Summary
Studies have examined mortality risk for metabolically healthy obesity, defined as zero or one metabolic risk factors but not as zero risk factors. Thus, we sought to determine the independent mortality risk associated with obesity or elevated glucose, blood pressure or lipids in isolation or clustered together. The sample included 54 089 men and women from five cohort studies (follow‐up = 12.8 ± 7.2 years and 4864 [9.0%] deaths). Individuals were categorized as having obesity or elevated glucose, blood pressure or lipids alone or clustered with obesity or another metabolic factor. In our study sample, 6% of individuals presented with obesity but no other metabolic abnormalities. General obesity (hazard ratios [HR], 95% CI = 1.10, 0.8–1.6) and abdominal obesity (HR = 1.24, 0.9–1.7) in the absence of metabolic risk factors were not associated with mortality risk compared to lean individuals. Conversely, diabetes, hypertension and dyslipidaemia in isolation were significantly associated with mortality risk (HR range = 1.17–1.94, P < 0.05). However, when using traditional approaches, obesity (HR = 1.12, 1.02–1.23) is independently associated with mortality risk after statistical adjustment for the other metabolic risk factors. Similarly, metabolically healthy obesity, when defined as zero or one risk factor, is also associated with increased mortality risk (HR = 1.15, 1.01–1.32) as compared to lean healthy individuals. Obesity in the absence of metabolic abnormalities may not be associated with higher risk for all‐cause mortality compared to lean healthy individuals. Conversely, elevation of even a single metabolic risk factor is associated with increased mortality risk.
Keywords: Body mass index, hypertension, metabolic syndrome, waist circumference
What is already known about this subject
Obesity is associated with several metabolic disorders.
Obesity is associated with mortality risk after statistical adjustment of several risk factors.
When Metabolic Healthy Obesity (MHO) is defined as obesity with zero or one metabolic risk factor, MHO is associated with elevated mortality risk.
What does this study adds
MHO, defined as obesity with zero other metabolic risk factors, is not associated with increased mortality risk.
MHO, defined as abdominal obesity with zero other metabolic risk factors, is not associated with increased mortality risk.
Elevations in metabolic risk factors are much more strongly associated with mortality risk than obesity.
Introduction
Obesity often presents in conjunction with several metabolic risk factors, and these factors have been independently associated with mortality risk 1, 2, 3, 4, 5, 6, 7, 8, 9. Current weight management guidelines recommend that all individuals with obesity should be prescribed weight loss, suggesting that obesity even without other risk factors carries health risk 10. Recently, metabolically healthy obesity has become a topic of interest, but its association with mortality risk is under debate 11, 12, 13, 14, 15, 16. One of the issues with the definition of ‘healthy’, used in most existing studies suggesting that metabolically healthy obesity is associated with increased mortality risk, is that they may not in fact be healthy as they allow for obesity in the presence of up to one of the metabolic risk factors 11, 12, 13, 14, 15. This would mean that individuals with obesity and hypertension, for example, could have been categorized as healthy. The recent study by Caleyachetty et al. 16, which examined metabolically health obesity risk for cardiovascular events in 3.5 million adults, defined healthy as the absence of hypertension, diabetes and dyslipidaemia but did not exclude individuals with one or more preclinically elevated risk factors from being defined as healthy. This means that these ‘healthy’ individuals may present with the metabolic syndrome, which is also associated with increased mortality risk 17. Using the most stringent definition, wherein metabolically healthy obesity is defined as no clinical or preclinical risk factors, studies have suggested that metabolically healthy obesity would account for only 1.3% of the U.S. population 14. To our knowledge, there are no studies that examine the mortality risk for metabolically healthy obesity when defined as having no other clinical or preclinical metabolic risk factors.
Similarly, while it is clear that a clustering of metabolic risk factors with or without obesity is associated with elevated mortality risk 18, 19, a single metabolic risk factor may 20, 21 or may not 18. Previous research suggesting an independent association between risk factors and mortality has mainly used statistical adjustment for the other metabolic risk factors 1, 2, 3, 4, 5, 6, 7, 8, 9, 18, 20, 21 as opposed to restricting the sample to only individuals classified as healthy by the absence of risk factor(s). Sample restriction or categorization may also be the more clinically relevant approach to risk assessment but requires a very large sample size as the prevalence at which obesity and cardiometabolic risk factors occur in isolation are quite low (<10%) 22. Thus, it is unclear whether these cardiometabolic risk factors are associated with mortality risk when they occur in isolation.
