Abstract
Objectives
Health disparities between individuals of African and European ancestry are well documented. The disparities in bipolar disorder may be driven by racial bias superimposed on established factors contributing to misdiagnosis, including: evolving empirically based diagnostic criteria (International Classification of Diseases [ICD], Research Diagnostic Criteria [RDC] and Diagnostic and Statistical Manual [DSM]), multiple symptom domains (i.e. mania, depression and psychosis), and multimodal medical and additional psychiatric comorbidity.
Methods
For this paper, we reviewed the phenomenological differences between bipolar individuals of African and European ancestry in the context of diagnostic criteria and clinical factors that may contribute to a potential racial bias.
Results
Published data show that bipolar persons of African ancestry, compared with bipolar persons of non‐African ancestry, are more often misdiagnosed with a disease other than bipolar disorder (i.e. schizophrenia). Additionally, studies show that there are disparities in recruiting patients of African ancestry to participate in important genomic studies. This gap in biological research in this underrepresented minority may represent a missed opportunity to address potential racial differences in the risk and course of bipolar illness.
Conclusion
A concerted effort by the research community to increase inclusion of diverse persons in studies of bipolar disorder through community engagement may facilitate fully addressing these diagnostic and treatment disparities in bipolar individuals of African ancestry.
Keywords: bipolar disorder, African ancestry, health/racial disparities, minority research participation
1. INTRODUCTION
Bipolar disorder is the sixth leading cause of disability worldwide, and its early onset and chronic nature underscore its cumulative illness burden and the importance of early intervention and optimal disease management strategies.1, 2 However, lack of access to and minimal utilization of healthcare coupled with low socioeconomic status continue to drive disease‐related disability worldwide, including in the USA. In comparison to the general US population, Americans with mental illness have decreased life expectancy; for people with severe mental disorders (i.e. schizophrenia, depression, and bipolar disorder), this life expectancy reduction ranges from 10 to 20 years.3, 4
There is a general recognition that the increased morbidity and mortality of people with serious mental illness may be magnified by racial disparities in access to, or provision of healthcare. African‐American individuals with bipolar disorder, in comparison to white individuals with bipolar disorder, have been reported to have significantly higher rates of receiving an initial clinical diagnosis other than bipolar disorder; this misdiagnosis may impede treatment strategies that can directly address illness morbidity.5, 6, 7
The patient advocacy group Depression Bipolar Support Alliance (formerly known as the National Depressive & Manic Depressive Association) conducted membership surveys, both in 1994 and nearly 10 years later, that continue to suggest lengthy delays (10+ years) in receiving an accurate diagnosis.8, 9 Misdiagnosis has significant implications. A misdiagnosis of bipolar depression as unipolar major depressive disorder with subsequent antidepressant treatment increases the likelihood of treatment non‐response and/or antidepressant‐induced mania/mood destabilization, while a misdiagnosis of schizophrenia limits the opportunity for treatment with lithium and/or mood‐stabilizing anticonvulsants, as well as access to bipolar evidence‐based psychotherapies. With these considerations in mind, we reviewed psychiatry disparity literature to gain a better understanding of racial diagnostic differences and explore methods for addressing this disparity, with a focus on biological and genetic studies inclusive of individuals of African ancestry.
To accomplish this goal, we reviewed literature pertaining to diagnosis and treatment of bipolar disorder (either type I or type II) in people of African ancestry compared with people of non‐African ancestry. Barriers to research inclusion and participation are also discussed, especially in the context of genetic research, underscoring the need to address potential racial biases during diagnosis and treatment, and the potential hazards of not doing so. We first present the literature chronologically to evaluate how recognition of this problem has evolved over the last 50 years. We then explore the potential effects of misdiagnosis on outcome and prognosis, and posit genomic studies of bipolar disorder as a possible method of addressing this disparity.
2. METHODS
Literature for this descriptive review was selected using key search terms to target studies describing diagnostic, treatment, and outcome differences between individuals of European and African ancestry, and research participation in biological research, specifically genetic studies, among people of African and European ancestry. The cited literature came from PubMed and Google Scholar searches with the following key terms: bipolar disorder African Americans, bipolar disorder African Americans treatment, bipolar disorder African ancestry, bipolar disorder African Americans lithium, and bipolar disorder blacks. To identify genetic studies, these keywords were searched for: bipolar disorder African ancestry genetics. A total of 28 publications were excluded from the initial search, and 20 more were excluded based on pertinent content, the details of which are shown in Figure 1.
Figure 1.
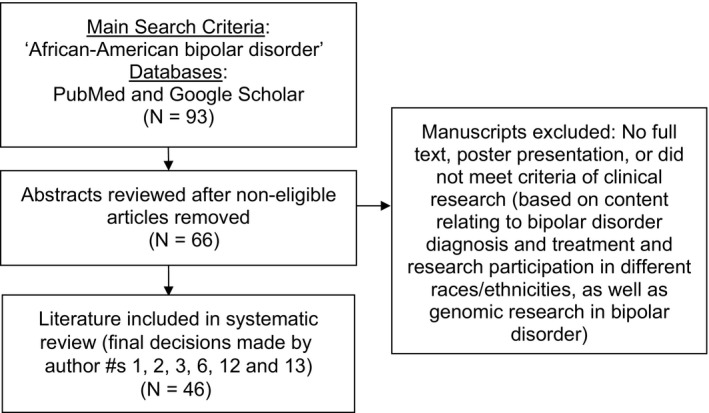
Flow diagram for literature selection and inclusion
We retained the language used in the original publications to describe various racial and ethnic identifications; for example, if a publication described patients of African ancestry as ‘African American’, the term ‘African American’ was used when discussing the publication. In addition, race and ethnicity are terms often used interchangeably. The Oxford Dictionary defines ethnicity as ‘the fact or state of belonging to a social group that has a common national or cultural tradition’, while defining race as ‘each of the major divisions of humankind, having distinct physical characteristics… a group of people sharing the same culture, history, language, etc.; an ethnic group.’10 These definitions are very similar to each other and we therefore use the terms interchangeably and retain the language used (either race or ethnicity) in the cited studies.
