Abstract
Aim
To comparatively evaluate the morbidity following maxillary sinus floor elevation according to either transcrestal (tSFE) or lateral (lSFE) approach with concomitant implant placement.
Materials & Methods
Patients with ≥1 edentulous maxillary posterior site with residual bone height (RBH) of 3–6 mm were enrolled. tSFE was performed in association with a xenograft and a collagen matrix. For lSFE, the sinus was grafted with the xenograft, and the antrostomy was covered with a membrane. Implants were inserted concomitantly. The postoperative course was assessed through questionnaires. Pain level (VAS pain) was recorded using a 100‐mm visual analogue scale.
Results
Twenty‐nine and 28 patients were included in tSFE and lSFE group, respectively. On the day of surgery, VAS pain was significantly higher for tSFE compared to lSFE, and similar from day 1 to 14. tSFE was characterized by significantly lower incidence of swelling, bruising and nasal discharge/bleeding. Significantly less severe limitation in swallowing, continuing daily activities, eating, speaking, opening the mouth and going to school/work was found for tSFE only at specific postsurgery intervals.
Conclusions
lSFE was associated with lower pain on the day of surgery, and tSFE revealed lower postoperative morbidity as well as more tolerable postoperative course.
Keywords: alveolar process, bone regeneration, bone resorption, dental implants, maxillary sinus, minimally invasive, spiral cone‐beam computed tomography, surgical procedures, tooth extraction
1. INTRODUCTION
Maxillary sinus floor elevation with a lateral (lSFE) or transcrestal (tSFE) approach represent two surgical options to vertically enhance the available bone in the edentulous posterior maxilla (Lundgren et al., 2017). Both augmentation techniques are clinically effective in achieving a vertical increase in crest dimension and are associated with high implant survival rates (Corbella, Taschieri, & Del Fabbro, 2015; Del Fabbro, Corbella, Weinstein, Ceresoli, & Taschieri, 2012; Del Fabbro, Wallace, & Testori, 2013; Pjetursson, Tan, Zwahlen, & Lang, 2008; Tan, Lang, Zwahlen, & Pjetursson, 2008).
To date, some studies have comparatively evaluated the efficacy and safety of tSFE and lSFE (Al‐Almaie, Kavarodi, & Al Faidhi, 2013; Cannizzaro, Felice, Leone, Viola, & Esposito, 2009; Jurisic, Markovic, Radulovic, Brkovic, & Sándor, 2008; Kim, Park, Suh, Sohn, & Lee, 2011; Krennmair, Krainhöfner, Schmid‐Schwap, & Piehslinger, 2007; Temmerman et al., 2017; Tetsch, Tetsch, & Lysek, 2010; Yu & Qiu, 2017; Zitzmann & Schärer, 1998). Overall, both techniques were shown to have the potential to achieve substantial vertical bone augmentation with a varying degree of intra‐ and postoperative morbidity. Although some differences in morbidity were reported between the two approaches, these studies either lack of a randomized design or refer to different surgical conditions (including residual bone height, one‐two stage procedure, number of implants placed per patient) between treatments. When considering these methodological issues, it becomes difficult to extrapolate clear information on the intra‐ and postoperative morbidity of tSFE and lSFE when applied in similar clinical scenarios.
Therefore, the present randomized controlled study was performed to comparatively evaluate the postsurgery morbidity following either tSFE or lSFE with concomitant implant placement in presence of limited (3–6 mm) residual bone.
2. MATERIALS AND METHODS
2.1. Experimental design
The study is a bi‐centre, parallel‐arm, single‐blind, randomized controlled clinical trial comparing the morbidity of tSFE and lSFE. The study is part of a larger project which comparatively evaluated tSFE and lSFE under a broader perspective (including implant survival, radiographic outcomes, cost‐benefit ratio and quality of life). The experimental protocol was prepared in full accordance with guidelines for reporting randomized controlled studies (CONSORT) (http://www.consort-statement.org/), and the project was registered in http://www.clinicaltrials.gov (study ID: NCT02415946). The results of the project which are not strictly pertinent to morbidity of the investigated interventions will be published in companion papers.
2.2. Ethical aspects
The experimental protocol was approved by the Local Ethical Committees of Ferrara (protocol number: 140386) and Modena‐Reggio Emilia, Italy (protocol number: 144/14). Each patient provided a written informed consent before participation.
2.3. Study population
Patients were consecutively treated at two University‐Hospitals (Ferrara and Modena, Italy) according to the inclusion and exclusion criteria listed in Table 1. When both maxillary posterior sextants in the same patient were eligible for the study, only one quadrant was randomly selected and, therefore, regarded as “experimental.” The surgical procedures in the non‐experimental quadrant were performed at least 1 month before or after the surgical procedure in the experimental quadrant.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Patient specific
|
|
Site specific For a site to be considered as experimental (and thus included for analysis), the following criteria had to be fulfilled:
| |
| Exclusion criteria | Patient‐specific
|
Site specific
|
2.4. Treatment allocation and allocation concealment
Each eligible patient was randomly assigned to receive tSFE or lSFE according to a computer‐generated randomization list. Block randomization was applied to ensure an equal distribution of (a) treatments within each centre and (b) number of implants placed per patient among treatment groups. The assignment of eligible patients to treatment was recorded using sealed envelopes and was disclosed to each clinical operator at the end of the screening appointment. The examiners were kept blinded as to treatment allocation.
2.5. Experimental procedures
2.5.1. Pre‐surgery procedures (week −12/−1)
All included patients underwent a full‐mouth screening, and restorative, endodontic and/or periodontal treatments were performed as needed. Each patient underwent a conventional or cone‐beam computed tomography (CT and CBCT, respectively) while wearing a radiological stent fabricated on the diagnostic wax‐up of the maxillary cast. The stent included 4‐mm‐thick radiopaque indicators in the centre of each tooth and extending for the entire apico‐coronal crown height. All acquired data were saved in Digital Imaging and Communications in Medicine (DICOM) format.
Antibiotic premedication was administered to each patient 1 hr prior to the initiation of the surgical procedure.
2.5.2. Surgical procedures (day 0)
Surgical procedures were performed by trained and calibrated surgeons expert in sinus lift procedures (L.T., O.R., R.F., G.F., L.M., G.P.S., D.T.).
After infiltration anaesthesia, access to the bone crest (for patients undergoing tSFE) or to the lateral sinus wall (for patients undergoing lSFE) was accomplished via a full‐thickness mucoperiosteal flap originating from the midcrestal area or slightly to the palate if a minimal amount of keratinized tissue was present. Anterior and posterior releasing incisions were made if needed, at operator discretion.
