 mmol/L; P < 0.001) levels.
Figure 2.
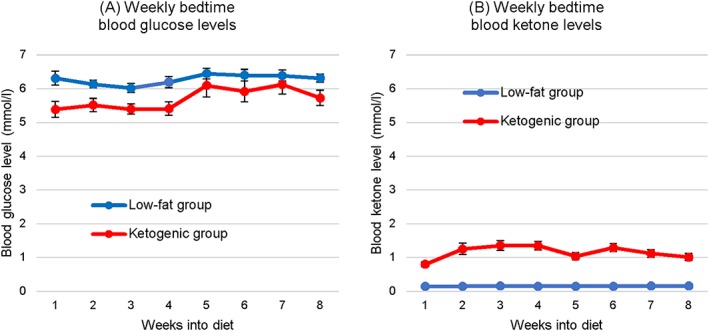
Mean weekly bedtime blood (A) glucose and (B) ketone (beta‐hydroxybutyrate) levels (n = 23 for the low‐fat diet group, n = 24 for the ketogenic group). Data were missing for 16.3% of the recordings (1.8% of the 38 completer recordings, 77% of the 9 withdrawal recordings). Days missing data were left blank, with the weekly mean calculated using the remaining days of the week. Error bars indicate standard error.
Changes in MDS‐UPDRS Parts 1 to 4 and Metabolic Parameters
We confirmed that patients in both diet groups took their l‐dopa at the same time before each visit by measuring the time interval from the last clinically relevant (taken within the previous 8 hours) l‐dopa dose to MDS‐UPDRS assessment, which did not significantly differ between any two clinical visits within either group (low‐fat group: 128 ± 56 minutes at baseline, 126 ± 58 minutes in week 4, 131 ± 79 minutes in week 8, P > 0.05; ketogenic group: 93 ± 50 minutes at baseline, 92 ± 61 minutes in week 4, 88 ± 51 minutes in week 8, P > 0.05).
Changes in MDS‐UPDRS Parts 1 to 4 are shown in Table 2. Both diet groups significantly decreased in Part 1 (low‐fat group: P = 0.030 vs. ketogenic group: P < 0.001), Part 2 (P = 0.011 vs. P < 0.001), and Part 3 (P < 0.001 vs. P < 0.001); the low‐fat group showed no change in Part 4 (P = 0.13) whereas the ketogenic group decreased in Part 4 (P = 0.005). The ketogenic group showed a larger magnitude of decrease in Part 1 (P < 0.001), with the largest between‐group decreases observed for urinary problems (–0.70 points difference), pain and other sensations (–0.64 points difference), fatigue (–0.50 points difference), daytime sleepiness (–0.45 points difference), and cognitive impairment (–0.27 points difference). There were no between‐group differences in the magnitude of decrease for Part 2 (P = 0.11), Part 3 (P = 0.055), or Part 4 (P = 0.32). The within‐ and between‐group comparisons of changes in Parts 1 to 4 remained similar, and the statistical conclusions unchanged, in all four sensitivity analyses (for details, see Table S1 in the Supplementary Appendix).
Table 2.
Changes in MDS‐UPDRS Parts 1 to 4 and metabolic parameters.