To our knowledge, there has been no research examining the mortality risk associated with preclinical and clinically elevated levels of obesity, dysglycaemia, dyslipidaemia and hypertension in isolation. This research is important to understand risk stratification for treatment. Thus, the main objective of this study is to examine the association between obesity and cardiometabolic risk factors when they occur in isolation and to compare this with other commonly used approaches in the literature.
Methods
Participants
This sample includes a merged dataset from the ACLS (Updated December 31st, 2003), Coronary Artery Risk Development in Young Adults (CARDIA – Updated April 27th, 2017), Multi‐Ethnic Study of Atherosclerosis (MESA – Updated November 15th, 2016) and National Health and Nutrition Examination Survey (NHANES III and continuous 1999–2008). All study participants gave their informed written consent as required by the relevant ethics boards for each survey. Institutional ethics approval to analyse this merged dataset was obtained from York University's Research Ethics Board (e2017–364).
ACLS was available through a research collaboration with study investigators. Limited data access for CARDIA and MESA was obtained through the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH). This manuscript was prepared using research materials obtained from the NHLBI Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the study survey investigators or the NHLBI. NHANES is available publicly online.
The initial merged dataset contained 129 915 participants with complete mortality follow‐up data. Participants were included if they were over 18 years of age (n = 114 956) and had information on age, gender, body mass index (BMI), ethnicity and smoking status (n = 78 664) and information for all of the risk factors (BMI, waist, glucose, systolic blood pressure [SBP], diastolic blood pressure [DBP], high‐density lipoprotein [HDL] and triglycerides) (n = 54 857). Participants were excluded if they had a BMI less than 18.5 kg m−2, leaving a final sample of 54 089 individuals with complete baseline and mortality follow‐up data.
Datasets
Aerobics Center Longitudinal Study (ACLS) includes a cohort of participants who attended the Cooper Clinic (Dallas, TX) for periodic self‐ or physician‐referred medical examinations between 1987 and 2001. Mortality follow‐up till December 31, 2003 was used in this analysis.
Coronary Artery Risk Development in Young Adults (CARDIA) is a multicentre, longitudinal study conducted in 1985–1986 on 5115 participants aged 18–20 years from Birmingham, AL; Chicago, IL; Minneapolis, MN; and Oakland, CA 23. Mortality follow‐up till December 31, 2011 was used in this analysis.
Multi‐Ethnic Study of Atherosclerosis (MESA) is a longitudinal study of 6800 ethnically diverse (White, Black, Hispanic and Asian) men and women beginning in 1999. Participants were followed up every 9–12 months to ascertain medical events and mortality status through to examination 5 (August 2015) was used for these analyses. Mortality status was confirmed using medical records, death certificates, interviews, questionnaires and other procedures 24.
National Health and Nutrition Examination Survey (NHANES) III and Continuous are a series of nationally representative cross‐sectional surveys collected using a stratified, multistage, probability cluster design. NHANES III was conducted between 1988 and 1994 on 33 994 persons, aged 2 months and older. NHANES continuous cycles are released biannually, with 1999–2000 (n = 9965), 2001–2002 (n = 11 039), 2003–2004 (n = 10 122), 2005–2006 (n = 9950) and 2007–2008 (n = 9762) included in these analyses. Public access Mortality Linkage data file with follow‐up through December 31st, 2011 was used for these analyses.
Survey methods
Age, gender, ethnicity (White or other), smoking status (non or current), self‐reported medical history and medications were assessed by questionnaire. BMI (kg m−2) was calculated from measured weight and height. Standard BMI cut‐offs for normal weight (NW: 18.5 to 24.9 kg m−2), overweight (OW: 25 to 29.9 kg m−2) and obese (OB: ≥30 kg m−2) were used. Waist circumference (WC) was assessed by trained technicians.
Obesity and metabolic factor measurements
The following cut‐off points were used to classify factors into low, moderate and higher risk categories using standard Normal, Preclinical and Clinical cut‐offs, respectively (Table 1) 25, 26. The ‘No RF’ group was defined as being low risk for all other metabolic risk factor categories and was free of obesity. Hypertension was categorized as exceeding the clinical cut‐offs for blood pressure, as given in Table 1. Diabetes was categorized as exceeding the clinical cut‐offs for glucose, as given in Table 1. Dyslipidaemia was categorized as exceeding the clinical cut‐offs for HDL and triglycerides, as given in Table 1.
Table 1.