In patients assigned to receive tSFE, the preparation of the implant site/s was performed according to the standardized sequence of instruments of the Smart Lift technique (Franceschetti, Farina, Minenna, Franceschetti, & Trombelli, 2015; Franceschetti et al., 2014, 2017; Trombelli, Franceschetti, Trisi, & Farina, 2015; Trombelli, Minenna, Franceschetti, Farina, & Minenna, 2008; Trombelli, Minenna, Franceschetti, Minenna, & Farina, 2010; Trombelli, Minenna, Franceschetti, Minenna, Itro et al., 2010; Trombelli et al., 2012, 2014) (Supporting Information Appendix Figures S1 and S2). In case of multiple, adjacent implant sites, each step of the Smart Lift technique was performed at all sites before proceeding to the next step of the sequence. After placing a plug of collagen matrix (Mucograft Seal®; Geistlich Pharma, AG, Wolhusen, Switzerland), the trephined bone core was condensed and malleted with a calibrated osteotome (Smart Lift Elevator) to fracture the sinus floor. Membrane perforation was assessed by the Valsalva manoeuvre. If no perforation was detected, a pre‐determined amount of deproteinized bovine bone mineral (DBBM; Bio‐Oss® spongiosa granules, particle size 0.25–1.0 mm; Geistlich Pharma, AG, Wolhusen, Switzerland), which was related to the programmed extent of implant penetration into the sinus (Supporting Information Appendix Table S1), was pushed through each implant site by gradual increments with the Smart Lift Elevator. When membrane perforation was detected, it was treated with repeated insertions of plugs trimmed from a collagen matrix (Mucograft Seal®; Geistlich Pharma AG, Wolhusen, Switzerland) in the apical portion of the crestal access (Supporting Information Appendix Table S2). The Valsalva manoeuvre was then re‐assessed: if negative, the grafting procedure was completed and the implant was inserted; if positive, the patient exited the study, and tSFE and concomitant implant placement were postponed at 4 months following first surgery.
In patients assigned to receive lSFE (Supporting Information Appendix Figure S3), the instruments used to create the lateral access to the maxillary sinus (i.e. rotating diamond bur, piezoelectric instruments or a combination of the two), the management of the lateral window (complete abrasion, removal or introflection into the sinus cavity), and the dimensions of the lateral window were left at operator discretion. The grafting procedure was performed with DBBM (Bio‐Oss® spongiosa granules, particle size 0.25–1.0 mm or 1–2 mm; Geistlich Pharma, AG, Wolhusen, Switzerland) immediately after the elevation of the sinus membrane with manual instruments (Hu‐Friedy, Chicago, US). The particle size and amount of graft material were left at operator discretion. Implant bed preparation was, then, performed according to the sequence of burs recommended by the implant manufacturer (Thommen Medical AG; Grenchen, Switzerland). The window in the lateral wall was filled with DBBM as well and covered with a resorbable collagen membrane (Bio‐Gide; Geistlich Pharma, AG, Wolhusen, Switzerland). When membrane perforation (as visually detected) occurred, it was treated according to Fugazzotto and Vlassis (2003) (Supporting Information Appendix Table S2), and the grafting procedure was completed. Implant placement was performed concomitantly with the sinus lift procedure if a type I or IIa perforation had occurred. Differently, in case of type IIb and III perforations, the patient exited the study, and implant placement was delayed at 9 months following surgery.
In both tSFE and lSFE groups, implants (SPI Inicell Element©; Thommen Medical AG, Grenchen, Switzerland) were inserted immediately after the completion of the grafting procedure with the 1.0‐mm polished collar above the bone crest. The healing protocol (submerged or transmucosal) was left at the operator's discretion. Flaps were closed by means of 4–0, 5–0 or 6–0 Vycril© (Ethicon, Sommerville, NY) internal mattress and interrupted sutures.
2.5.3. Postsurgery procedures
Immediately after surgery, patients undergoing lSFE received a single intra‐muscular injection of 8 mg of dexamethasone (Decadron® 8 mg, VISUFARMA S.p.A., Rome, Italy) in the masseter omolateral to the surgical procedure.
At the completion of either tSFE or lSFE, a peri‐apical radiograph was obtained. All patients were asked to abstain from self‐performed mechanical plaque control at teeth adjacent to the surgical area for 2 weeks. A mouthrinse containing chlorhexidine, an anti‐discoloration system and 0.2% hyaluronic acid (Curasept ADS Trattamento Rigenerante®; Curaden Healthcare, Saronno, Italy) was prescribed (3 rinses per day for 2 weeks). A rescue anti‐inflammatory drug (i.e. ibuprofen 600 mg tablets) was prescribed immediately after surgery, then pro re nata for the following postoperative days. Patients continued the same antibiotic regimen used for premedication up to the sixth day postsurgery. Sutures were removed at 2 weeks following surgery.
Implants placed with a submerged healing protocol at day 0 were surgically exposed (with either the elevation of a flap or a mucosal pouch performed with a mucotome) at 20 weeks postsurgery, and a healing abutment was positioned.
Implants were loaded with a provisional or definitive restoration (according to their treatment plan) between week +24 and week +32. A periapical radiograph was obtained for each implant area at the time of the prosthetic rehabilitation.
2.6. Outcome measures
2.6.1. Postsurgery complications
During the entire follow‐up period, the operator recorded the occurrence of postsurgical complications associated with the sinus lift procedure, including early implant failure, Benign Paroxysmal Positional Vertigo (BPPV), postoperative infection and haemorrhage.
2.6.2. Patient‐reported outcomes
The postoperative course was self‐reported by each patient in terms of:
level of pain (VASpain), as recorded in the evening at day 0, +1, +2, +3, +4, +7 and +14 on a 100‐mm visual analogue scale (VAS) ranging from “0–no pain” to “100–worst pain imaginable”;
dosage of rescue anti‐inflammatory drug (i.e. number of ibuprofen 600 mg tablets) and other types of medications taken from the day of surgery to the 14th postoperative day, as recorded daily on a medication diary;
level of discomfort, as recorded at day 0 (evening), +1, +2, +3, +4, +7 and +14 on a 5‐point rating scale ranging from “no discomfort” to “very high discomfort”;
limitations in daily functions (i.e., swallowing, breathing, continuing daily activities, eating, speaking, opening the mouth), as recorded at day 0 (evening), +1, +2, +3, +4, +7 and +14 on a 5‐point rating scale ranging from “no limitations” to “unable to eat a lot of types of food” (for the “eating” item), and from “not at all difficult” to “extremely difficult” (for the other items);
incidence of postoperative signs and symptoms (i.e., swelling, nausea, bruising, nasal discharge/bleeding, relevant obliteration of nostril air flow, bad taste/smell, nasal discharge and/or bleeding) at day 0 (evening), +1, +2, +3, +4, +7 and +14;
willingness to undergo the same type of surgery, recorded at day +14 on a 4‐point rating scale ranging from “no problem to repeat surgery if needed” to “I will never undergo this type of surgery again.”