| Low‐fat group (n = 23) | Ketogenic group (n = 24) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | Week 8 | Change | Baseline | Week 8 | Change | P Value (between groups) | |
| Part 1 (nonmotor daily living experiences) | 8.96 ± 4.34 | 7.96 ± 6.56 | −0.99 ± 3.63 | 11.15 ± 4.15 | 6.57 ± 4.09 | −4.58 ± 2.17 | <0.001 |
| Part 2 (motor daily living experiences) | 11.13 ± 5.59 | 9.80 ± 6.81 | −1.33 ± 3.28 | 12.75 ± 5.30 | 9.62 ± 5.64 | −3.13 ± 4.01 | 0.11 |
| Part 3 (motor examination) | 34.93 ± 13.40 | 26.36 ± 13.58 | −8.58 ± 5.50 | 36.48 ± 13.29 | 30.20 ± 12.88 | −6.27 ± 4.07 | 0.055 |
| Part 4 (motor complications) | 4.33 ± 4.44 | 3.54 ± 4.86 | −0.79 ± 2.71 | 4.90 ± 3.95 | 3.33 ± 3.02 | −1.56 ± 2.45 | 0.32 |
| Weight (kg) | 78.13 ± 19.45 | 73.26 ± 17.99 | −4.87 ± 2.47 | 83.71 ± 19.38 | 79.34 ± 17.13 | −4.37 ± 3.00 | 0.55 |
| BMI | 26.96 ± 6.35 | 25.28 ± 5.95 | −1.67 ± 0.79 | 27.77 ± 5.29 | 26.31 ± 4.46 | −1.46 ± 1.01 | 0.30 |
| HbA1C (mmol/mol) | 34.67 ± 5.22 | 34.18 ± 3.74 | −0.49 ± 2.72 | 36.15 ± 8.60 | 34.72 ± 5.68 | −1.42 ± 4.12 | 0.095 |
| Triglycerides (mmol/L) | 1.71 ± 0.88 | 1.45 ± 0.55 | −0.26 ± 0.74 | 1.81 ± 1.18 | 1.71 ± 0.81 | −0.10 ± 0.63 | 0.46 |
| HDL (mmol/L) | 1.51 ± 0.47 | 1.40 ± 0.38 | −0.11 ± 0.22 | 1.50 ± 0.44 | 1.89 ± 0.75 | +0.40 ± 0.59 | <0.001 |
| LDL (mmol/L) | 2.74 ± 0.82 | 2.35 ± 0.77 | −0.40 ± 0.30 | 2.73 ± 1.06 | 3.42 ± 1.37 | +0.70 ± 0.67 | <0.001 |
| Total cholesterol (mmol/L) | 5.03 ± 1.00 | 4.40 ± 0.93 | −0.63 ± 0.51 | 5.03 ± 1.05 | 5.98 ± 1.39 | +0.94 ± 0.80 | <0.001 |
| Urate (mmol/L) | 0.30 ± 0.08 | 0.28 ± 0.08 | −0.02 ± 0.03 | 0.30 ± 0.08 | 0.33 ± 0.08 | +0.03 ± 0.05 | <0.001 |
| CRP (mmol/L) | 2.95 ± 2.99 | 1.95 ± 1.79 | −1.00 ± 3.57 | 1.95 ± 1.59 | 2.24 ± 1.26 | +0.29 ± 1.93 | 0.10 |
Values are presented as mean ± standard deviation.
Bolded values highlight changes in scores and parameters; due to round‐off, some change values differ by 0.01 from the absolute value differences.
P values refer to changes in scores and parameters between (not within) groups.
Changes in metabolic parameters are also shown in Table 2. Both diet groups significantly decreased in weight and BMI (P < 0.001 for all within‐group comparisons), but there were no between‐group differences in the magnitude of weight loss (P = 0.55) or BMI decrease (P = 0.30). The low‐fat group did not change in HbA1C (P = 0.92) whereas the ketogenic group decreased in HbA1C (P = 0.032), although with the exclusion of 1 outlier, a patient with type 1 diabetes whose HbA1C decreased by –19.5 mmol/mol, the magnitude of HbA1C decrease in the ketogenic group lessened considerably (from –1.42 ± 4.12 mmol/mol to –0.64 ± 1.49 mmol/mol). There were no between‐group differences in the magnitude of change in HbA1C (P = 0.095). There were no within‐group changes in triglycerides (low‐fat group: P = 0.19 vs. ketogenic group: P = 0.55), nor were there any between‐group differences (P = 0.46). The low‐fat group decreased in HDL (P = 0.027), LDL (P < 0.001), total cholesterol (P < 0.001), and urate (P = 0.004) whereas the ketogenic group increased in HDL (P < 0.001), LDL (P < 0.001), total cholesterol (P < 0.001), and urate (P = 0.004), with notable between‐group differences observed for all four parameters (P < 0.001 for all between‐group comparisons). There were no within‐group changes in CRP (P = 0.47 vs. P = 0.12), nor were there any between‐group differences (P = 0.10).
