Abstract
Normothermic machine perfusion (NMP) is a technique that utilizes extracorporeal membrane oxygenation to recondition and repair kidneys at near body temperature prior to transplantation. The application of this new technology has been fueled by a significant increase in the use of the kidneys that were donated after cardiac death, which are more susceptible to ischemic injury. Preliminary results indicate that NMP itself may be able to repair marginal organs prior to transplantation. In addition, NMP serves as a platform for delivery of therapeutics. The isolated setting of NMP obviates problems of targeting a particular therapy to an intended organ and has the potential to reduce the harmful effects of systemic drug delivery. There are a number of emerging therapies that have shown promise in this platform. Nutrients, therapeutic gases, mesenchymal stromal cells, gene therapies, and nanoparticles, a newly explored modality, have been successfully delivered during NMP. These technologies may be effective at blocking multiple mechanisms of ischemia‐ reperfusion injury (IRI) and improving renal transplant outcomes. This review addresses the mechanisms of renal IRI, examines the potential for NMP as a platform for pretransplant allograft modulation, and discusses the introduction of various therapies in this setting.
Keywords: drug interaction, Ischemia‐reperfusion injury (IRI), kidney transplantation/nephrology, organ perfusion and preservation, translational research/science
Short abstract
Normothermic machine perfusion of kidneys, an upcoming method for reconditioning and repair, has the potential to serve as a platform for pretransplant allograft modulation in which various therapies can be introduced to alleviate ischemia‐reperfusion injury.
Abbreviations
- AKI
acute kidney injury
- DCD
donation after cardiac death
- DGF
delayed graft function
- ECD
extended criteria donor
- ICAM‐1
intracellular adhesion molecule‐1
- IRI
ischemia‐reperfusion injury
- JAK/STAT
Janus kinase/signal transducer and activator of transcription
- MPS
mononuclear phagocyte system
- NF‐κB
nuclear factor kappa B
- NMP
normothermic machine perfusion
- NPs
nanoparticles
- PLA‐PEG
poly(lactic acid)‐poly(ethylene) glycol
- Pmp
per million population
- RCT
randomized control trial
- ROS
reactive oxygen species
- TNF
tumor necrosis factor
1. INTRODUCTION
With a growing incidence of kidney transplants using marginal kidneys, susceptibility to ischemia‐reperfusion injury (IRI) is becoming an increasingly prominent problem.1 Normothermic machine perfusion (NMP) is a new method introduced to recondition and assess function in kidneys prior to transplantation.2 Preliminary results have suggested that NMP can reduce delayed graft function, perhaps by mitigating the severity of IRI.3 In addition, NMP is emerging as a platform for the delivery of therapies aimed at reducing the negative effects of ischemic injury. Although attempts to achieve anatomic specificity of systemically administered drugs have been largely unsuccessful in humans, NMP circumvents any systemic side effects via direct delivery to an isolated kidney.4, 5, 6 A number of therapeutic strategies have been tested in this system, mostly addressing singular mechanisms of IRI. Nanoparticles (NPs), with their small size and large surface area for modification, are a particularly advantageous vehicle for drug encapsulation and delivery. Nanoparticle‐targeted therapies can address multiple mechanisms of IRI. In a range of experimental studies, nanoparticles have been used to target the endothelium and tubular epithelial cells.7 More recently, nanoparticle targeting to vascular endothelium has been successfully demonstrated in human kidneys during NMP.8 In this review, we discuss the role of a variety of therapies, ending with nanoparticles, in an isolated, pretransplant setting to prevent complications of IRI.
2. STATE OF RENAL TRANSPLANTATION
The shortage of suitable donor organs remains one of the leading issues facing renal transplantation today. In an attempt to close this growing gap, the demographics of accepted kidney donations have changed over the past 15 years. Kidneys from extended criteria donors (ECDs) are being considered more frequently. ECDs are estimated to last an average of 5.1 years as compared to standard‐criteria donor kidneys, which are projected to function on average for more than 10 years.9 In addition to ECDs, there has been a significant increase in the use of donation after cardiac death (DCD) kidneys. However, there is some hesitation in using marginal DCD kidneys due to exposure to conditions of warm ischemia prior to procurement. Prolonged warm ischemia has been associated with worsened long‐term outcomes in DCD kidney transplants.10 These kidneys are thought to be markedly more susceptible to the deleterious effects of cold storage and as a result typically have higher rates of delayed graft function (DGF).11 Due to this concern, around 13% of DCD kidneys retrieved are discarded, even though some of these organs may have yielded successful long‐term results.12 There remains a need for new pretransplant therapies to address the increased susceptibility associated with ECD and DCD kidneys. Optimizing the use of these marginal organs will require developing comprehensive IRI therapies.
3. ISCHEMIA‐REPERFUSION INJURY
Clinically, IRI is defined as a sudden period of disrupted blood flow followed by the restoration of an oxygenated blood supply (Figure 1).1 Histologically, IRI results in tubular necrosis, endothelial and epithelial damage, widespread inflammation, loss of structure, and cyst formation.13 An ischemic organ will also typically have reduced metabolic function and microvascular damage. IRI is thought to be a factor in the development of acute kidney injury (AKI) and DGF.14, 15 The severity of IRI can worsen depending on cytokine release at the time of death of the donor and through issues in general management of the organ resulting from the complex logistical nature of transplantation.16
Figure 1.