Normal, preclinical and clinical cut‐offs for obesity, blood pressure, glucose and lipids
| Factors | Normal | Preclinical | Clinical |
|---|---|---|---|
| Obesity | BMI: 18.5–24.9 kg m−2 | BMI: 25–29.9 kg m−2 | BMI ≥ 30 kg m−2 |
| Ab obesity | WC: <80 cm (♀) | WC: 80–87.9 cm (♀) | WC: ≥88 cm (♀) |
| WC: <94 cm (♂) | WC: 94–101.9 cm (♂) | WC: ≤102 cm (♂) | |
| Blood pressure | SBP < 130 mmHg | SBP: 130–139 mmHg | SBP ≥ 140 mmHg |
| DBP ≥ 90 mmHg | |||
| BP meds, or | |||
| SR hypertension | |||
| DBP < 85 mmHg | DBP: 85–89 mmHg | ||
| Glucose | Glucose < 5.6 mM | Glucose: 5.6–6.9 mM | Glucose ≥ 7 mM |
| T2D meds, or | |||
| SR diabetes | |||
| Lipids | Trig: <1.69 mM | Trig: 1.69–2.25 mM | Trig ≥ 2.26 mM |
| Chol ≥ 6.2 mM | |||
| Lipid meds, or | |||
| SR hyperlipidaemia | |||
| HDL ≥ 1.29 mM (♀) | HDL < 1.29 mM (♀) | ||
| HDL ≥ 1.04 mM (♂) | HDL < 1.04 mM (♂) | ||
| Chol: <5.2 mM | Chol: 5.2–6.1 mM |
BMI, body mass index; DBP, diastolic blood pressure; Chol, cholesterol; HDL: high density lipoprotein; SBP, systolic blood pressure; SR, self‐report; T2D, type 2 diabetes; Trig, triglycerides; meds, medications; WC, waist circumference.
Statistical analysis
Generalized linear mixed models (Proc Glimmix, SAS v9.4, Cary, NC) were used to examine differences in means and prevalence. Cox proportional hazards regression was used to estimate hazard ratios (HR) to examine differences in all‐cause mortality across metabolic risk groups adjusted for age, gender, smoking status and ethnicity. For each model, the groups were divided into healthy, preclinical and clinically elevated risk factors according to BMI, glucose, blood pressure or lipids and were then further divided into the presence or absence of the other risk factors, with the healthy–no risk factors (lowest risk) category being the referent group (HR = 1.00). Proportionality assumptions for the mortality analyses were assessed using graphical methods. All analyses allowed for random intercepts to account for variation between study samples.
To allow for comparisons with previous studies, two sets of analyses were conducted. First, the relationship between metabolic factors and mortality was assessed when metabolically healthy was defined as having zero or only one risk factor, with men and women collapsed while adjusting for age, smoking status, ethnicity and follow‐up time. Second, a single model examining the independent associations between high‐risk glucose, lipids, blood pressure and obesity was conducted. Statistical significance was set at alpha = 0.05.
Results
Characteristics of participants stratified by risk profile are shown in Table 2. Within the entire sample, 16.7% of participants were free from all preclinical or clinical metabolic abnormalities and obesity, while 52.0% of the sample had more than one metabolic risk factor or obesity. Among those with obesity, 5.8% of individuals did not present with any other risk factors. Participants who were classified as having one preclinical or clinical risk factor (i.e. glucose, blood pressure or lipid) or obesity alone ranged from 1.2 to 21.7%. During the 12.8 ± 7.2‐year follow‐up, there were 4864 (9.0%) deaths. The NoRF and Obesity Only groups tended to be younger and had the lowest incidences of death of all groups.
Table 2.
