Abstract
Objective
The aim was to investigate whether vitamin D supplementation, combined with a hypocaloric diet, could have an independent effect on insulin sensitivity in subjects with both overweight and hypovitaminosis D. Changes from baseline in anthropometric parameters, body composition, glucose tolerance, and insulin secretion were considered as secondary outcomes.
Methods
Eighteen volunteers who were nondiabetic and vitamin D deficient and had BMI > 25 kg/m2 were randomized (1:1) in a double‐blind manner to a hypocaloric diet + either oral cholecalciferol at 25,000 IU/wk or placebo for 3 months. Hyperinsulinemic‐euglycemic clamp to measure insulin sensitivity was performed at baseline and after intervention.
Results
Body weight in both groups decreased significantly (−7.5% in the vitamin D group and −10% in the placebo group; P < 0.05 for both), with no between‐group differences. Serum 25‐hydroxyvitamin D levels in the vitamin D group increased considerably (from 36.7 ± 13.2 nmol/L to 74.8 ± 18.7 nmol/L; P < 0.001). Insulin sensitivity in the vitamin D group improved (from 4.6 ± 2.0 to 6.9 ± 3.3 mg·kg−1·min−1; P < 0.001), whereas no changes were observed in the placebo group (from 4.9 ± 1.1 to 5.1 ± 0.3 mg·kg−1·min−1; P = 0.84).
Conclusions
Cholecalciferol supplementation, combined with a weight loss program, significantly improves insulin sensitivity in healthy subjects with obesity and might represent a personalized approach for insulin‐resistant subjects with obesity.
Introduction
Serum 25‐hydroxyvitamin D (25[OH]D) concentration has been reported to be inversely associated with impaired glucose regulation, insulin resistance, β‐cell dysfunction, and risk of metabolic syndrome 1, 2. Although vitamin D insufficiency, defined as 25(OH)D levels under 75 nmol/L, is common in subjects with obesity 3, there is still controversy as to the mechanisms underlying vitamin D deficiency in obesity. Enhanced adipose tissue uptake 4, altered vitamin D metabolism in adipose tissue 5, sunlight underexposure, and low consumption of dairy products have all been postulated as possible causes. Moreover, it is still unclear whether the concurrence of vitamin D deficiency and insulin resistance in obesity is a causality or merely an independent finding.
Several cross‐sectional clinical studies have associated low vitamin D status with insulin resistance in adults 6 and children 7. Higher basal levels of 25(OH)D have been found to predict better β‐cell function and lower glycemia in subjects at risk for type 2 diabetes mellitus (T2DM) 8. Vitamin D may have favorable effects on insulin sensitivity through a series of mechanisms: it increases transcriptional activation and expression of the insulin receptor gene, facilitating both basal and insulin‐stimulated glucose oxidation and thus improving insulin sensitivity 9; enhances insulin action and signal transduction by regulating extracellular calcium 10; and modulates cytokine‐mediated β‐cell apoptosis, an important factor in the development and progression of T2DM 11.
In a previous study 12, we reported a direct correlation between 25(OH)D concentration and insulin sensitivity, evaluated by hyperinsulinemic‐euglycemic clamp, suggesting that vitamin D deficiency could worsen obesity‐related insulin resistance. Since then, further studies on the effects of vitamin D supplementation on insulin resistance have produced inconsistent data, with results from trials showing either no effect 13 or improvements in insulin action 14 in a wide range of patient populations. The inconsistency of the findings is most probably due to differences in the methods used to assess insulin resistance, as indirect indexes of insulin resistance derived from fasting insulin and glucose mostly reflect hepatic insulin sensitivity, whereas post‐oral glucose tolerance tests (OGTTs) do not account for all the variables influencing the results, including insulin secretion.
Our aim was therefore to evaluate whether vitamin D supplementation could ameliorate insulin sensitivity in patients at a high risk for diabetes; we designed a randomized, double‐blind, placebo‐controlled trial in which subjects with obesity were supplemented with vitamin D or placebo on top of a hypocaloric diet to clarify the relationship between hypovitaminosis D and glucose metabolism, with a particular focus on insulin resistance, as evaluated by hyperinsulinemic‐euglycemic clamp.
Methods
Study protocol
This was a double‐blind, randomized, placebo‐controlled trial (http://ClinicalTrials.gov registration: NCT02020694). The primary outcome measure was a change from baseline in insulin sensitivity after an intervention of 3 months.
Secondary outcomes included a change from baseline in glucose tolerance and insulin secretion and/or a change from baseline in body composition, anthropometric parameters, and phosphocalcic metabolism.