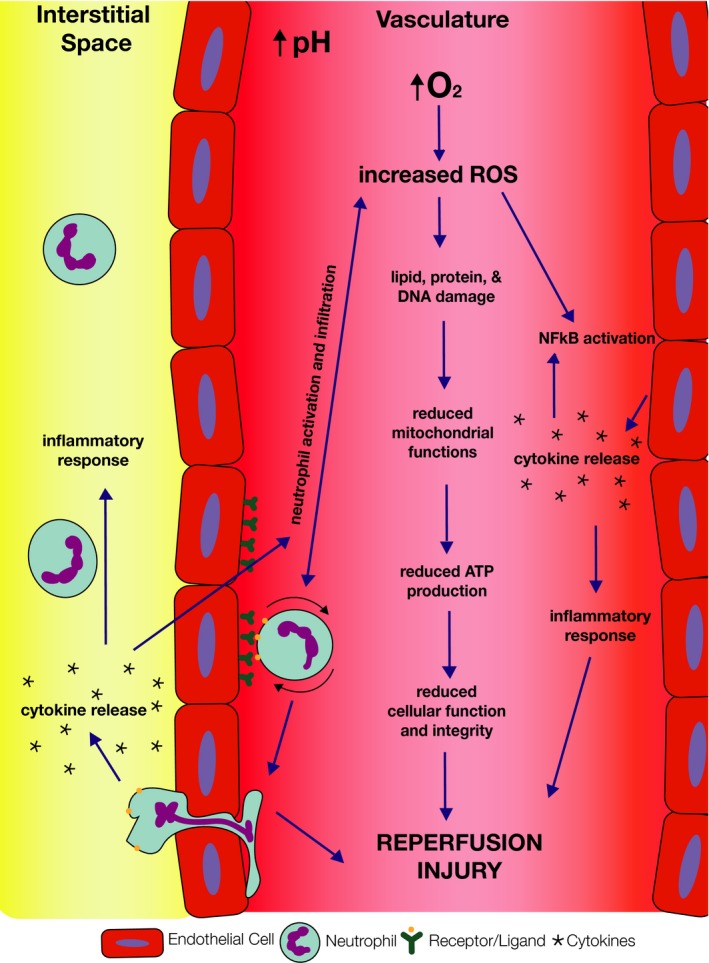
Mechanisms of ischemia‐reperfusion injury. Although the mechanisms of IRI are not entirely understood, there are some main components of the injury that are well established
It is imperative to understand the components behind IRI to develop an appropriate treatment. The period following transplantation, when conditions are being restored to normal, results in a dynamic process of neutrophil activation, increased production of cytokines, expression of adhesion molecules, and release of reactive oxygen species (ROS), resulting in inflammation and cellular necrosis (Figure 1).17, 18 Although the physiology of this process is not entirely understood, reperfusion is known to exacerbate any damage acquired during the period of ischemia. Given that the pathophysiology of IRI is multifactorial, it is likely that effective therapeutic treatments will need to be capable of addressing the various aspects of vascular biology and immune responses involved.
However, both rodent and human studies to date have typically focused on treating singular mechanisms of the injury. As the inflammatory signaling pathways associated with IRI have become clearer, studies have been conducted in rodent models to investigate blocking singular events in these pathways. The Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway plays a critical role in cytokine production mediating IRI.19 Dexmedetomidine, an α2‐adrenoreceptor agonist, inhibits the phosphorylation of JAK/STAT proteins, and has proved to be an effective agent in slowing the progression of renal IRI in rodents.20 Upstream of the JAK/STAT pathway lies nuclear factor kappa B (NF‐κB), a transcription factor that has also been shown to regulate the inflammatory cascade in IRI. Rodent studies have demonstrated that the inhibition of NF‐κB correlates with the extent of tubular injury, apoptosis, and inflammation within the model.21 Alternatively, instead of blocking the inflammatory cascade one could administer anticellular adhesion molecules to compete with and inhibit binding between neutrophils and endothelium.22 Although progress has been made in these model systems, clinical translation remains a challenge.
To date, only a handful of clinical trials have started to investigate methods of ameliorating IRI (NCT00298168, NCT01442337, and NCT00802347). However, these clinical trials did not replicate the same successful outcomes previously demonstrated in rodent models. All the studies in question have faced similar challenges associated with systemic drug delivery and have been discontinued.23, 24, 25, 26 Traditionally, treatments for IRI (as well as for most other renal diseases) are delivered systemically following transplantation.27, 28 The primary challenge of the systemic delivery stem is largely driven by the mononuclear phagocyte system (MPS). The MPS consists primarily of monocytes and macrophages present in the liver, spleen, and lymph nodes. This system works to remove foreign bodies, which can include therapeutics, from the circulation, and this often limits the effectiveness of systemically administered drugs.23 Thus, there remains a significant need for highly specific renal therapies that can overcome the challenges of systemic delivery and adequately alleviate IRI.