Characteristics of participants by metabolic status group
| Variable | No RF | Obesity only | Glucose only | BP only | Dyslipidaemia only | Multiple RF |
|---|---|---|---|---|---|---|
| Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| N | 9 018 | 656 | 1 702 | 2 891 | 11 712 | 28 110 |
| Age (years) | 40.2 (5.3) | 39.6 (5.3) | 44.6 (5.3)* | 48.1 (5.3)* | 42.5 (5.3)* | 49.6 (5.3)* |
| Gender (% M) | 45.0 (6.2) | 43.7 (6.4) | 58.0 (6.2)* | 59.1 (6.2)* | 48.9 (6.2)* | 59.5 (6.1)* |
| White (%) | 56.4 (10.9) | 42.3 (10.9)* | 54.6 (10.9)* | 54.9 (10.9)* | 57.6 (10.9)* | 54.9 (10.9)* |
| Smoker (%) | 20.8 (3.0) | 18.5 (3.3) | 18.9 (3.1) | 18.7 (3.0)* | 25.1 (3.0)* | 22.0 (2.9)* |
| Waist (M‐cm) | 86.4 (2.2) | 107.2 (2.3)* | 89.1 (2.2)* | 89.5 (2.2)* | 90.7 (2.2)* | 99.5 (2.2)* |
| Waist (F‐cm) | 77.7 (3.6) | 98.9 (3.6)* | 80.6 (3.6)* | 81.1 (3.6)* | 81.2 (3.6)* | 95.1 (3.6)* |
| Death (%) | 6.6 (4.5) | 4.9 (4.7) | 10.8 (4.6)* | 14.1 (4.6)* | 8.4 (4.5)* | 16.3 (4.5)* |
| Follow‐up (years) | 14.2 (3.1) | 14.4 (3.1) | 13.9 (3.1)* | 13.3 (3.1)* | 15.2 (3.1)* | 13.9 (3.1)* |
Significantly different from NoRF (P < 0.05).
F, female; M, male; RF, risk factor.
The associations between obesity, metabolic status and mortality risk are shown in Fig. 1. Obesity (HR, 95% CI = 1.10, 0.8–1.6) without other metabolic risk factors was not associated with increased mortality risk as compared to lean healthy individuals, but obesity was associated with higher risk for mortality when in combination with at least one other factor (HR range: 1.33–1.80, P < 0.05, Fig. 1) as compared to lean healthy individuals. In contrast, diabetes (HR = 1.94, 1.2–3.1), preclinical hypertension (HR = 1.36, 1.1–1.7), hypertension (HR = 1.64, 1.4–1.9) and dyslipidaemia (HR = 1.17, 1.0–1.3) alone were associated with increased HR of all‐cause mortality as compared to their healthy counterparts. Furthermore, the mortality risk for these metabolic factors in combination with other factors mortality risk was even higher (HR range: 1.22–2.58, P < 0.05). When obesity was defined using WC, abdominal obesity alone was also not significantly associated with mortality (HR = 1.24, 0.9–1.7, P = 0.16, Fig. 2) as compared to healthy low‐waist individuals.
Figure 1.
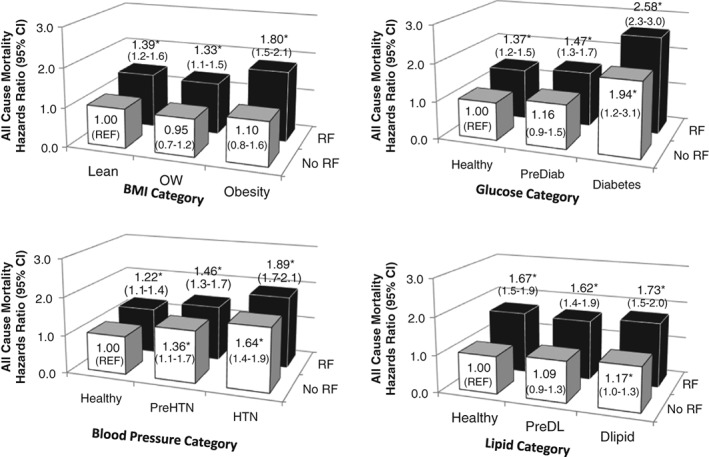
Hazards ratio of all‐cause mortality by health status. *HR significantly different from No RF (Referent, P < 0.05). Figures are adjusted for age, gender, white ethnicity, smoking status and follow‐up time. No RF = low risk for the other metabolic variables and no obesity. RF = at least one additional preclinical or clinical metabolic risk factor or obesity (Table 1). Dlipid, dyslipidaemia; HTN, hypertension; OW, overweight; PreDiab, preclinical diabetes; PreDL, preclinical Dyslipidaemia; PreHTN, preclinical hypertension.
Figure 2.