Adverse Effects
Adverse effects are shown in Table 3. In the low‐fat diet group, the most common adverse effect was excessive hunger, which occurred in 5 patients in weeks 1 to 4 and 6 patients in weeks 5 to 8. In the ketogenic group, the most common adverse effect was exacerbated tremor and/or rigidity, which occurred in 12 patients in weeks 1 to 4 and 7 patients in weeks 5 to 8.
Table 3.
Adverse effects experienced at any time during the study.
| Low‐fat group (n=23) | Ketogenic group (n=24) | |||
|---|---|---|---|---|
| Weeks 1‐4 | Weeks 5‐8 | Weeks 1‐4 | Weeks 5‐8 | |
| Exacerbated tremor and/or rigidity | 3 (13%) | 5 (22%) | 12 (50%) | 7 (29%) |
| Increased irritability | 4 (17%) | 3 (13%) | 8 (33%) | 2 (8%) |
| Excessive hunger | 5 (22%) | 6 (26%) | 4 (17%) | 1 (4%) |
| Excessive thirst | 1 (4%) | 3 (13%) | 6 (25%) | 5 (21%) |
| Feeling lightheaded | 2 (9%) | 4 (17%) | 2 (8%) | 2 (8%) |
| Nausea | 0 | 0 | 7 (29%) | 3 (13%) |
| Sugar cravings | 2 (9%) | 1 (4%) | 3 (13%) | 3 (13%) |
| Palpitations | 1 (4%) | 3 (13%) | 1 (4%) | 2 (8%) |
| Headache | 1 (4%) | 1 (4%) | 2 (8%) | 2 (8%) |
Daily medication doses were altered in 6 patients. In the low‐fat diet group, 1 patient self‐decreased l‐dopa attributed to improved motor symptoms and another with pre‐existing postural hypotension and falls required increased midodrine attributed to ongoing falls. In the ketogenic group, 1 patient required decreased l‐dopa and ropinirole attributed to agitation (resolved), another required decreased pramipexole attributed to visual hallucinations (resolved), another required decreased l‐dopa, ropinirole, and apomorphine attributed to exacerbated dyskinesias (partially resolved), and another with type 1 diabetes self‐decreased insulin attributed to improved blood glucose control.
Discussion
We have shown, in a general neurology hospital clinic of PD patients, that it is plausible and safe to maintain a low‐fat or ketogenic diet for 8 weeks. Both diet groups significantly improved in motor and nonmotor symptoms, but the ketogenic group showed greater improvements in nonmotor symptoms. Adverse effects were generally mild and differed between the two groups.
Several study strengths should be highlighted. First, we utilized a one‐phase design, strictly enforced consistent timing, diet‐blinded neurologists, and the MDS‐UPDRS (a validated rating scale with high internal consistency in measuring motor and nonmotor symptoms)18 to minimize assessment bias. Second, our diet plans utilized recipe ingredients readily available at local supermarkets, with an emphasis on affordability and palatability—thus, we chose not to use regular supplements in either plan (in particular, we chose not to supplement the ketogenic diet with medium‐chain triglyceride supplements, which are not available at some supermarkets, are relatively costly, and can produce adverse gastrointestinal effects). Third, protein intake was approximately 1 g per kg of body weight per day within each diet group and equal between groups; this is critical, given that low‐protein diets containing 0.5 to 0.8 g of protein per kg of body weight per day enhance L‐dopa absorption compared to high‐protein diets, which may improve motor symptoms and exacerbate dyskinesias in some people with PD.24, 25, 26 Fourth, our protocol involved multimedia supports, such as weekly educational videos, designed to mitigate the patient losses traditionally associated with studies involving ketogenic diets in adults,23 which probably contributed to the low number of patient withdrawals attributed to diet‐related difficulties (3 patients).
