Abstract
Undernutrition is associated with maternal morbidity and poor pregnancy outcomes. This qualitative study seeks to understand the multilevel factors influencing maternal dietary practices in Niger, including the impact of pregnancy illnesses on diet. Criterion‐based, purposive sampling was used to select pregnant women and household members from 24 villages in a rural district of the Maradi Region in south‐central Niger. Semistructured interviews (n = 153) and focus group discussions (n = 38) explored 4 primary themes: (a) perceptions of ideal diet during pregnancy, (b) barriers to consuming the ideal diet, (c) coping strategies including dietary responses related to pregnancy illnesses, and (d) changes in perceptions from early to late pregnancy. Longitudinal data collection allowed for repeated interviews of pregnant women to document changes in dietary practices throughout pregnancy. Transcripts were coded using an inductive approach informed by grounded theory methodology. Participants categorized foods into 4 primary dietary taxonomies when discussing ideal maternal diets but cited constraints related to accessibility and availability impeding routine consumption of these foods. Perceptions of “modern,” urban foods as healthy, coupled with key structural barriers such as food costs, were identified. Maternal morbidity influenced food consumption, as women reported reducing food intake early in pregnancy in response to illness episodes. Although awareness of optimal foods for supporting healthy pregnancies was moderately high, some misconceptions were observed and multilevel barriers to food security restricted opportunities for consuming these foods. Nutrition‐specific and nutrition‐sensitive interventions could improve access and availability of acceptable foods for supporting increased dietary intake during pregnancy.
Keywords: diet, maternal nutrition, Niger, nutrition during pregnancy, pregnancy outcomes, qualitative research
Key Messages.
Pregnant women, household members, and health workers reported awareness of increased nutritional requirements during pregnancy but cited food unavailability, high food prices, gendered dynamics of household food purchasing, and maternal illnesses as barriers to consuming nutritive foods during pregnancy.
Maternal morbidity influenced food consumption, as women reduced or substituted food intake early in pregnancy in response to illness episodes.
Findings highlight a need for complementary interventions to increase availability and access to locally acceptable nutritious foods in this setting.
1. INTRODUCTION
Despite measurable improvements in public health care provision over the last two decades, poor pregnancy outcomes and high reproduction rates persist in Niger (ICF International, Institut National de la Statistique [INS], 2013; Marquez, Holschneider, Broughton, & Hiltebeitel, 2014; Maternal Mortality Estimation Inter‐Agency Group, United Nations Population Division, 2016; UNICEF, 2016; United Nations Population Fund, 2016). Adequate nutrition is essential for optimizing pregnancy outcomes (Imdad & Bhutta, 2012; Ota, Hori, Mori, Tobe‐Gai, & Farrar, 2015; World Health Organization [WHO], 2016), and maternal malnutrition associated with food insecurity, infections, and increased nutrient requirements throughout gestation poses a significant challenge to improving maternal and child health (Christian, 2010; Dalmiya, Darnton‐Hill, Schultink, & Shrimpton, 2009; Suchdev, Peña‐Rosas, & De‐Regil, 2014). In Niger, 13% of pregnant women have a low pre‐pregnancy body mass index (based on the WHO criteria, <18 kg/m2), and over half of them experience anaemia (Requejo, Victoria, & Bryce, 2015; WHO, 2015).
Long‐standing local dietary practices can affect food intake during different life stages, including pregnancy. Local dietary taxonomies, referring to the segmentation of food items into categories based on social functions rather than nutritional profiles, can dictate the quantities and types of food women consume during pregnancy. Many of these taxonomies emerge from sociocultural associations of specific foods with poor health outcomes in pregnancy, such as miscarriage and complicated delivery (Lennox, Petrucka, & Bassendowski, 2017; Mothupi, 2014; Riang'a, Broerse, & Nangulu, 2017; Wulandari & Whelan, 2011). Across country settings, perceptions of nutrient‐dense foods as harmful to the developing fetus or undervaluing of locally available nutritive food items have been linked to poor maternal and newborn nutrition (Heidkamp, Ayoya, Teta, Stoltzfus, & Marhone, 2015; Maimbolwa, Yamba, Diwan, & Ransjö‐Arvidson, 2003; Ogbeide, 1974; Onyesom, Onyesom, Ofili, Anyanwu, & Uzuegbu, 2008).
There is some evidence for the contribution of these local dietary taxonomies to suboptimal maternal dietary practices. Studies conducted in South Asia have attributed particular maternal food consumption behaviours, including intentional food intake restrictions (referred to as “eating down” in pregnancy), to both physiological and sociocultural factors, such as food aversions during pregnancy, fears of an oversized fetus resulting from excessive food intake, and perceived pregnancy/childbirth complications associated with unhealthy pregnancy diets (Christian et al., 2006; Gittelsohn, Thapa, & Landman, 1997; Harding et al., 2017; Hutter, 1996; Nichter & Nichter, 1983). Similarly, research in Nigeria has documented the influence of culturally bound food proscriptions on intentional reductions in food consumption during pregnancy (Maduforo, 2010; Ojofeitimi, Elegbe, & Babafemi, 1982; Ojofeitimi & Tanimowo, 1980; Ugwa, 2016). Less attention, however, has been devoted to exploring the contribution of multilevel factors, including maternal morbidity, to dietary practices and preferences during pregnancy.
This paper presents findings from a longitudinal qualitative study in south‐central Niger exploring maternal food consumption practices and their underlying determinants during pregnancy. Determinants operating at the structural, community, household, and physiological levels were characterized and assessed using semistructured interviews and focus group discussions with pregnant women, household members, and health workers. A better understanding of maternal diets and their domains of influence in this setting could inform strategies to promote consumption of nutritive foods by pregnant women vulnerable to malnutrition and pregnancy complications.
2. METHODS
This qualitative study was nested within a cluster randomized trial assessing the effectiveness of prenatal supplementation on infant immunogenicity to oral rotavirus vaccination (“Efficacy and Safety of a Pentavalent Rotavirus Vaccine Against Severe Rotavirus Gastroenteritis in Niger,” http://clinicaltrials.gov Identifier: NCT02145000), conducted in the Madarounfa Health District within Maradi Region of south‐central Niger (Isanaka et al., 2017). In 2012, 17.9% of women of reproductive age in this region were underweight (body mass index < 18.5 kg/m2), with 11.8% of babies born with low birthweight (less than 2,500 g) and an estimated fertility rate of 8.4 (ICF International, INS, 2013).