4. REPAIR DURING NORMOTHERMIC MACHINE PERFUSION
Normothermic machine perfusion is a technique that utilizes extracorporeal membrane oxygenation to recondition and repair kidneys prior to transplantation. Leading centers in the UK, Canada, The Netherlands, and the United States have all begun to move NMP technology forward8, 24, 25, 26 (Table 1). Normothermic perfusion technologies have begun to make their way into the clinic in an attempt to recondition marginal organs and reverse aspects of IRI. Preliminary results in a small series of kidneys from older deceased donors in the UK suggested that NMP may reduce DGF rates.3 This has led to the establishment of a UK multicenter randomized control trial (RCT) of NMP versus cold storage in DCD kidneys (ISRCTN15821205).24 It is possible that NMP can improve some of the deleterious effects of IRI on its own, perhaps by a pre‐conditioning mechanism in the setting of a protective, closely monitored environment. In addition to these benefits, NMP serves as a platform to deliver potential treatments to further IRI treatments.
Table 1.
State of experimental NMP studies
| Year | Author | Model | Methods | WI time (m) | Outcome |
|---|---|---|---|---|---|
| 1980 | Van der Wijk et al; Groningen, The Netherlands | Canine heterotopic autotransplantation | DBD kidneys subjected to HMP for 72 h, then NMP (in situ) for 1‐4 h and HMP for 72 h more | N/A | Kidneys can be preserved for up to 144 h using intermediate NMP |
| 1984 | Rijkmans et al; Groningen, The Netherlands | Canine heterotopic autotransplantation | DBD kidneys preserved for 6 d HMP, interrupted at 3 d for 3 h of NMP | N/A | Intermittent, brief NMP yielded superior results to HMP alone |
| 1989 | Maessen et al; Groningen, The Netherlands | Canine heterotopic autotransplantation | DCD or DBD kidneys with 24 or 48 h of CS w/or w/o 30 m WIT with intermediate NMP | 30 | Intermittent, brief NMP yielded superior results to that of CS alone |
| 2000 | Brasile et al; Maastricht, The Netherlands | Canine heterotopic autotransplantation | DBD kidneys either reimplanted immediately, on HMP for 18 h, or transitioned to 18 h NMP before implantation | 120 | 18 h of NMP is feasible and results in immediate function following transplant |
| 2002 | Brasile et al; Maastricht, The Netherlands | Canine kidneys | 4 h of NMP accompanied with GFP transfection | N/A | Transfections during NMP were successful and supported de novo synthesis of GFP |
| 2003 | Brasile et al; Maastricht, The Netherlands | Canine kidneys | HBD or DCD kidneys on HMP for 4‐24 h followed by 0 or 6 h of NMP | 30 | HO‐1 activity can be induced during NMP |
| 2007 | Kay et al.; Leicester, UK | Porcine kidneys | Kidneys flushed at with warm AQIXRS‐I and given either CS or NMP | 5‐10 | Renal viability was maintained following 6 h of NMP |
| 2008 | Bagul et al; Leicester, UK | Porcine kidneys | 2 h CS, 18 h CS, 18 h HMP, or 16 h CS + 2 h NMP followed by 3 h reperfusion | 10 | NMP was able to restore depleted ATP levels and reverse some of the effects of CS |
| 2008 | Bagul et al; Leicester, UK | Porcine kidneys | 18 h CS followed by 3 h NMP with carbon monoxide compared to control | 10 | Carbon monoxide, when delivered during NMP, may protect against IRI |
| 2009 | Hosgood & Nicholson; Leicester, UK | Porcine kidneys | 18 h CS followed by 3 h NMP with hydrogen sulfide compared to control | 25 | Hydrogen sulfide, when delivered during NMP, may protect against IRI |
| 2011 | Hosgood & Nicholson; Leicester, UK | Human transplant | 11 h CS followed by 35 m NMP immediately prior to transplant | 30 | NMP is a feasible method of preservation; first case conducted without compromising results of transplant kidney |
| 2013 | Nicholson & Hosgood; Leicester, UK | Human transplant | 18 kidneys given ~60 m of NMP compared to a Ctrl group of 47 recipients of ECD kidneys that underwent CS | Pt dependent | Significantly more predialysis pts and more receiving hemodialysis in NMP group compared to CS alone |
| 2013 | Hosgood et al.; Leicester, UK | Porcine kidneys | DCD kidneys treated with CS for 24 h or CS for 23 h followed by 1 h NMP | 10 | NMP kidneys had improved metabolic function and less tubular injury compared to CS kidneys |
| 2014 | Hosgood & Nicholson; Leicester, UK | Human transplant | 10.5 h of CS followed by 60 m NMP and then 5.3 h CS | 0 | Intermediate NMP is feasible and safe and may reduce effects of CI injury and extend length of preservation |
| 2015 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | 3 h of CS followed by 10 h NMP with leukocyte‐depleted blood | 0 | Prolonged NMP is feasible in DCD kidneys |
| 2015 | Hosgood et al.; Cambridge, UK | Human discard kidneys | 74 discard kidneys were given NMP for 60 m and assigned an NMP score | Pt dependent | A high percentage of viable kidneys are being discarded unnecessarily as assessed through NMP |