3. EVOLUTION OF DIAGNOSTIC CRITERIA AND HISTORICAL STUDIES OF POTENTIAL RACIAL BIAS
Psychiatric diagnostic classification has been achieved globally through the World Health Organization (WHO) International Classification of Diseases (ICD), starting with the ICD6 in 1948, and the Diagnostic and Statistical Manual (DSM), published in the USA by the American Psychiatric Association (APA).11 Primarily based on dynamic formulation, manic‐depressive ‘reaction’ and ‘illness’ were first classified in DSM I (1952) and DSM II (1968), respectively. Informed by earlier work used to develop the Research Diagnostic Criteria (RDC) and DSM III (1980), empirically based, contemporary diagnostic categorization based on specific, descriptive, and reliable inclusion/exclusion criteria with inter‐observer reliability and stability was introduced in DSM IIIR (1987). DSM IIIR marked a fundamental shift away from a predominantly psychodynamic theoretical influence toward a biomedical model.12
A second diagnostic debate, during this time of ICD and DSM classification revision, was whether psychosis in affective disorder represented a separate disease process from schizophrenia.13 Internationally, more so than nationally in the USA, there was a movement to adapt a broader concept or spectrum of affective psychosis. For example, a UK vs USA comparative study by Cooper et al. in 1972 found that, despite similar clinical presentations, there was a significant difference in the diagnostic rates of schizophrenia (New York, 62%; London, 34%), psychotic depression (five times higher in London), and mania (12 times higher in London).14 The late entrance of lithium carbonate into the US pharmacopoeia in 1970 (the USA was the 50th country to admit lithium to the world marketplace) as well as further refinement of diagnostic criteria (DSM III, RDC and DSM IIIR) began to slowly shift the US diagnostic practice and started to address the misdiagnosis of bipolar disorder.15 However, the misdiagnosis remains prevalent, especially in African‐American and African‐Caribbean individuals.5, 16 The evolution of biomedical diagnostic criteria, increasing recognition of an affective spectrum concept including psychosis, and the first Food and Drug Administration (FDA)‐approved medication for bipolar disorder introduced in the USA more than 15 years after FDA approval of antipsychotic chlorpromazine (1954) and nearly 10 years after FDA approval of antidepressant amitriptyline (1961) are relevant historical events to better understand the African‐American bipolar patient experience. Additional clinical factors that may be associated with the misdiagnosis of bipolar disorder have included: stage of mania when seeking treatment, hospital setting where diagnosis is made, symptom presentation, and clinical interpretation of symptom presentation.
A case series of three African‐American people with psychotic bipolar disorder (two male and one female) diagnosed as schizophrenic at a university hospital in New Jersey suggested that misdiagnosis was, in part, related to delays in seeking care. The investigators observed that hypomanic or manic behavior ‘may be more easily tolerated than it would be in a higher socioeconomic area’ (vs the low socioeconomic area reported in the study).17 Classic Kraepelinian observations have suggested that, as an episode of mania progresses, euphoria decreases and risk of psychosis increases.18 Therefore, individuals with bipolar disorder who delay seeking treatment may be more likely to display psychotic symptoms once they present to a medical center for a cross‐sectional, non‐longitudinal assessment. Of note, there have been no systematic studies of the contribution of treatment‐seeking delay to misdiagnosis in bipolar individuals.
A 1983 medical record review compared rates of misdiagnosis among 76 bipolar individuals (Hispanic, 18 [23.4%]; black, 21 [27.3%]; white, 37 [48.1%]) treated in an outpatient department of an inner‐city New York hospital. A greater proportion of black and Hispanic individuals with bipolar disorder were previously misdiagnosed with schizophrenia compared to white individuals (schizophrenia: 85.7% and 83.3% vs 51.4%; paranoid schizophrenia: 66.7% and 33.3% vs 18.9% for black, Hispanic and white individuals, respectively; P < .0005 and P < .005, respectively). As none of the individuals had a history of clinical diagnoses of a non‐affective psychosis, ethnicity was concluded to be a significant factor in their misdiagnosis.6 A larger 2004 study explored the relationship between ethnicity, symptom presentation, and diagnosis. African‐Americans were four times as likely to have a schizophrenia diagnosis when compared to otherwise similar white Americans (odds ratio [OR] = 4.05, 95% confidence interval [CI]: 3.91‐4.19)7 in analyses of the Veteran's Administration Medical Center National Psychosis Registry (n = 134 523; 48 443 [36.9%] bipolar, 14 717 [10.9%] schizoaffective) that adjusted for potential demographic confounds. Furthermore, the lack of significant ethnic differences in positive and negative moderate symptom severity also suggests equivalent symptom burden but different clinical interpretation of diagnostic information. While these historical studies identify racial/ethnic bias as a contributor to misdiagnosis, more contemporary research (with enhanced study methodology) may suggest strategies to correct for these differences.