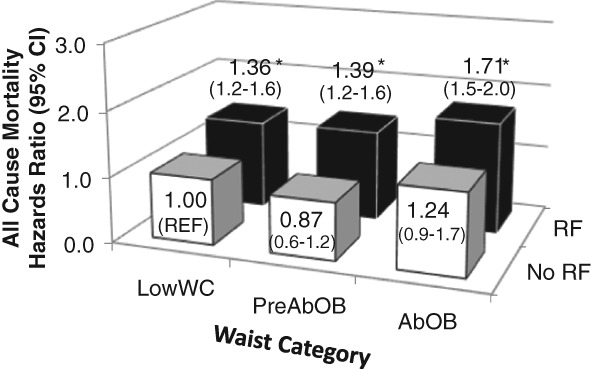
Association between waist circumference and metabolic status and all‐cause mortality risk. *HR significantly different from No RF (Referent, P < 0.05). Figures are adjusted for age, gender, white ethnicity, smoking status and follow‐up time. No RF = low risk for the metabolic variables. RF = at least one additional preclinical or clinical metabolic risk factor. PreAbOB = preclinical abdominal obesity (Men: waist circumference: 94–101.9 cm; Women: waist circumference: 80–87.9 cm). AbOB = abdominal obesity (Men: waist circumference: ≥102 cm; Women: waist circumference: ≥88 cm).
When metabolically healthy was defined as zero or one metabolic risk factor as done in previous research, metabolically healthy obesity was associated with increased mortality risk as compared to healthy lean (HR = 1.15, 1.01–1.32). In a model with all of the risk factors, obesity (HR = 1.12, 1.02–1.23), diabetes (HR = 1.13, 1.05–1.21), hypertension (HR = 1.21, 1.12–1.32) and dyslipidaemia (HR = 1.17, 1.07–1.28) were independently associated with all‐cause mortality.
Discussion
Results of this analysis illustrate that obesity in the absence of metabolic risk factors may not be associated with higher mortality risk than lean healthy individuals. This is in contrast to previous studies that have suggested that obesity is independently associated with mortality risk 14, 15, 27, 28. These differences may, in part, be related to how metabolically ‘healthy’ has been defined in the past due to constraints related to sample size as obesity in the absence of other metabolic risk factors is rare 14. In contrast with obesity, we observe that other common metabolic risk factors in isolation are associated with all‐cause mortality risk.
In the literature, the association between obesity and mortality risk independent of commonly observed comorbidities such as diabetes, hypertension or dyslipidaemia is most commonly demonstrated using a single model with statistical adjustment for the other health risk factors as continuous variables in the same model 18, 19, 20, 21, 29, 30 or as categorical variables (High and low risk) 20, 21, 29, 30. When we used statistical adjustment, we also observe that obesity, hypertension, dyslipidaemia and diabetes were all independently associated with mortality risk. When using the stratified analysis, which is more akin to risk stratification approaches in clinical care, we demonstrate that the metabolic variables in isolation, but not obesity, were associated with all‐cause mortality as compared to their healthy counterparts. We also demonstrate that preclinically elevated risk factors in isolation were not associated with mortality risk with the exception of pre‐hypertension. This is in line with the metabolic syndrome concept 17 in that the clustering of preclinical risk factors is associated with increased risk.
A systematic review and meta‐analysis by Kramer et al. 15 report that metabolically healthy obesity is associated with increased mortality risk. However, one of the major limitations of our previous work and other published research on metabolically healthy obesity is that individuals with obesity and one (or more) metabolic risk factor(s) were still considered healthy 11, 12, 13, 14, 15. This is clearly problematic as the current study demonstrates that diabetes, dyslipidaemia, hypertension and even pre‐hypertension in isolation are associated with increased mortality risk. Thus, allowing for individuals with risk factors to be included in the definition of healthy may have incorrectly inflated the mortality risk associated with this group.
Previous studies have reported that metabolically healthy obesity, defined as no risk factors, may be as low as 0.4% 22, and thus, those studies may have elected to use this more lenient definition of healthy due to sample size issues. In our study, only 1.2% of individuals presented with obesity and remained free of all clinical and preclinical risk factors. Thus, this study with over 50 000 individuals and nearly 5000 mortality events represents one of the first few sufficiently large studies to date to properly examine mortality risk in metabolically healthy obesity. In contrast to previous research, we demonstrate that obesity alone, without the presence of other preclinical or clinical metabolic risk factors, is not associated with elevated mortality risk in men or women. It is suggested by others 15 that individuals with metabolically healthy obesity are at increased mortality risk as they are more likely than their normal‐weight counterparts to transition to unhealthy over time. Our study used a single risk assessment, and changes in obesity and the risk profile over the 13 year follow‐up were not captured. However, changes in metabolic status would theoretically elevate the mortality risk with the metabolic healthy obesity group. That we saw no differences in mortality risk by BMI in the healthy group may suggest that the true mortality risk for the metabolically healthy obesity group that remains free of metabolic risk factors over time could be lower than what we report: the obesity paradox 31.