| 2015 | Hosgood et al.; Cambridge, UK | Human discard kidneys | 22 kidneys put on NMP for 60 m | Pt dependent | NMP restores function and enables a platform for quality assessment of the kidney |
| 2015 | Hosgood et al.; Cambridge, UK | Porcine kidneys | Kidneys with variable WIT, CS for 2 h followed by 3 h NMP | 15, 60, 90, or 120 | NMP allows for assessment and potential recover from Wl injury |
| 2016 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | HBD kidneys either stored for 8 h in cold HTK or preserved with 8 h of NMP | 0 | NMP is feasible and safe in good quality HBD kidney grafts |
| 2016 | Hosgood et al.; Cambridge, UK | Human transplant | 60 m of NMP following procurement from 35 y/o donor | 13 | NMP has the potential to rescue kidneys deemed “untransplantable,” reducing their discard rate |
| 2017 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | 8 h of CS followed by 0, 1, 8 or 16 h of NMP with leukocyte‐depleted blood | 30 | Prolonged NMP is optimal for reconditioning; no CS produces better functioning kidneys |
| 2017 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | Kidneys with variable WIT underwent NMP for 8 h | 0, 30, or 60 | Perfusion characteristics (eg, metabolism markers, functional parameters) correlate with injury |
| 2017 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | DCD kidneys were either stored for 8 h in cold HTK or preserved with 8 h of NMP | 30 | NMP improves renal graft function compared to CS |
| 2017 | Kaths et al.; Toronto, Canada | Porcine heterotopic autotransplantation | DCD kidneys given 16 h, 15 h, 8 h, or 0 h of CS with 0 h, 1 h, 8 h, or 16 h of NMP followed by 60 m reperfusion | 30 | NMP provides superior outcome in DCD kidney transplant when compared to CS |
| 2017 | Blum et al.; Cleveland, Ohio | Porcine kidneys | DCD kidneys with 5 h of CS followed by 8 h NMP | 45 | NMP provided comparable preservation of renal function as HMP and minimized AP and GGT release |
| 2017 | Adams et al.; Cambridge, UK | Porcine kidneys | 24 h of CS or 23 h of CS with by 1 h of NMP using leukocyte‐depleted blood prior to reperfusion | 10 | Tubular and renal functions were better preserved after 1 h NMP compared to warm perfusion or CS |
| 2017 | Hosgood & Nicholson; Cambridge, UK | Human discard kidneys | 56 discard kidneys given 60 m NMP, scored based on the macroscopic appearance, RBF, and UO | 13 | Urinary biomarkers (ie, NGAL), functional perfusion parameters, and EVKP score provides informative measure of kidney quality for transplant decisions |
| 2017 | Tietjen et al.; New Haven, Connecticut | Human discard kidneys | Targeted nanoparticles administered and circulating for up to 8 h of NMP | Pt dependent | Endothelial cells can be successfully targeted with nanoparticles during NMP |
A summary of experimental normothermic perfusion work was compiled following a comprehensive literature review using keyword searches in PubMed. Keywords used to identify relevant studies included normothermic machine perfusion, ex vivo normothermic perfusion, kidney perfusion, and renal perfusion. A methods review was then performed to identify all relevant studies. Experimental NMP work is highlighted in bold. ATP, adenosine triphosphate; CS, cold storage; DBD, donation after brain death; DCD, Donation After Cardiac Death; GFP, green fluorescent protein; GGT, Gamma‐Glutamyltransferase; HBD, heart beating donor; HTK, Histidine‐tryptophan‐ketoglutarate; NGAL, Neutrophil gelatinase‐associated lipocalin; UO, urine output; WIT, warm ischemia time.
NMP presents a unique opportunity to deliver therapies in isolation from the phagocytic system that rapidly eliminates systemically delivered drugs from circulation. Any treatments administered during NMP have direct contact with the organ vasculature and avoid most complications derived from systemic delivery. A handful of therapies, although not directly tested in human kidney NMP, have begun to emerge as promising treatments to protect renal grafts from complications of transplantation.
4.1. Perfusate composition
Although major deviations from the original perfusate used by Hosgood et al have yet to be implemented, optimizing perfusate conditions may be a critical step in ensuring properly reconditioned organs. Vasodilators, antiinflammatory and antihypertension drugs, insulin, glucose, and a nutrient solution are delivered during clinical cases of renal NMP to help establish strong renal blood flow and better prepare the kidney. Yet, there have been no studies examining the necessity or benefit of these additions to the perfusate. Other groups, mostly working with porcine kidneys, have used the acellular STEEN solution to help dilute the perfusate—a solution that was initially optimized for normothermic machine perfusion of the lung. Again, the benefit of this diluent has not been properly assessed in renal NMP. However, it may be impactful for a study to be conducted that systematically examines and optimizes perfusate composition, to maximize all therapeutic results from NMP.