The Epidemiologic Catchment Area Study (ECA) was a landmark study that examined the utilization of a structured diagnostic interview suitable for administration by lay interviewers in a community‐based setting. The diagnostic interview was administered to five sites of different sizes and resident characteristics (n = 20 000; New Haven, CT, Baltimore, MD, St. Louis, MO, Durham, NC, and Los Angeles, CA). While the percentage of black respondents at each site ranged from 4% to 34%, there was no significant difference in rates of bipolar disorder by race, suggesting the value of a highly structured research diagnostic interview as a data source to reduce clinical interpretative differences among persons of different races and ethnicities.19
Critical investigations from the University of Cincinnati First‐Episode Psychosis and Mania Projects not only used structured diagnostic interviews, as done in the ECA study, but also used a multi‐racial expert‐consensus diagnostic panel of psychiatrists blind to ethnicity to reveal possible limitations, or biases, when clinical diagnoses are the sole source of patient data.5, 20 The first project enrolled 100 people (46% African‐American and 54% Caucasian) from inpatient psychiatry services. The investigators evaluated the differences in clinical diagnoses made at the initial point of care (the Psychiatric Emergency Service) vs diagnoses made once patients were admitted to the inpatient unit where they underwent a research Structural Clinical Interview for DSM‐III‐R. African‐American bipolar individuals, in comparison to Caucasian bipolar individuals, were more likely to be diagnosed in the Emergency Department with non‐affective psychosis in general (i.e. schizophrenia + psychosis not otherwise specified [NOS]; 33% vs 13%, respectively; P = .03) and, in particular, with schizophrenia (20% in African‐Americans vs 7% in white individuals; P = .07).5 In the second project, 195 African‐American and white individuals with at least one psychotic symptom were recruited between 1998 and 2001. Of these, 79 (39 African‐Americans and 40 white individuals) received a bipolar disorder diagnosis by an expert consensus blind to ethnicity. After controlling for demographic variables and comorbid drug use, African‐American men with bipolar disorder had significantly higher rates of clinical schizophrenia diagnoses (25% vs 7%, respectively; P = .02) and higher rates of schizophrenia diagnosis by structured interview (29% vs 15%; P < .03) when compared to the other patient groups. Rates of first‐rank psychotic symptoms did not differ by ethnic group, suggesting that the patient's race and sex were primary factors for schizophrenia diagnosis.20 The use of an expert panel reviewing diagnostic criteria, in comparison to both clinical diagnosis and structured interview, appeared to yield less misdiagnosis.
The evolution of bipolar disorder diagnostic criteria coincides with these inaugural efforts to understand differences in diagnosis between bipolar individuals of European and African ancestry. Reports suggest that these differences are mainly attributable to racial/ethnic bias and/or misattribution of psychotic symptoms. These historical and distinctive studies are key to understanding the development of this disparity and its impact on subsequent treatment (Figure 2).
Figure 2.
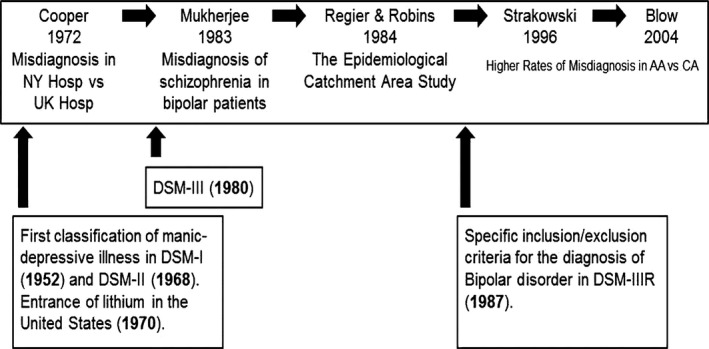
Timeline of historical clinical and diagnostic studies addressing racial differences and potential bias. AA, African‐American; CA, Caucasian; Hosp, hospital; NY, New York
4. TREATMENT, RESPONSE, AND PROGNOSIS
A 2002 prospective longitudinal study reported that 24 African‐Americans with bipolar I disorder received antipsychotics at a greater percentage of follow‐up visits (44%/70 visits) than 34 white individuals (40%/34 visits; P < .007). The prescription of typical antipsychotics in African‐American vs white bipolar individuals was significantly higher (38% vs 15%, respectively; P < .05).21 A larger 2003 cross‐sectional study of 535 hospitalized individuals with bipolar disorder confirmed this earlier observation as African‐Americans, in comparison to white patients, were prescribed antipsychotic medications at a higher rate (92% vs 62%, respectively); there was no difference, however, in the use of atypical vs typical antipsychotics.22 While first‐generation or typical antipsychotics are well known to be very effective in treating acute mania, they have a higher incidence (in comparison to FDA second‐generation or atypical antipsychotics) of mood destabilization (i.e. post manic depression) and extrapyramidal side effects including tardive dyskinesia.23 Adverse effects such as these warrant strong efforts toward understanding the root cause of misdiagnosis and inevitable suboptimal treatment.
A study that examined 34 bipolar persons taking lithium carbonate demonstrated that African‐Americans had a mean lithium red blood cell (RBC)/plasma ratio (39.70 ± 17.8; n = 12) that was significantly higher than that of white individuals (26.12 ± 10.9; n = 22; P < .05). The side effect burden was similarly higher in African‐Americans vs white patients even though the two groups did not significantly differ in mean daily dose (1131 mg/day vs 1159 mg/day, respectively) and average plasma level (0.58 ± 0.27 vs 0.57 ± 0.17, respectively). The RBC measurement has been proposed to be a better measure of brain lithium level than conventional plasma levels, and earlier research has suggested that African‐Americans, in comparison to Caucasians, have reduced efficiency in the RBC lithium sodium counter transport pathway.24, 25, 26 Lower dose lithium proved to have positive results in a more recent study from 2015 that examined 283 bipolar patients who participated in a 6‐month, randomized, double‐blind, placebo‐controlled trial of adjunctive low‐dose lithium (600 mg) with optimized treatment (LiTMUS). Compared to white participants, African‐Americans had a greater reduction of depression symptoms (P = .04) and improved quality of life (P = .03). Although the study showed promising support for low‐dose lithium in African‐Americans, larger sample sizes in future studies are necessary to confirm these significant findings.27 Underutilization of mood stabilizers or suboptimal dosing of mood stabilizers may negatively affect disease progression.
