Abstract
Aims
Individuals with both diabetes mellitus (DM) and atherosclerotic cardiovascular disease (ASCVD) are at very high risk of cardiovascular events. This post‐hoc analysis evaluated efficacy and safety of the PCSK9 inhibitor alirocumab among 984 individuals with DM and ASCVD pooled from 9 ODYSSEY Phase 3 trials.
Materials and methods
Changes in low‐density lipoprotein cholesterol (LDL‐C) and other lipids from baseline to Week 24 were analysed (intention‐to‐treat) in four pools by alirocumab dosage (150 mg every 2 weeks [150] or 75 mg with possible increase to 150 mg every 2 weeks [75/150]), control (placebo/ezetimibe) and background statin usage (yes/no).
Results
At Week 24, LDL‐C changes from baseline in pools with background statins were −61.5% with alirocumab 150 (vs −1.0% with placebo), −46.4% with alirocumab 75/150 (vs +6.3% with placebo) and −48.7% with alirocumab 75/150 (vs −20.6% with ezetimibe), and −54.9% with alirocumab 75/150 (vs +4.0% with ezetimibe) without background statins. A greater proportion of alirocumab recipients achieved LDL‐C < 70 and < 55 mg/dL at Week 24 vs controls. Alirocumab also resulted in significant reductions in non‐high‐density lipoprotein cholesterol, apolipoprotein B and lipoprotein(a) vs controls. Alirocumab did not appear to affect glycaemia over 78‐104 weeks. Overall safety was similar between treatment groups, with a higher injection‐site reaction frequency (mostly mild) with alirocumab.
Conclusion
Alirocumab significantly reduced LDL‐C and other atherogenic lipid parameters, and was generally well tolerated in individuals with DM and ASCVD.
Keywords: cardiovascular disease, dyslipidaemia, lipid‐lowering therapy, clinical trial
1. INTRODUCTION
Diabetes mellitus (DM) is associated with a high prevalence of atherosclerotic cardiovascular disease (ASCVD), including coronary heart disease, ischaemic stroke and peripheral arterial disease, and ASCVD is the main cause of mortality and morbidity among those with DM.1, 2, 3 Furthermore, individuals with both DM and ASCVD represent a particularly high‐risk group, with a higher risk of further ASCVD events compared with individuals with ASCVD but without DM.4, 5, 6
International guidelines for ASCVD risk management place individuals with DM and ASCVD in the highest risk category and recommend treatment with maximally tolerated statin therapy to reduce levels of low‐density lipoprotein cholesterol (LDL‐C), thereby reducing ASCVD risk.7, 8, 9, 10 This is supported by data from randomized clinical trials and meta‐analyses showing that treatment with statins reduces LDL‐C levels and ASCVD risk in individuals with DM.11, 12, 13 DM is commonly associated with diabetic dyslipidaemia, including elevated triglycerides and reduced levels of high‐density lipoprotein cholesterol (HDL‐C), and with an increased number of small dense LDL particles and apolipoprotein (apo) B‐containing particles, which is thought to contribute to the increased risk level associated with DM.3, 14 Because of this, some guidelines have suggested using non‐HDL‐C, representative of the sum total of all atherogenic cholesterol‐containing particles, as an alternative or secondary treatment target for LDL‐C.8, 9, 15 However, despite recent increases in the use of high‐intensity statin therapy in practice, recent evidence indicates that many individuals with ASCVD and/or DM are not achieving LDL‐C and non‐HDL‐C goals.16
Further reduction in LDL‐C and ASCVD events has been observed in individuals with DM and ASCVD when non‐statin therapies, ezetimibe17 or the proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor evolocumab,18 were added to statin therapy, compared with statins alone. The proportion of participants in these trials who experienced adverse events was comparable with that of controls. Based on these data, guidelines have been updated and now propose that adding ezetimibe and/or a PCSK9 inhibitor should be considered if the individual does not attain sufficient LDL‐C reduction with maximally tolerated statins alone, for example, if they have insufficient response to statin therapy or are unable to tolerate high or any doses of statins.7, 8, 9, 10, 15
Alirocumab is a PCSK9 inhibitor that signficantly reduced LDL‐C and other atherogenic lipid parameters in participants with hypercholesterolaemia in Phase 3 ODYSSEY trials,19, 20, 21, 22, 23, 24, 25, 26 including dedicated trials involving individuals with DM who were receiving insulin therapy27 or with mixed dyslipidaemia,28 with a safety profile comparable to controls. Alirocumab has also been demonstrated to reduce major adverse cardiovascular events vs placebo in patients with recent acute coronary syndrome in the ODYSSEY OUTCOMES trial.29 Subgroup analyses have suggested similar efficacy and tolerability of alirocumab in individuals with and without DM.26, 30, 31, 32, 33 However, it is important to examine the effects of alirocumab in the specific subgroup of individuals with both DM and ASCVD who are at particularly high risk and may benefit from additional lipid‐lowering therapy beyond a statin.7, 8, 9, 10, 15 This post‐hoc analysis used pooled data from 9 ODYSSEY Phase 3 trials to evaluate the efficacy and safety of alirocumab in individuals with both DM and ASCVD.
2. METHODS
2.1. Study designs and participants
This post‐hoc pooled analysis included individuals with a medical history of Type 1 or Type 2 DM and ASCVD who participated in 9 randomized, double‐blind, placebo‐ or ezetimibe‐controlled ODYSSEY Phase 3 trials with subcutaneous alirocumab administered every 2 weeks (Q2W), with trial durations of 24‐104 weeks (LONG TERM [NCT01507831],26 FH I [NCT01623115],23 FH II [NCT01709500],23 HIGH FH [NCT01617655],22 COMBO I [NCT01644175],24 COMBO II [NCT01644188],20 OPTIONS I [NCT01730040],19 OPTIONS II [NCT01730053]21 and ALTERNATIVE [NCT01709513]).25 Individual trial results have been published previously. Trial protocols were approved by appropriate independent ethics committees or institutional review boards at study centres. All studies were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization guidelines for Good Clinical Practice. All participants provided written informed consent before trial enrolment.
For all but one of the trials included in this analysis, eligible participants with a history of ASCVD were required to have LDL‐C levels ≥70 mg/dL at screening; in one trial (HIGH FH), eligible individuals had heterozygous familial hypercholesterolaemia with LDL‐C levels ≥160 mg/dL at screening. ASCVD was defined as coronary heart disease, ischaemic stroke or peripheral arterial disease.34 Coronary heart disease included acute/silent myocardial infarction, unstable angina, prior coronary revascularization procedures and other clinically significant coronary heart disease.
In seven trials, alirocumab was administered at a dosage of 75 mg Q2W with possible dose increase to 150 mg Q2W at Week 12 (denoted by 75/150 mg in the text) if LDL‐C was ≥ 70 mg/dL at Week 8 in the FH I, FH II, COMBO I and COMBO II trials or was ≥70 or ≥ 100 mg/dL, depending on cardiovascular risk, in the OPTIONS I, OPTIONS II and ALTERNATIVE trials. Two trials, LONG TERM and HIGH FH, used alirocumab 150 mg Q2W throughout the treatment period. Alirocumab and control treatments were administered with background statin therapy in eight trials, at the maximally tolerated dose in LONG TERM, FH I, FH II, COMBO I, COMBO II and HIGH FH, and in addition to atorvastatin 20‐40 mg in the OPTIONS I trial and to rosuvastatin 10‐20 mg in the OPTIONS II trial. Maximally tolerated statin was defined as atorvastatin 40‐80 mg, rosuvastatin 20‐40 mg or simvastatin 80 mg, or lower doses with an investigator‐approved reason. Participants in the ALTERNATIVE trial had documented statin intolerance and, therefore, were not receiving background statin therapy.