Diabetes, dyslipidaemia and hypertension have been widely accepted and regularly reviewed clinical criteria and cut‐offs for diagnosis. However, obesity has only been recently recognized as a chronic disease by the World Health Organization 32 and the American Medical Association 33. This change acknowledges the unique aetiology and health consequences of obesity that necessitates the development of appropriate medical interventions and treatments. In these reports, obesity is generally described as the presence of abnormal or excess body fat that impairs health 33. However, many current weight management guidelines do not make the distinction between excess body fat that does or does not impair health and simply diagnose obesity using a BMI greater than or equal to 30 kg m−2. There is much research suggesting that the deleterious effects of obesity are more closely associated with abdominal obesity 34. However, even when using WC, the association between obesity and mortality risk remained non‐significant, and the magnitude of the risk estimates were not substantially different using WC versus BMI. Thus, whether abdominal obesity should be central to risk criteria, such as some metabolic syndrome criteria 35 or the hypertriglyceridemic waist 36, is unclear.
Current clinical weight management guidelines prescribe weight loss for all individuals with obesity as defined by a BMI > 30 kg m−2 regardless of their metabolic status 10. If the deleterious effects of obesity occur predominantly through changes in these other metabolic risk factors, then this would support risk algorithms such as the Edmonton Obesity Staging System, which suggest a triaged approach that only recommends weight loss for individuals with obesity who have physical, functional, or psychological obesity‐related comorbidities 37. However, if abdominal or overall obesity does carry independent risk, then this would support current guidelines suggesting that weight loss should be recommended for all individuals with obesity regardless of their health profile 38. In our study sample, 6% of individuals with obesity presented without any other risk factors and were not at significantly elevated mortality risk as compared to healthy lean individuals. Thus, it is unclear whether these individuals with metabolically healthy obesity would benefit from weight loss. Furthermore, given the low success rates for obesity reduction 39, 40 and the stigma and bias experienced by those struggling with obesity 41, it may be particularly important to confirm whether obesity itself is associated with increased morbidity and mortality risk or reduced quality of life outcomes. Furthermore, whether metabolically healthy individuals with obesity benefit from weight loss in terms of physical, functional, psychological and metabolic outcomes needs to be confirmed in future research.
The strengths and limitations of this study warrant mention. First, this study uses a harmonized sample from five separate well‐established cohort studies. With this large sample, we were able to examine the mortality risk associated with obesity, glucose, blood pressure and lipids in isolation or clustered together. Although the methods used between studies were not identical and were conducted over a long time span, they did use standardized and clinically accepted methodologies for the measures used here, and the statistical analyses used accommodated for some of the potential differences between the studies. However, socioeconomic status, medications, physical activity and diet were not consistently captured between surveys and may have confounded results observed. It is unclear whether individuals with metabolically healthy obesity also had better lifestyle factors, higher socioeconomic status, better medical care or other factors that may have confounded the results. Although the models were adjusted for white ethnicity, we did not examine other ethnicities as it was not always captured in a way that would allow us to use ethnic‐specific guidelines for obesity or metabolic risk factors. Finally, this study was limited to the examination of all‐cause mortality, and it may be expected that the associations may be stronger with cardiovascular disease mortality or quality of life measures.
Summary
In conclusion, we suggest that obesity in the absence of metabolic abnormalities is not associated with increased risk for all‐cause mortality as compared to normal weight individuals. In contrast, diabetes, hypertension and dyslipidaemia in isolation and in combination are more strongly associated with increased mortality risk.
Author contributions
Each author made substantial contributions to conception and design. JLK and MR were responsible for the analysis and interpretation of data. All authors were involved in revising the manuscript critically for important intellectual content and gave final approval of the version to be published. Each author takes responsibility for the content and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of Interest Statement
No conflict of interest was declared.