In the parent trial (beginning March 2015), 3,000 pregnant women across 53 villages were randomized to receive one of three nutritional supplements during pregnancy—iron and folic acid (IFA) pills, multiple micronutrient (MMN) capsules, or medium‐quantity lipid‐based nutrient supplement (MQ‐LNS; a 40‐g fortified, ready‐to‐use paste made of peanuts, oil, dried skimmed milk powder, sugar, and 22 micronutrients) sachets. Mothers and children participating in the trial receive health care free of charge, including prenatal supplements. No food assistance is provided to villages in the study catchment area. Women were included in the parent trial if they were less than 30 weeks of gestation at time of enrolment; intended to remain in the study area through delivery and for 2 years thereafter; and did not display or report chronic health conditions, severe illness, pregnancy complications, or peanut allergy. Community leaders provided consent for their villages to participate in the study prior to randomization and recruitment of participants.
The qualitative substudy purposively sampled pregnant women participating in the parent trial and household members (husbands and in‐laws) in 24 villages. Pregnant women in selected villages were sampled across age groups, gestational ages, and parent trial supplement arm assignment. As most women initiated prenatal supplementation during the first trimester, gestational age provides a proxy for duration of prenatal supplementation usage at the time of qualitative data collection. Midwives and health assistants affiliated with the parent trial were also sampled, given their influence on pregnant women's health behaviours.
2.1. Data collection
Qualitative data were collected in two phases: July–August and November–December 2016. Using semistructured interviews and focus group discussions, we sought to understand local food consumption preferences, dietary intake during pregnancy, experiences with illnesses throughout pregnancy, and other related health‐seeking behaviours. Questions gauged participant perceptions of ideal maternal diets and the extent to which these diets deviated from typical nonpregnancy diets; sources of information for dietary decision‐making in pregnancy; and factors perceived to influence dietary outcomes in pregnancy, such as food availability/access as well as maternal illness. The longitudinal design aimed to capture changes in the experiences, including health and nutrition‐related behaviours and perceptions, of women as they progressed through their pregnancies.
In the first phase of data collection, semistructured interviews and focus group discussions were conducted with participating women across all three trimesters, their husbands and mothers‐in‐law, and study health staff. To capture women's changing perceptions and attitudes during their pregnancies, semistructured interviews and focus groups in Phase 2 were conducted with a subset of Phase 1 participants in the later stages of their pregnancies (≥20 weeks of gestation) or who had recently delivered. These women were sampled across parent trial supplement arms based on their gestational age and their willingness to participate in follow‐up data collection activities. Compared with Phase 1 participants, women participating in Phase 2 data collection activities were prompted to discuss any changes to their food consumption patterns and illness experiences in the later stages of their pregnancy using modified interview and focus group discussion guides based on emergent findings from Phase 1. Participant sampling within the parent study arms across study phases (interviews: IFA = 50, MMN = 54, LNS = 49; focus groups: IFA = 9, MMN = 10, LNS = 19) was intended to achieve representation among participants consuming different prenatal supplements, because supplement properties could influence food intake patterns differentially, and to ensure data saturation was reached across groups (Morse, Barrett, Mayan, Olson, & Spiers, 2002).
2.2. Data analysis
Study staff conducted interviews and led focus group discussions in Hausa. Interview and focus group recordings were transcribed verbatim and translated into English. Transcripts were uploaded into Dedoose (version 7.5.27, Los Angeles, CA) to facilitate data management. In alignment with the inductive approach underpinning data collection, two principal coders drew from key tenets of grounded theory to identify and capture salient themes emerging from the transcripts (Charmaz, 2006).
A multistep analytic process was followed. First, topics included in the interview and focus group discussion guides served as the 23 topical codes during the preliminary open coding process and memo taking (Creswell & Miller, 2000). Emergent themes informed the development of a more refined codebook that included 38 response codes and served as the analytic framework for the remainder of the coding process. This final list of codes was applied to the transcripts and included a combination of categories identified a priori and emergent themes identified during the open coding process. Third, themes and subthemes were reassembled through axial coding and memos using narrative matrices and chronological arrays, through which coded textual data were organized into various tabulated presentations, stratified by participant categories and noteworthy topics (Yin, 2016). This method of reconstructing coded data enabled identification of salient themes across participant groups and constant comparison of emerging categories across the study phases (Charmaz, 2006). Phase 1 transcripts were coded in full prior to coding of Phase 2 interview and focus group transcripts, a process that allowed data collection and analysis to be iterative (Morse et al., 2002).
2.3. Ethical approval
The study was approved by the Comité Consultatif National d'Ethique (Niger); the Comité de Protection des Personnes (France); the Commission d'Ethique de la Recherche sur l'Etre Humain, Hôpitaux Universitaires de Genève (Switzerland); Research Ethics Review Committee of the WHO (Switzerland); and the Western Institutional Review Board (United States). Individual written informed consent was obtained from all study participants in the local language (Hausa) prior to data collection activities.
3. RESULTS
One hundred and fifty‐three semistructured interviews and 38 focus groups, with two to five participants per group, were conducted from July to December 2016. The number of interviews and focus groups stratified by participant category and data collection phase is shown in Table 1. In Phase 1, 114 interviews and 26 focus groups were conducted among pregnant women, husbands, mothers‐in‐law, study midwives, and health assistants. Phase 2 included 39 interviews and 12 focus groups with pregnant women participating in Phase 1, enabling longitudinal data collection from these participants. The results comprise multilevel thematic areas, but they can be organized into three primary domains pertinent to maternal health: typical and ideal diets during pregnancy, constraints to maternal diets, and changes to maternal diets over time.
Table 1.