2.2. Analysis pools
For analysis of baseline characteristics and lipid efficacy, data from the nine trials were pooled based on alirocumab dosage, control treatment (placebo or ezetimibe) and whether background statin was used: Pool 1: alirocumab 150 mg Q2W vs placebo with background statin (LONG TERM, HIGH FH); Pool 2: alirocumab 75 mg Q2W vs placebo with background statin (FH I, FH II, COMBO I); Pool 3: alirocumab 75 mg Q2W vs ezetimibe with background statin (COMBO II, OPTIONS I, OPTIONS II); Pool 4: alirocumab 75 mg Q2W vs ezetimibe without background statin (ALTERNATIVE only). Safety data were analysed in two pools based on control: a placebo‐controlled pool and an ezetimibe‐controlled pool.
2.3. Endpoints
The primary efficacy endpoint was percentage change from baseline in LDL‐C at Week 24, as in the primary trial analyses;26 secondary endpoints included changes in non‐HDL‐C, lipoprotein(a) [Lp(a)], apoB, HDL‐C and triglycerides from baseline to Week 24. LDL‐C was calculated using the Friedewald formula; LDL‐C values were excluded from analysis if triglyceride levels were > 400 mg/dL at that time point. Safety was assessed via reporting of treatment‐emergent adverse events (TEAEs) and laboratory values for the placebo‐ and ezetimibe‐controlled pools. Adverse events were classed as TEAEs if they were reported from the first dose of study treatment up to the last dose plus 70 days.
2.4. Statistical analyses
Data were analysed using the same statistical approaches as those used for the primary trial analyses.26 Efficacy was analysed using an intent‐to‐treat (ITT) approach, including all patients with a baseline and at least one post‐baseline LDL‐C value, regardless of adherence to treatment, in pools as described above. Least‐squares mean lipid values were derived from a mixed‐effects model with repeated measures for lipids assumed to follow a normal distribution, and adjusted mean values were calculated from a multiple imputation, followed by robust regressions for lipids not following a normal distribution (ie, Lp(a) and triglycerides) as described previously.26 The proportion of individuals achieving an LDL‐C level < 70 or < 55 mg/dL was analysed using a modified ITT approach, including only on‐treatment lipid values, using multiple imputation followed by a logistic regression. LDL‐C < 55 mg/dL is a goal not previously specified for the ODYSSEY trials but is assessed here following recent guideline updates from the American Association of Clinical Endocrinologists.9 Descriptive statistics only were used for baseline and safety analyses; no formal statistical inference was planned in the original study protocols. The effects of treatment on glycated haemoglobin (HbA1c) and fasting plasma glucose (FPG) are presented for the placebo‐ and ezetimibe‐controlled pools using descriptive statistics and graphs during the treatment period (ie, up to 21 days after the last injection). Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, North Carolina).
3. RESULTS
3.1. Baseline characteristics
A total of 984 participants with DM and ASCVD from 9 ODYSSEY Phase 3 clinical trials were included in the analysis. Most had Type 2 DM (n = 969, 98.5%), with few having Type 1 DM (n = 15, 1.5%). The most common type of ASCVD was coronary heart disease (85%‐100% of patients across the groups); most individuals (83%‐94%) had hypertension (Table 1). Baseline characteristics were generally well balanced between alirocumab and control groups within the pools of studies using background statins (Table 1). However, there was more variability between the alirocumab and ezetimibe groups in the pool with no background statin; for example, mean baseline LDL‐C levels were 157.6 and 194.4 mg/dL, and mean age was 70.3 and 63.0 years, with alirocumab and ezetimibe, respectively. The number of patients in this pool (one study) was relatively small (n = 23 for alirocumab and n = 12 for ezetimibe). Mean baseline LDL‐C, non‐HDL‐C, apoB and triglyceride levels overall were highest in the pool with no background statin (Table 1).
Table 1.
Baseline characteristics of randomized patients with DM and ASCVD
| Study pools | Alirocumab 150 mg Q2W vs placebo (with statin) | Alirocumab 75/150 mg Q2W vs placebo (with statin) | Alirocumab 75/150 mg Q2W vs ezetimibe (with statin) | Alirocumab 75/150 mg Q2W vs ezetimibe (without statin) | ||||
|---|---|---|---|---|---|---|---|---|
| Treatment groups | Alirocumab (n = 346) | Placebo (n = 176) | Alirocumab (n = 93) | Placebo (n = 46) | Alirocumab (n = 176) | Ezetimibe (n = 112) | Alirocumab (n = 23) | Ezetimibe (n = 12) |
| Age, years, mean ± SD | 63.2 ± 8.7 | 62.0 ± 9.5 | 64.0 ± 8.9 | 62.7 ± 9.1 | 64.2 ± 9.3 | 64.6 ± 8.8 | 70.3 ± 7.1 | 63.0 ± 7.6 |
| Males, n (%) | 218 (63.0) | 104 (59.1) | 60 (64.5) | 29 (63.0) | 122 (69.3) | 78 (69.6) | 14 (60.9) | 9 (75.0) |