Acknowledgements
The NHANES III study with mortality follow‐up was funded and conducted, and the data were made publicly available by the Centers for Disease Control and Prevention. This manuscript was prepared using research materials obtained from the NHLBI Data Repository Information Coordinating Center and does not necessarily reflect the opinions or views of the study investigators or the NHLBI. The ACLS was supported by National Institutes of Health Grants AG06945, HL62508, and DK088195 to Dr. Steven Blair. This study was supported in part by a research grant from the Canadian Institutes of Health Research to Dr. Jennifer Kuk and Dr. Chris Ardern (#131594). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1. Jacobs DR Jr, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow‐up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol 1990; 131: 32–47. [DOI] [PubMed] [Google Scholar]
- 2. Bengtsson C, Bjorkelund C, Lapidus L, Lissner L. Associations of serum lipid concentrations and obesity with mortality in women: 20 year follow up of participants in prospective population study in Gothenburg, Sweden. BMJ 1993; 307: 1385–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goldbourt U, Yaari S, Medalie JH. Isolated low HDL cholesterol as a risk factor for coronary heart disease mortality. A 21‐year follow‐up of 8000 men. Arterioscler Thromb Vasc Biol 1997; 17: 107–113. [DOI] [PubMed] [Google Scholar]
- 4. Cui Y, Blumenthal RS, Flaws JA et al Non‐high‐density lipoprotein cholesterol level as a predictor of cardiovascular disease mortality. Arch Intern Med 2001; 161: 1413–1419. [DOI] [PubMed] [Google Scholar]
- 5. Wahab NN, Cowden EA, Pearce NJ et al Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol 2002; 40: 1748–1754. [DOI] [PubMed] [Google Scholar]
- 6. von Muhlen D, Langer RD, Barrett‐Connor E. Sex and time differences in the associations of non‐high‐density lipoprotein cholesterol versus other lipid and lipoprotein factors in the prediction of cardiovascular death (The Rancho Bernardo Study). Am J Cardiol 2003; 91: 1311–1315. [DOI] [PubMed] [Google Scholar]
- 7. Hjerkinn EM, Sandvik L, Hjermann I, Arnesen H. Fasting triglycerides as a predictor of long‐term mortality in middle‐aged men with combined hyperlipidaemia. Scand J Clin Lab Invest 2003; 63: 273–278. [DOI] [PubMed] [Google Scholar]
- 8. Assmann G, Schulte H. The importance of triglycerides: results from the Prospective Cardiovascular Munster (PROCAM) Study. Eur J Epidemiol 1992; 8: 99–103. [DOI] [PubMed] [Google Scholar]
- 9. Keys A, Karvonen MJ, Punsar S et al HDL serum cholesterol and 24‐year mortality of men in Finland. Int J Epidemiol 1984; 13: 428–435. [DOI] [PubMed] [Google Scholar]
- 10. National Institutes of Health . Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res 1998; 6 Suppl.: 51S–209S. [PubMed] [Google Scholar]
- 11. Brochu M, Tchernof A, Dionne IJ et al What are the physical characteristics associated with a normal metabolic profile despite a high level of obesity in postmenopausal women? J Clin Endocrinol Metab 2001; 86: 1020–1025. [DOI] [PubMed] [Google Scholar]
- 12. Sims EA. Are there persons who are obese, but metabolically healthy? Metab Clin Exp 2001; 50: 1499–1504. [DOI] [PubMed] [Google Scholar]
- 13. Stefan N, Kantartzis K, Machann J et al Identification and characterization of metabolically benign obesity in humans. Arch Intern Med 2008; 168: 1609–1616. [DOI] [PubMed] [Google Scholar]
- 14. Kuk JL, Ardern CI. Are metabolically normal but obese individuals at lower risk for all‐cause mortality? Diabetes Care 2009; 32: 2297–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kramer CK, Zinman B, Retnakaran R. Are metabolically healthy overweight and obesity benign conditions?: a systematic review and meta‐analysis. Ann Intern Med 2013; 159: 758–769. [DOI] [PubMed] [Google Scholar]
- 16. Caleyachetty R, Thomas GN, Toulis KA et al Metabolically healthy obese and incident cardiovascular disease events among 3.5 million men and women. J Am Coll Cardiol 2017; 70: 1429–1437. [DOI] [PubMed] [Google Scholar]
- 17. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA 2001; 285: 2486–2497. [DOI] [PubMed] [Google Scholar]
- 18. Eberly LE, Prineas R, Cohen JD et al Metabolic syndrome: risk factor distribution and 18‐year mortality in the multiple risk factor intervention trial. Diabetes Care 2006; 29: 123–130. [DOI] [PubMed] [Google Scholar]
- 19. Butler J, Rodondi N, Zhu Y et al Metabolic syndrome and the risk of cardiovascular disease in older adults. J Am Coll Cardiol 2006; 47: 1595–1602. [DOI] [PubMed] [Google Scholar]
- 20. Salonen JT, Puska P. Relation of serum cholesterol and triglycerides to the risk of acute myocardial infarction, cerebral stroke and death in eastern Finnish male population. Int J Epidemiol 1983; 12: 26–31. [DOI] [PubMed] [Google Scholar]
- 21. Tverdal A, Foss OP, Leren P et al Serum triglycerides as an independent risk factor for death from coronary heart disease in middle‐aged Norwegian men. Am J Epidemiol 1989; 129: 458–465. [DOI] [PubMed] [Google Scholar]
- 22. Kuk JL, Ardern CI. Age and sex differences in the clustering of metabolic syndrome factors: association with mortality risk. Diabetes Care 2010; 33: 2457–2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. National Institutes of Health—National Heart, Lung, and Blood Institute . Coronary Artery Risk Development in Young Adults Study (CARDIA): 2014. (updated December 30, 2014). URL https://biolincc.nhlbi.nih.gov/studies/cardia/ (accessed June 1, 2016).