The study protocol was approved by the Ethics Committee of the Catholic University of the Sacred Heart. All subjects provided written informed consent.
Subjects
Participants were recruited from patients attending the outpatient clinic of the Center for Endocrine and Metabolic Diseases of our hospital, for follow‐up of thyroid nodules or overweight or obesity. Male and female subjects aged 18 to 70 years with BMI ≥ 30 kg/m2 and vitamin D deficiency (serum 25[OH]D < 75 nmol/L), who wished to be included in a weight loss program were enrolled. The exclusion criteria are discussed in the Supporting Information Methods.
Intervention
Subjects were blindly randomized (1:1), in a parallel manner, to a standardized hypocaloric diet + either oral cholecalciferol at 25,000 IU/wk or placebo once a week for 3 months. Cholecalciferol was provided by Abiogen (Dibase cod.A11CC05, 25,000 IU/2.5 mL oral solution), whereas the placebo was manufactured by our hospital pharmacy and was similar to the study medication in color, smell, and taste. The study medications were dispensed in identical boxes, sequentially numbered as per the random allocation list (by the pharmacist who was not involved in data collection or analysis and who kept the list linking the randomization code to the participants' identity and to the delivered box number). Randomization was carried out in blocks of six by gender and month of recruitment to ensure gender balance and minimize seasonal changes in vitamin D levels. The principal investigator, the co‐investigators, and the health care providers had no access to these code breaks and were all blinded to treatment allocation. Compliance to treatment was assessed by interview and inspection of vitamin D/placebo bottles supplied to participants and returned at each visit.
In addition, all subjects received dietary counseling from the same nutritionist, who calculated dietary energy composition by subtracting 500 kcal from the usual individual energy intake, evaluated using the diet history method and a 3‐day recall questionnaire. Diet compliance was evaluated through changes in anthropometric parameters via food frequency questionnaires during a monthly nutritional visit.
Assessments
Data on demographics, coexisting illnesses, family history of diabetes, and medications used were collected at the first visit. Anthropometric values (body weight, height, and waist and hip circumferences), a hormonal assessment (parathyroid hormone, 25[OH]D, thyroid function), measurement of electrolytes (calcium, phosphorus), a serum lipid profile (triglycerides, total cholesterol, high‐density lipoprotein and low‐density lipoprotein cholesterol), and a comprehensive metabolic assessment (OGTT, hyperinsulinemic‐euglycemic clamp) were performed at baseline and after 3 months of intervention. A detailed description of metabolic assessments has been added in the Supporting Information Methods.
Body composition
Whole body fat mass, whole body lean mass, and trunk fat mass were assessed by dual x‐ray absorptiometry (Delphi‐W densitometer; Hologic, Marlborough, Massachusetts) at the beginning and at the end of the intervention.
Statistical analyses
From previous data on a similar cohort of patients with obesity studied at our Center 13 showing a difference in the mean glucose infusion rate between the upper and the lower part of serum 25(OH)D (25[OH]D ≥ 75 and ≤ 50 respectively) of 2.0 ± 1.5 mg·kg−1·min−1, we determined that a sample size of 18 subjects for each arm was necessary to detect a difference of 1 m/kg per minute in insulin‐mediated glucose uptake on the basis of a type I error of 0.05 and a type II error of 0.20 (power of 80%).
Statistical analyses were performed with SPSS (version 20; SPSS, Chicago, Illinois), and statistical significance was set at P < 0.05.Normality was assessed by using the Shapiro‐Wilk test. Results are reported as mean ± SD for normally distributed data sets or median for nonnormally distributed variables. Differences in baseline and follow‐up characteristics between the two groups were tested by using independent t tests for normally distributed continuous variables, Mann‐Whitney U tests for skewed variables, and Pearson's χ2 tests for categorical variables.
Results
Of a total of 45 subjects screened from October 2013 to March 2014, only 42 were considered eligible for the study. During the first visit, two subjects were excluded because of high serum levels of vitamin D and another was excluded because of desire to attempt pregnancy. Six subjects were diagnosed with diabetes after the baseline OGTT and were excluded from the study before randomization. The remaining 36 subjects were randomly assigned to either the vitamin D group or the placebo group. A total of 18 subjects (9 participants for both placebo and vitamin D group) were included in the analysis. At follow‐up, three participants refused to sign the informed consent, while an additional eight subjects were lost because of lack of compliance in attending visits or taking medication, and other seven subjects were excluded because of noncompliance with the dietary regimen. A flowchart (Supporting Information Figure S1) shows the study progression.