| Race, white, n (%) | 302 (87.3) | 158 (89.8) | 72 (77.4) | 36 (78.3) | 147 (83.5) | 90 (80.4) | 21 (91.3) | 11 (91.7) |
| BMI, kg/m2, mean ± SD | 31.9 ± 5.7 | 32.1 ± 5.2 | 33.2 ± 6.5 | 33.5 ± 7.2 | 31.7 ± 6.3 | 32.8 ± 5.8 | 31.9 ± 6.3 | 29.5 ± 3.7 |
| HbA1c, %, median (Q1:Q3) | 6.7 (6.1:7.8) | 6.9 (6.1:8.0) | 6.6 (6.1:7.3) | 6.4 (5.9:7.2) | 6.8 (6.2:7.5) | 6.7 (6.1:7.4) | 6.5 (5.9:6.8) | 6.2 (6.0:7.0) |
| FPG, mg/dL, median (Q1:Q3) | 127.9 (108.1:154.9) | 129.7 (108.1:162.5) | 121.0 (104.0:151.3) | 119.0 (104.0:138.0) | 131.0 (110.5:149.5) | 122.3 (102.8:142.3) | 127.9 (101.0:145.0) | 117.6 (101.0:150.8) |
| HeFH, n (%) | 23 (6.6) | 17 (9.7) | 23 (24.7) | 20 (43.5) | 1 (0.6) | 0 | 0 | 0 |
| Insulin usage, n (%) | 85 (24.6) | 48 (27.3) | 22 (23.7) | 5 (10.9) | 42 (23.9) | 19 (17.0) | 8 (34.8) | 5 (41.7) |
| Statin usage, n (%) | 345 (99.7) | 176 (100.0) | 93 (100.0) | 46 (100.0) | 176 (100.0) | 112 (100.0) | 1 (4.3) | 0 |
| High‐intensity statina usage, n (%) | 151 (43.6) | 83 (47.2) | 62 (66.7) | 32 (69.6) | 111 (63.1) | 65 (58.0) | 0 | 0 |
| ASCVD history, n (%) | ||||||||
| CHD | 304 (87.9) | 149 (84.7) | 84 (90.3) | 44 (95.7) | 161 (91.5) | 105 (93.8) | 21 (91.3) | 12 (100.0) |
| Acute coronary syndromeb | 195 (56.4) | 101 (57.4) | 52 (55.9) | 33 (71.7) | 108 (61.4) | 66 (58.9) | 10 (43.5) | 8 (66.7) |
| Coronary revascularization procedure | 202 (58.4) | 101 (57.4) | 69 (74.2) | 32 (69.6) | 108 (61.4) | 77 (68.8) | 13 (56.5) | 10 (83.3) |
| Other clinically significant CHD | 118 (34.1) | 57 (32.4) | 27 (29.0) | 17 (37.0) | 77 (43.8) | 60 (53.6) | 12 (52.2) | 7 (58.3) |
| Peripheral arterial disease | 29 (8.4) | 21 (11.9) | 3 (3.2) | 2 (4.3) | 18 (10.2) | 6 (5.4) | 0 | 1 (8.3) |
| Ischaemic stroke | 48 (13.9) | 24 (13.6) | 12 (12.9) | 2 (4.3) | 23 (13.1) | 12 (10.7) | 2 (8.7) | 1 (8.3) |
| Hypertension, n (%) | 314 (90.8) | 157 (89.2) | 86 (92.5) | 43 (93.5) | 159 (90.3) | 104 (92.9) | 21 (91.3) | 10 (83.3) |
| Lipid parameters, mean ± SD, mg/dL | ||||||||
| Calculated LDL‐C | 117.3 ± 36.1 | 120.6 ± 39.6 | 105.2 ± 34.3 | 114.2 ± 47.1 | 105.6 ± 32.3 | 97.8 ± 29.5 | 157.6 ± 33.6 | 194.4 ± 84.3 |
| Non‐HDL‐C | 152.0 ± 41.5 | 153.0 ± 45.5 | 135.8 ± 38.1 | 143.0 ± 53.7 | 139.3 ± 41.0 | 129.2 ± 31.8 | 196.9 ± 42.6 | 253.0 ± 72.7 |
| ApoB | 102.3 ± 26.1 | 102.6 ± 28.2 | 95.4 ± 25.4 | 96.0 ± 30.2 | 95.7 ± 23.8 | 88.3 ± 17.4 | 125.7 ± 23.2 | 150.1 ± 37.3 |
| Lp(a), median (Q1:Q3) | 20.1 (6.0:54.1) | 17.2 (6.0:61.9) | 38.5 (7.0:86.0) | 43.5 (12.5:105.5) | 27.0 (8.0:63.0) | 19.5 (9.5:50.0) | 28.0 (7.0:71.0) | 8.0 (3.0:26.0) |
| Triglycerides, median (Q1:Q3) | 154.9 (111.5:215.0) | 148.7 (105.2:205.3) | 133.0 (100.0:173.0) | 120.0 (101.0:188.0) | 146.5 (109.0:208.5) | 146.5 (112.5:188.0) | 201.0 (125.0:272.0) | 247.0 (140.5:346.5) |
| HDL‐C | 47.9 ± 10.8 | 47.5 ± 12.4 | 44.8 ± 13.2 | 46.0 ± 12.5 | 43.5 ± 11.2 | 43.6 ± 10.7 | 43.7 ± 12.5 | 45.3 ± 11.1 |
Abbreviations: Apo, apolipoprotein; ASCVD, atherosclerotic cardiovascular disease; BMI, body mass index; CHD, coronary heart disease; DM, diabetes mellitus; FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; HDL‐C, high‐density lipoprotein cholesterol; HeFH, heterozygous familial hypercholesterolaemia; LDL‐C, low‐density lipoprotein cholesterol; Lp(a), lipoprotein(a); MI, myocardial infarction; Q2W, every 2 weeks; SD, standard deviation.
Atorvastatin 40 or 80 mg, rosuvastatin 20 or 40 mg or simvastatin 80 mg daily.
Includes silent MI, acute MI and unstable angina.
3.2. Efficacy
Significant reductions from baseline in LDL‐C with alirocumab treatment vs control were observed at Week 24 in all analysis pools in this population of individuals with DM and ASCVD (Figure 1A). At Week 24, in the pools with background statins, changes from baseline in LDL‐C were −61.5% with alirocumab 150 mg Q2W (vs −1.0% with placebo), −46.4% with alirocumab 75/150 mg Q2W (vs +6.3% with placebo) and −48.7% with alirocumab 75/150 mg Q2W (vs −20.6% with ezetimibe) (Figure 1A). In the pool with no background statin, the change from baseline to Week 24 in LDL‐C was −54.9% with alirocumab (vs +4.0% with ezetimibe) (Figure 1A). LDL‐C reductions with alirocumab were maintained over time, with changes from baseline of −51.1% with 150 mg Q2W (vs +3.8% with placebo) and −43.1% with 75/150 mg Q2W (vs −0.3% with placebo) at Week 78 in the placebo‐controlled pools, and −40.0% with 75/150 mg Q2W (vs −23.1% with ezetimibe) at Week 104 in the ezetimibe‐controlled pool with background statin (Figure S1). A greater proportion of alirocumab recipients achieved LDL‐C < 70 and < 55 mg/dL at Week 24 vs controls (Figure 1B,C). Compared with the ezetimibe‐controlled pool, where background statins were used, the proportion achieving LDL‐C < 55 mg/dL was lower in the pool with no background statin in both alirocumab and ezetimibe groups (Figure 1C); this can be explained by the relatively high baseline LDL‐C levels at baseline in this pool (Figure 1A).
Figure 1.