2.7. Statistical analysis
2.7.1. Statistical power
As reported in the Materials and Methods, this study was part of a larger project comparing tSFE and lSFE under several aspects. As sample size calculation was based on a radiographic outcome not considered in this study, details regarding sample size calculation will be reported elsewhere. For this study, VASpain was considered as the primary outcome, and a post hoc verification of the statistical power was performed. Based on unpublished data from the study by Temmerman et al. (2017) on the current level of pain as assessed on a 100‐mm VAS at 4 hr postoperatively (standard deviation for lSFE: 16.6; standard deviation for tSFE: 29.79), a sample of 27 subjects in each treatment group had a power of 96.8% in detecting an expected inter‐group difference in VASpain of 25 with a two‐tailed test at α level of 0.05.
2.7.2. Descriptive and inferential statistics
An intention‐to‐treat (ITT) analysis was performed, including all randomized patients. The patient was regarded as the statistical unit. Therefore, for patients rehabilitated with more than one implant in the experimental quadrant, all eligible implant sites were considered for analysis and RBH was averaged to obtain a single value representative of the patient. Categorical variables were described using count and percentage. As all numerical variables showed a non‐normal and non‐symmetric distribution, they were expressed as median and interquartile range (IR). Treatment groups were compared for patient and implant characteristics, surgical aspects and study outcomes using Fisher's exact test (for categorical variables with no‐ordered categories) and Mann–Whitney U test (for numerical and ordinal variables). Moreover, a within‐group comparison was performed to evaluate the variation in VASpain with time using Friedman's test followed by a series of Wilcoxon signed rank tests for matched pairs. For each postsurgery observation interval, VASpain was compared with either its preceding value or VASpain at day 0. Bonferroni's correction for multiple comparisons was applied. The level of statistical significance was fixed at 0.05, and the analysis was performed using Stata 13 for Windows (StataCorp, College Station, TX).
3. RESULTS
3.1. Study population
Surgeries were performed between March, 2015 and December, 2016. The ITT study population consisted of 29 patients (25 receiving one implant, four receiving two adjacent implants) in the tSFE group and 28 patients (23 receiving one implant, five receiving two adjacent implants) in the lSFE group (Figure 1). No difference in age, gender and smoking status were observed between groups (Table 2).
Figure 1.
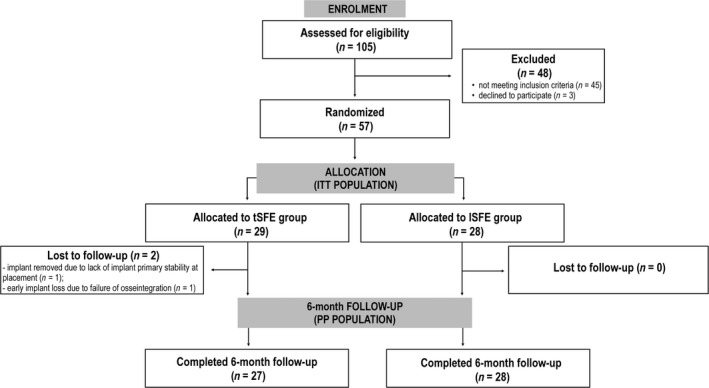
Flow chart of patient inclusion and follow‐up
Table 2.
Patient and implant characteristics in tSFE and lSFE groups
| No patients | No implants | No implants placed concomitantly with SFE per patient | Age (years) | Gender | Smoking status | RBH (mm) | Implant diameter (mm) | Implant length (mm) | |
|---|---|---|---|---|---|---|---|---|---|
| n | n | 1 implant/2 implants | Median (IR) | No males/No females | No current smokers/former smokers/never smoked | Median (IR) | Median (IR) | Median (IR) | |
| tSFE group | 29 | 33 | 25/4 | 51.0 (47.0–58.0) | 18/11 | 4/3/22 | 4.5 (4.0–5.3) | 4.0 (4.0–4.0) | 9.5 (9.5–9.5) |
| lSFE group | 28 | 33 | 23/5 | 53.0 (48.5–59.0) | 11/17 | 3/2/23 | 4.1 (4.0–4.4) | 4.0 (4.0–4.0) | 9.5 (9.5–11.0) |
| p value | 0.730 | 0.554 | 0.114 | 1 | 0.191 | 0.087 | 0.352 |
IR: inter‐quartile range; lSFE: lateral sinus floor elevation; RBH: residual bone height; tSFE: transcrestal sinus floor elevation (Smart Lift technique).
3.2. Surgical aspects of experimental procedures
RBH was 4.5 (IR 4.0–5.3) mm and 4.1 (IR 4.0–4.4) mm in the tSFE and lSFE group, respectively (p = 0.191) (Table 2). The surgical aspects of tSFE and lSFE are reported in Table 3. The dimensions of the bony window in the lSFE group were 7.0 (IR 6.0–8.0) mm (apico‐coronal height) × 10.0 (IR 9.0–12.5) mm (mesio‐distal width). When compared to tSFE, lSFE was associated with more frequent use of releasing incisions (p < 0.0001), greater dose of anaesthetic (3 vials and 2 vials; p = 0.001), greater amount of DBBM (1,975 mg and 420 mg; p < 0.0001) and longer duration of either the surgical procedure (86.0 min and 54.0 min; p < 0.0001) or the SFE procedure (54.5 min and 32.0 min; p = 0.0001) (Table 3). Implant length was 9.5 (IR 9.5–9.5) mm and 9.5 (IR 9.5–11.0) mm in the tSFE and lSFE group, respectively (p = 0.352) (Table 2).
Table 3.