More females than males were included. Baseline characteristics were comparable for both groups (Table 1). In particular, serum levels of vitamin D were similar for both groups (vitamin D: 36.7 ± 13.2 nmol/L, placebo: 34.7 ± 21.2 nmol/L; P = 0.80) as was insulin sensitivity, expressed as milligrams of glucose consumed per minute per kilogram of body weight (glucose uptake) during hyperinsulinemic‐euglycemic clamp (vitamin D: 4.6 ± 2.1, placebo: 4.9 ± 1.1 mg·kg‐1·min‐1 mg·kg−1·min−1; P = 0.75).
Table 1.
Clinical and biochemical characteristics of all subjects at baseline
| Placebo group (n = 9) | Vitamin D group (n = 9) | P | |
|---|---|---|---|
| Age (y) | 35.3 ± 11.0 | 45.5 ± 11.1 | 0.06 |
| Gender (F/M) | 6/3 | 8/1 | |
| Body weight (kg) | 111 ± 12.2 | 100 ± 15.8 | 0.22 |
| BMI (kg/m2) | 38.7 ± 9.75 | 36.6 ± 5.14 | 0.50 |
| WHR | 0.94 ± 0.03 | 0.86 ± 0.08 | 0.13 |
| 25(OH)D (nmol/L) | 34.7 ± 21.1 | 36.7 ± 13.2 | 0.80 |
| Serum calcium (mmol/L) | 2.32 ± 0.09 | 2.31 ± 0.09 | 0.90 |
| PTH (ng/L) | 46.5 ± 1.41 | 47.8 ± 19.5 | 0.90 |
| Fasting glucose (mmol/L) | 4.78 ± 0.22 | 5.08 ± 0.55 | 0.18 |
| Fasting insulin (µUI/mL) | 16.8 ± 1.69 | 13.7 ± 8.59 | 0.49 |
| Total cholesterol (mmol/L) | 4.57 ± 0.66 | 5.12 ± 0.49 | 0.22 |
| HDL cholesterol (mmol/L) | 1.21 ± 0.27 | 1.47 ± 0.42 | 0.22 |
| LDL cholesterol (mmol/L) | 2.69 ± 0.22 | 3.16 ± 0.43 | 0.22 |
| Triglycerides (mmol/L) | 1.43 ± 0.36 | 1.19 ± 0.60 | 0.55 |
| AUC glycemia 0′‐120′(mmol/L·min·104) | 85.9 ± 7.70 | 82.5 ± 19.6 | 0.62 |
| AUC insulin 0′‐120′ (µU/mL·min·106) | 12.3 ± 8.77 | 9.77 ± 7.90 | 0.52 |
| Glucose uptake (mg·kg−1·min−1) | 4.93 ± 1.13 | 4.62 ± 2.08 | 0.75 |
| Total fat (%) | 38.8 ± 7.42 | 39.7 ± 7.06 | 0.81 |
| Trunk lean mass (kg) | 32.3 ± 1.04 | 28.3 ± 5.23 | 0.20 |
| Trunk fat mass (kg) | 23.9 ± 1.10 | 21.1 ± 7.99 | 0.46 |
Data are expressed as mean ± SD. P value is for interaction between the two groups.
25(OH)D, 25‐hydroxyvitamin D; AUC, area under the curve; F, female; HDL: high‐density lipoprotein; LDL: low‐density lipoprotein; M, male; PTH, parathyroid hormone; WHR, waist‐hip ratio.
After 3 months of intervention with hypocaloric diet + cholecalciferol or placebo, body weight decreased significantly in both groups (vitamin D: −7.5%, placebo: −10% reduction in body weight; P = 0.0007 and P = 0.001); while no other between‐group differences were observed (P = 0.68) (Figure 1A).
Figure 1.
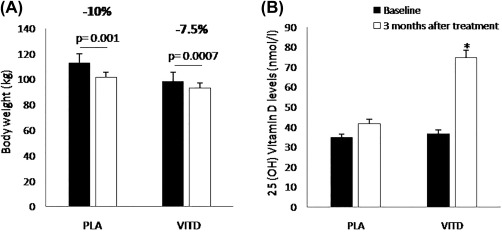
(A) Body weight and (B) 25‐hydroxyvitamin D (25[OH]D) levels at baseline (black bars) and after 3 months of treatment (white bars); *P < 0.001.