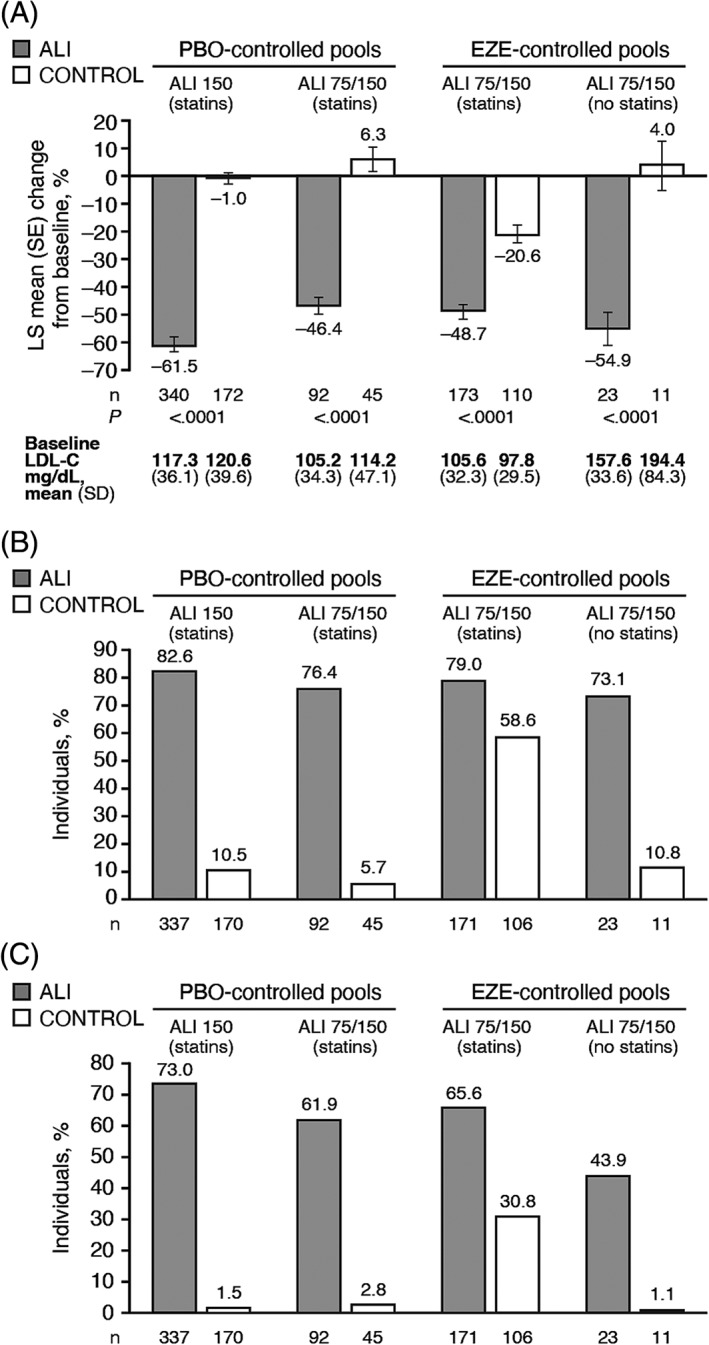
A, Percentage change from baseline to week 24 in LDL‐C and proportion achieving B, LDL‐C < 70 mg/dL or C, <55 mg/dL at week 24 among individuals with both DM and ASCVD, by analysis pool. Baseline values are from the randomized population. LS means (SE) in panel A derived from a mixed‐effect model with repeated measures (ITT analysis). Proportions in panel B and C estimated from multiple imputation (modified ITT analysis). Abbreviations: ALI 150, alirocumab 150 mg Q2W; ALI 75/150, alirocumab 75 mg Q2W with possible increase to 150 mg Q2W at Week 12 based on Week 8 LDL‐C; ASCVD, atherosclerotic cardiovascular disease; DM, diabetes mellitus; EZE; ezetimibe; ITT, intent‐to‐treat; LDL‐C, low‐density lipoprotein cholesterol; LS, least‐squares; ND, not derivable (proportion in control group too small); PBO, placebo; Q2W, every 2 weeks; SE, standard error
In the pools allowing for blinded alirocumab dose increase from 75 to 150 mg Q2W at Week 12, based on achievement of pre‐specified LDL‐C levels at Week 8, the alirocumab dose was increased in 16.3% of patients in the pool of alirocumab 75/150 vs placebo (on statins), in 15.6% of patients in the pool of alirocumab vs ezetimibe (on statins) and in 34.8% of patients in the pool of alirocumab 75/150 vs ezetimibe (no statins). In comparison, for the overall trial populations, the dose was increased in 32.9%, 16.7% and 38.2%, respectively (data from ITT population).
Across all pools, alirocumab significantly reduced non‐HDL‐C and apoB levels compared with control (Figure 2A,B). As with LDL‐C, reductions in non‐HDL‐C and apoB were maintained to Week 78 in the placebo‐controlled pools and to Week 104 in the ezetimibe‐controlled pool with background statin (Figures S2 and S3). Significant reductions in Lp(a) from baseline were seen with alirocumab at Week 24 in all pools with the exception of the pool of alirocumab 75/150 mg vs placebo (Figure 2C). Moderate increases in HDL‐C and moderate reductions in triglycerides were also observed with alirocumab, which were significant vs placebo, but not vs ezetimibe (Figure S5).
Figure 2.
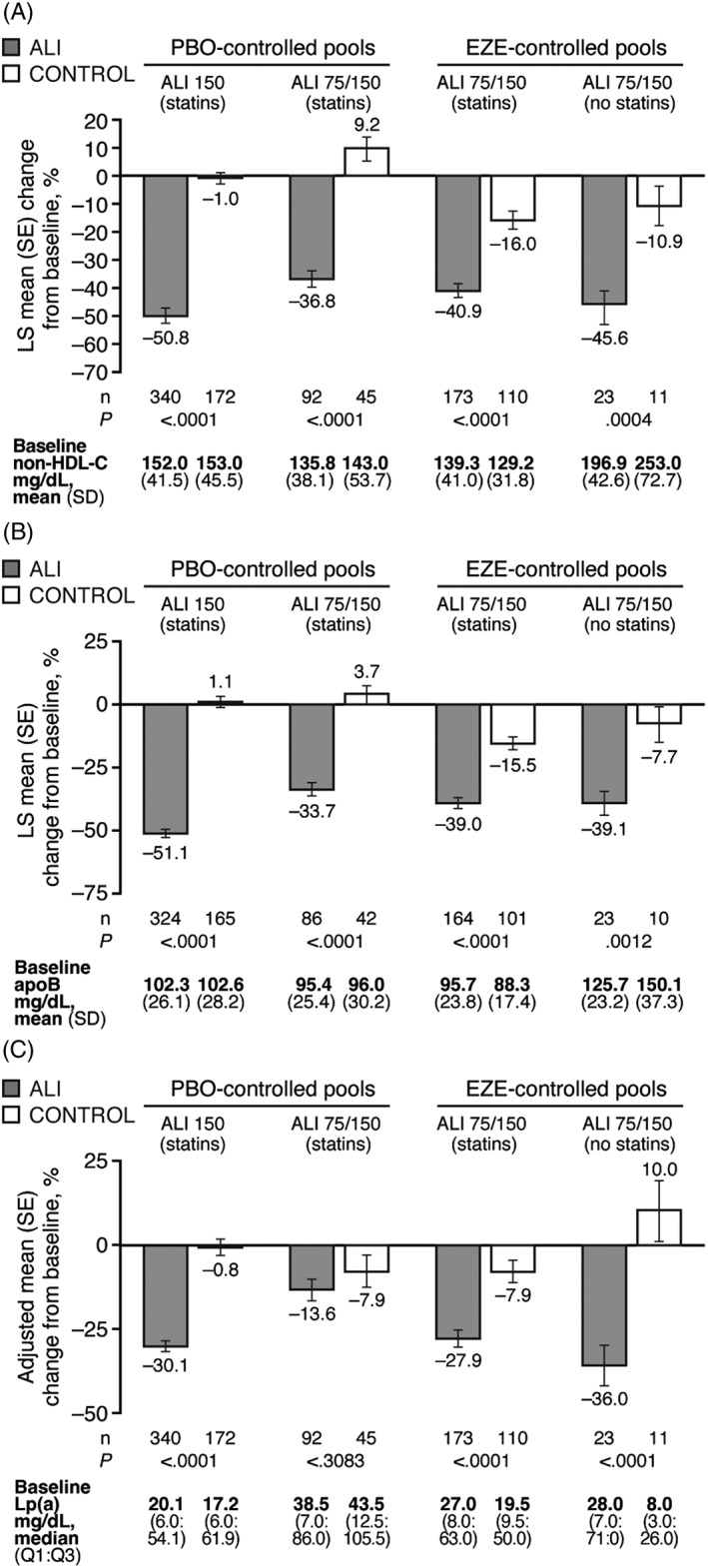
Percentage change from baseline to week 24 in A, non‐HDL‐C, B, apoB and C, Lp(a) among individuals with both DM and ASCVD, by analysis pool. Baseline values are from the randomized population. LS means (SE) in panels A and B derived from a mixed‐effect model with repeated measures (ITT analysis). Adjusted means (SE) in panel C from multiple imputation followed by robust regression (ITT analysis). Abbreviations: ALI 150, alirocumab 150 mg Q2W; ALI 75/150, alirocumab 75 mg Q2W with possible increase to 150 mg Q2W at Week 12 based on Week 8 LDL‐C; apo, apolipoprotein; ASCVD, atherosclerotic cardiovascular disease; DM, diabetes mellitus; EZE; ezetimibe; HDL‐C, high‐density lipoprotein cholesterol; ITT, intent‐to‐treat; Lp(a), lipoprotein (a); LS, least‐squares; PBO, placebo; SE, standard error
HbA1c levels were stable up to 78 weeks in both alirocumab and placebo arms in the placebo‐controlled pool of studies (Figure 3A). In the ezetimibe‐controlled pool, stable HbA1c levels were maintained up to Week 104 in both alirocumab and ezetimibe arms (Figure 3B). Similar trends were seen in FPG (Figures 3C,D). In addition, stability in HbA1c and FPG levels with alirocumab and control was seen in all patients, irrespective of insulin use (Figure S6).