4.2. Gases
As a baseline, 95% oxygen is delivered during NMP and rates of oxygen consumption are used to measure the health of the organ. Oxygen consumption is maximized at normothermic temperatures, making NMP an ideal system for reconditioning.25 Nevertheless, there are other gases that, when delivered at low doses, have shown some evidence of having protective effects in the kidney. Carbon monoxide, nitric oxide, and hydrogen sulfide have all been investigated as therapeutic agents to protect against IRI, promote vasodilation, and inhibit apoptosis. Both carbon monoxide and hydrogen sulfide have been shown to inhibit IRI throughout early functional outcome measures in porcine kidneys during NMP.26, 29 Because gases can be easily absorbed into the blood, they work as ideal therapeutics in this closed, circulating system. Although little work has been done in clinically relevant systems for renal transplant, expanding and translating this work to a human model may offer kidneys additional benefit and protection prior to transplantation.
4.3. Stem cell therapies
Mesenchymal stromal cells (MSCs) can adapt to their microenvironment and differentiate into several different lineages, all while locally releasing a range of modulatory cytokines. After encouraging results from animal studies, MSC administration has been adapted for preclinical studies, delivered via intravenous infusion after renal transplantation. Although there were no adverse effects noted from these studies, MSCs were shown to be trapped in lung microcapillaries and could not reach the kidneys.30 At this point no full work has been published on delivering MSCs during any form of NMP; however, it is hypothesized that MSCs will be more effective in this system as they are able to avoid the complications of intravenous delivery.
4.4. Gene therapies
The prospect of altering gene expression in isolated organs ex vivo opens the door for more effective, personalized therapies. In porcine models of ex vivo lung perfusion, adenoviral vector delivery has been demonstrated to be safe and effective. This therapy resulted in a decreased inflammatory response with better graft functionality after transplantation.31 In the area of renal research, Brasile et al previously demonstrated the ability to target an exogenous gene to the vascular endothelium of a kidney during NMP in a proof‐of‐concept experiment.32 To protect the graft from complications of transplantation, gene therapies can be tailored for HLA silencing or to block transcriptional mechanisms of IRI. Introducing other methods of genetic manipulation, for example, siRNA, into the perfusate may require the utilization of stable vehicles for delivery. Nanoparticles have properties that make them ideally suited to this role. In contrast to other theorized treatments, nanoparticles have long‐lasting effects and well‐established slow‐release profiles that extend beyond the period of normothermic perfusion. Recently and for the first time, endothelial targeted nanoparticles have been delivered in an NMP setting.8 With all of the benefits of this system, NMP is emerging as a device to deliver therapies within an opportunistic window in an attempt to reduce the injurious effects of ischemic injury.
Any drug that is typically delivered systemically can also be introduced during NMP for more concentrated, short‐term treatments. However, when treating IRI, a more prolonged, protective action is desired, as its effects persist long after the initial period of perfusion after transplantation. Each theorized therapy for this system has its own setbacks. What is needed is a modular system that can work across multiple mechanisms of IRI for an extended period.
5. NANOPARTICLE THERAPIES IN NMP
Polymeric nanoparticles (NPs) have a long history of safe use in humans as drug delivery vehicles for the treatment of a wide variety of diseases.33, 34, 35 One of the primary benefits of NPs is their modularity. The polymer type can be varied to enable encapsulation of various classes of therapeutics from small molecules to nucleic acid–based drugs.35, 36 In addition, NPs can be formulated to provide sustained therapeutic release with the rate of release controlled by polymer molecular weight. Finally, the polymer surface can also be modified in a variety of ways to suppress unwanted cellular interactions and/or enhance association with a target cell type. Despite all the potential of NP drug carriers, clinical translation remains a challenge, largely due to issues with controlling NP localization following systemic administration.
Following systemic delivery, most varieties of NPs are rapidly eliminated via phagocytes in the liver and spleen (via the aforementioned MPS).37 This results in a relatively low accumulation of NPs at the desired site of therapeutic delivery and creates the potential for off‐target toxicity.38 To achieve higher accumulation at sites of disease, NPs have more recently been manufactured to have “stealth” properties in order to evade the immune system and thereby extend circulation times.39 However, this method is not entirely effective and can still result in relatively short circulation half‐lives and high levels of off‐target accumulation. Another popular strategy utilized in an attempt to introduce more reliable anatomic specificity is “molecular targeting.” This approach employs conjugation of ligands to the NP surface, with the ligand selected on the basis of specificity for a receptor expressed on the target cell type of interest. Although this strategy seems logical, it is important to note that the forces that govern ligand‐receptor interactions (eg, hydrogen bonds, electrostatics) only operate over a distance of ~0.3‐0.5 nm.40 Thus targeting is not capable of equipping NPs with a capacity to “home” to sites of interest,5, 33, 34 but rather can only enhance the likelihood that an NP will be retained by a cell it happens to contact. Consequently, molecular targeting has not been a reliable approach for controlling the anatomic localization of systemically administered NPs.