Gonzalez et al. compared 1‐year treatment outcomes from the US Systematic Treatment Enhancement Program for Bipolar Disorder (STEP‐BD) among African‐American, Hispanic, and white individuals. African‐Americans (n = 155) with psychotic symptoms at baseline, in comparison to non‐Hispanic white individuals (n = 729) with psychotic symptoms at baseline, had a significantly lower response rate (50% reduction in Montogomery‐Asberg Depression Rating Scale [MADRS]) and recovery rate defined as remission of symptoms over the 1‐year period (38% vs 53%, respectively; P < .07). The investigators did note that symptom reports, during the clinical assessment, from some African‐Americans may have been misattributed to psychopathology instead of sociocultural background. For example, the authors proposed that a persecutory delusion classified as a psychotic symptom may have been more accurate to view as an anxiety symptom when sociocultural context was considered.28 This misattribution could fuel unsuitable treatment recommendations (i.e. using antipsychotic vs anti‐anxiety treatment). This study suggests that culturally competent treatment regimens in populations with different sociocultural background may help address racial bias and aid in yielding more appropriate treatment recommendations.
Consideration of sociocultural background may also be crucial regarding continuity of outpatient care. A 2005 Veterans Affairs (VA) National Patient Care Database study of veterans with bipolar disorder (n = 2316; African‐Americans = 303) revealed that African‐Americans were significantly less likely to have an outpatient follow‐up visit within 90 days of their diagnosis compared to white individuals (13% African‐Americans vs 87% white individuals; P = .009). The investigators suggested that the reduced likelihood of African‐Americans receiving adequate outpatient care compared to white individuals may be due to lack of culturally competent providers, particularly in urban facilities.29 A study from 2014 using the National Comorbidity Survey Replication (NSC‐R; a US study of mental health) further examined the difference in treatment of bipolar disorder between black (n = 30) and white (n = 137) Americans. No black patients received minimally adequate treatment (defined as use of a mood stabilizer alone or in combination with an antipsychotic) in the previous year, compared to 17% of white patients who did (P < .05). Their findings suggest that, in general, people with bipolar disorder receive inadequate treatment that is then further confounded by race.30
Disparities in treatment regimens and subsequent lower quality outcomes warrant targeted treatment models aimed at improving outcomes and reducing health disparities. Specialized Care for Bipolar Disorder (SCBD) and Enhanced Clinical Intervention (ECI) are examples of such treatment regimens. The study that developed these treatment models sought to reduce health disparities in three groups often underrepresented in clinical trials: the young and elderly, African Americans, and rural residents with bipolar disorder. The ECI component was intensive case management adapted to the specific needs of each subpopulation focused on education about the mood disorder itself and treatment strategies. A total of 463 bipolar individuals (68 African‐American and 385 Caucasian) were randomly assigned to SCBD alone or SCBD + ECI for up to 3 years. While the study results showed that improvement in quality of life was greater in the SCBD + ECI group, there were no significant differences by race, suggesting the benefit of culturally competent case management and standardized treatment protocols.31 If personalized treatment protocols are to be developed, understanding the patient's sociocultural background is just as important as the diagnostic criteria of bipolar disorder in African‐Americans (Table 1). Biologically based definitions of bipolar disorder and psychotic symptoms are also valuable, but early in validation and development. Further advancement of clinically relevant biomarkers (through biological and/or genetic studies of the neurobiology of the disease) is an important step to addressing these disparities.32
Table 1.
Studies addressing treatment and drug response in bipolar patients of African ancestry
| Study | Sample size (N or % total) | Major conclusions |
|---|---|---|
| Szarek et al.22 | Total = 535 hospitalized inpatients | Both AA and HIS were more likely to have antipsychotics prescribed (92% and 85%, respectively) compared with CA (62.2%) |
| Fleck et al.21 |
Total = 58 outpatients AA = 24 (41.3%) CA = 34 (58.6%) |
AA received antipsychotics during a greater percentage of follow‐up treatments compared with CA (mean = 70 [44%] vs mean = 34 [40%]; P < .007) |
| Fagiolini et al.31 |
Total N = 463 AA = 68 (14.7%) CA = 385 (83.2%) |
There was no significant difference found between participants of different race. However, adding ECI to SCBD showed benefits of greater QOL |
| Gonzalez et al.28 |
Total = 1858 AA = 155 (8.3%) CA = 1551 (83.5%) |
For depression response (measured by the MADRS), AA with psychotic symptoms at baseline had poorer outcomes compared with non‐HIS CA with psychotic symptoms at baseline (total recovered/responded: AA = 38 vs CA = 241; P = .339) (recovered/responded = 50% improvement over baseline) |
| Kilbourne et al.29 |
Total BD I = 2316 AA = 303 (13.1%) |
AA patients were less likely to receive suitable outpatient care within 90 days of the index bipolar diagnosis compared with CA patients (202 vs 1351; P = .009) |
| Johnson et al.30 |
Total = 167 AA = 30 (18%) CA = 137 (82%) |
Minimally adequate treatment (defined as use of a mood stabilizer alone or in combination with an antipsychotic) was significantly different in AA vs CA (0% vs 17%; P < .05) |
| Strickland et al.24 |
Total = 34 AA = 12 CA = 22 |
There were higher lithium red blood cell/plasma ratios and side effects in AA vs CA (39.70 ± 17.84 vs 26.12 ± 10.95; P < .05) |
| Gonzalez Arnold et al.27 |
Total = 283 AA = 47 (19.7%) CA = 175 (61.8%) HIS = 39 (13.8%) (cohort included those with self‐identified race) |
AA on low‐dose lithium (600 mg average dosage), compared with CA, had greater improvement on depression symptoms (P = .04) and improved QOL scores (P = .03) |
AA, African‐American; CA, Caucasian; ECI, enhanced clinical intervention; HIS, Hispanic; MADRS; the Montgomery‐Åsberg Depression Rating Scale; QOL, quality of life; SCBD, specialized care for bipolar disorder.