Figure 3.
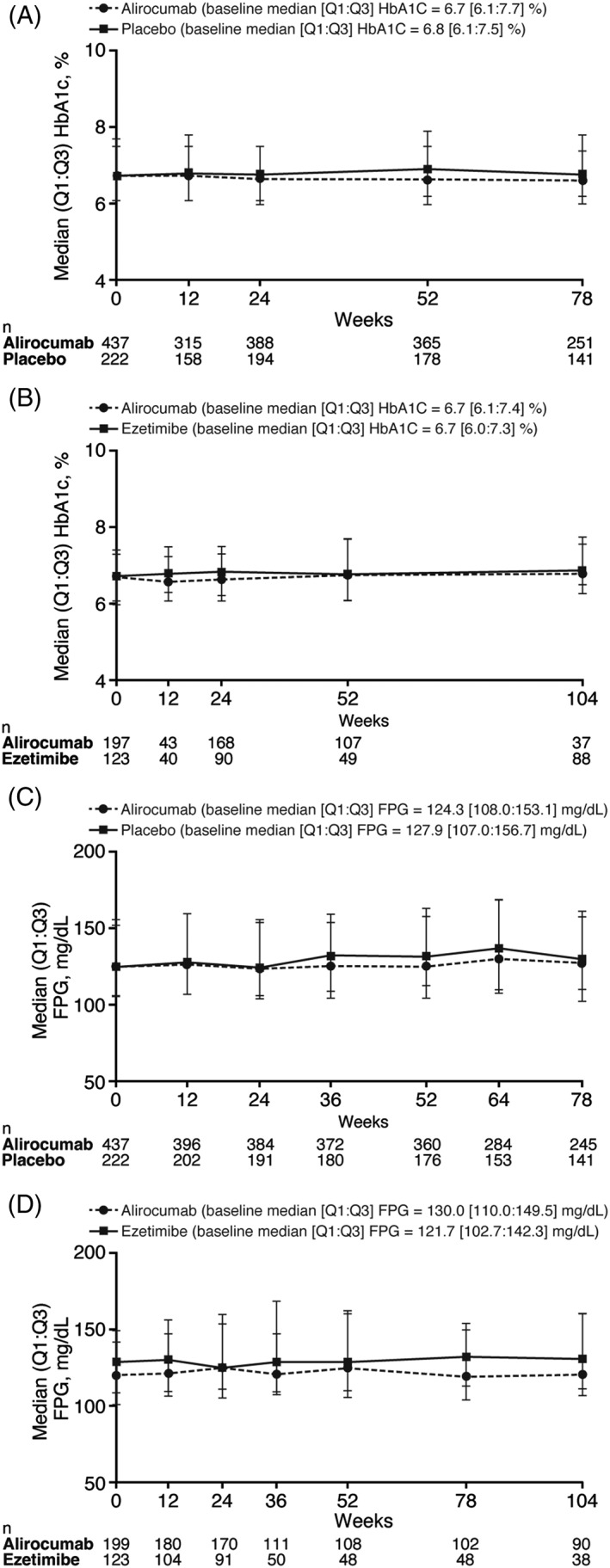
Median HbA1c values over time in A, placebo‐controlled and B, ezetimibe‐controlled pools and FPG values over time in C, placebo‐controlled and D, ezetimibe‐controlled pools. Analysed in the safety population. Abbreviations: FPG, fasting plasma glucose; HbA1c, glycated haemoglobin; Q, quartile
3.3. Safety
Overall safety was generally similar between alirocumab and control groups in the placebo‐ and ezetimibe‐controlled pools (Table 2). Myalgia and other muscle‐related TEAEs occurred in <5% of alirocumab‐treated patients, and occurred with a similar frequency in the control groups (Table 2). Injection‐site reactions were reported by 5.0% and 2.7% of alirocumab‐ and placebo‐treated patients in the pool of placebo‐controlled studies, and by 2.5% and 0.8% of alirocumab and ezetimibe recipients in the pool of ezetimibe‐controlled studies; these events were mostly mild and rarely led to treatment discontinuation (Table 2).
Table 2.
Safety data for patients with DM and ASCVD in placebo‐controlled and ezetimibe‐controlled pools
| Placebo‐controlled pools (n = 656) | Ezetimibe‐controlled pool (n = 322) | |||
|---|---|---|---|---|
| n (%) | Alirocumab (n = 437) | Placebo (n = 222) | Alirocumab (n = 199) | Ezetimibe (n = 123) |
| TEAEs | 358 (81.9) | 179 (80.6) | 162 (81.4) | 93 (75.6) |
| Treatment‐emergent SAEs | 110 (25.2) | 67 (30.2) | 45 (22.6) | 22 (17.9) |
| TEAEs leading to death | 5 (1.1) | 4 (1.8) | 2 (1.0) | 2 (1.6) |
| TEAEs leading to discontinuation | 33 (7.6) | 13 (5.9) | 22 (11.1) | 18 (14.6) |
| TEAEs in ≥5% of individuals | ||||
| Nasopharyngitis | 53 (12.1) | 21 (9.5) | 8 (4.0) | 5 (4.1) |
| Upper respiratory tract infection | 38 (8.7) | 25 (11.3) | 11 (5.5) | 11 (8.9) |
| Urinary tract infection | 30 (6.9) | 16 (7.2) | 6 (3.0) | 2 (1.6) |
| Hypertension | 22 (5.0) | 7 (3.2) | 13 (6.5) | 5 (4.1) |
| Influenza | 22 (5.0) | 11 (5.0) | 10 (5.0) | 9 (7.3) |
| Injection‐site reaction | 22 (5.0) | 6 (2.7) | 5 (2.5) | 1 (0.8) |
| Bronchitis | 23 (5.3) | 19 (8.6) | 9 (4.5) | 7 (5.7) |
| Arthralgia | 16 (3.7) | 16 (7.2) | 7 (3.5) | 4 (3.3) |
| Myalgia | 14 (3.2) | 8 (3.6) | 8 (4.0) | 8 (6.5) |
| Osteoarthritis | 13 (3.0) | 7 (3.2) | 7 (3.5) | 7 (5.7) |
| Pain in extremity | 13 (3.0) | 13 (5.9) | 5 (2.5) | 4 (3.3) |
| Fatigue | 12 (2.7) | 13 (5.9) | 6 (3.0) | 1 (0.8) |
| Accidental overdosea | 7 (1.6) | 4 (1.8) | 17 (8.5) | 5 (4.1) |
| Muscle‐related TEAEs | ||||
| Myalgia | 14 (3.2) | 8 (3.6) | 8 (4.0) | 8 (6.5) |
| Musculoskeletal pain | 12 (2.7) | 4 (1.8) | 0 | 1 (0.8) |
| Muscle spasms | 12 (2.7) | 6 (2.7) | 5 (2.5) | 3 (2.4) |
| Muscle strain | 2 (0.5) | 5 (2.3) | 1 (0.5) | 2 (1.6) |
| Injection‐site reactions | ||||
| Leading to treatment discontinuation | 1/22 (4.5) | 1/6 (16.7) | 1/5 (20.0) | 1/1 (100.0) |
| Severityb | ||||
| Mild | 20/22 (90.9) | 5/6 (83.3) | 4/5 (80.0) | 0/1 (0.0) |
| Moderate | 2/22 (9.1) | 1/6 (16.7) | 1/5 (20.0) | 0/1 (0.0) |
| Severe | 0/22 (0.0) | 0/6 (0.0) | 0/5 (0.0) | 1/1 (100.0) |
Abbreviations: ASCVD, atherosclerotic cardiovascular disease; DM, diabetes mellitus; SAE, serious adverse event; TEAE, treatment‐emergent adverse event.