Although enhanced retention through the conjugation of ligands may not always be effective in systemic delivery, it is in many ways perfectly suited for an isolated NMP setting.6, 33 In the context of an isolated organ, NPs can largely avoid the challenges associated with systemic delivery. An oxygenated plasma‐free red cell solution is used to perfuse the organ, which obviates any complications that may arise from opsonization by serum proteins or tissue‐resident phagocytes. Moreover, studies have shown that targeting will typically lead to internalization of NPs, which then provides an intracellular depot effect for effective drug delivery.41 Preliminary work with targeted, dye‐loaded poly(lactic acid)‐poly(ethylene) glycol (PLA‐PEG) nanoparticles has provided evidence that enhanced retention in specific vascular beds in kidneys is possible during NMP.8 Performing studies of this nature in discard human kidneys opens new possibilities in the development of therapeutics.
Polymeric NPs provide a venue for the encapsulation of siRNAs and hydrophobic drugs.35, 36 Meeting this criteria, small molecules like BAY 11‐7082, Parthenolide, and BOT‐64 have previously been shown to inhibit NF‐κB activity.42, 43 If one of these drugs was to be encapsulated in polymeric NPs and delivered to kidneys prior to transplantation, it could alleviate the detrimental effects of IRI after transplant.44 Targeted, drug‐loaded NPs if delivered prior to transplant, could block mechanisms leading to inflammatory cascades, thereby limiting damage from IRI (Figure 2). Testing the effects of these drugs during NMP helps us to mitigate any challenges that arise from cell culture or rodent models. Results from Tietjen et al highlighted that while there was an 80‐fold benefit of targeting observed in cell culture, this result translated to an average 2‐ to 4‐fold benefit in target specificity when applied to an NMP setting.8 This phenomenon has been observed in a plethora of studies investigating the efficacy of NPs as therapeutic agents and necessitates more therapeutically relevant models.
Figure 2.
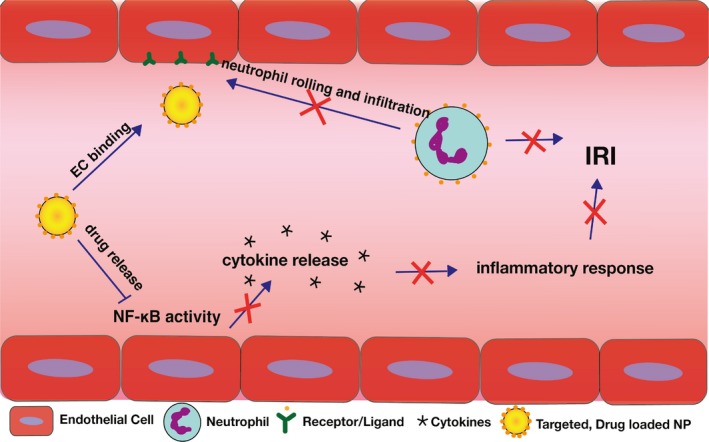
Using NPs as a therapeutic agent in IRI. The use of targeted, drug‐loaded NPs can serve as a therapy that blocks more than one mechanism of IRI (eg, neutrophil binding to endothelial cells and NF‐κB activity)
6. REMAINING CHALLENGES
Because NMP is a new technique in renal transplantation, optimal conditionings are still evolving. Current studies have focused on determining the ideal time for perfusion in addition to deciding about an optimal perfusate composition. Although Hosgood et al are currently running a clinical trial investigating the effects of 1 hour periods of NMP prior to transplantation, Kaths et al have shown in a porcine experimental model that prolonged periods of NMP can further improve the reconditioning effect.24, 45, 46 To properly assess the efficacy of a therapeutic treatment, like extended perfusion, more comprehensive diagnostic tools must come to fruition. Although the number of marginal kidney transplants has increased, there has been little progress in the development of a rigorous quality assessment for marginal kidneys. Currently, NMP also allows us to monitor to the organ and assess its quality. Outcome measures typically include renal blood flow, urine output, mean arterial pressure, oxygen consumption, creatinine clearance, ion concentrations, metabolic markers, and histologic analysis. A variety of biomarkers are assessed to determine the health of the organ, but a specific biomarker for IRI has yet to be identified. Most of these measures are providing clinicians with only superficial, regional information. It is likely that the quality of a perfusion is not uniform throughout the entire organ and that biopsies may only give insight into a certain section of the tissue. To properly gauge how well a kidney has reperfused, and how therapies are affecting the microenvironment, we must be able to look inside of the organ.
7. CONCLUSION
As the number of ECD and DCD kidney transplants rises, IRI is becoming an increasingly prominent issue. Current renal therapies face classic challenges of systemic delivery and there is a scarcity of clinical trials investigating the matter. NMP serves as an ideal platform for isolated treatments. Targeted, drug‐loaded NPs delivered during a period of NMP may be the future of IRI therapy in renal transplantation, providing long‐lasting, stable therapies. Before any significant advances can be made with therapeutic treatments, more comprehensive diagnostic tools for this setting must be realized.