- 24. National Heart Lung and Blood Institute ‐ National Institutes of Health BioLINCC. Multi‐Ethnic Study of Atherosclerosis (MESA). 2018. URL https://biolincc.nhlbi.nih.gov/studies/mesa/ (accessed 2018).
- 25. Executive summary of the clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults. Arch Intern Med 1998; 158: 1855–1867. [DOI] [PubMed] [Google Scholar]
- 26. Matthews DR, Hosker JP, Rudenski AS et al Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28: 412–419. [DOI] [PubMed] [Google Scholar]
- 27. Ärnlöv J, Ingelsson E, Sundström J, Lind L. Impact of body mass index and the metabolic syndrome on the risk of cardiovascular disease and death in middle‐aged men. Circulation 2010; 121: 230–236. [DOI] [PubMed] [Google Scholar]
- 28. Hinnouho G‐M, Czernichow S, Dugravot A et al Metabolically healthy obesity and the risk of cardiovascular disease and type 2 diabetes: the Whitehall II cohort study. Eur Heart J 2015; 36: 551–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pitsavos C, Panagiotakos DB, Menotti A et al Forty‐year follow‐up of coronary heart disease mortality and its predictors: the Corfu cohort of the seven countries study. Prev Cardiol 2003; 6: 155–160. [DOI] [PubMed] [Google Scholar]
- 30. Bogers RP, Bemelmans WE, Hoogenveen RT et al Association of overweight with increased risk of coronary heart disease partly independent of blood pressure and cholesterol levels: a meta‐analysis of 21 cohort studies including more than 300 000 persons. Arch Intern Med 2007; 167: 1720–1728. [DOI] [PubMed] [Google Scholar]
- 31. Brown RE, Kuk JL. Consequences of obesity and weight loss: a devil's advocate position. Obes Rev 2015; 16: 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. James WP. WHO recognition of the global obesity epidemic. Int J Obes (Lond) 2008; 32: S120–S126. [DOI] [PubMed] [Google Scholar]
- 33. Stoner L, Cornwall J. Did the American Medical Association make the correct decision classifying obesity as a disease? Australas Med J 2014; 7: 462–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Janssen I, Katzmarzyk PT, Ross R. Waist circumference and not body mass index explains obesity‐related health risk. Am J Clin Nutr 2004; 79: 379–384. [DOI] [PubMed] [Google Scholar]
- 35. Alberti KG, Zimmet P, Shaw J. The metabolic syndrome—a new worldwide definition. Lancet 2005; 366: 1059–1062. [DOI] [PubMed] [Google Scholar]
- 36. Lemieux I, Pascot A, Couillard C et al Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation 2000; 102: 179–184. [DOI] [PubMed] [Google Scholar]
- 37. Sharma AM, Kushner RF. A proposed clinical staging system for obesity. Int J Obes (Lond) 2009; 33: 289–295. [DOI] [PubMed] [Google Scholar]
- 38. American College of Cardiology/American Heart Association Task Force on Practice Guidelines, Obesity Expert Panel. Expert panel report: guidelines (2013) for the management of overweight and obesity in adults. Obesity 2014; 22: S41–S410. [DOI] [PubMed] [Google Scholar]
- 39. Kuk JL, Wharton S. Differences in weight change trajectory patterns in a publicly funded adult weight management centre. Obes Sci Pract 2016; 2: 215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hall KD, Kahan S. Maintenance of lost weight and long‐term Management of Obesity. Med Clin North Am 2018; 102: 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Puhl RM, Moss‐Racusin CA, Schwartz MB, Brownell KD. Weight stigmatization and bias reduction: perspectives of overweight and obese adults. Health Educ Res 2008; 23: 347–358. [DOI] [PubMed] [Google Scholar]