Accidental or intentional administration of study drug at a frequency higher than that allowed by study protocol, if associated with an adverse event.
Local injection‐site reactions were graded by severity and were characterized by related signs and symptoms such as (but not limited to) redness and pain. Severity was highest if an individual experienced several local injection site reactions.
4. DISCUSSION
Individuals with both DM and ASCVD have a particularly high risk of events, compared with individuals with either DM alone or ASCVD alone, yet are often sub‐optimally treated in clinical practice and may benefit from additional lipid‐lowering therapy beyond statins, because of elevated numbers of atherogenic particles.7, 8, 9, 10, 15 In this analysis of alirocumab Phase 3 trials in a population of very high‐risk patients with both ASCVD and DM, alirocumab treatment was shown to significantly reduce levels of LDL‐C and other atherogenic lipid parameters compared with placebo or ezetimibe controls; reductions were maintained throughout the duration of the trials (24‐104 weeks depending on trial) and overall safety was comparable to controls. The magnitude of LDL‐C and other lipid percentage reductions, as well as the safety profile, were consistent with previous post‐hoc analyses of alirocumab trials in individuals with or without DM.26, 30, 31, 32, 33 The overall efficacy and safety of alirocumab observed in this sub‐analysis of individuals with both DM and ASCVD was also consistent with that reported for the overall patient population in alirocumab Phase 2 and 3 clinical trials.35, 36
Recommended LDL‐C targets for high‐risk individuals have become stricter over the years with the development of more efficacious lipid‐lowering drugs and new evidence regarding the cardiovascular benefit and safety of reducing LDL‐C to lower levels. Most recently, The American Association of Clinical Endocrinologists (AACE) has proposed an LDL‐C goal of < 55 mg/dL for “extreme risk” individuals, which includes those with both DM and ASCVD.9 Achievement of such LDL‐C levels may only be possible for many individuals via treatment with a statin plus a PCSK9 inhibitor, as demonstrated in the current analysis where 61.9%‐73.0% of individuals treated with alirocumab plus statin achieved LDL‐C < 55 mg/dL, from mean baseline levels of 105.2‐117.3 mg/dL, compared with 1.5%‐2.8% of individuals treated with statin plus placebo and 30.8% treated with statin plus ezetimibe. Among individuals who were not receiving background statin, the proportion of individuals who achieved LDL‐C <55 mg/dL was 43.9% with alirocumab, from a mean baseline LDL‐C of 157.6 mg/dL, and 1.1% with ezetimibe, from a mean baseline LDL‐C of 194.4 mg/dL.
The reductions in non‐HDL‐C and apoB observed with alirocumab in the present analysis may be particularly relevant for this population of individuals with both DM and ASCVD as these lipid parameters are considered to provide a better estimate of cardiovascular risk than LDL‐C among individuals with DM, because they more closely reflect the true number of atherogenic particles compared with LDL‐C.37, 38 Alirocumab also produced significant reductions in Lp(a), which has been proposed to be an independent cardiovascular risk factor; however, other commonly used lipid‐lowering strategies such as statins or ezetimibe have little or no effect on Lp(a).39 The percentage reduction in Lp(a) observed in individuals with DM and ASCVD who were treated with alirocumab was similar to that observed in the overall alirocumab‐treated patient populations in the ODYSSEY trials,39 with the exception of the pool without statins. For the DM and ASCVD population, Lp(a) changes from baseline were −36.0% with alirocumab and +10.0% with ezetimibe, compared with −25.9% with alirocumab and −7.3% with ezetimibe in the overall population.25 These differences are possibly a result of the small number of individuals with DM and ASCVD in that pool.
Although statins have been shown consistently to reduce cardiovascular events, statin use is associated with a small but significant increased risk of developing Type 2 DM.40, 41 Similarly, Mendelian randomization studies have indicated that individuals with loss‐of‐function mutations in PCSK9 have low levels of LDL‐C and low rates of cardiovascular events, but have an increased propensity for developing Type 2 DM.42, 43, 44 In this analysis of patients with existing DM, treatment with alirocumab had no effect on FPG or HbA1c levels compared with placebo over 78 weeks of treatment and compared with ezetimibe over 104 weeks of follow‐up, including comparison of individuals receiving insulin vs those not receiving insulin. These findings are consistent with those of the ODYSSEY DM‐INSULIN trial27 and previous sub‐analyses that revealed no effect of alirocumab on glycaemic parameters31, 33, 45 or no increase in new‐onset DM compared with controls.45 Furthermore, in a recent meta‐analysis of 15 randomized controlled trials with PCSK9 inhibitors, including alirocumab, there was no increase in glycaemic parameters in those without DM or pre‐existing DM (crude rate, 5.6% vs 5.9%; odds ratio, 1.05 [95% confidence interval, 0.95‐1.17], P = .32, I2 = 0%, heterogeneity P = .86).46 More evidence has recently become available from the ODYSSEY OUTCOMES trial, involving 18 924 patients with recent acute coronary syndrome, using alirocumab vs placebo, with exposure for up to 5 years (median exposure, 2.8 years).29
Comparing corresponding pools from the population with DM and ASCVD vs the overall trial population, the alirocumab dose was increased in a lower proportion of individuals with DM and ASCVD compared with the overall population (16.3% compared with 32.9%; pool of alirocumab 75/150 vs placebo [on statins]), but proportions were similar for the other two pools. The requirement for dose increase was LDL‐C goal‐based and was largely driven by baseline LDL‐C levels.47 The lower proportion of patients with dose increase in the DM and ASCVD population vs the overall population (in the alirocumab 75/150 vs placebo pool) may be explained, therefore, by the lower baseline LDL‐C levels in the DM and ASCVD population (105.2 vs 129.0 mg/dL in the overall population). The proportion of HeFH patients in the DM and ASCVD population in this pool was also lower than that in the overall population (24.7% vs 70.1%).