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
ACKNOWLEDGMENTS
The research was funded by the National Institute for Health Research Blood and Transplant Research Unit (NIHR BTRU) in Organ Donation and Transplantation at the University of Cambridge in collaboration with Newcastle University and in partnership with NHS Blood and Transplant (NHSBT). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, the Department of Health, or NHSBT.
DiRito JR, Hosgood SA, Tietjen GT, Nicholson ML. The future of marginal kidney repair in the context of normothermic machine perfusion. Am J Transplant. 2018;18:2400–2408. 10.1111/ajt.14963
REFERENCES
- 1. Malek M, Nematbakhsh M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J Renal Inj Prev. 2015;4(2):20‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hosgood SA, van Heurn E, Nicholson ML. Normothermic machine perfusion of the kidney: better conditioning and repair? Transpl Int. 2015;28(6):657‐664. [DOI] [PubMed] [Google Scholar]
- 3. Hosgood SA, Patel M, Nicholson ML. The conditioning effect of ex vivo normothermic perfusion in an experimental kidney model. J Surg Res. 2013;182(1):153‐160. [DOI] [PubMed] [Google Scholar]
- 4. Cheng CJ, Tietjen GT, Saucier‐Sawyer JK, Saltzman WM. A holistic approach to targeting disease with polymeric nanoparticles. Nat Rev Drug Discov. 2015;14(4):239‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Von Hoff DD, Mita MM, Ramanathan RK, et al. Phase I study of PSMA‐targeted docetaxel‐containing nanoparticle BIND‐014 in patients with advanced solid tumors. Clin Cancer Res. 2016;22(13):3157‐3163. [DOI] [PubMed] [Google Scholar]
- 6. Zuckerman JE, Gritli I, Tolcher A, et al. Correlating animal and human phase Ia/Ib clinical data with CALAA‐01, a targeted, polymer‐based nanoparticle containing siRNA. Proc Natl Acad Sci U S A. 2014;111(31):11449‐11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khoshnejad M, Shuvaev VV, Pulsipher KW, et al. Vascular accessibility of endothelial targeted ferritin nanoparticles. Bioconjug Chem. 2016;27(3):628‐637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tietjen GT, Hosgood SA, Dirito J, et al. Nanoparticle targeting to the endothelium during normothermic machine perfusion of human kidneys. Sci Transl Med. 2017;9(418):eaam6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rao PS, Ojo A. The alphabet soup of kidney transplantation: SCD, DCD, ECD‐fundamentals for the practicing nephrologist. Clin J Am Soc Nephrol. 2009;4(11):1827‐1831. [DOI] [PubMed] [Google Scholar]
- 10. Tennankore KK, Kim SJ, Alwayn IP, Kiberd BA. Prolonged warm ischemia time is associated with graft failure and mortality after kidney transplantation. Kidney Int. 2016;89(3):648‐658. [DOI] [PubMed] [Google Scholar]
- 11. Chaumont M, Racape J, Broeders N, et al. Delayed graft function in kidney transplants: time evolution, role of acute rejection, risk factors, and impact on patient and graft outcome. J Transplant. 2015;2015:163757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Summers DM, Watson CJ, Pettigrew GJ, et al. Kidney donation after circulatory death (DCD): state of the art. Kidney Int. 2015;88(2):241‐249. [DOI] [PubMed] [Google Scholar]
- 13. Le Clef N, Verhulst A, D'Haese PC, Vervaet BA. Unilateral renal ischemia‐reperfusion as a robust model for acute to chronic kidney injury in mice. PLoS ONE. 2016;11(3):e0152153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2(2):1303‐1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ponticelli C. Ischaemia‐reperfusion injury: a major protagonist in kidney transplantation. Nephrol Dial Transplant. 2014;29(6):1134‐1140. [DOI] [PubMed] [Google Scholar]
- 16. Kosieradzki M, Rowinski W. Ischemia/reperfusion injury in kidney transplantation: mechanisms and prevention. Transplant Proc. 2008;40(10):3279‐3288. [DOI] [PubMed] [Google Scholar]
- 17. Kalogeris T, Baines CP, Krenz M, Korthuis RJ. Cell biology of ischemia/reperfusion injury. Int Rev Cell Mol Biol. 2012;298:229‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kezic A, Stajic N, Thaiss F. Innate immune response in kidney ischemia/reperfusion injury: potential target for therapy. J Immunol Res. 2017;2017:6305439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yang N, Luo M, Li R, et al. Blockage of JAK/STAT signalling attenuates renal ischaemia‐reperfusion injury in rat. Nephrol Dial Transplant. 2008;23(1):91‐100. [DOI] [PubMed] [Google Scholar]
- 20. Si Y, Bao H, Han L, et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the JAK/STAT signaling activation. J Transl Med. 2013;11:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xin YL, Li G, Liu H, Ai D. AS‐IV protects against kidney IRI through inhibition of NF‐κB activity and PUMA upregulation. Int J Clin Exp Med. 2015;8(10):18293‐18301. [PMC free article] [PubMed] [Google Scholar]