Surgical aspects of the experimental procedures
| Maxillary posterior sextant | Experimental site/s | Dose of anaesthetic | Releasing incisions | Amount of graft inserted into the sinus (g)a | Technique for antrostomy | Management of the lateral bony window | Antrostomy dimensions (mm) | Duration of the surgical procedureb (min) | Duration of the experimental procedurec(min) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No right/No left | 1st premolar/2nd premolar/1st molar/2nd molar | No of vials median (IR) | No releasing incision/1 releasing incision/2 releasing incisions | Median (IR) | Rotating bur/piezoelectric instruments/combination | Abrasion/removal/introflection | Width Median (IR) | Height Median (IR) | Median (IR) | Median (IR) | |
| tSFE group (29 patients) | 14/15 | 1/6/23/3 | 2.0 (2.0–2.5) | 8/3/18 | 420.0 (350.0–500.0) | ‐ | ‐ | ‐ | ‐ | 54.0 (45.0–60.0) | 32.0 (24.0–38.0) |
| lSFE group (28 patients) | 11/17 | 0/5/25/3 | 3.0 (2.0–4.0) | 0/10/18 | 1,975.0 (1,450.0–2,500.0) | 7/18/3 | 20/0/8 | 10.0 (9.0–12.5) | 7.0 (6.0–8.0) | 86.0 (65.8–98.0) | 54.5 (39.8–65.0) |
| p value | 0.596 | 0.918 | 0.001 | <0.0001 | <0.0001 | <0.0001 | 0.0001 | ||||
IR: inter‐quartile range; lSFE: lateral sinus floor elevation; tSFE: transcrestal sinus floor elevation (Smart lift technique).
aIn the lSFE group, DBBM was used in the small (0.25–1 mm) or large (1–2 mm) granule size, at operator discretion. bAs assessed from the first incision to the completion of the suturing phase. cAs assessed from the cortical perforation with the Locator Drill to implant placement (for tSFE) or from the creation of the osteotomy on the lateral wall of the sinus to the placement of the resorbable membrane to cover the grafted sinus, thus including the grafting procedure and implant placement (for lSFE).
3.3. Postsurgery complications
In the tSFE group, one implant was immediately removed after placement due to the lack of primary stability, while one implant in another patient failed to osseointegrate and was removed at 2 months after insertion. When these complications were pooled, no significant difference in their incidence was observed between treatment groups (p = 0.491). In both cases, an implant of same dimensions was inserted 6 months later without additional bone augmentation. Periapical radiographs taken at 6 months following surgery showed that, in all cases, the endosinusal portion of the implant was completely surrounded by a radiopaque area. All implants were successfully loaded at 6 months after insertion.
The incidence of membrane perforation in the tSFE group (n = 2; 6.9%) was not significantly different from that in lSFE group (n = 5; 17.9%) (p = 0.253). In the lSFE group, three perforations were type I and two perforations were type IIa (Fugazzotto & Vlassis, 2003). In both groups, membrane perforations were treated as described in Supporting Information Appendix Table S2, and the sinus lift procedure was followed by implant placement.
In the lSFE group, one patient experienced orbital and periorbital sub‐cutaneous emphysema (OPE), which occurred immediately after roughly blowing the nose a few hours after surgery. OPE and its management have been described in details in a previous case report (Farina, Zaetta, Minenna, & Trombelli, 2016), and complete resolution was observed at 10 days after manifestation of the event.
3.4. Patient‐reported outcomes
No centre effect on postsurgery VASpain was found. A significant effect of time on VASpain was observed (p < 0.001 for both groups; Figure 2). VASpain significantly decreased compared to postsurgery from day +1 in tSFE group and from day +7 in lSFE group (Figure 2). At day 0, tSFE group showed significantly higher VASpain (p = 0.041; Figure 2) and significantly lower proportion of patients with low VASpain (p = 0.032; Supporting Information Appendix Table S3) compared to lSFE group.
Figure 2.
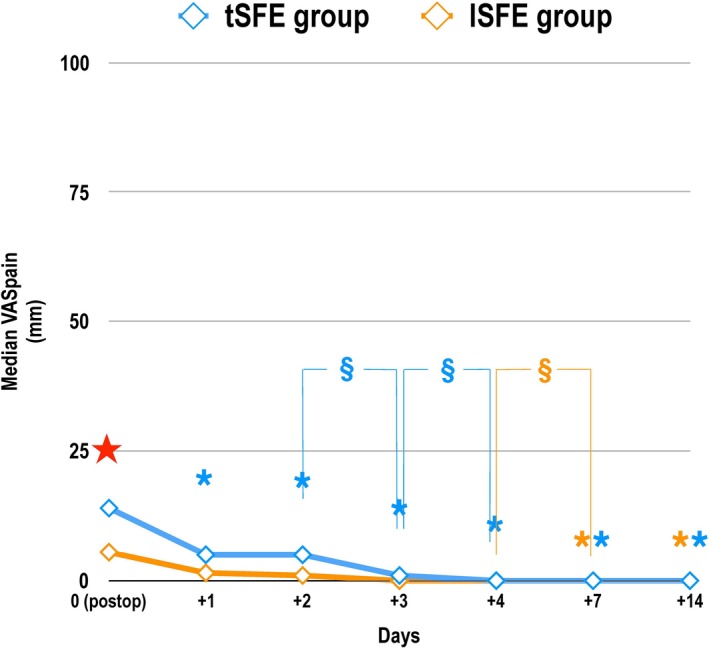
Mean severity of pain (VAS pain) as self‐reported during the first 14 postoperative days using a 100‐mm visual analogue scale (VAS) (ranging from “0–no pain” to “100–worst pain imaginable”). tSFE group: transcrestal sinus floor elevation (Smart Lift technique); lSFE: lateral sinus floor elevation. Effect of time (Friedman's test, post hoc comparisons with Wilcoxon matched pairs signed rank test and Bonferroni's correction): time had a significant effect on VAS pain in each treatment group (p < 0.001). * (orange): significant difference in VAS pain compared to day 0 within lSFE group: day +7 (p < 0.001); day +14 (p = 0.0001); * (blue): significant difference in VAS pain compared to day 0 within tSFE group: day +1 (p = 0.0009); day +2 (p = 0.0041); day +3; (p < 0.0001); day +4 (p < 0.0001); day +7 (p < 0.0001); day +14 (p < 0.0001). § (orange): significant difference in VAS pain between day +4 and day+7 (p = 0.0039) within lSFE group; § (blue): significant difference in VAS pain between day +2 and day+3 (p = 0.0013) and between day +3 and day +4 (p = 0.0018) within tSFE group. Effect of treatment (Friedman's test, Wilcoxon signed rank tests for matched pairs and Bonferroni's correction for multiple comparisons). ★ significant difference in VAS pain between groups at day 0 (p = 0.041)
The total number of analgesics during the first 2 postoperative weeks was 2.0 (IR 1.0–4.0) in the tSFE group and 3.5 (IR 1.0–6.3) in the lSFE group (p = 0.398). At day 0, a significantly different patient distribution according to the dose of analgesics was observed at day 0 (where tSFE group showed more frequent use of 2–4 tablets than lSFE group; p < 0.05) and day +3 (where lSFE group showed more frequent use of 1–3 tablets than tSFE group; p < 0.05) (Figure 3).