Certain study weaknesses warrant mention. First, the study population and duration were small; a larger sample size or longer diet intervention would have increased the statistical power or provided additional time, either of which may have enabled the detection of further significant differences between the diet groups. Thus, our findings should be viewed as preliminary. Second, there were 9 patient withdrawals; imputation techniques were used to replace the missing data. To assess the robustness of our primary outcome findings, we additionally performed sensitivity analyses on all primary outcomes using mean imputation, baseline observation carried forward, median imputation, and complete case analyses.
Patient adherence to each diet plan was monitored directly and indirectly. Rather than use food diaries, which may be perceived as burdensome,27 we directly monitored diet adherence by simply ensuring that patients ticked the day‐to‐day completion of each set meal as they proceeded. Perhaps more important, we indirectly monitored diet adherence by training patients to self‐monitor their daily blood glucose and ketone levels throughout the study; we chose blood pinprick testing as it is easier, more specific, and more accurately reflects ketone levels compared to urine dipstick testing.28 Using blood monitors, the low‐fat diet group measured negligible ketone levels, whereas the ketogenic group demonstrated a mean weekly blood ketone level of 1.15 ± 0.59 mmol/L consistent with a state of physiological ketosis, a coordinated metabolic response in which the liver provides ketones as an alternative, fat‐derived fuel source when body glucose reserves are in short supply.29
Both diet groups significantly improved their MDS‐UPDRS scores over the 8‐week diet intervention. It is tempting to speculate that the low‐fat diet increased brain dopamine levels and/or gut short‐chain fatty acid production and that the ketogenic diet enhanced central and peripheral neuron energy metabolism. However, despite being reminded to eat until satiated, the average patient in both groups lost 4 to 5 kg. It is well documented that overweight patients on unrestricted low‐fat and low‐carbohydrate diets can still experience significant weight loss, which may relate to altered levels of energy‐regulating hormones such as insulin.30, 31 Although weight loss is often associated with a negative impact on PD severity,32 a recent study has shown that patients with metabolic syndrome experience greater PD progression over time, mainly as a result of increased motor scores, compared to patients without metabolic syndrome.33 Given that the average patient in both groups lost 4 to 5 kg, yet remained overweight at week 8, the observed weight loss may have contributed to the improved motor scores in both groups. In addition, the placebo effect may significantly impact motor symptoms in PD patients.34, 35 Since our protocol included multimedia supports designed to mitigate patient withdrawals, it is also possible that a placebo effect contributed to the improved motor scores in both groups. The potential effects of both weight loss and a placebo effect must be kept in mind when interpreting the within‐group comparisons.
Both diet groups improved in Part 1 (nonmotor daily living experiences), but the ketogenic group improved more; every single patient in the ketogenic group improved in Part 1, resulting in a substantial 41% reduction in baseline Part 1 scores (as opposed to 11% in the low‐fat group) over the 8‐week diet intervention. The robustness of this between‐group finding to various imputation conditions was confirmed with multiple sensitivity analyses, including the conservative baseline observation carried forward method, which assumed that none of the 6 patient withdrawals from the ketogenic group would have improved in Part 1 at all. This is a potentially important finding, given that nonmotor symptoms ultimately represent the most disabling aspect of PD—for example, depression alone may have over twice the impact of motor symptoms on health.4 Moreover, nonmotor symptoms, such as urinary problems, pain, fatigue, daytime sleepiness, and cognitive impairment, are among those least responsive to l‐dopa,2, 36 yet these are the nonmotor symptoms that improved the most in the ketogenic group compared to the low‐fat group in our study. Since the magnitude of weight loss and BMI decrease were similar between groups, and the protocol and assessments were applied equally to both groups excepting the diets, neither weight loss nor a placebo effect explains the between‐group differences in nonmotor symptom improvements.