- 22. Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine‐adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P‐selectin ligand. J Clin Invest. 1997;99(11):2682‐2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18(1):49‐53. [DOI] [PubMed] [Google Scholar]
- 24. Hosgood SA, Saeb‐Parsy K, Wilson C, Callaghan C, Collett D, Nicholson ML. Protocol of a randomised controlled, open‐label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017;7(1):e012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Adams TD, Patel M, Hosgood SA, Nicholson ML. Lowering perfusate temperature from 37°C to 32°C diminishes function in a porcine model of ex vivo kidney perfusion. Transplant Direct. 2017;3(3):e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bagul A, Hosgood SA, Kaushik M, Nicholson ML. Carbon monoxide protects against ischemia‐reperfusion injury in an experimental model of controlled nonheartbeating donor kidney. Transplantation. 2008;85(4):576‐581. [DOI] [PubMed] [Google Scholar]
- 27. Bidwell GL 3rd, Mahdi F, Shao Q, et al. A kidney‐selective biopolymer for targeted drug delivery. Am J Physiol Renal Physiol. 2017;312(1):F54‐F64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deelman LE, Decleves AE, Rychak JJ, Sharma K. Targeted renal therapies through microbubbles and ultrasound. Adv Drug Deliv Rev. 2010;62(14):1369‐1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hunter JP, Hosgood SA, Patel M, Rose R, Read K, Nicholson ML. Effects of hydrogen sulphide in an experimental model of renal ischaemia‐reperfusion injury. Br J Surg. 2012;99(12):1665‐1671. [DOI] [PubMed] [Google Scholar]
- 30. Sierra‐Parraga JM, Eijken M, Hunter J, et al. Mesenchymal stromal cells as anti‐inflammatory and regenerative mediators for donor kidneys during normothermic machine perfusion. Stem Cells Dev. 2017;26(16):1162‐1170. [DOI] [PubMed] [Google Scholar]
- 31. Yeung JC, Wagnetz D, Cypel M, et al. Ex vivo adenoviral vector gene delivery results in decreased vector‐associated inflammation pre‐ and post‐lung transplantation in the pig. Mol Ther. 2012;20(6):1204‐1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Brasile L, Stubenitsky BM, Booster MH, Arenada D, Haisch C, Kootstra G. Transfection and transgene expression in a human kidney during ex vivo warm perfusion. Transplant Proc. 2002;34(7):2624. [DOI] [PubMed] [Google Scholar]
- 33. Hubbell JA, Langer R. Translating materials design to the clinic. Nat Mater. 2013;12(11):963‐966. [DOI] [PubMed] [Google Scholar]
- 34. Davis ME, Zuckerman JE, Choi CH, et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464(7291):1067‐1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cui J, Qin L, Zhang J, et al. Ex vivo pretreatment of human vessels with siRNA nanoparticles provides protein silencing in endothelial cells. Nat Commun. 2017;8(1):191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185‐198. [DOI] [PubMed] [Google Scholar]
- 37. Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505‐515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gaspar R. Nanoparticles: pushed off target with proteins. Nat Nanotechnol. 2013;8(2):79‐80. [DOI] [PubMed] [Google Scholar]
- 39. Amoozgar Z, Yeo Y. Recent advances in stealth coating of nanoparticle drug delivery systems. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2012;4(2):219‐233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu D, Tsai CJ, Nussinov R. Hydrogen bonds and salt bridges across protein‐protein interfaces. Protein Eng. 1997;10(9):999‐1012. [DOI] [PubMed] [Google Scholar]
- 41. Muzykantov VR. Targeted drug delivery to endothelial adhesion molecules. ISRN Vascular Medicine. 2013;2013:1‐27. [Google Scholar]
- 42. Kis A, Yellon DM, Baxter GF. Role of nuclear factor‐kappa B activation in acute ischaemia‐reperfusion injury in myocardium. Br J Pharmacol. 2003;138(5):894‐900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rauert‐Wunderlich H, Siegmund D, Maier E, et al. The IKK inhibitor Bay 11‐7082 induces cell death independent from inhibition of activation of NFkappaB transcription factors. PLoS ONE. 2013;8(3):e59292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kim YS, Kim JS, Kwon JS, et al. BAY 11‐7082, a nuclear factor‐kappaB inhibitor, reduces inflammation and apoptosis in a rat cardiac ischemia‐reperfusion injury model. Int Heart J. 2010;51(5):348‐353. [DOI] [PubMed] [Google Scholar]
- 45. Kaths JM, Cen JY, Chun YM, et al. Continuous normothermic ex vivo kidney perfusion is superior to brief normothermic perfusion following static cold storage in donation after circulatory death pig kidney transplantation. Am J Transplant. 2017;17(4):957‐969. [DOI] [PubMed] [Google Scholar]
- 46. Kaths JM, Echeverri J, Linares I, et al. Normothermic ex vivo kidney perfusion following static cold storage‐brief, intermediate, or prolonged perfusion for optimal renal graft reconditioning? Am J Transplant. 2017;17(10):2580‐2590. [DOI] [PubMed] [Google Scholar]