Figure 3.
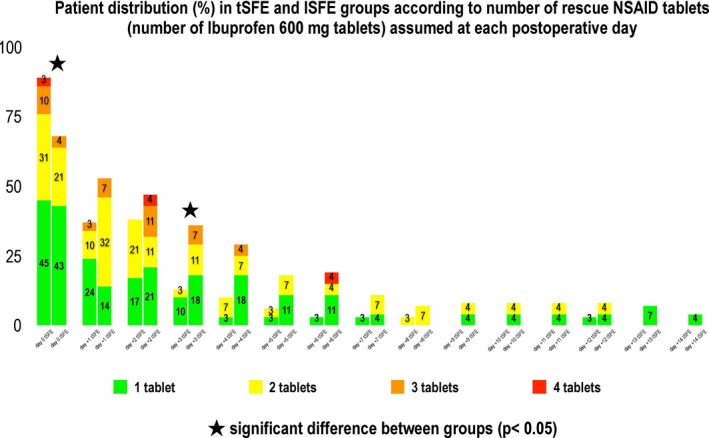
Patient distribution (%) in tSFE and lSFE groups according to number of tablets of rescue analgesics (ibuprofen 600 mg tablets) used at each postoperative day. tSFE group: transcrestal sinus floor elevation (Smart Lift technique); lSFE: lateral sinus floor elevation
A significantly different patient distribution according to the level of postoperative discomfort was observed between groups at day +2 (p = 0.014), with high or very high discomfort occurring only in lSFE group (Figure 4a).
Figure 4.
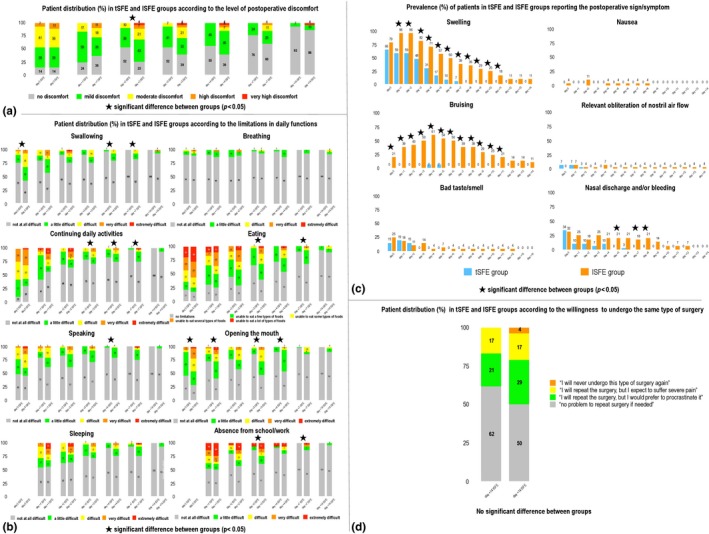
Patient distribution (%) in tSFE and lSFE groups according to the self‐reported level of postoperative discomfort (a), limitations in daily functions (b), postoperative signs and symptoms (c) and willingness to undergo the same type of surgery if needed (d). tSFE group: transcrestal sinus floor elevation (Smart Lift technique); lSFE: lateral sinus floor elevation
Patient distribution according to postoperative limitations in daily functions, symptoms and willingness to undergo the same type of surgery are reported in Figure 4b,c,d, respectively. In tSFE group, a significantly lower limitation in swallowing (day 0, +4 and +7), continuing daily activities (day +3, +4 and +7), eating (day +3 and +7), speaking (day +4), opening the mouth (day 0, +1, +3 and +4) and continuing school/work activities (day +3 and +7) was observed when compared to lSFE (p < 0.05 for all comparisons) (Figure 4b). Also, a significantly lower incidence of swelling between day +1 and +11, bruising between day 0 and +11, and nasal discharge/bleeding at day +5, +7 and +8, was self‐reported by patients in tSFE group compared to patients in lSFE group (p < 0.05 for all comparisons) (Figure 4c). No inter‐group significant difference was found in patient distribution according to their willingness to undergo the same type of surgery if needed (Figure 4d).
4. DISCUSSION
In the present study, tSFE and lSFE were compared under similar local conditions in terms of RBH (3–6 mm). To date, the wide heterogeneity (from 1 mm to >7 mm) in RBH evident in clinical trials on sinus floor elevation (Corbella et al., 2015; Del Fabbro et al., 2012) reflects the absence of clear indications in terms of available native bone for this surgical procedure, particularly when combined with simultaneous implant placement. In this study, no sites with RBH > 6 mm were included, as at these sites the placement of short implants entirely in native bone was shown to be preferable to sinus floor elevation with simultaneous placement of standard (>8 mm) implants due to a lower incidence of complications and similarly high survival rates (Fan, Li, Deng, Wu, & Zhang, 2017). Differently, while the evidence on very short (≤5 mm) implants in native bone is still insufficient, the performance of sinus floor elevation with concomitant implant placement at sites with RBH ≤ 6 mm is well documented and currently represents a standard of care in implant therapy (Lundgren et al., 2017). No sites with RBH < 3 mm were included as well, as the technique used for tSFE (i.e. the Smart Lift technique) requires a minimum RBH of 3 mm to be applied according to a modification (Trombelli et al., 2015) of the standardized sequence of instruments (Trombelli, Minenna, Franceschetti, Minenna, Itro et al., 2010; Trombelli, Minenna, Franceschetti, Minenna, & Farina, 2010).