Plasma 25(OH)D levels increased from 36.7 ± 13.2 to 74.8 ± 18.7 nmol/L in the vitamin D group (P < 0.001) and from 34.7 ± 21.1 to 41.7 ± 7.7 nmol/L in the placebo group (P = 0.41) (Figure 1B).
Total trunk fat mass, evaluated by dual x‐ray absorptiometry, decreased significantly only in the vitamin D group after treatment (vitamin D group decreased from 21.1 ± 7.9 to 17.9 ± 7.8 kg, P = 0.003; placebo group from 23.9 ± 1.1 to 21.5 ± 5.9 kg, P = 0.32), as reported in Table 2. After 3 months of treatment, a substantial improvement in insulin sensitivity was observed in the vitamin D group (from 4.6 ± 2.1 to 6.9 ± 3.3 mg·kg−1·min−1; P < 0.001), whereas the change from baseline in glucose uptake during the clamp procedure was not significant in the placebo group (from 4.9 ± 1.0 to 5.1 ± 0.3 mg·kg−1·min−1; P = 0.84) (Figure 2). Seventy‐five percent of subjects in the vitamin D group achieved a 20% improvement in insulin sensitivity, versus 33.3% in the placebo group. Nevertheless, the improvement in insulin sensitivity achieved in the supplemented group was not significant compared with the placebo group (P for interaction = 0.16).
Table 2.
Biochemical parameters, β‐cell function (HOMA2‐B, insulinogenic index, disposition index), and body composition at baseline and after 3 months of treatment in both groups
| Placebo group (n = 9) | Vitamin D group (n = 9) | |||||
|---|---|---|---|---|---|---|
| Baseline | Follow‐up | P | Baseline | Follow‐up | P | |
| 25(OH)D (nmol/L) | 34.7 ± 21.1 | 41.7 ± 7.7 | 0.41 | 36.7 ± 13.2 | 74.8 ± 18.7 | 0.001 |
| Serum calcium (mmol/L) | 2.32 ± 0.09 | 2.32 ± 0.10 | 0.85 | 2.31 ± 0.09 | 2.39 ± 0.08 | 0.01 |
| PTH (ng/L) | 46.5 ± 1.41 | 40.9 ± 13.6 | 0.44 | 47.8 ± 19.5 | 47.6 ± 20.1 | 0.97 |
| HOMA2‐B | 299 ± 107 | 287 ± 105 | 0.29 | 182 ± 84.2 | 190 ± 107 | 0.67 |
| Insulinogenic index | 1.30 ± 0.36 | 1.07 ± 0.33 | 0.51 | 2.99 ± 5.40 | 1.07 ± 0.62 | 0.32 |
| Disposition index | 3.38 ± 0.56 | 3.89 ± 0.04 | 0.51 | 4.89 ± 56.0 | 4.91 ± 5.84 | 0.65 |
| Total trunk mass (kg) | 56.9 ± 9.50 | 57.2 ± 10.4 | 0.35 | 50.0 ± 11.3 | 37.4 ± 20.5 | 0.05 |
| Trunk fat mass (kg) | 23.9 ± 11.0 | 21.5 ± 5.88 | 0.32 | 21.1 ± 7.98 | 17.9 ± 78.3 | 0.003 |
Data are expressed as mean ± SD. P value is for interaction between baseline and follow‐up in each group.
25(OH)D, 25‐hydroxyvitamin D; HOMA2‐B, homeostatic model assessment 2 B; PTH, parathyroid hormone.
Figure 2.
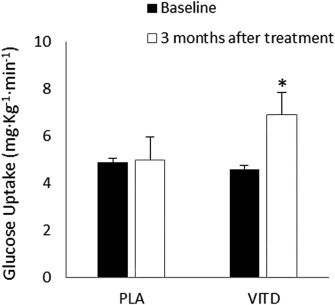
Insulin sensitivity (glucose uptake) at baseline (black bars) and after 3 months of treatment (white bars) in the placebo (PLA) and vitamin D (VITD) groups; *P = 0.02.
Changes from baseline in glucose and insulin areas under the curve were nonsignificant and plasma glucose and insulin levels did not differ at any OGTT time point (Figure 3).
Figure 3.