Number of participants enrolled by population and data collection method, July–December 2016
| Data collection method | Semistructured interviews | Focus group discussions | ||
|---|---|---|---|---|
| Study phase | Phase 1 | Phase 2 | Phase 1 | Phase 2 |
| Pregnant women (<20 weeks of gestation) | 40 | ‐ | 12 | ‐ |
| Pregnant women (≥20 weeks of gestation) | 44 | 39 | 5 | 12 |
| Husbands | 18 | ‐ | ‐ | ‐ |
| Mothers‐in‐law | 9 | ‐ | ‐ | ‐ |
| Community health assistants | ‐ | ‐ | 9 | ‐ |
| Midwives | 3 | ‐ | ‐ | ‐ |
| Totals | 114 | 39 | 26 | 12 |
Hyphens represent the value zero ‐ they indicate no data collection activities were performed for specific populations during a particular study phase.
3.1. Typical and ideal diets during pregnancy
Figure 1 summarizes local dietary taxonomies described by study participants, who classified foods into four principal categories: traditional/local foods, modern/city foods, body‐building foods, and items to avoid during pregnancy (food proscriptions). Participants described the typical meals consumed in their villages as predominantly plant‐based and locally available. Millet‐ and maize‐based food items (e.g., fura and tuwo) were cited as the dietary staples of the traditional local diet. This local diet was readily distinguished from “modern” or “city” food items available in urban areas, such as bread, meat, and macaroni, and from “body‐building” foods, including fruits and protein‐rich foods such as beans, eggs, and meat—all of which were perceived to provide the most nutrition to pregnant women. In some cases, foods considered to be “body‐building” overlapped with “modern/city” foods, specifically eggs, meat, and spaghetti. Although participants frequently described foods providing adequate nutrition during pregnancy, they also classified some foods with limited nutritive properties (specifically “modern/city” foods, such as spaghetti) as nutritious, and thus highly desirable, during pregnancy.
Figure 1.
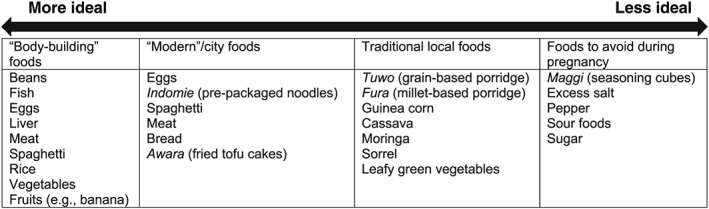
Food items enumerated by study participants related to ideal maternal diets, by dietary taxonomy
Participants recognized the limitations of resorting to traditional diets when ideal foods for consumption during pregnancy were unavailable. As this pregnant woman states, foods traditionally consumed in local diets, such as maize‐ and millet‐based porridges, do not provide acceptable levels of nutrition to pregnant women:
Some of the foods, we just eat them because it is part of our tradition, like fura [millet‐based porridge prepared with milk or water]. It does not build up a person, unlike vegetables, which are said to build up an individual. (Pregnant woman, 20 weeks gestation)
The benefits of consuming nutritious foods during pregnancy, including positive birth outcomes, prevention of maternal illness, and protection of newborn health status, were widely reported. Participants also perceived an ideal pregnancy diet as leading to improved strength and general well‐being, with specific food prescriptions identified during interviews. As this pregnant woman explains,
The kinds of food a pregnant woman is supposed to be eating include fish, liver, meat, banana, orange, pineapple, egg, beans, and rice or spaghetti … If a pregnant woman is eating them, she will be healthy, her baby will develop very well in the womb, and she will deliver a healthy baby. (Pregnant woman, 35 weeks gestation)
Consensus around the types of foods associated with positive pregnancy outcomes was observed among different participant groups, with most characterizing healthy pregnancies as ones devoid of maternal illnesses and concluding in uncomplicated births of normal‐weight babies, free from illness.
Many women specifically alluded to protein‐rich foods as protective against the development of anaemia in pregnancy and cited an improvement in energy these foods brought to women at early gestational ages. Protein‐rich diets were noted to specifically “build blood” in the body to improve pregnancy outcomes. As women continued performing labour‐intensive domestic tasks during pregnancy, foods that provided energy for overcoming the malaise frequently reported in early pregnancy were desirable:
If you have healthy blood, and you can do your work very well, like pounding [grain]. If you don't have healthy blood, there is no work [to be done]. (Pregnant woman, 12 weeks gestation)
Complementing these dietary taxonomies related to ideal maternal diets, participants framed specific foods and behaviours related to food preparation, storage, and consumption as dietary proscriptions due to their perceived association with maternal illness or pregnancy complications. Participants insisted upon deliberately reducing intake of, if not complete abstention from, items such as excess salt, spicy/peppery foods, sugar, and “sour foods.” Although some of the justifications shared by participants were based on biomedical knowledge aligned with messages frequently disseminated in health facilities, other explanations provided for avoiding specific foods were informed by ethnomedical paradigms:
If a woman consumes such foods in excess, her breastmilk will be very watery after delivery. That is why we normally adjust the consumption of such foods. (Pregnant woman, 8 weeks gestation)
Similarly, household members discussed the importance of appropriate handling and storage practices of food to be consumed by pregnant women. Numerous respondents highlighted the importance of protecting food from flies, which were understood as vectors of diarrhoeal disease:
Food that has been touched by flies cannot be consumed by a pregnant woman. The result of consuming this kind of food is a serious diarrhea that may turn to cholera … which can drain water from the woman's body. (Pregnant woman, <20 weeks gestation)
3.2. Constraints to ideal maternal diet
In spite of broad participant awareness of the benefits nutritious foods offered pregnant women, participants described multilevel barriers to acquiring the foods associated with positive pregnancy outcomes. Participants described the latsamniya (“struggle”) to acquiring and consuming foods corresponding to their ideal maternal diet. We have situated these reported barriers in a socioecological framework (Figure 2) and present findings in alignment with the framework's components: structural, community, household, and physiological.
Figure 2.