5. GENOMIC STUDIES OF BIPOLAR DISORDER: UNDERREPRESENTATION OF POPULATIONS OF AFRICAN ANCESTRY
Understanding the genetic basis of bipolar disorder could greatly advance knowledge of its neurobiology and etiology. Bipolar disorder is a complex genetic disorder, with heritability estimated to be between 60% and 85%, indicating that a large proportion of disease risk is potentially attributed to inter‐individual genetic variation.33 Numerous studies have attempted to identify genetic factors contributing to the risk of bipolar disorder to uncover the underlying pathophysiology and pathogenesis of the disease. While genomic research could aid in resolving health disparities, others have argued that knowledge of genetic factors that contribute to illness or treatment outcomes will not itself reduce health disparities. Kashyap and colleagues note that, although the role of genomics in health disparities is quite complex, it is critical to understand how genetic variation influences the health and well‐being of at‐risk communities to eliminate health disparities in the USA.34 On the other hand, West et al.35 argue that clarification of genetic contributors to disease etiology will not help to provide ways to address disparities, as they are rooted in social, material, and environmental conditions. Nevertheless, there is recognition that genetic studies should include diverse populations to enable identification of a wide range of genetic variation contributing to health outcomes and ensure that knowledge gained from these studies is applicable to all populations.
Numerous studies have been conducted to investigate the genetics of bipolar disorder, including many candidate gene studies, a growing number of genome‐wide association studies (GWASs), and recently introduced whole exome and whole genome sequencing studies. These genetic association studies and the efforts of large international consortia, particularly the Psychiatric Genomics Consortium (PGC), have led to the discovery of several bipolar disorder risk variants with genome‐wide significant evidence of association.36 While these discoveries constitute important progress toward a better understanding of the neurobiology of the disease, the studies that produced these results were performed almost exclusively in populations of European ancestry. Very few studies of the genetics of bipolar disorder, and only one published GWAS, have included samples of African ancestry. That GWAS included only 345 African‐American cases, a small number compared with the 1001 European‐American cases in the same study, providing inadequate power to detect genetic associations in the African‐American subset.37 The small sample size of the published African‐American GWAS of bipolar disorder is in stark contrast to the large number of European ancestry cases that the PGC has accumulated (n = 9784, to date), leading to the discovery of more than a dozen genetic variants contributing to bipolar disorder risk in European populations38 (Figure 3). Similarly, in reviewing GWASs of psychiatric pharmacogenomics, Murphy and McMahon noted that ‘non‐European groups were underrepresented in these studies’.39
Figure 3.
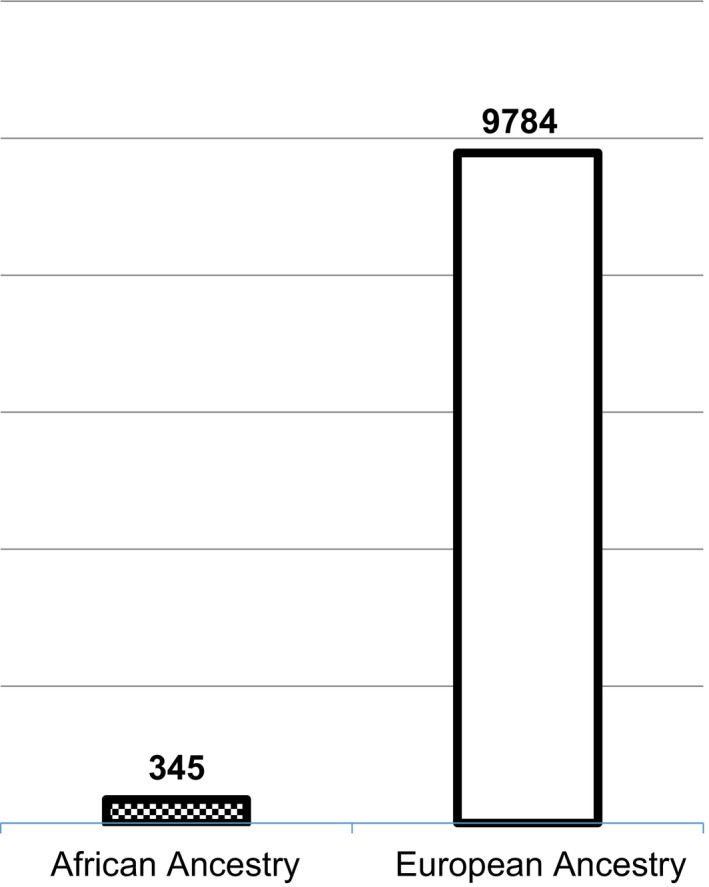
Sample sizes of the largest African‐American and European‐American bipolar disorder genome‐wide association study published to date; Based on reference numbers 36 and 37 [Colour figure can be viewed at http://wileyonlinelibrary.com]
The underrepresentation of individuals of African ancestry in genetic studies is a two‐fold issue stemming from both the small numbers of African‐ancestry participants and the frequent exclusion of participating minorities from analyses to promote sample homogeneity and prevent confounding by population stratification. The low participation rate of African‐Americans in bipolar disorder genetic research speaks to the need to increase engagement of these populations in research; however, recruitment of African‐Americans for genetic research (and bipolar disorder studies more specifically) is challenging40 (Table 2). For example, the Mayo Clinic Bipolar Biobank has about 2148 individuals enrolled, but only 3.7% are of African ancestry.41 Even with policy initiatives from the National Institute of Mental Health (NIMH) aimed at increasing minority participation, results have been mixed. Nwulia et al.42 assessed participants in the US Bipolar Genome Study to identify concerns that influence individual participation in psychiatric genetic studies, and found that there is an increased perception of harmful consequences among black individuals, when compared to white individuals. The authors also reported that another main concern among black individuals when compared to white individuals was racial discrimination (34% of African‐Americans were ‘very concerned’ compared to 13% of white individuals; P < .0001), and noted that there may be additional factors contributing to the decision to participate in research. To better understand what factors influence patient participation, Hartz et al.40 used a large, population‐based sample from a genetic study of nicotine dependence (Collaborative Study on the Genetics of Nicotine Independence) to investigate differences between European‐American (n = 705) and African‐American (n = 352) participation. They examined three critical steps of study recruitment: establishment of initial contact, participation in screening, and recruitment into the genetic study (with blood draw). Surprisingly, the participation rate was lower in European‐Americans than in African‐Americans (57% vs 71%, respectively; P < .0001). This difference was because willingness to participate was not seen as a major barrier; once reached, minorities were more likely to participate. Locating minority participants and establishing contact were the key barriers, suggesting that recruitment efforts should focus on areas with a high frequency of individuals of African ancestry.