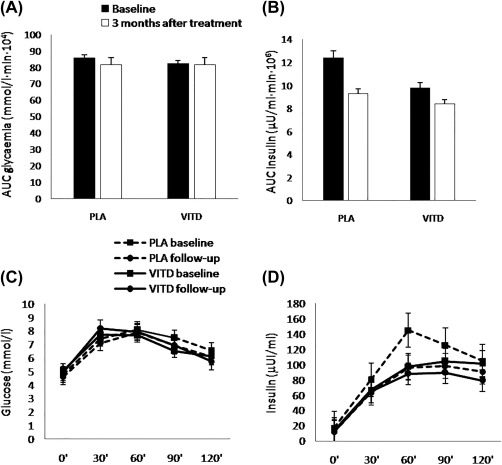
Areas under the curve (AUC) for (A) glycemia and, (B) insulin and (C) glucose and (D) insulin curves during oral glucose tolerance tests at baseline and after 3 months of treatment in both groups.
Indirect indexes of β‐cell function (i.e., homeostatic model assessment 2 B, insulinogenic index, disposition index) remained consistent in both groups. A clinically irrelevant but statistically significant increment in serum calcium levels was observed in patients treated with vitamin D (from 2.31 ± 0.09 to 2.39 ± 0.08 mmol/L; P = 0.01), while parathyroid hormone levels and lipid profiles remained unchanged in both groups (Table 2).
We noticed that only 60% of subjects in the vitamin D group reached the target serum 25(OH)D concentration of 75 nmol/L after treatment. To confirm the relationship between the improvement in insulin sensitivity and vitamin D supplementation, we performed a further subanalysis on the group treated with Vitamin D between subjects who reached a 25(OH)D concentration of 75 nmol/L and those who did not. At follow‐up, both subgroups showed a significant increase in vitamin D levels (Supporting Information Figure S2A), but only subjects with vitamin D higher than 75 nmol/L reached the cutoff of normal serum levels of vitamin D.
Although the subclassification limits the power of analysis, which doesn't reach statistical significance, both groups showed an increase in insulin sensitivity following vitamin D supplementation (25 [OH]D > 75 nmol/L, P = 0.10; 25[OH]D < 75 nmol/L, P = 0.18). Further, no difference was observed between groups at follow‐up (P = 0.73) (Supporting Information Figure S2B).
Discussion
This randomized controlled trial compared the effects of a hypocaloric diet combined with 3 months of vitamin D supplementation (25,000 IU weekly) or placebo on insulin sensitivity, measured using a hyperinsulinemic‐euglycemic clamp, in 18 adults with both obesity and vitamin D deficiency. The study revealed that cholecalciferol supplementation combined with weight loss is associated with a significant improvement in insulin sensitivity in vitamin D deficient subjects with obesity. However, we failed to demonstrate an unequivocal effect of cholecalciferol supplementation on insulin sensitivity, as the improvements observed in the vitamin D group were not significant versus placebo.
A systematic meta‐analysis found that vitamin D supplementation had a bland effect on the reduction of fasting glucose and improvement of insulin resistance in T2DM or impaired glucose tolerance, but no effect on these variables was noted in patients with obesity or with normal glucose tolerance 15. In a recently published randomized controlled trial, Gulseth et al. 16 also observed no changes in insulin sensitivity or insulin secretion in T2DM patients after vitamin D3 supplementation. Similarly, Kampmann et al. 17 found no differences in insulin sensitivity, glycemic control, inflammatory parameters, or blood pressure in patients with established T2DM after 12 weeks of vitamin D supplementation, but they suggested a positive effect on insulin secretion and C‐peptide levels in the vitamin D‐treated group.
However, previous reports have suggested that vitamin D can improve insulin sensitivity both in adolescents and in adults. To date, very few studies have used the gold standard technique to measure insulin sensitivity (i.e., euglycemic‐hyperinsulinemic clamp) in order to assess the effect of vitamin D supplementation in subjects with overweight or obesity, and it is therefore difficult to compare these results with data from other studies using surrogate indexes. In a small group of healthy male volunteers (n = 18), insulin sensitivity, measured using the clamp procedure, was similar after either calcitriol or placebo treatment, but these subjects had normal body weight and vitamin D levels 18. The findings of a recent randomized controlled trial 19 conducted on subjects with overweight or obesity and with no history of diabetes and vitamin D deficiency contrast with our findings, showing no difference in insulin sensitivity measured with the use of a hyperinsulinemic‐euglycemic clamp and insulin secretion measured with the use of an intravenous glucose tolerance test after vitamin D supplementation compared to placebo, despite a significant increase in 25(OH)D concentrations in the vitamin D group. However, in our study, subjects showed a reduction in body weight following a hypocaloric diet, suggesting that supplementation with vitamin D may have a greater effect than lifestyle intervention alone in improving insulin sensitivity in vitamin D deficient subjects with obesity.