No difference in the incidence of membrane perforation was observed between tSFE and lSFE groups. Although a higher incidence for tSFE (10%) compared to lSFE (5.6%) was previously reported in one study (Yu & Qiu, 2017), the majority of comparative clinical trials showed a lower frequency of this complication for the transcrestal approach. In particular, incidence for tSFE vs. lSFE was 21% vs. 58% (Krennmair et al., 2007), 0% vs. 10% (Cannizzaro et al., 2009), 1.5% vs. 13.4% (Tetsch et al., 2010), 4.16% vs. 6.45% (Al‐Almaie et al., 2013), and 0% vs. 15.4% (Temmerman et al., 2017), respectively. Difference in the incidence of perforations among studies and treatment groups can be explained by methodological aspects, including the extent of implant penetration and the technique to perform sinus lift (with tSFE being more relevant, as several different techniques have been used). However, it must also be considered that, except two studies where the method to assess membrane integrity consisted of probing (Cannizzaro et al., 2009) or was not explicitly stated (Tetsch et al., 2010), all the other studies mentioned above (including the present one) evaluated membrane integrity through a Valsalva manoeuvre. Although the diagnostic accuracy of the Valsalva manoeuvre (as evaluated in vivo with endoscopy) in detecting membrane perforations during tSFE procedures is presently not known, it is reasonable to speculate that the incidence of perforations during tSFE may have been to some extent underestimated as the result of some false negatives.
tSFE and lSFE resulted in low pain levels (i.e. VASpain < 15) during a 2‐week postoperative period. Pain levels recorded in lSFE group are consistent with those reported at 7 and 14 days postsurgery in the randomized trial by Baldini et al. (2017), where similar dimensions of the antrostomy (8 × 10 mm) were adopted in one study arm, and those reported during the first week postsurgery in another randomized trial (Temmerman et al., 2017). Also, consistently with the study by Temmerman et al. (2017), on the day of surgery pain levels associated with tSFE were significantly higher than those reported in the lSFE arm. Interestingly, tSFE in this study was much less painful when compared to either the osteotome group of the study by Temmerman et al. (2017), with a difference of about 40–50 on a 100‐mm VAS scale, while produced similar pain levels compared to the “intralift” group of the same study where sinus floor elevation was obtained with a combination of piezosurgery and controlled hydrodynamic pressure. It is reasonable to hypothesize that differences in pain levels are explained, at least in part, by the different technique used to perform tSFE. In particular, the tSFE technique used in the present study was associated with low VAS pain scores for pain and limited postoperative use of analgesics (Franceschetti et al., 2017; Trombelli et al., 2012, 2015). Interestingly, the pain experienced during the first postoperative week and the dose of rescue analgesics reported for tSFE were shown to be not significantly different from those reported for implant placement entirely in native bone (Franceschetti et al., 2017).
When compared to lSFE, tSFE was associated with a significantly lower incidence of swelling, bruising and nasal discharge/bleeding. The inter‐group difference in swelling was evident even though patients in lSFE group received a postoperative intra‐muscular injection of 8 mg of dexamethasone, which has been shown to exert significant effects on the control of postoperative pain and swelling following oral surgery (Nandini, 2016; Rocha‐Neto, Nogueira, Borba, Laureano‐Filho, & Vasconcelos, 2017). Swelling, bruising and nasal discharge/bleeding are frequently reported signs following sinus floor elevation (Lundgren et al., 2017; Pjetursson & Lang, 2014; Pjetursson, Rast, Bragger, Zwahlen, & Lang, 2009). The observed inter‐group difference in the incidence of swelling and bruising can be partly explained by the less frequent use of releasing incisions and the shorter duration of the surgical procedure in the tSFE group compared to the lSFE group. In this respect, previous randomized studies on the surgical extraction of third molars showed that the additional use of vertical incisions is significantly associated with increased postoperative swelling and limitation in mouth opening (Alqahtani, Khaleelahmed, & Desai, 2017; Baqain, Al‐Shafii, Hamdan, & Sawair, 2012; Kirk, Liston, Tong, & Love, 2007), and implant surgeries lasting 60 min or longer were found to be associated with significantly higher VAS scores for postoperative swelling and bruising compared to surgeries lasting less than 60 min (Tan, Krishnaswamy, Ong, & Lang, 2014). Also, the greater incidence of nasal discharge/bleeding in lSFE group can be motivated by the higher (although not significantly) incidence of membrane perforation (17.9%) observed in the same group compared to the tSFE group (7.4%).
In our study, postoperative limitations in daily functions were also recorded and analysed. At specific observation intervals, a significantly less sever limitation in swallowing, continuing daily activities, eating, speaking, opening the mouth and continuing school/work activities was found for tSFE compared to lSFE. Although the adopted items and evaluation scale may be useful to capture some relevant aspects of the morbidity of the investigated interventions and are of immediate understanding, it must be kept in consideration that this instrument has neither previous validation nor underwent a reliability assessment (e.g. test–retest) within our study population. Therefore, these findings must be considered with caution. Other validated instruments might have been used. More specifically, the Oral Health Impact Profile (OHIP) in either its short (Allen & Locker, 2002; Awad et al., 2003) or original extended version (Slade & Spencer, 1994) was designed to evaluate the impacts of oral health conditions on several domains, including functional limitation. Although the OHIP is currently accepted as one of the most powerful and validated tools for the assessment of oral health‐related quality of life and shares some items (e.g. eating, continuing daily activities) with the daily functions investigated here, the choice to design and adopt a personalized questionnaire was based on the need to punctually evaluate aspects typically related to the postoperative course of sinus lift surgery (e.g. swallowing, breathing, opening the mouth) and not specifically captured by OHIP.
Two implant failures (1 immediately after placement due to the lack of primary stability, 1 due to failure of osseointegration at 2 months) occurred in tSFE group. Although the inter‐group difference in the incidence of implant failure was not significant, it must be considered that the study is probably underpowered (due to insufficient sample size and low incidence of the complication) to detect a difference, if any, in the incidence of implant failure between groups. Therefore, these data can only be considered confirmatory of the high implant survival rates that were reported for both techniques (Corbella et al., 2015; Del Fabbro et al., 2012, 2013; Pjetursson et al., 2008; Tan et al., 2008). Also, the fact that failed implants were replaced without additional bone augmentation seems to suggest that implant failure due to absence of stability or failure to osseointegrate does not compromise the outcomes of tSFE when the latter is performed concomitantly with implant placement.
In conclusion, the results of the present study showed that, at edentulous maxillary posterior sites with residual bone height of 3–6 mm, lSFE was associated with lower pain on the day of surgery, and tSFE revealed lower postoperative morbidity (consisting of significantly lower incidence of swelling, bruising and nasal discharge/bleeding) as well as more tolerable postoperative course (characterized by significantly less severe limitation in swallowing, continuing daily activities, eating, speaking, opening the mouth and going to school/work).
CONFLICT OF INTEREST
The authors declare they have no conflict of interest related to the present study.
5.
CLINICAL RELEVANCE.