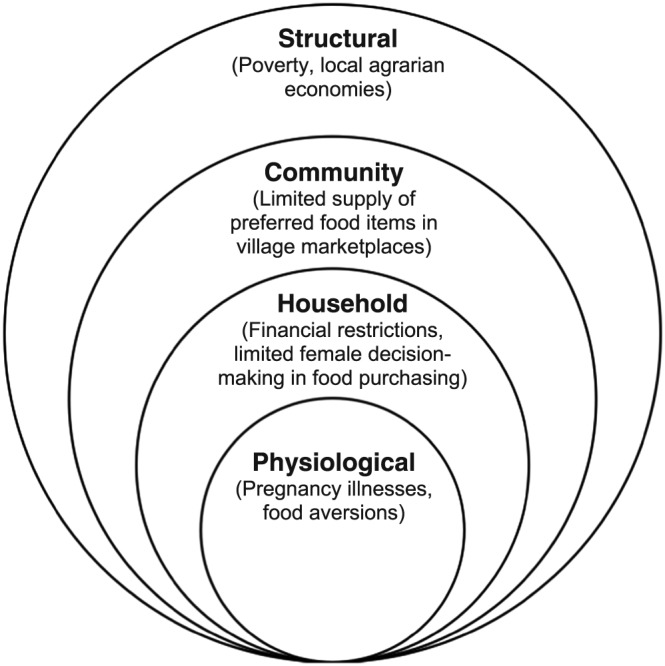
Socioecological model summarizing participant‐reported constraints to consuming ideal pregnancy diets
3.2.1. Structural/community: Food availability
Many participants quickly drew contrasts between the foods available in urban settings and those available in their villages. As this mother‐in‐law describes, limited availability of these food items in village marketplaces restricted diet modification during pregnancy:
Here in the village, honestly it is hard, because we are in the bush, not like you people living in the cities. Because where you are, one can get things like spaghetti, indomie [instant noodles], macaroni, meat, eggs, and so on, while here we only have millet‐based porridge, maize‐based foods, and vegetables. (Mother‐in‐law, 80 years old)
The agrarian economy in the study setting, thus, was perceived to play a central role in defining the parameters of local maternal diets. As one husband explains,
Each and every man in the village goes to the farm and grows crops. So, at the end of the day when he harvests his crops, he would have enough food to give to his family … We eat to survive most of the time. (Husband, 30 years old)
The limited availability of specific foods drove reliance on subsistence farming for sustaining dietary intake during pregnancy, with many of these locally cultivated foods perceived as insufficient for supporting increased nutritional requirements during pregnancy. Subsequently, health worker messaging regarding maternal dietary intake emphasized locally available substitutes for nutrient‐rich foods unavailable in rural settings:
Women in the village do not have opportunities like women in the city, but we tell them to eat cooked moringa leaves and beans, or if her husband is a middle‐class man, he should be buying her liver [meat]. (Community health agent)
3.2.2. Household: Food access
Even where nutritious foods were available for consumption, accessing them was inhibited by lack of affordability and other household‐level factors. Numerous participants noted that limited household finances restricted dietary options for pregnant women. Foods perceived to maximize pregnancy outcomes were often unaffordable, preventing pregnant women from supplementing their traditional diets with nutrient‐rich foods. As a result, many participants noted that women retained traditional diets during pregnancy with minimal modifications, consuming ideal pregnancy foods only infrequently—as permitted by household finances.
Women also alluded to male authority over household food procurement restricting their ability to consume an ideal maternal diet. Limited financial autonomy of women in this setting reportedly restricted their ability to inform food‐purchasing decisions. Pregnant women commonly referenced their husband's authority over financial matters as a primary influence on the foods they prepared at home and consumed during their pregnancies:
That is the rule here in the village: to eat any food that a husband provides, because that is the food he can afford to buy. Foods like maize‐based porridge, fura [millet‐based porridge with milk], and green leafy vegetables are the kind of foods … my husband provides. (Pregnant woman, 14 weeks gestation)
Husbands confirmed this decision‐making authority that pregnant women commonly described:
When my wallet is filled with money, my wife eats four to five times a day, and when I don't have money, she eats three times a day. (Husband, 28 years old)
For pregnant women, it was ultimately the combination of concentrated dietary decision‐making authority among male heads of household and limited financial resources that restricted opportunities to diversify their traditional diets during pregnancy. A few participants noted that women with income of their own could have greater purchasing autonomy to obtain the food items they desired; yet, as many participants lamented, the price of these items at markets often rendered these items unaffordable and, thus, inaccessible even to women with independent finances.
Overall, the barriers to consuming ideal maternal diets identified by pregnant women were described as unavoidable due to the aforementioned constraints on food availability and access, many of which manifested across socioecological domains—as presented in Figure 2. In contrast to personal preference or food aversions that may contribute to dietary practices, participants in this setting emphasized constraints influenced by multilevel factors, from availability to access, seen to be operating largely outside of their control or influence.
3.2.3. Physiological: Pregnancy illness and food aversions
Participants discussed concerns regarding illness and potential complications during pregnancy, explaining how these symptoms impacted dietary intake during pregnancy. Nausea and vomiting were specifically noted to influence dietary intake and were frequently discussed as concerns for pregnant women and their family members. Women acknowledged vomiting to be commonplace during the early stages of pregnancy, and the severity of their symptoms sometimes resulted in prolonged food aversions, lasting as long as the duration of the pregnancy, perceived as harmful to the developing fetus:
That is why I cannot eat, because of this vomiting, because it's a suffering to me, and I will suffer [as will] my baby … I don't want to eat maize‐based porridge because if I eat it, I will vomit it out … If I continue vomiting, I can affect my baby, God forbid, I can even have a miscarriage. That is why I cannot eat it, because I don't want a problem. (Pregnant woman, 5 weeks gestation)
In such cases, food intake was directly associated with pregnancy‐related illness and presented as potentially harmful to pregnancy outcomes. Modifying diets to consume food more amenable to their sensitivities, or reducing their food intake altogether, allowed women to cope with specific presentations of maternal illness. In many instances, pregnant women preferred to restrict their food consumption in the hopes of mitigating illness symptoms and protecting the developing fetus. As this midwife suggests, health worker messaging supported food restrictions in pregnant women in order to protect fetal health:
We advise them [pregnant women] that whenever they have problems eating, they should stop consuming that food. But if they are undisturbed, then let them continue eating that kind of food. (Midwife)
Participants also described other illnesses commonly experienced during pregnancy, by which diets or dietary intake was unaffected. General malaise—or what participants described as “body weakness,” possibly related to anaemia—was articulated repeatedly as an illness commonly experienced during pregnancy. For many women, the weakness they described was debilitating, even for women with previous pregnancies. They expressed concern over this weakness because it prevented them from performing necessary household tasks:
When I feel weakness of the body, I cannot even do work. Standing up and fetching water is not possible. Even if I stand up, I cannot pull the wheelbarrow, because I feel dizzy if I stand; I have to assign children to fetch the water. (Pregnant woman, 30 weeks gestation)
For many women, resting and abstaining from exhausting physical labour served as chief strategies to respond to their malaise. No dietary prescription was identified for such symptoms.