In our study, the improvement in insulin resistance following vitamin D supplementation could be related to the important reduction in trunk fat mass observed only in the vitamin D group after intervention. Despite controversial findings on how vitamin D supplementation acts on body weight or fat mass composition, increases in visceral fat mass or trunk fat mass were correlated with pathological obesity and features of metabolic syndrome in this population 20. A well‐designed randomized controlled trial combining weight loss intervention with placebo or vitamin D supplementation in 218 women with overweight or obesity revealed that a 12‐month daily supplementation with 2,000 IU cholecalciferol was associated with greater reduction in BMI and waist circumference only in those subjects whose 25(OH)D levels rose to 80 nmol/L or higher, suggesting that the beneficial effects of supplementation only kick in at elevated plasma 25(OH)D levels 21. Other studies have reported that calcium and/or vitamin D supplementation contributes to a beneficial reduction in visceral adiposity 22 and have described the mechanism with which such supplements influence the expression of genes correlated with adipogenesis and fatty acid oxidation 23. In vitro studies have also shown that the differentiation of preadipocytes into mature adipocytes is halted by 1,25‐dihydroxyvitamin D3 24. Some of these vitamin D effects could be explained by the expression and activation of different variants of vitamin D receptors in adipose tissue 25.
An additional factor to consider is the increase in serum calcium levels reported in subjects treated with vitamin D; indeed, a significant inverse correlation between high calcium levels and insulin resistance has been reported in the literature. This relationship is probably explained by the interaction between serum calcium levels and cellular function such as the regulation of glucose uptake in muscle cells, modulating the insulin receptor and insulin sensitivity of these cells 26.
We observed a modest, nonsignificant increase in 25(OH)D levels in the placebo group and no significant improvement in insulin sensitivity despite a clinically relevant 10% weight loss. Serum 25(OH)D concentrations have been shown to increase following weight loss 27, although in a large, controlled, retrospective study 28, this increase did not contribute to improvements in insulin sensitivity and β‐cell function. Furthermore, the improvement in insulin sensitivity in the vitamin D–supplemented group was not significant versus placebo. Only 60% of subjects in the vitamin D group reached the target serum 25(OH)D concentration of 75 nmol/L (30 ng/dL), identified as the optimal value for vitamin D homeostasis in epidemiological studies 29. However, when we divided the subjects treated with vitamin D in two subgroups, on the basis of serum 25(OH)D concentration reached after supplementation, we found no significant difference in insulin sensitivity, glucose tolerance, or insulin secretion between groups, while both groups showed an increase in insulin sensitivity following vitamin D supplementation, suggesting that the effect of vitamin D supplementation on insulin sensitivity might be independent of the achievement of the cutoff level of Vitamin D.
Furthermore, we found no improvements in β‐cell function due to vitamin D supplementation, at least as assessed by the disposition index. Similarly, glucose and insulin areas under the curve during 120‐min OGTT did not apparently improve after treatment; this result is only in apparent contrast with the increase in insulin sensitivity following vitamin D supplementation. An explanation may be that the sample size is probably too small to catch possible changes in glucose tolerance; therefore, an improvement in insulin secretion cannot be excluded. A limited number of interventional studies have examined the effect of vitamin D supplementation on measures of β‐cell function 30; these studies have, however, yielded inconsistent results. In a randomized clinical trial, Mitri et al. showed that 16 weeks of cholecalciferol (2,000 IU daily) supplementation improved β‐cell function in adults at high risk of diabetes 31.
The strengths of our study are the rigorous assessment of both insulin sensitivity and insulin secretion and the use of gold standard methodology for measuring insulin sensitivity; moreover, the study is well designed, and the participants are well characterized in terms of body composition and anthropometric measures, despite the small sample size, which could lead to insufficient power in detecting differences in study outcomes. Indeed, the main limitation of our study lies in the number of dropouts due to difficulty in following the hypocaloric diet. Despite this limitation, the estimated effect size was higher than anticipated and statistical significance in the primary outcomes changes was detected, even with the reduced final sample analyzed at the end of the study.
In conclusion, our data suggest that vitamin D supplementation, combined with dietary lifestyle intervention, favorably affects insulin sensitivity and body composition, at least in vitamin D deficient subjects with obesity whose serum levels of vitamin D significantly increase after treatment. Our data lay the grounds for future, larger studies aimed at confirming the effect of vitamin D supplementation on peripheral and hepatic insulin resistance, with the long‐run objective to investigate personalized strategies for obesity.
Supporting information
Supporting Information 1
Supporting Information Figure S1
Acknowledgments
The authors wish to thank all the people involved in the study and all the trial participants for their time.