Perhaps unsurprisingly, the low‐fat diet group showed decreases, whereas the ketogenic group showed increases, in HDL, LDL, total cholesterol, and urate. The health benefits of lower versus higher LDL and total cholesterol in PD are debatable, with some studies correlating higher LDL and total cholesterol levels with a lower risk of PD.37, 38 The association between urate levels and PD is less controversial, with higher serum urate levels predicting a slower rate of PD progression.39
Interestingly, the adverse effect profile differed between the two diet groups. The most common adverse effect in the low‐fat group was excessive hunger. In contrast, the most common adverse effect in the ketogenic group was an intermittent exacerbation of the PD tremor and/or rigidity, which resulted in 2 patient withdrawals from this group at the end of week 1. Although this adverse effect improved or resolved in many patients in weeks 5 to 8, suggesting that it was a largely transient phenomenon, it may still have impacted the Part 3 (motor examination) scores to some extent at week 8. We can only speculate as to the reason the ketogenic diet exacerbated the PD tremor and/or rigidity in some patients, but it is conceivable that the abrupt increase in fat intake temporarily augmented dopamine depletion and/or oxidative stress in the SN, followed by an adaptive response over time.40
In conclusion, this pilot randomized, controlled trial shows that modified diets based on readily available ingredients, with normal protein levels, are plausible and safe treatment approaches in PD, with the ketogenic diet leading to greater improvements in many of the more disabling, less l‐dopa‐responsive nonmotor symptoms. It is possible that a ketogenic diet could play a complementary role alongside l‐dopa in the treatment of PD, but due to the preliminary nature of our findings, larger and longer randomized, controlled studies are needed before this can be stated with confidence.
Author Roles
(1) Research Project: A. Conception, B. Organization, C. Execution; (2) Statistical Analysis: A. Design, B. Execution, C. Review and Critique; (3) Manuscript Preparation: A. Writing of the First Draft, B. Review and Critique.
M.C.L.P.: 1A, 1B, 1C, 2A, 2B, 3A, 3B
D.K.J.M.: 1A, 1C
L.J.G.: 1C, 3B
F.J.S.A.: 1C, 3B
C.D.P.L.: 1C, 3B
Financial Disclosures
D.K.J.M. runs a whole‐foods coaching business, however none of her recipes were used in this study. Both study diets were based on recipes from sources with no personal or financial affiliation to any of the authors.
Supporting information
Supplementary information 1 ߚ Supplementary Appendix
Supplementary information 2 ߚ LowߚFat Standard Diet Plan
Supplementary information 3 ߚ Ketogenic Standard Diet Plan
Supplementary Information 4 ߚ CONSORT Checklist
Acknowledgments
We sincerely thank the patients and family members that participated in this study. We also thank Craig Clarke, creator of Ruled.Me, for freely licensing many of the recipes that formed the basis of the ketogenic diet, and Dr. Lyn Hunt, statistician, for her input into the statistical analysis.
Funding agencies: Waikato Hospital Neurology Research Fund.
Relevant conflicts of interest/financial disclosures: Nothing to report.
Full financial disclosures and author roles may be found in the online version of this article.
Registry: Australia New Zealand Clinical Trials ACTRN12617000027314.