Study participants also cited malaria and other febrile illnesses as highly problematic during pregnancy. Despite participants' assertion of fever as commonplace (frequently called malaria, irrespective of origin), pregnancy complications attributed to fever rendered these febrile illnesses worrisome for participants. Pregnant women directly implicated fever and malaria in severe pregnancy complications, including eclampsia, miscarriage, and “draining blood from the body.” For participants who reported high fever or malaria during previous pregnancies or who observed these illnesses in family or community members, they unanimously discussed seeking biomedical care from a health clinic or hospital as the optimal coping strategy for persistent symptoms. No particular dietary prescription was identified for fever or malaria.
3.3. Changes in pregnancy illnesses over time
Follow‐up data collection activities revealed changes to women's diets accompanying pregnancy progression. During interviews and focus groups at later gestational ages or after childbirth (Phase 2), women explained that many of their illness symptoms waned over time, specifically nausea and vomiting, abdominal pain, headache, and fever. Participants often reported that their food sensitivities subsided along with symptoms of morning sickness.
I experienced vomiting when the pregnancy was three months, before I started antenatal [care] visits. Now that the pregnancy has matured, I am not experiencing any problem—just being healthy. (Pregnant woman, 33 weeks gestation)
With reductions in symptoms that deterred women from maintaining desired food consumption over time, participants reported returning to their routine dietary intake patterns.
I found it very difficult to eat food during my previous pregnancies. [Now] that I am using this [MQ‐LNS], I feel dizzy when I do not eat breakfast. I have to buy food or eat if it is available within the house. Then, I feel well and satisfied. (Pregnant woman, 21 weeks gestation)
Many participants recognized that these symptoms dissipated naturally as their pregnancies progressed; however, some women attributed improvements in their morning sickness symptoms to prenatal supplement consumption. Women, like the participant quoted above, attributed reductions in nausea and food aversions to consumption of prenatal supplements, while reporting increased symptoms of hunger (e.g., dizziness), which were alleviated with increased food intake.
4. DISCUSSION
This study examined maternal dietary practices and preferences among pregnant women and their household members, in addition to exploring the relationship between maternal illness and food consumption during pregnancy. Participant responses highlighted disparities between their description of an ideal maternal diet and the typical food consumption patterns in study communities. Pregnant women across prenatal supplement assignment consistently reported these perceptions. Although most participants recognized the contribution of nutrient‐rich foods such as legumes and fruits to healthy pregnancies, they highlighted numerous food insecurity‐related barriers to incorporating desirable foods into their pregnancy diets. These barriers included challenges related to food availability and access, specifically limited supply of nutritious foods in village marketplaces, financial constraints, and women's limited decision‐making authority in household food purchasing.
Participants articulated four primary dietary taxonomies in their depictions of an ideal maternal diet: traditional/local foods, modern/city foods, body‐building foods, and food proscriptions. The categories presented a conflation of food items classified as “modern,” costly, and predominantly available in urban areas with those perceived as ideal for pregnancy. Representations of spaghetti, for example, as nutritious for pregnant women are noteworthy, because this food was described as largely inaccessible to rural populations and, thus, consumed almost exclusively by wealthier city dwellers, who in Niger tend to have better pregnancy and overall health outcomes (ICF International, INS, 2013). The observed overlap of urban, “modern” foods with diets perceived to optimize pregnancy outcomes is an important finding of this study, as it highlights opportunities for increased availability and promoting consumption of imported, specialized nutrient‐dense foods, such as MQ‐LNS—whose presentation can facilitate effective product marketing towards these rural consumers who favour pre‐packaged foods.
The undervaluing of nutritious local food items available for maternal consumption in low‐income rural settings has been documented elsewhere. One study in southern India reported numerous locally available agricultural products whose nutritive properties were either overlooked or undervalued in pregnancy and the post‐partum period as a result of culturally bound food proscriptions (Varadarajan, 2010). Another study in Haiti attributed the underconsumption of widely accessible, nutrient‐rich produce items to “low social valorization” despite the affordability of these foods (Heidkamp et al., 2015). In this study setting, specific local foods may have been undervalued, for example, leafy green vegetables, which were frequently described as readily available and staples of the local diet but overlooked in nutritional value. Even so, local foods were relatively undervalued in comparison with more expensive, inaccessible food items with fewer nutritive properties, such as spaghetti and bread.
For the majority of participants, modifying diets during pregnancy in order to accommodate food prescriptions was challenged by financial, familial, and supply‐side barriers operating at various socioecological levels, as summarized in Figure 2. Limited financial capital to purchase more expensive items, coupled with gendered food‐purchasing patterns in the household, were primary access‐related barriers to incorporating particular foods into women's diets during pregnancy. Women described specific symptoms associated with morning sickness as constraints to a healthy prenatal diet, but these symptoms typically resulted in aversions to food overall, rather than to specific food items—as documented in other studies (Young & Pike, 2012). The financial inaccessibility of nutritious foods during pregnancy and gender norms related to household food procurement have been reported extensively in low‐ and middle‐income countries (Christian et al., 2006; Girard, Dzingina, Akogun, Mason, & McFarland, 2012; Hartini, Padmawati, Lindholm, Surjono, & Winkvist, 2005; Huybregts, Roberfroid, Kolsteren, & Van Camp, 2009; Levay, Mumtaz, Rashid, & Willows, 2013; Scorgie et al., 2015). However, documenting the impact of these social and market forces on nutritional and pregnancy outcomes has been less of a priority for research conducted in Niger, where studies measuring maternal micronutrient deficiency burdens and other pregnancy‐related outcomes dominate the current research agenda (Brindle et al., 2017; Kay, Idrissa, & Hampton, 2014; Seim et al., 2014).