Scientific background: Limited comparative information is currently available on the intra‐ and postoperative morbidity of tSFE and lSFE when applied in similar clinical scenarios.
Principal findings: On the day of surgery, pain was significantly higher for tSFE compared to lSFE. tSFE showed significantly lower: chair time; incidence of swelling, bruising and nasal discharge/bleeding; and limitation in swallowing, continuing daily activities, eating, speaking, opening the mouth and going to school/work.
Practical implications: When compared to lSFE, tSFE may require higher doses of analgesics on the day of surgery, but is expected to have a more rapid and eventless postoperative course.
Supporting information
ACKNOWLEDGEMENTS
The study was supported by a research grant by Regione Emilia‐Romagna (Programma di Ricerca Regione‐Università, Area 1 “Ricerca Innovativa,” Bando Giovani Ricercatori “Alessandro Liberati” 2013; project PRUA1GR‐2013‐00000168), and by a research grant of the Osteology Foundation, Lucerne, Switzerland (project #13‐063).
Regenerative devices were kindly provided by Geistlich Biomaterials Italia, Thiene, Italy. Dental implants were kindly provided by Dental Trey, Fiumana‐Predappio, Italy. Mouthrinses were kindly provided by Curaden Healthcare, Saronno, Italy.
The Authors wish to thank Dr. Andy Temmerman, Section Periodontology & Oral Microbiology, Department of Oral Health Sciences, University Hospital of Leuven Belgium, for kindly providing additional information on his study (Temmerman et al., 2017).
Farina R, Franceschetti G, Travaglini D, et al. Morbidity following transcrestal and lateral sinus floor elevation: A randomized trial. J Clin Periodontol. 2018;45:1128–1139. 10.1111/jcpe.12985
Funding information
The study was supported by a research grant by Regione Emilia‐Romagna Programma di Ricerca Regione‐Università Area 1 “Ricerca Innovativa” Bando Giovani Ricercatori “Alessandro Liberati”’ 2013; project PRUA1GR‐2013‐00000168, and by a research grant of the Osteology Foundation, Lucerne, Switzerland (project #13‐063).
ClinicalTrials.gov ID: NCT02415946
REFERENCES
- Al‐Almaie, S. , Kavarodi, A. M. , & Al Faidhi, A. (2013). Maxillary sinus functions and complications with lateral window and osteotome sinus floor elevation procedures followed by dental implants placement: A retrospective study in 60 patients. The Journal of Contemporary Dental Practice, 14, 405–413. 10.5005/jp-journals-10024 [DOI] [PubMed] [Google Scholar]
- Allen, F. , & Locker, D. (2002). A modified short version of the oral health impact profile for assessing health‐related quality of life in edentulous adults. The International Journal of Prosthodontics, 15, 446–450. [PubMed] [Google Scholar]
- Alqahtani, N. A. , Khaleelahmed, S. , & Desai, F. (2017). Evaluation of two flap designs on the mandibular second molar after third molar extractions. Journal of Oral and Maxillofacial Pathology, 21, 317–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Society of Anesthesiologists . (2010). Continuum of Depth of Sedation: Definition of general anesthesia and levels of sedation/analgesia. Approved October 27, 2004, amended October 21, 2009. Retrieved 2010‐11‐29.
- Awad, M. A. , Lund, J. P. , Shapiro, S. H. , Locker, D. , Klemetti, E. , Chehade, A. , … Feine, J. S. (2003). Oral health status and treatment satisfaction with mandibular implant overdentures and conventional dentures: A randomized clinical trial in a senior population. The International Journal of Prosthodontics, 16, 390–396. [PubMed] [Google Scholar]
- Baldini, N. , D'Elia, C. , Bianco, A. , Goracci, C. , de Sanctis, M. , & Ferrari, M. (2017). Lateral approach for sinus floor elevation: Large versus small bone window ‐ a split‐mouth randomized clinical trial. Clinical Oral Implants Research, 28, 974–981. 10.1111/clr.12908 [DOI] [PubMed] [Google Scholar]
- Baqain, Z. H. , Al‐Shafii, A. , Hamdan, A. A. , & Sawair, F. A. (2012). Flap design and mandibular third molar surgery: A split mouth randomized clinical study. International Journal of Oral and Maxillofacial Surgery, 41, 1020–1024. 10.1016/j.ijom.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Cannizzaro, G. , Felice, P. , Leone, M. , Viola, P. , & Esposito, M. (2009). Early loading of implants in the atrophic posterior maxilla: Lateral sinus lift with autogenous bone and Bio‐Oss versus crestal mini sinus lift and 8‐mm hydroxyapatite‐coated implants. A randomised controlled clinical trial. European Journal of Oral Implantology, 2, 25–38. [PubMed] [Google Scholar]
- Corbella, S. , Taschieri, S. , & Del Fabbro, M. (2015). Long‐term outcomes for the treatment of atrophic posterior maxilla: A systematic review of literature. Clinical Implant Dentistry and Related Research, 17, 120–132. 10.1111/cid.12077 [DOI] [PubMed] [Google Scholar]
- Del Fabbro, M. , Corbella, S. , Weinstein, T. , Ceresoli, V. , & Taschieri, S. (2012). Implant survival rates after osteotome‐mediated maxillary sinus augmentation: A systematic review. Clinical Implant Dentistry and Related Research, 14(Suppl 1), e159–e168. 10.1111/j.1708-8208.2011.00399.x [DOI] [PubMed] [Google Scholar]
- Del Fabbro, M. , Wallace, S. S. , & Testori, T. (2013). Long‐term implant survival in the grafted maxillary sinus: A systematic review. International Journal of Periodontics and Restorative Dentistry, 33, 773–783. https://doi.org/10.11607/prd.1288 [DOI] [PubMed] [Google Scholar]
- Fan, T. , Li, Y. , Deng, W. W. , Wu, T. , & Zhang, W. (2017). Short Implants (5 to 8 mm) Versus Longer Implants (>8 mm) with Sinus Lifting in Atrophic Posterior Maxilla: A Meta‐Analysis of RCTs. Clinical Implant Dentistry and Related Research, 19, 207–215. 10.1111/cid.12432 [DOI] [PubMed] [Google Scholar]
- Farina, R. , Zaetta, A. , Minenna, L. , & Trombelli, L. (2016). Orbital and periorbital emphysema following maxillary sinus floor elevation: A case report and literature review. Journal of Oral and Maxillofacial Surgery, 74, 2192 e1‐2192.e7. 10.1016/j.joms.2016.06.186 [DOI] [PubMed] [Google Scholar]