Table 2.
Participation in genetic studies among subjects of African ancestry
| Study | Sample size (N or % total) | Major conclusions |
|---|---|---|
| Nwulia et al.42 |
Total = 1253 AA = 188 (15%) CA = 1065 (85%) |
AA exhibited more concern about risks to procreation (27% of AA were ‘very concerned’ compared with 18% of CA; P < .004) and racial discrimination (34% of AA were ‘very concerned’ compared with 13% of CA; P < .0001) |
| Hartz et al.40 |
Total N = 28 658* AA = 352 cases, 152 controls EA = 705 cases, 710 controls |
The participation rate was lower in EA than in AA (57% vs 71%; P < .0001). Mistrust of medical research did not prove to be a barrier for minority participation. Critical barriers were locating minority subjects and establishing contact. Once reached, minorities were more likely to participate |
| Kogan et al.44 |
Total = 2848 (4.1% AA) Community sites: 157 (25.8% AA) Academic sites: 2691 (4.8% AA) |
Community sites had significantly higher minority enrollment than academic sites (45.2% vs 15.3%; P < .001) |
| Sanderson et al.43 |
Total = 13 000 AA = 1483 (12%) CA = 6521 (51%) |
AA participants expressed the lowest levels of willingness to participate compared to CA (56% vs 70%) |
AA, African‐American; CA, Caucasian; EA, European ancestry.*Number screened by phone and filtered through inclusion/exclusion criteria.
Another recent study43 assessed willingness to participate in a biobank, hypothesizing that willingness would be higher under more restrictive scenarios. Participants (n = 13 000; African‐Americans = 1483; white individuals = 6521) were randomized to receive a survey in one of three hypothetical biobank scenarios; all scenarios were the same except for consent type and data sharing approach. In this study, African‐American participants expressed lower levels of willingness to participate compared to white participants (56% vs 70%, respectively). However, few studies have aimed to understand how to overcome barriers to study participation and inclusion. The STEP‐BD created the Community Partner Program (CPP) to address the issue of underrepresented minorities in mental health research studies. Community sites enrolled higher percentages of minority participants when compared to collaborating academic sites (45.2% vs 15.3%, respectively; P < .001). The inception of such programs is essential and demonstrates that including community partners greatly enhances minority involvement in research studies. Moreover, community‐engaged participatory‐based research remains crucial to motivating individuals to consistently participate in research activities.44 These research activities are key to conducting impactful studies that will enhance understanding of the biological and genetic basis of bipolar disorder, which can possibly address previously observed symptomatic differences that lead to misdiagnosis of bipolar African‐Americans.
6. CONCLUSION AND FUTURE DIRECTIONS
This paper has reviewed the racial disparities in bipolar disorder diagnosis, treatment, and research participation, emphasizing the need for increased efforts by the scientific community to address these disparities. The reviewed literature suggests that people of African ancestry with bipolar disorder (either type I or II) have higher rates of misdiagnosis in comparison to people of non‐African ancestry with bipolar disorder. These disparities have developed and persisted despite revision of diagnostic criteria from a psychodynamic formulation to a biomedical model, increasing recognition of an affective spectrum, and a bipolar pharmacopoeia, at least in the USA, developed 10‐15 years later than treatments for schizophrenia and major depression.
This descriptive review has a number of limitations. While focused on biological research as a means to address health disparities, there needs to be recognition that many additional factors may contribute to a bipolar misdiagnosis and these factors may not be unique to patients of African ancestry. There are socioeconomic, cultural, and healthcare administrative aspects of access to and benefit from a bipolar diagnosis and treatment program that go beyond race and ethnicity. While this review focused on biological and genetic factors of bipolar disorder, other additional non‐biological and historical factors may contribute to this health disparity. Systematic issues such as access to the healthcare system and historical mistrust may also play a role. The mechanisms and processes contributing to this important issue likely involve slavery, institutional racism, discrimination, poverty, and segregation. The focus on genomic and community‐based participatory research is meant to be an alternative approach to address these disparities and not reduce the importance of other contributing factors.