This analysis is limited by its post‐hoc nature and by the non‐randomized nature of the subgroups. There were relatively few individuals in the pool with no background statin therapy (ie, the ALTERNATIVE study), which probably contributes to some discrepancies that were observed in this pool, including imbalances in baseline LDL‐C between alirocumab and ezetimibe groups, and an observed 4% increase in LDL‐C from baseline in the ezetimibe group; in the primary trial, ALTERNATIVE, LDL‐C reductions from baseline to Week 24 were 45.0% with alirocumab and 14.6% with ezetimibe.25 The analysis of glycaemic parameters is limited by the duration of the trials, the longest follow‐up being 104 weeks. None of the studies included in this analysis was prospectively designed or powered for analysis of the effect of alirocumab on cardiovascular events, which was assessed in the ODYSSEY OUTCOMES study,29 although data from the DM subgroup are not yet known. In addition, the efficacy of PCSK9 inhibition in reducing cardiovascular events in a sub‐population of very high‐risk patients from the evolocumab FOURIER trial has recently been demonstrated.48
The present analysis demonstrated that alirocumab significantly reduced LDL‐C and other atherogenic lipid parameters and was generally well tolerated in individuals with DM and ASCVD from phase 3 ODYSSEY trials. The efficacy and safety of alirocumab in this population was comparable to that of the overall ODYSSEY clinical programme. These data support the use of alirocumab as an effective lipid‐lowering option for high‐risk individuals with DM and ASCVD who require additional LDL‐C reductions beyond that provided by statins.
Supporting information
Figure S1. Percentage change in LDL‐C from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75/150 mg Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S2. Percentage change in non‐HDL‐C from baseline over time with (A) alirocumab 150 mg/Q2W versus placebo, (B) alirocumab 75/150 mg/Q2W versus placebo and (C) alirocumab 75/150 mg/Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S3. Percentage change in apoB from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75/150 mg Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S4. Percentage change in Lp(A) from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75 150 mg/Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using multiple imputation followed by robust regression).
Figure S5. Percentage change in (A) HDL‐C and (B) triglycerides, from baseline up to week 24.
Figure S6. Median (A) HbA1C and (B) FPG values over the study period, analysed based on background insulin treatment.
ACKNOWLEDGMENTS
The authors would like to thank the patients, their families and all investigators involved in this study. Additional statistical analysis was provided by Desmond Thompson, PhD, consultant to medical affairs at Regeneron Pharmaceuticals, Inc. The following people from the study sponsors provided editorial comments on the manuscript: Michael Howard, MBA, L. Veronica Lee, MD and Corinne Hanotin, MD (Sanofi), and Robert Pordy, MD, Carol Hudson, MS, and Eva‐Lynne Greene, MS (Regeneron Pharmaceuticals, Inc.). Medical writing support, under the direction of the authors, was provided by Aparna Shetty, PhD and Rob Campbell, PhD of Prime (Knutsford, UK), supported by Sanofi and Regeneron Pharmaceuticals, Inc., according to Good Publication Practice guidelines (Link).
Conflict of interest
O. P. G. has received a research grant from Amarin Pharma; has participated in lectures for Merck; has been a consultant/advisory board member for Sanofi, Regeneron Pharmaceuticals, Inc., Amgen, Merck and Novo Nordisk; has received honoraria from Merck, Novo Nordisk, Amgen, Sanofi and Regeneron Pharmaceuticals, Inc.; and was partially supported by NIDDK grant # P30‐DK036836. J. P. has received a research grant from AstraZeneca; and has been a consultant/advisory board member for Eli Lilly, CVS Caremark, Merck, Aegerion, Amgen, Janssen, Novo Nordisk, Sanofi and Vivus. M. B.‐B, A. K. and A. L. are employees of and shareholders in Sanofi. S. S. is an employee of and shareholder in Regeneron Pharmaceuticals, Inc. J. M. is a contractor for Sanofi. L. A. L. has received personal fees from Esperion; has received grants and personal fees from Amgen, AstraZeneca, Eli Lilly and Company, Merck, Regeneron Pharmaceuticals, Inc. and Sanofi; and has received grants from Kowa and the Medicines Company, outside the submitted work.
Author contributions
O. P. G., J. P., S. K. S., M. B.‐B., A. K., J. M., A. L. and L. A. L. were involved in interpretation of the data. L. A. L. was a trial investigator and involved in data acquisition. J. M. and A. L. performed the statistical analyses. All authors were involved in critical revision of the manuscript drafts, read and approved the final version, and are accountable for the accuracy and integrity of the manuscript.
Ganda OP, Plutzky J, Sanganalmath SK, et al. Efficacy and safety of alirocumab among individuals with diabetes mellitus and atherosclerotic cardiovascular disease in the ODYSSEY phase 3 trials. Diabetes Obes Metab. 2018;20:2389–2398. 10.1111/dom.13384
Funding information This research was supported by Sanofi and Regeneron Pharmaceuticals, Inc. The sponsors were involved in the study design, collection, analysis and interpretation of data, as well as in checking of information. The authors were responsible for all content and editorial decisions and received no honoraria related to the development of this publication.
REFERENCES
- 1. Emerging Risk Factors Collaboration , Sarwar N, Gao P, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta‐analysis of 102 prospective studies. Lancet. 2010;375:2215‐2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Leon BM, Maddox TM. Diabetes and cardiovascular disease: epidemiology, biological mechanisms, treatment recommendations and future research. World J Diabetes. 2015;6:1246‐1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Low Wang CC, Hess CN, Hiatt WR, Goldfine AB. Clinical update: cardiovascular disease in diabetes mellitus: atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus ‐ mechanisms, management, and clinical considerations. Circulation. 2016;133:2459‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhatt DL, Eagle KA, Ohman EM, et al. Comparative determinants of 4‐year cardiovascular event rates in stable outpatients at risk of or with atherothrombosis. JAMA. 2010;304:1350‐1357. [DOI] [PubMed] [Google Scholar]
- 5. Donahoe SM, Stewart GC, McCabe CH, et al. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298:765‐775. [DOI] [PubMed] [Google Scholar]
- 6. Krempf M, Parhofer KG, Steg PG, et al. Cardiovascular event rates in diabetic and nondiabetic individuals with and without established atherothrombosis (from the REduction of Atherothrombosis for continued health [REACH] registry). Am J Cardiol. 2010;105:667‐671. [DOI] [PubMed] [Google Scholar]
- 7. American Diabetes Association . 9. Cardiovascular disease and risk management: standards of medical care in diabetes‐2018. Diabetes Care. 2018;41((suppl)):S86‐S104. [DOI] [PubMed] [Google Scholar]
- 8. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;37:2999‐3058. [DOI] [PubMed] [Google Scholar]
- 9. Jellinger PS, Handelsman Y, Rosenblit PD, et al. American Association of Clinical Endocrinologists and American College of Endocrinology guidelines for management of dyslipidemia and prevention of cardiovascular disease. Endocr Pract. 2017;23:1‐87. [DOI] [PubMed] [Google Scholar]
- 10. Lloyd‐Jones DM, Morris PB, Ballantyne CM, et al. 2017 focused update of the 2016 ACC expert consensus decision pathway on the role of non‐statin therapies for LDL‐cholesterol lowering in the management of atherosclerotic cardiovascular disease risk: a report of the American College of Cardiology Task Force on expert consensus decision pathways. J Am Coll Cardiol. 2017;70:1785‐1822. [DOI] [PubMed] [Google Scholar]
- 11. Cholesterol Treatment Trialists’ (CTT) Collaboration , Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670‐1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the collaborative atorvastatin diabetes study (CARDS): multicentre randomised placebo‐controlled trial. Lancet. 2004;364:685‐696. [DOI] [PubMed] [Google Scholar]