These findings contribute to the growing body of literature exploring determinants of maternal food consumption practices and preferences in low‐income countries. Previous research has implicated food proscriptions in undermining the nutritive values of locally available foods for consumption during pregnancy (Bouchier, 1984; Demissie & Kogi‐Makau, 2017; Ojofeitimi et al., 1982; Ojofeitimi & Tanimowo, 1980; Varadarajan, 2010). Other studies have alternatively documented food aversions as chief drivers of food intake during pregnancy (Christian et al., 2006; Harding et al., 2017; Hutter, 1996). In this study, physiology (e.g., morning sickness) contributed to self‐reported changes in food consumption patterns among pregnant women; however, social and structural factors, including gendered food‐purchasing patterns and household financial restrictions, weighed most heavily in participant discourses on constraints to optimal maternal diets.
4.1. Limitations
There are limitations of this study. First, semistructured interview and focus group discussion guides covered a range of themes hypothesized to influence prenatal supplementation utilization and adherence, as well as maternal dietary practices and health care‐seeking behaviours. This enabled interviewers and focus group facilitators to collect large quantities of data but restricted opportunities for extensive probing on all specific topics presented in this analysis. In‐depth interviews, as part of an ethnographic approach, may have yielded richer narratives to understand the cultural constructions of diet during pregnancy. Second, although data collection guides were revised prior to Phase 2 data collection to address emergent themes from Phase 1, there were limited opportunities for study staff to adapt these guides during each data collection phase to explore these emerging phenomena and categories more in‐depth. One advantage to this approach, however, is that it enabled data collectors to cover numerous topics across a large study sample. Third, because data collectors were recognized by participants as employees of the organization providing health services, participants may have refrained from disclosing certain practices, attitudes, and preferences if inconsistent with study messages. Social desirability bias remains a chief concern in qualitative research (Bradley, 1993; Sturges & Hanrahan, 2004); providing adequate interview training to data collectors in detecting participant acquiescence, as well as building response probes into data collection guides, aided study staff in recognizing and appropriately responding to this issue.
5. CONCLUSIONS
Longitudinal qualitative research designs, such as the one employed in this study, can be useful to document evolving participant perspectives, attitudes, and perceptions during a prolonged health event such as pregnancy. A growing number of qualitative studies are employing this design to assess fluctuating experiences and perspectives throughout pregnancy and post‐partum (Garner, McKenzie, Devine, Thornburg, & Rasmussen, 2017; Goldade et al., 2008; Jardine, McLellan, & Dombrowski, 2016; Pletsch & Thornton Kratz, 2004). This study highlights the potential advantages of longitudinal qualitative research to capture meaningful progressions of perspectives influencing the nutritional status of women during pregnancy.
Identifying physiological and sociocontextual barriers to adequate dietary intake during pregnancy should be a priority for programme implementers and policymakers seeking to close gaps in maternal morbidity, micronutrient deficiencies during pregnancy, and child undernutrition. Future research in Niger would benefit from comparing maternal dietary practices and illness coping strategies between primigravida and multigravida. In a food‐insecure setting such as south‐central Niger, improvements in the nutritional status of pregnant women can be facilitated with increased availability and access to foods that are affordable, beneficial, and acceptable to women at all stages of their pregnancy. Efforts to increase the availability of imported fortified nutritious foods with high consumer acceptability (Young et al., 2015), coupled with increased access to these items in village marketplaces through targeted interventions such as conditional cash transfers, could improve maternal nutrition among food‐insecure, rural Nigerien women. Efforts to integrate salient terms and discourses identified in this study into programming strategies, for example, culturally appropriate social and behaviour change communications promoting increased consumption of leafy green vegetables and other local nutrient‐rich produce during pregnancy, could help amplify gains in maternal nutrition achieved by the previously recommended market‐level interventions (Lamstein et al., 2014). This study's identification of perceptions of and barriers to ideal pregnancy diets can be incorporated into social and behavioural communication strategies accompanying interventions for improving maternal diets and pregnancy outcomes.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
SI, SK, and RG led study design. AMS and AS supported the field data collection. JGR and AC conducted data analysis. JGR wrote the first draft of the manuscript. All authors read and approved the final manuscript.
ACKNOWLEDGMENTS
The authors would like to acknowledge Canice Ahearn and Meagan Harrison for coding of interview and focus group transcripts as well as the Epicentre Maradi team members for overseeing on‐site data collection activities and general study implementation activities.
Rosen JG, Clermont A, Kodish SR, et al. Determinants of dietary practices during pregnancy: A longitudinal qualitative study in Niger. Matern Child Nutr. 2018;14:e12629 10.1111/mcn.12629
REFERENCES
- Bouchier, V. A. (1984). Maternity care in the Sudd, southern Sudan. Tropical Doctor, 14, 32–33. [DOI] [PubMed] [Google Scholar]
- Bradley, J. (1993). Methodological issues and practices in qualitative research. The Library Quarterly, 63, 431–449. [Google Scholar]