Disclosure: The authors declared no conflict of interest.
Author contributions: CMAC generated the data; VAS, SM, AAGB and ES contributed in collecting data; CC analyzed data; CMAC and CC wrote manuscript; AG and TM edited manuscript; AG, GM, and GPS designed the study; AP and FC contributed to discussion; and AG and AP managed and coordinated responsibility for research activity planning and execution.
Clinical trial registration: http://ClinicalTrials.gov identifier NCT02020694.
Chiara M.A. Cefalo and Caterina Conte contributed equally to this work.
References
- 1. Forouhi NG, Luan J, Cooper A, Boucher BJ, Wareham NJ. Baseline serum 25‐hydroxy vitamin D is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely Prospective Study 1990‐2000. Diabetes 2008;57:2619‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kayaniyil S, Vieth R, Retnakaran R, et al. Association of vitamin D with insulin resistance and beta‐cell dysfunction in subjects at risk for type 2 diabetes. Diabetes Care 2010;33:1379‐1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pereira‐Santos M, Costa PR, Assis AM, Santos CA, Santos DB. Obesity and vitamin D deficiency: a systematic review and meta‐analysis. Obes Rev 2015;16:341‐349. [DOI] [PubMed] [Google Scholar]
- 4. Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF. Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 2000;72:690‐693. [DOI] [PubMed] [Google Scholar]
- 5. Wamberg L, Christiansen T, Paulsen SK, et al. Expression of vitamin D‐metabolizing enzymes in human adipose tissue‐the effect of obesity and diet‐induced weight loss. Int J Obes (Lond) 2012;37:651‐657. [DOI] [PubMed] [Google Scholar]
- 6. Barchetta I, De Bernardinis M, Capoccia D, et al. Hypovitaminosis D is independently associated with metabolic syndrome in obese patients. PLoS One 2013;31;8:e68689. doi: 10.1371/journal.pone.0068689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Olson ML, Maalouf NM, Oden JD, White PC, Hutchison MR. Vitamin D deficiency in obese children and its relationship to glucose homeostasis. J Clin Endocrinol Metab 2012;97:279‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kayaniyil S, Retnakaran R, Harris SB, et al. Prospective associations of vitamin D with beta‐cell function and glycemia: the prospective Metabolism and Islet cell Evaluation (PROMISE) cohort study. Diabetes 2011;60:2947‐2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dunlop TW, Vaisanen S, Frank C, Molnar F, Sinkkonen L, Carlberg C. The human peroxisome proliferator‐activated receptor delta gene is a primary target of 1alpha,25‐dihydroxyvitamin D3 and its nuclear receptor. J Mol Biol 2005;349:248‐260. [DOI] [PubMed] [Google Scholar]
- 10. Maiti A, Beckman MJ. Extracellular calcium is a direct effecter of VDR levels in proximal tubule epithelial cells that counter‐balances effects of PTH on renal Vitamin D metabolism. J Steroid Biochem Mol Biol 2007;103:504‐508. [DOI] [PubMed] [Google Scholar]
- 11. Dutta D, Mondal SA, Choudhuri S, et al. Vitamin‐D supplementation in prediabetes reduced progression to type 2 diabetes and was associated with decreased insulin resistance and systemic inflammation: an open label randomized prospective study from Eastern India. Diabetes Res Clin Pract 2014;103:e18‐e23. [DOI] [PubMed] [Google Scholar]
- 12. Muscogiuri G, Sorice GP, Prioletta A, et al. 25‐Hydroxyvitamin D concentration correlates with insulin‐sensitivity and BMI in obesity. Obesity (Silver Spring) 2010;18:1906‐1910. [DOI] [PubMed] [Google Scholar]
- 13. Grimnes G, Figenschau Y, Almas B, Jorde R. Vitamin D, insulin secretion, sensitivity, and lipids: results from a case‐control study and a randomized controlled trial using hyperglycemic clamp technique. Diabetes 2011;60:2748‐2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. von Hurst PR, Stonehouse W, Coad J. Vitamin D supplementation reduces insulin resistance in South Asian women living in New Zealand who are insulin resistant and vitamin D deficient ‐ a randomised, placebo‐controlled trial. Br J Nutr 2010;103:549‐555. [DOI] [PubMed] [Google Scholar]
- 15. George PS, Pearson ER, Witham MD. Effect of vitamin D supplementation on glycaemic control and insulin resistance: a systematic review and meta‐analysis. Diabet Med 2012;29:e142‐e150. [DOI] [PubMed] [Google Scholar]