- 13. Shepherd J, Barter P, Carmena R, et al. Effect of lowering LDL cholesterol substantially below currently recommended levels in patients with coronary heart disease and diabetes: the treating to new targets (TNT) study. Diabetes Care. 2006;29:1220‐1226. [DOI] [PubMed] [Google Scholar]
- 14. Adiels M, Olofsson SO, Taskinen MR, Boren J. Diabetic dyslipidaemia. Curr Opin Lipidol. 2006;17:238‐246. [DOI] [PubMed] [Google Scholar]
- 15. Jacobson TA, Maki KC, Orringer CE, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9:S1‐122.e121. [DOI] [PubMed] [Google Scholar]
- 16. Reiner Z, De Backer G, Fras Z, et al. Lipid lowering drug therapy in patients with coronary heart disease from 24 European countries ‐ findings from the EUROASPIRE IV survey. Atherosclerosis. 2016;246:243‐250. [DOI] [PubMed] [Google Scholar]
- 17. Giugliano RP, Cannon CP, Blazing MA, et al. Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with vs. without diabetes: results from IMPROVE‐IT. Circulation. 2018;137:1571‐1582. [DOI] [PubMed] [Google Scholar]
- 18. Sabatine MS, Leiter LA, Wiviott SD, et al. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941‐950. [DOI] [PubMed] [Google Scholar]
- 19. Bays H, Gaudet D, Weiss R, et al. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140‐3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cannon CP, Cariou B, Blom D, et al. Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186‐1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Farnier M, Jones P, Severance R, et al. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138‐146. [DOI] [PubMed] [Google Scholar]
- 22. Ginsberg HN, Rader DJ, Raal FJ, et al. Efficacy and safety of alirocumab in patients with heterozygous familial hypercholesterolemia and LDL‐C of 160 mg/dl or higher. Cardiovasc Drugs Ther. 2016;30:473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kastelein JJ, Ginsberg HN, Langslet G, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996‐3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kereiakes DJ, Robinson JG, Cannon CP, et al. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906‐915.e13. [DOI] [PubMed] [Google Scholar]
- 25. Moriarty PM, Thompson PD, Cannon CP, et al. Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758‐769. [DOI] [PubMed] [Google Scholar]
- 26. Robinson JG, Farnier M, Krempf M, et al. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489‐1499. [DOI] [PubMed] [Google Scholar]
- 27. Leiter LA, Cariou B, Muller‐Wieland D, et al. Efficacy and safety of alirocumab in insulin‐treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM‐INSULIN randomized trial. Diabetes Obes Metab. 2017;19:1781‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ray KK, Leiter LA, Muller‐Wieland D, et al. Alirocumab vs usual lipid‐lowering care as add‐on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM‐DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20:1479‐1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schwartz GG, Szarek M, Bhatt DL, et al. Cardiovascular outcomes with alirocumab after acute coronary syndrome: results of the ODYSSEY outcomes trial. Presented at the 67th Annual Scientific Session of the American College of Cardiology (ACC), Orlando, Florida: 10‐12 March 2018 (Presentation number 401‐08). 2018. https://accscientificsession.acc.org/features/2018/03/video-sanofi-regeneron. Accessed June 22, 2018.
- 30. Ginsberg HN, Farnier M, Robinson JG, et al. Abstract 17070: efficacy and safety of alirocumab: pooled analyses of 1048 individuals with diabetes mellitus from five placebo‐controlled phase 3 studies of at least 52 weeks duration. Circulation. 2015;132:A17070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leiter LA, Muller‐Wieland D, Baccara‐Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med. 2018;35:121‐130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leiter LA, Tinahones FJ, Karalis D, et al. Alirocumab safety in individuals with and without diabetes mellitus: pooled data from 14 ODYSSEY trials. J Am Coll Cardiol. 2017;69:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, et al. Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989‐996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stone NJ, Robinson JG, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol. 2014;63:2889‐2934. [DOI] [PubMed] [Google Scholar]
- 35. Farnier M, Gaudet D, Valcheva V, Minini P, Miller K, Cariou B. Efficacy of alirocumab in high cardiovascular risk populations with or without heterozygous familial hypercholesterolemia: pooled analysis of eight ODYSSEY phase 3 clinical program trials. Int J Cardiol. 2016;223:750‐757. [DOI] [PubMed] [Google Scholar]
- 36. Jones PH, Bays HE, Chaudhari U, et al. Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol. 2016;118:1805‐1811. [DOI] [PubMed] [Google Scholar]
- 37. Modi KD, Chandwani R, Ahmed I, Kumar KV. Discordance between lipid markers used for predicting cardiovascular risk in patients with type 2 diabetes. Diabetes Metab Syndr. 2016;10:S99‐S102. [DOI] [PubMed] [Google Scholar]
- 38. Verbeek R, Hovingh GK, Boekholdt SM. Non‐high‐density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. 2015;26:502‐510. [DOI] [PubMed] [Google Scholar]
- 39. Gaudet D, Watts GF, Robinson JG, et al. Effect of alirocumab on lipoprotein(a) over ≥1.5 years (from the phase 3 ODYSSEY program). Am J Cardiol. 2017;119:40‐46. [DOI] [PubMed] [Google Scholar]
- 40. Ridker PM, Pradhan A, MacFadyen JG, Libby P, Glynn RJ. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: an analysis from the JUPITER trial. Lancet. 2012;380:565‐571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sattar N, Preiss D, Murray HM, et al. Statins and risk of incident diabetes: a collaborative meta‐analysis of randomised statin trials. Lancet. 2010;375:735‐742. [DOI] [PubMed] [Google Scholar]
- 42. Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144‐2153. [DOI] [PubMed] [Google Scholar]
- 43. Lotta LA, Sharp SJ, Burgess S, et al. Association between low‐density lipoprotein cholesterol‐lowering genetic variants and risk of type 2 diabetes: a meta‐analysis. JAMA. 2016;316:1383‐1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Schmidt AF, Swerdlow DI, Holmes MV, et al. PCSK9 genetic variants and risk of type 2 diabetes: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2017;5:97‐105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Colhoun HM, Ginsberg HN, Robinson JG, et al. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY phase 3 studies. Eur Heart J. 2016;37:2981‐2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Karatasakis A, Danek BA, Karacsonyi J, et al. Effect of PCSK9 inhibitors on clinical outcomes in patients with hypercholesterolemia: a meta‐analysis of 35 randomized controlled trials. J Am Heart Assoc. 2017;6(12):pii: e006910 10.1161/JAHA.117.006910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kastelein JJ, Kereiakes DJ, Cannon CP, et al. Effect of alirocumab dose increase on LDL lowering and lipid goal attainment in patients with dyslipidemia. Coron Artery Dis. 2017;28:190‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabatine MS, De Ferrari GM, Giugliano RP, et al. Clinical benefit of Evolocumab by severity and extent of coronary artery disease: an analysis from FOURIER. Circulation. 2018. CIRCULATIONAHA.118.034309. 10.1161/CIRCULATIONAHA.118.034309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Percentage change in LDL‐C from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75/150 mg Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S2. Percentage change in non‐HDL‐C from baseline over time with (A) alirocumab 150 mg/Q2W versus placebo, (B) alirocumab 75/150 mg/Q2W versus placebo and (C) alirocumab 75/150 mg/Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S3. Percentage change in apoB from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75/150 mg Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using mixed‐effect model with repeated measures).
Figure S4. Percentage change in Lp(A) from baseline over time with (A) alirocumab 150 mg Q2W versus placebo, (B) alirocumab 75/150 mg Q2W versus placebo and (C) alirocumab 75 150 mg/Q2W versus ezetimibe with background statins and (D) alirocumab 75/150 mg Q2W versus ezetimibe without background statins (intent‐to‐treat population analysed using multiple imputation followed by robust regression).
Figure S5. Percentage change in (A) HDL‐C and (B) triglycerides, from baseline up to week 24.
Figure S6. Median (A) HbA1C and (B) FPG values over the study period, analysed based on background insulin treatment.