- Brindle, E. , Lillis, L. , Barney, R. , Hess, S. Y. , Wessells, K. R. , Ouédraogo, C. T. , … Lyman, C. (2017). Simultaneous assessment of iodine, iron, vitamin A, malarial antigenemia, and inflammation status biomarkers via a multiplex immunoassay method on a population of pregnant women from Niger. PLoS One, 12, e0185868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmaz, K. (2006). Constructing grounded theory: A practical guide through qualitative analysis. London; Thousand Oaks, CA: Sage Publications. [Google Scholar]
- Christian, P. (2010). Micronutrients, birth weight, and survival. Annual Review of Nutrition, 30, 83–104. [DOI] [PubMed] [Google Scholar]
- Christian, P. , Bunjun Srihari, S. , Thorne‐Lyman, A. , Khatry, S. K. , LeClerq, S. C. , & Ram Shrestha, S. (2006). Eating down in pregnancy: Exploring food‐related beliefs and practices of pregnancy in rural Nepal. Ecology of Food and Nutrition, 45, 253–278. [Google Scholar]
- Creswell, J. W. , & Miller, D. L. (2000). Determining validity in qualitative inquiry. Theory Into Practice, 39, 124–130. [Google Scholar]
- Dalmiya, N. , Darnton‐Hill, I. , Schultink, W. , & Shrimpton, R. (2009). Multiple micronutrient supplementation during pregnancy: A decade of collaboration in action. Food and Nutrition Bulletin, 30, S477–S479. [DOI] [PubMed] [Google Scholar]
- Demissie, T. , & Kogi‐Makau, W. (2017). Food taboos among pregnant women in Hadiya Zone, Ethiopia. The Ethiopian Journal of Health Development, 12, 45–49. [Google Scholar]
- Garner, C. D. , McKenzie, S. A. , Devine, C. M. , Thornburg, L. L. , & Rasmussen, K. M. (2017). Obese women experience multiple challenges with breastfeeding that are either unique or exacerbated by their obesity: Discoveries from a longitudinal, qualitative study. Maternal & Child Nutrition, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard, A. W. , Dzingina, C. , Akogun, O. , Mason, J. B. , & McFarland, D. A. (2012). Public health interventions, barriers, and opportunities for improving maternal nutrition in Northeast Nigeria. Food and Nutrition Bulletin, 33, S51–S70. [DOI] [PubMed] [Google Scholar]
- Gittelsohn, J. , Thapa, M. , & Landman, L. T. (1997). Cultural factors, caloric intake and micronutrient sufficiency in rural Nepali households. Social Science & Medicine, 44, 1739–1749. [DOI] [PubMed] [Google Scholar]
- Goldade, K. , Nichter, M. , Nichter, M. , Adrian, S. , Tesler, L. , & Muramoto, M. (2008). Breastfeeding and smoking among low‐income women: Results of a longitudinal qualitative study. Birth, 35, 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding, K. L. , Matias, S. L. , Mridha, M. K. , Vosti, S. A. , Hussain, S. , Dewey, K. G. , & Stewart, C. P. (2017). Eating down or simply eating less? The diet and health implications of these practices during pregnancy and postpartum in rural Bangladesh. Public Health Nutrition, 20, 1928–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartini, T. N. S. , Padmawati, R. S. , Lindholm, L. , Surjono, A. , & Winkvist, A. (2005). The importance of eating rice: Changing food habits among pregnant Indonesian women during the economic crisis. Social Science & Medicine, 61, 199–210. [DOI] [PubMed] [Google Scholar]
- Heidkamp, R. A. , Ayoya, M. A. , Teta, I. N. , Stoltzfus, R. J. , & Marhone, J. P. (2015). Complementary feeding practices and child growth outcomes in Haiti: An analysis of data from Demographic and Health Surveys. Maternal & Child Nutrition, 11, 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutter, I. (1996). Reduction of food intake during pregnancy in rural South India. Tropical Medicine & International Health, 1, 399–405. [DOI] [PubMed] [Google Scholar]
- Huybregts, L. F. , Roberfroid, D. A. , Kolsteren, P. W. , & Van Camp, J. H. (2009). Dietary behaviour, food and nutrient intake of pregnant women in a rural community in Burkina Faso. Maternal & Child Nutrition, 5, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICF International, Institut National de la Statistique (INS) . (2013). Enquête démographique et de santé et à indicateurs multiples du Niger 2012. Retrieved from: http://dhsprogram.com/pubs/pdf/FR277/FR277.pdf
- Imdad, A. , & Bhutta, Z. A. (2012). Maternal nutrition and birth outcomes: Effect of balanced protein‐energy supplementation. Paediatric and Perinatal Epidemiology, 26, 178–190. [DOI] [PubMed] [Google Scholar]
- Isanaka, S. , Guindo, O. , Langendorf, C. , Matar Seck, A. , Plikaytis, B. D. , Sayinzoga‐Makombe, N. , … Jochum, B. (2017). Efficacy of a low‐cost, heat‐stable oral rotavirus vaccine in Niger. New England Journal of Medicine, 376, 1121–1130. [DOI] [PubMed] [Google Scholar]
- Jardine, E. E. , McLellan, J. , & Dombrowski, S. U. (2016). Is being resolute better than being pragmatic when it comes to breastfeeding? Longitudinal qualitative study investigating experiences of women intending to breastfeed using the Theoretical Domains Framework. Journal of Public Health (Oxf.), 1–7. [DOI] [PubMed] [Google Scholar]
- Kay, A. , Idrissa, A. , & Hampton, B. S. (2014). Epidemiologic profile of women presenting to the National Hospital of Niamey, Niger for vaginal fistula repair. International Journal of Gynecology & Obstetrics, 126, 136–139. [DOI] [PubMed] [Google Scholar]
- Lamstein, S. , Stillman, T. , Koniz‐Booher, P. , Aakesson, A. , Collaiezzi, B. , Williams, T. , Beall, K. , & Anson, M. (2014). Evidence of effective approaches to social and behavior change communication for preventing and reducing stunting and anemia: Report from a systematic literature review. Arlington, VA: USAID/ Strengthening Partnerships, Results, and Innovations in Nutrition Globally (SPRING) Project. Retrieved from: https://www.spring-nutrition.org/sites/default/files/publications/series/spring_sbcc_lit_review.pdf
- Lennox, J. , Petrucka, P. , & Bassendowski, S. (2017). Eating practices during pregnancy: Perceptions of select Maasai women in Northern Tanzania. Global Health Research and Policy, 2, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levay, A. V. , Mumtaz, Z. , Rashid, S. F. , & Willows, N. (2013). Influence of gender roles and rising food prices on poor, pregnant women's eating and food provisioning practices in Dhaka, Bangladesh. Reproductive Health, 10, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduforo, A. N. (2010). Superstitions and nutrition among pregnant women in Nwangele local government area of Imo state, Nigeria. Journal of Research in National Development, 8, 1–7. [Google Scholar]