We proposed a plan of action to address these disparities that involves understanding the evolution of the problem, and identifying the contribution of associated clinical and biological risk factors of bipolar disorder, particularly through genomic studies. Targeted, biologically based research focused on these differences has the potential to clarify the issues and effect change in the psychiatric care of minority populations.44 However, low rates of research participation among minority populations compound the problem because low numbers preclude comprehensive evaluation of potential biologic and cultural factors that may contribute to possible differences in clinical presentation and disease progression. Low research participation is best addressed through increased understanding of the barriers to engagement with minority communities as well as strong efforts from the scientific community to include minority persons in studies of bipolar disorder, especially genetic and other etiologic studies.45 Examples of active engagement efforts include, but are not limited to: community‐based participatory research (CBPR) focused on patient and family education, working with faith‐based organizations to disseminate impactful and educational research findings, focused efforts to train more psychiatrists in cultural competency, and overall training of more psychiatrists of African ancestry.46
The complexities of the factors that contribute to misdiagnosis of bipolar disorder in individuals of African ancestry and minimal participation from minority samples are critical disparities that warrant attention and action from the scientific community and facilitators.
DISCLOSURES
None declared.
Akinhanmi MO, Biernacka JM, Strakowski SM, et al. Racial disparities in bipolar disorder treatment and research: a call to action. Bipolar Disord. 2018;20:506–514. 10.1111/bdi.12638
Funding information
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH), and support from the Marriott Foundation and the Mayo Foundation. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH.
Presented in part at the Annual Meeting of the International Society of Bipolar Disorder, 4‐8 May 2017, Washington, DC, USA.
REFERENCES
- 1. Merikangas KR, Jin R, He JP, et al. Prevalence and correlates of bipolar spectrum disorder in the world mental health survey initiative. Arch Gen Psychiatry. 2011;68:241‐251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Friedrich MJ. Depression is the leading cause of disability around the world. JAMA. 2017;317:1517. [DOI] [PubMed] [Google Scholar]
- 3. Carliner H, Collins PY, Cabassa LJ, McNallen A, Joestl SS, Lewis‐Fernández R. Prevalence of cardiovascular risk factors among racial and ethnic minorities with schizophrenia spectrum and bipolar disorders: a critical literature review. Compr Psychiatry. 2014;55:233‐247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu NH, Daumit GL, Dua T, et al. Excess mortality in persons with severe mental disorders: a multilevel intervention framework and priorities for clinical practice, policy and research agendas. World Psychiatry. 2017;16:30‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strakowski SM, McElroy SL, Keck PE Jr, West SA. Racial influence on diagnosis in psychotic mania. J Affect Disord. 1996;39:157‐162. [DOI] [PubMed] [Google Scholar]
- 6. Mukherjee S, Shukla S, Woodle J, Rosen AM, Olarte S. Misdiagnosis of schizophrenia in bipolar patients: a multiethnic comparison. Am J Psychiatry. 1983;140:1571‐1574. [DOI] [PubMed] [Google Scholar]
- 7. Blow FC, Zeber JE, McCarthy JF, Valenstein M, Gillon L, Bingham CR. Ethnicity and diagnostic patterns in veterans with psychoses. Soc Psychiatry Psychiatr Epidemiol. 2004;39:841‐851. [DOI] [PubMed] [Google Scholar]
- 8. Lish JD, Dime‐Meenan S, Whybrow PC, Price RA, Hirschfeld RMA. The National Depressive and Manic‐depressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281‐294. [DOI] [PubMed] [Google Scholar]
- 9. Hirschfeld RM, Lewis L, Vornik LA. Perceptions and impact of bipolar disorder: how far have we really come? Results of the national depressive and manic‐depressive association 2000 survey of individuals with bipolar disorder. J Clin Psychiatry. 2003;64:161‐174. [PubMed] [Google Scholar]
- 10. Morning A. Ethnic classification in global perspective: a cross‐national survey of the 2000 census round In: Simon P, Piché V, Gagnon AA, eds. Social Statistics and Ethnic Diversity: Cross‐National Perspectives in Classifications and Identity Politics. Cham: Springer International Publishing; 2015:17‐37. [Google Scholar]
- 11. Bramer GR. International statistical classification of diseases and related health problems. Tenth revision. World health statistics quarterly. Rapport trimestriel de statistiques sanitaires mondiales. 1988;41:32‐36. [PubMed] [Google Scholar]
- 12. Mason BL, Brown ES, Croarkin PE. Historical underpinnings of bipolar disorder diagnostic criteria. Behav Sci. 2016;6:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heckers S. Bleuler and the neurobiology of schizophrenia. Schizophr Bull. 2011;37:1131‐1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cooper JE Psychiatric Diagnosis in New York and London; A Comparative Study of Mental Hospital Admissions, Vol. xi London, New York: Oxford University Press; 1972:152p. [Google Scholar]
- 15. Shorter E. The history of lithium therapy. Bipolar Disord. 2009;11(Suppl 2):4‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Neighbors HW, Trierweiler SJ, Ford BC, Muroff JR. Racial differences in DSM diagnosis using a semi‐structured instrument: the importance of clinical judgment in the diagnosis of African Americans. J Health Soc Behav. 2003;44:237‐256. [PubMed] [Google Scholar]
- 17. Roukema R, Fadem B, James B, Rayford F. Bipolar disorder in a low socioeconomic population. Difficulties in diagnosis. J Nerv Ment Dis. 1984;172:76‐79. [DOI] [PubMed] [Google Scholar]
- 18. Kraepelin E. Manic depressive insanity and paranoia. J Nerv Ment Dis. 1921;53:350. [Google Scholar]