References
- 1. Cotzias GC, Papavasiliou PS, Gellene R. Modification of Parkinsonism‐chronic treatment with L‐dopa. N Engl J Med 1969;280:337‐345. [DOI] [PubMed] [Google Scholar]
- 2. Sethi K. Levodopa unresponsive symptoms in Parkinson disease. Mov Disord 2008;23(Suppl 3):S521‐S533. [DOI] [PubMed] [Google Scholar]
- 3. Hu M, Cooper J, Beamish R, et al. How well do we recognise non‐motor symptoms in a British Parkinson's disease population? J Neurol 2011;258:1513‐1517. [DOI] [PubMed] [Google Scholar]
- 4. Hinnell C, Hurt CS, Landau S, Brown RG, Samuel M; PROMS‐PD Study Group . Nonmotor versus motor symptoms: how much do they matter to health status in Parkinson's disease? Mov Disord 2012;27:236‐241. [DOI] [PubMed] [Google Scholar]
- 5. Müller B, Assmus J, Herlofson K, Larsen JP, Tysnes OB. Importance of motor vs. non‐motor symptoms for health‐related quality of life in early Parkinson's disease. Parkinsonism Relat Disord 2013;19:1027‐1032. [DOI] [PubMed] [Google Scholar]
- 6. Hely MA, Morris JG, Reid WG, Trafficante R. Sydney multicentre study of Parkinson's disease: non‐L‐dopa‐responsive problems dominate at 15 years. Mov Disord 2005;20:190‐199. [DOI] [PubMed] [Google Scholar]
- 7. Mischley LK, Lau RC, Bennett RD. Role of diet and nutritional supplements in Parkinson's disease progression. Oxid Med Cell Longev 2017;2017:6405278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Seidl SE, Santiago JA, Bilyk H, Potashkin JA. The emerging role of nutrition in Parkinson's disease. Front Aging Neurosci 2014;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ. Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios. Am J Clin Nutr 2003;77:128‐132. [DOI] [PubMed] [Google Scholar]
- 10. Murakami K, Miyake Y, Sasaki S, et al.; Fukuoka Kinki Parkinson's Disease Study Group . Dietary glycemic index is inversely associated with the risk of Parkinson's disease: a case‐control study in Japan. Nutrition 2010;26:515‐521. [DOI] [PubMed] [Google Scholar]
- 11. Unger MM, Spiegel J, Dillmann KU, et al. Short chain fatty acids and gut microbiota differ between patients with Parkinson's disease and age‐matched controls. Parkinsonism Relat Disord 2016;32:66‐72. [DOI] [PubMed] [Google Scholar]
- 12. Mulak A, Bonaz B. Brain‐gut‐microbiota axis in Parkinson's disease. World J Gastroenterol 2015;21:10609‐10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging 2012;33:425.e19‐425.e27. [DOI] [PMC free article] [PubMed]
- 14. Davis Parker W, Jr. , Parks JK, Swerdlow RH. Complex I deficiency in Parkinson's disease frontal cortex. Brain Res 2008;1189:215‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tieu K, Perier C, Caspersen C, et al. D‐beta‐hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Investig 2003;112:892‐901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bough KJ, Wetherington J, Hassel B, et al. Mitochondrial biogenesis in the anticonvulsant mechanism of the ketogenic diet. Ann Neurol 2006;60:223‐235. [DOI] [PubMed] [Google Scholar]
- 17. Vanitallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet‐induced hyperketonemia: a feasibility study. Neurology 2005;64:728‐730. [DOI] [PubMed] [Google Scholar]
- 18. Goetz CG, Tilley BC, Shaftman SR, et al.; Movement Disorder Society UPDRS Revision Task Force . Movement Disorder Society‐Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS‐UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129‐2170. [DOI] [PubMed] [Google Scholar]
- 19. Abbott Diabetes Care. Website. Available at: http://myfreestyle.com.au/products/freestyle-optium-neo-blood-glucose-ketone-monitoring-system/. Accessed December 18, 2016.
- 20. http://Randomization.com. Website. Available at: http://www.randomization.com. Accessed June 20, 2017.