- Maimbolwa, M. C. , Yamba, B. , Diwan, V. , & Ransjö‐Arvidson, A. B. (2003). Cultural childbirth practices and beliefs in Zambia. Journal of Advanced Nursing, 43, 263–274. [DOI] [PubMed] [Google Scholar]
- Marquez, L. , Holschneider, S. , Broughton, E. , & Hiltebeitel, S. (2014). Improving health care: The results and legacy of the USAID Health Care Improvement Project. Final report. Retrieved from: https://www.usaidassist.org/sites/assist/files/improving_health_care_resultslegacy_hci_sept2014_0.pdf
- Maternal Mortality Estimation Inter‐Agency Group, United Nations Population Division . (2016). Maternal mortality in 1990–2015, Niger. Retrieved from: http://www.who.int/gho/maternal_health/countries/ner.pdf
- Morse, J. M. , Barrett, M. , Mayan, M. , Olson, K. , & Spiers, J. (2002). Verification strategies for establishing reliability and validity in qualitative research. International Journal of Qualitative Methods, 1, 13–22. [Google Scholar]
- Mothupi, M. C. (2014). Use of herbal medicine during pregnancy among women with access to public healthcare in Nairobi, Kenya: A cross‐sectional survey. BMC Complementary and Alternative Medicine, 14, 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichter, M. , & Nichter, M. (1983). The ethnophysiology and folk dietetics of pregnancy: A case study from South India. Human Organization, 42, 235–246. [DOI] [PubMed] [Google Scholar]
- Ogbeide, O. (1974). Nutritional hazards of food taboos and preferences in Mid‐West Nigeria. The American Journal of Clinical Nutrition, 27, 213–216. [DOI] [PubMed] [Google Scholar]
- Ojofeitimi, E. O. , Elegbe, I. , & Babafemi, J. (1982). Diet restriction by pregnant women in Nigeria. International Journal of Gynecology & Obstetrics, 20, 99–103. [DOI] [PubMed] [Google Scholar]
- Ojofeitimi, E. O. , & Tanimowo, C. M. (1980). Nutritional beliefs among pregnant Nigerian women. International Journal of Gynecology & Obstetrics, 18, 66–69. [DOI] [PubMed] [Google Scholar]
- Onyesom, I. , Onyesom, C. , Ofili, M. I. , Anyanwu, B. E. , & Uzuegbu, U. (2008). Effect of cultural beliefs and forbidden foods on the ABCD parameters of nutrition among some children in Nigeria. Middle‐East Journal of Scientific Research, 3, 53–56. [Google Scholar]
- Ota, E. , Hori, H. , Mori, R. , Tobe‐Gai, R. , & Farrar, D. (2015). Antenatal dietary education and supplementation to increase energy and protein intake. Cochrane Database of Systematic Reviews, 6, CD0000032. [DOI] [PubMed] [Google Scholar]
- Pletsch, P. , & Thornton Kratz, A. (2004). Why do women stop smoking during pregnancy? Cigarettes taste and smell bad. Health Care for Women International, 25, 671–679. [DOI] [PubMed] [Google Scholar]
- Requejo, J. , Victoria, C. , & Bryce, J. (2015). A decade of tracking progress for maternal, newborn and child survival: The 2015 report. 2015. Retrieved from: http://countdown2030.org/documents/2015Report/CDReport_2015_profiles_N-Z.pdf [DOI] [PMC free article] [PubMed]
- Riang'a, R. M. , Broerse, J. , & Nangulu, A. K. (2017). Food beliefs and practices among the Kalenjin pregnant women in rural Uasin Gishu County, Kenya. Journal of Ethnobiology and Ethnomedicine, 13, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorgie, F. , Blaauw, D. , Dooms, T. , Coovadia, A. , Black, V. , & Chersich, M. (2015). “I get hungry all the time”: Experiences of poverty and pregnancy in an urban healthcare setting in South Africa. Globalization and Health, 11, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seim, A. R. , Alassoum, Z. , Bronzan, R. N. , Mainassara, A. A. , Jacobsen, J. L. , & Gali, Y. A. (2014). Pilot community‐mobilization program reduces maternal and perinatal mortality and prevents obstetric fistula in Niger. International Journal of Gynecology & Obstetrics, 127, 269–274. [DOI] [PubMed] [Google Scholar]
- Sturges, J. E. , & Hanrahan, K. J. (2004). Comparing telephone and face‐to‐face qualitative interviewing: A research note. Qualitative Research, 4, 107–118. [Google Scholar]
- Suchdev, P. S. , Peña‐Rosas, J. P. , & De‐Regil, L. M. (2014). Multiple micronutrient powders for home (point‐of‐use) fortification of foods in pregnant women. Cochrane Database of Systematic Reviews, 6, CD011158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugwa, E. A. (2016). Nutritional practices and taboos among pregnant women attending antenatal care at general hospital in Kano, Northwest Nigeria. Annals of Medical and Health Sciences Research, 6, 109–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Children's Fund . (2016). The state of the world's children, 2016. Retrieved from: https://www.unicef.org/publications/files/UNICEF_SOWC_2016.pdf
- United Nations Population Fund . (2016). World population dashboard: Total fertility rate, per woman, 2015–2020—Niger. Retrieved from: http://www.unfpa.org/world-population-dashboard
- Varadarajan, A. (2010). Food practices and food taboos during pregnancy and lactation among tribals of Andhra Pradesh. International Research Journal Social Sciences, 3, 147–155. [Google Scholar]
- World Health Organization . (2015). Global anemia prevalence and trends, 1995–2011. Retrieved from: http://apps.who.int/iris/bitstream/10665/177094/1/9789241564960_eng.pdf
- World Health Organization . (2016). WHO recommendations on antenatal care for a positive pregnancy experience. 2016. Retrieved from: http://apps.who.int/iris/bitstream/10665/250796/1/9789241549912-eng.pdf?ua=1 [PubMed]
- Wulandari, L. P. L. , & Whelan, A. K. (2011). Beliefs, attitudes and behaviours of pregnant women in Bali. Midwifery, 27, 867–871. [DOI] [PubMed] [Google Scholar]
- Yin, R. K. (2016). Qualitative research from start to finish (2nd ed.). New York, NY: The Guilford Press. [Google Scholar]
- Young, A. G. , & Pike, I. L. (2012). A biocultural framework for examining maternal cravings and aversions among pastoral women in East Africa. Ecology of Food and Nutrition, 51, 444–462. [DOI] [PubMed] [Google Scholar]
- Young, S. , Natamba, B. , Luwedde, F. , Nyafwono, D. , Okia, B. , Osterbauer, B. , … Robine, M. (2015). “I have remained strong because of that food”: Acceptability and use of lipid‐based nutrient supplements among pregnant HIV‐infected Ugandan women receiving combination antiretroviral therapy. AIDS and Behavior, 19, 1535–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]