- 19. Regier DA, Myers JK, Kramer M, et al. The NIMH Epidemiologic Catchment Area program. Historical context, major objectives, and study population characteristics. Arch Gen Psychiatry. 1984;41:934‐941. [DOI] [PubMed] [Google Scholar]
- 20. Strakowski SM, Keck PE Jr, Arnold LM, et al. Ethnicity and diagnosis in patients with affective disorders. J Clin Psychiatry. 2003;64:747‐754. [DOI] [PubMed] [Google Scholar]
- 21. Fleck DE, Hendricks WL, DelBello MP, Strakowski SM. Differential prescription of maintenance antipsychotics to African American and white patients with new‐onset bipolar disorder. J Clin Psychiatry. 2002;63:658‐664. [DOI] [PubMed] [Google Scholar]
- 22. Szarek BL, Goethe JW. Racial differences in use of antipsychotics among patients with bipolar disorder. J Clin Psychiatry. 2003;64:614‐615; author reply 5. [DOI] [PubMed] [Google Scholar]
- 23. Holder SD, Edmunds AL, Morgan S. Psychotic and bipolar disorders: antipsychotic drugs. FP Essent. 2017;455:23‐29. [PubMed] [Google Scholar]
- 24. Strickland TL, Lin KM, Fu P, Anderson D, Zheng Y. Comparison of lithium ratio between African‐American and Caucasian bipolar patients. Biol Psychiatry. 1995;37:325‐330. [DOI] [PubMed] [Google Scholar]
- 25. Bunker CH, Mallinger AG, Adams LL, Kuller LH. Red blood cell sodium‐lithium countertransport and cardiovascular risk factors in black and white college students. J Hypertens. 1987;5:7‐15. [DOI] [PubMed] [Google Scholar]
- 26. Trevisan M, Ostrow D, Cooper RS, Sempos C, Stamler J. Sex and race differences in sodium‐lithium countertransport and red cell sodium concentration. Am J Epidemiol. 1984;120:537‐541. [DOI] [PubMed] [Google Scholar]
- 27. Gonzalez Arnold J, Salcedo S, Ketter TA, et al. An exploratory study of responses to low‐dose lithium in African Americans and Hispanics. J Affect Disord. 2015;178:224‐228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gonzalez JM, Bowden CL, Berman N, et al. One‐year treatment outcomes of African‐American and hispanic patients with bipolar I or II disorder in STEP‐BD. Psychiatr Serv. 2010;61:164‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kilbourne AM, Bauer MS, Han X, et al. Racial differences in the treatment of veterans with bipolar disorder. Psychiatr Serv. 2005;56:1549‐1555. [DOI] [PubMed] [Google Scholar]
- 30. Johnson KR, Johnson SL. Inadequate treatment of black Americans with bipolar disorder. Psychiatr Serv. 2014;65:255‐258. [DOI] [PubMed] [Google Scholar]
- 31. Fagiolini A, Frank E, Axelson DA, et al. Enhancing outcomes in patients with bipolar disorder: results from the Bipolar Disorder Center for Pennsylvanians Study. Bipolar Disord. 2009;11:382‐390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ivleva EI, Clementz BA, Dutcher AM, et al. Brain structure biomarkers in the psychosis biotypes: findings from the bipolar‐schizophrenia network for intermediate phenotypes. Biol Psychiat. 2017;82:26‐39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kerner B. Genetics of bipolar disorder. Appl Clin Genet. 2014;7:33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kashyap MV, Nolan M, Sprouse M, et al. Role of genomics in eliminating health disparities. J Carcinog. 2015;14:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. West KM, Blacksher E, Burke W. Genomics, health disparities, and missed opportunities for the nation's research agenda. JAMA. 2017;317:1831‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Donovan MC. What have we learned from the Psychiatric Genomics Consortium. World Psychiatry. 2015;14:291‐293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Smith EN, Bloss CS, Badner JA, et al. Genome‐wide association study of bipolar disorder in European American and African American individuals. Mol Psychiatry. 2009;14:755‐763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hou L, Bergen SE, Akula N, et al. Genome‐wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum Mol Genet. 2016;25:3383‐3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Murphy E, McMahon FJ. Pharmacogenetics of antidepressants, mood stabilizers, and antipsychotics in diverse human populations. Discov Med. 2013;16:113‐122. [PMC free article] [PubMed] [Google Scholar]
- 40. Hartz SM, Johnson EO, Saccone NL, Hatsukami D, Breslau N, Bierut LJ. Inclusion of African Americans in genetic studies: what is the barrier? Am J Epidemiol. 2011;174:336‐344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Frye MA, McElroy SL, Fuentes M, et al. Development of a bipolar disorder biobank: differential phenotyping for subsequent biomarker analyses. Int J Bipolar Disord. 2015;3:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nwulia EA, Hipolito MM, Aamir S, Lawson WB, Nurnberger JI; BiGS Consortium . Ethnic disparities in the perception of ethical risks from psychiatric genetic studies. Am J Med Genet B Neuropsychiatr Genet. 2011;156:569‐580. [DOI] [PubMed] [Google Scholar]
- 43. Sanderson SC, Brothers KB, Mercaldo ND, et al. Public attitudes toward consent and data sharing in biobank research: a large multi‐site experimental survey in the US. Am J Hum Genet. 2017;100:414‐427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kogan JN, Bauer MS, Dennehy EB, et al. Increasing minority research participation through collaboration with community outpatient clinics: the STEP‐BD Community Partners Experience. Clin Trials. 2009;6:344‐354. [DOI] [PubMed] [Google Scholar]
- 45. Heller C, Balls‐Berry JE, Nery JD, et al. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp Clin Trials. 2014;39:169‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brewer LC, Balls‐Berry JE, Dean P, Lackore K, Jenkins S, Hayes SN. Fostering African‐American Improvement in Total Health (FAITH!): an Application of the American Heart Association's Life's Simple 7 among Midwestern African‐Americans. J Racial Ethn Health Disparities. 2017;4:269‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]