- 21. Horváth K, Aschermann Z, Kovács M, et al. Minimal clinically important differences for the experiences of daily living parts of movement disorder society‐sponsored unified Parkinson's disease rating scale. Mov Disord 2017;32:789‐793. [DOI] [PubMed] [Google Scholar]
- 22. Horváth K, Aschermann Z, Ács P, et al. Minimal clinically important difference on the Motor Examination part of MDS‐UPDRS. Parkinsonism Relat Disord 2015;21:1421‐1426. [DOI] [PubMed] [Google Scholar]
- 23. Klein P, Tyrlikova I, Mathews GC. Dietary treatments in adults with refractory epilepsy: a review. Neurology 2014;83:1978‐1985. [DOI] [PubMed] [Google Scholar]
- 24. Pincus JH, Barry K. Influence of dietary protein on motor fluctuations in Parkinson's disease. Arch Neurol 1987;44:270‐272. [DOI] [PubMed] [Google Scholar]
- 25. Pincus JH, Barry K. Plasma levels of amino acids correlate with motor fluctuations in parkinsonism. Arch Neurol 1987;44:1006‐1009. [DOI] [PubMed] [Google Scholar]
- 26. Tsui JK, Ross S, Poulin K, et al. The effect of dietary protein on the efficacy of L‐dopa: a double‐blind study. Neurology 1989;39:549‐552. [DOI] [PubMed] [Google Scholar]
- 27. Krikorian R, Shidler MD, Dangelo K, Couch SC, Benoit SC, Clegg DJ. Dietary ketosis enhances memory in mild cognitive impairment. Neurobiol Aging 2012;33:425.e19‐e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arora S, Henderson SO, Long T, Menchine M. Diagnostic accuracy of point‐of‐care testing for diabetic ketoacidosis at emergency‐department triage: {beta}‐hydroxybutyrate versus the urine dipstick. Diabetes Care 2011;34:852‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gibson AA, Seimon RV, Lee CM, et al. Do ketogenic diets really suppress appetite? A systematic review and meta‐analysis. Obes Rev 2015;16:64‐76. [DOI] [PubMed] [Google Scholar]
- 30. Saris WHM, Astrup A, Prentice AM, et al. Randomized controlled trial of changes in dietary carbohydrate/fat ratio and simple vs complex carbohydrates on body weight and blood lipids: the CARMEN study. Int J Obes 2000;24:1310‐1318. [DOI] [PubMed] [Google Scholar]
- 31. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low‐carbohydrate, Mediterranean, or low‐fat diet. N Engl J Med 2008;359:229‐241. [DOI] [PubMed] [Google Scholar]
- 32. Sharma JC, Lewis A. Weight in Parkinson's disease: phenotypical significance. Int Rev Neurobiol 2017;134:891‐919. [DOI] [PubMed] [Google Scholar]
- 33. Leehey M, Luo S, Sharma S, et al. Association of metabolic syndrome and change in Unified Parkinson's Disease Rating Scale scores. Neurology 2017;89:1789‐1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goetz CG, Leurgans S, Raman R; Parkinson Study Group . Placebo‐associated improvements in motor function: comparison of subjective and objective sections of the UPDRS in early Parkinson's disease. Mov Disord 2002;17:283‐288. [DOI] [PubMed] [Google Scholar]
- 35. Warren Olanow C, Bartus RT, Baumann TL, et al. Gene delivery of neurturin to putamen and substantia nigra in Parkinson disease: a double‐blind, randomized, controlled trial. Ann Neurol 2015;78:248‐257. [DOI] [PubMed] [Google Scholar]
- 36. Fabbri M, Coelho M, Guedes LC, et al. Response of non‐motor symptoms to levodopa in late‐stage Parkinson's disease: results of a levodopa challenge test. Parkinsonism Relat Disord 2017;39:37‐43. [DOI] [PubMed] [Google Scholar]
- 37. de Lau LM, Koudstaal PJ, Hofman A, Breteler MM. Serum cholesterol levels and the risk of Parkinson's disease. Am J Epidemiol 2006;164:998‐1002. [DOI] [PubMed] [Google Scholar]
- 38. Huang X, Abbott RD, Petrovitch H, Mailman RB, Ross GW. Low LDL cholesterol and increased risk of Parkinson's disease: prospective results from Honolulu‐Asia Aging Study. Mov Disord 2008;23:1013‐1018. [DOI] [PubMed] [Google Scholar]
- 39. Ascherio A, LeWitt PA, Xu K et al.; Parkinson Study Group DATATOP Investigators . Urate as a predictor of the rate of clinical decline in Parkinson disease. Arch Neurol 2009;66:1460‐1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Milder JB, Liang LP, Patel M. Acute oxidative stress and systemic Nrf2 activation by the ketogenic diet. Neurobiol Dis 2010;40:238‐244. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information 1 ߚ Supplementary Appendix
Supplementary information 2 ߚ LowߚFat Standard Diet Plan
Supplementary information 3 ߚ Ketogenic Standard Diet Plan
Supplementary Information 4 ߚ CONSORT Checklist