- 16. Gulseth HL, Wium C, Angel K, Eriksen EF, Birkeland KI. Effects of vitamin D supplementation on insulin sensitivity and insulin secretion in subjects with type 2 diabetes and vitamin D deficiency: a randomized controlled trial. Diabetes Care 2017;40:872‐878. [DOI] [PubMed] [Google Scholar]
- 17. Kampmann U, Mosekilde L, Juhl C, et al. Effects of 12 weeks high dose vitamin D3 treatment on insulin sensitivity, beta cell function, and metabolic markers in patients with type 2 diabetes and vitamin D insufficiency ‐ a double‐blind, randomized, placebo‐controlled trial. Metabolism 2014;63:1115‐1124. [DOI] [PubMed] [Google Scholar]
- 18. Fliser D, Stefanski A, Franek E, Fode P, Gudarzi A, Ritz E. No effect of calcitriol on insulin‐mediated glucose uptake in healthy subjects. Eur J Clin Invest 1997;27:629‐633. [DOI] [PubMed] [Google Scholar]
- 19. Mousa A, Naderpoor N, de Courten MP, et al. Vitamin D supplementation has no effect on insulin sensitivity or secretion in vitamin D‐deficient, overweight or obese adults: a randomized placebo‐controlled trial. Am J Clin Nutr 2017;105:1372‐1381. [DOI] [PubMed] [Google Scholar]
- 20. Liu P, Ma F, Lou H, Liu Y. The utility of fat mass index vs. body mass index and percentage of body fat in the screening of metabolic syndrome. BMC Public Health 2013;13:629. doi: 10.1186/1471-2458-13-629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mason C, Xiao L, Imayama I, et al. A. Vitamin D3 supplementation during weight loss: a double‐blind randomized controlled trial. Am J Clin Nutr 2014;99:1015‐1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Salehpour A, Hosseinpanah F, Shidfarf F, et al. A 12‐week double‐blind randomized clinical trial of vitamin D3 supplementation on body fat mass in healthy overweight and obese women. Nutr J 2012;11:78. doi: 10.1186/1475-2891-11-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Marcotorchino J, Tourniaire F, Astier J, et al. Vitamin D protects against diet‐induced obesity by enhancing fatty acid oxidation. J Nutr Biochem 2014;25:1077‐1083. [DOI] [PubMed] [Google Scholar]
- 24. Kim JH, Kang S, Jung YN, Choi HS. Cholecalciferol inhibits lipid accumulation by regulating early adipogenesis in cultured adipocytes and zebrafish. Biochembiophys Res Commun 2016;469:646‐653. [DOI] [PubMed] [Google Scholar]
- 25. Khan RJ, Riestra P, Gebreab SY, et al. Vitamin D receptor gene polymorphisms are associated with abdominal visceral adipose tissue volume and serum adipokine concentrations but not with body mass index or waist circumference in African Americans: the Jackson Heart Study. J Nutr 2016;146:1476‐1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams PF, Caterson ID, Cooney GJ, Zilkens RR, Turtle JR. High affinity insulin binding and insulin receptor‐effector coupling: modulation by Ca2+. Cell Calcium 1990;11:547‐556. [DOI] [PubMed] [Google Scholar]
- 27. Rock CL, Emond JA, Flatt SW, et al. Weight loss is associated with increased serum 25‐hydroxyvitamin D in overweight or obese women. Obesity (Silver Spring) 2012;20:2296‐2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Thibault V, Morisset AS, Brown C, et al. The increase in serum 25‐hydroxyvitamin D following weight loss does not contribute to the improvement in insulin sensitivity, insulin secretion and beta‐cell function. Br J Nutr 2015;114:161‐168. [DOI] [PubMed] [Google Scholar]
- 29. Holick MF. Vitamin D deficiency. N Engl J Med 2007;357:266‐281. [DOI] [PubMed] [Google Scholar]
- 30. Jorde R, Figenschau Y. Supplementation with cholecalciferol does not improve glycaemic control in diabetic subjects with normal serum 25‐hydroxyvitamin D levels. Eur J Nutr 2009;48:349‐354. [DOI] [PubMed] [Google Scholar]
- 31. Mitri J, Dawson‐Hughes B, Hu FB, Pittas AG. Effects of vitamin D and calcium supplementation on pancreatic beta cell function, insulin sensitivity, and glycemia in adults at high risk of diabetes: the Calcium and Vitamin D for Diabetes Mellitus (CADDM) randomized controlled trial. Am J Clin Nutr 2011;94:486‐494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information Figure S1