- Franceschetti, G. , Farina, R. , Minenna, L. , Franceschetti, G. , & Trombelli, L. (2015). Learning curve of a minimally invasive technique for transcrestal sinus floor elevation: A split‐group analysis in a prospective case series with multiple clinicians. Implant Dentistry, 24, 517–526. 10.1097/ID.0000000000000270 [DOI] [PubMed] [Google Scholar]
- Franceschetti, G. , Farina, R. , Stacchi, C. , Di Lenarda, R. , Di Raimondo, R. , & Trombelli, L. (2014). Radiographic outcomes of transcrestal sinus floor elevation performed with a minimally invasive technique in smoker and non‐smoker patients. Clinical Oral Implants Research, 25, 493–499. 10.1111/clr.12188 [DOI] [PubMed] [Google Scholar]
- Franceschetti, G. , Rizzi, A. , Minenna, L. , Pramstraller, M. , Trombelli, L. , & Farina, R. (2017). Patient‐reported outcomes of implant placement performed concomitantly with transcrestal sinus floor elevation or entirely in native bone. Clinical Oral Implants Research, 28, 156–162. 10.1111/clr.12774 [DOI] [PubMed] [Google Scholar]
- Fugazzotto, P. A. , & Vlassis, J. (2003). A simplified classification and repair system for sinus membrane perforations. Journal of Periodontology, 74, 1534–1541. 10.1902/jop.2003.74.10.1534 [DOI] [PubMed] [Google Scholar]
- Jurisic, M. , Markovic, A. , Radulovic, M. , Brkovic, B. M. , & Sándor, G. K. (2008). Maxillary sinus floor augmentation: Comparing osteotome with lateral window immediate and delayed implant placements. An interim report. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics, 106, 820–827. 10.1016/j.tripleo.2008.04.025 [DOI] [PubMed] [Google Scholar]
- Kim, S. M. , Park, J. W. , Suh, J. Y. , Sohn, D. S. , & Lee, J. M. (2011). Bone‐added osteotome technique versus lateral approach for sinus floor elevation: A comparative radiographic study. Implant Dentistry, 20, 465–470. 10.1097/ID.0b013e31823545b2 [DOI] [PubMed] [Google Scholar]
- Kirk, D. G. , Liston, P. N. , Tong, D. C. , & Love, R. M. (2007). Influence of two different flap designs on incidence of pain, swelling, trismus, and alveolar osteitis in the week following third molar surgery. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics, 104, e1–e6. 10.1016/j.tripleo.2007.01.032 [DOI] [PubMed] [Google Scholar]
- Krennmair, G. , Krainhöfner, M. , Schmid‐Schwap, M. , & Piehslinger, E. (2007). Maxillary sinus lift for single implant‐supported restorations: A clinical study. International Journal of Oral and Maxillofacial Implants, 22, 351–358. [PubMed] [Google Scholar]
- Lundgren, S. , Cricchio, G. , Hallman, M. , Jungner, M. , Rasmusson, L. , & Sennerby, L. (2017). Sinus floor elevation procedures to enable implant placement and integration: Techniques, biological aspects and clinical outcomes. Periodontology 2000, 73, 103–120. 10.1111/prd.12165 [DOI] [PubMed] [Google Scholar]
- Nandini, G. D. (2016). Eventuality of dexamethasone injected intra‐massetrically on post‐operative sequel following the surgical extraction of impacted mandibular third molars: A prospective study. Journal of Maxillofacial and Oral Surgery, 15, 456–460. 10.1007/s12663-015-0847-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pjetursson, B. E. , & Lang, N. P. (2014). Sinus floor elevation utilizing the transalveolar approach. Periodontology 2000, 66, 59–71. 10.1111/prd.12043 [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Rast, C. , Bragger, U. , Zwahlen, M. , & Lang, N. P. (2009). Maxillary sinus floor elevation using the osteome technique with or without grafting material. Part I – Implant survival and patient's perception. Clinical Oral Implants Research, 20, 667–676. 10.1111/j.1600-0501.2009.01704.x [DOI] [PubMed] [Google Scholar]
- Pjetursson, B. E. , Tan, W. C. , Zwahlen, M. , & Lang, N. P. (2008). A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Journal of Clinical Periodontology, 35(8 Suppl), 216–240. 10.1111/j.1600-051X.2008.01272.x [DOI] [PubMed] [Google Scholar]
- Rocha‐Neto, A. M. , Nogueira, E. F. , Borba, P. M. , Laureano‐Filho, J. R. , & Vasconcelos, B. C. (2017). Application of Dexamethasone in the Masseter muscle during the surgical removal of lower third molars. Journal of Craniofacial Surgery, 8, e43–e47. 10.1097/SCS.0000000000003188 [DOI] [PubMed] [Google Scholar]
- Slade, G. D. , & Spencer, A. J. (1994). Development and evaluation of the Oral Health Impact Profile. Community Dental Health, 11, 3–11. [PubMed] [Google Scholar]
- Tan, W. C. , Krishnaswamy, G. , Ong, M. M. , & Lang, N. P. (2014). Patient‐reported outcome measures after routine periodontal and implant surgical procedures. Journal of Clinical Periodontology, 41, 618–624. 10.1111/jcpe.12248 [DOI] [PubMed] [Google Scholar]
- Tan, W. C. , Lang, N. P. , Zwahlen, M. , & Pjetursson, B. E. (2008). A systematic review of the success of sinus floor elevation and survival of implants inserted in combination with sinus floor elevation. Part II: Transalveolar technique. Journal of Clinical Periodontology, 35(8 Suppl), 241–254. 10.1111/j.1600-051X.2008.01273.x [DOI] [PubMed] [Google Scholar]
- Temmerman, A. , Van Dessel, J. , Cortellini, S. , Jacobs, R. , Teughels, W. , & Quirynen, M. (2017). Volumetric changes of grafted volumes and the Schneiderian membrane after transcrestal and lateral sinus floor elevation procedures: A clinical, pilot study. Journal of Clinical Periodontology, 44, 660–671. 10.1111/jcpe.12728 [DOI] [PubMed] [Google Scholar]
- Tetsch, J. , Tetsch, P. , & Lysek, D. A. (2010). Long‐term results after lateral and osteotome technique sinus floor elevation: A retrospective analysis of 2190 implants over a time period of 15 years. Clinical Oral Implants Research, 21, 497–503. 10.1111/j.1600-0501.2008.01661.x [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Franceschetti, G. , Rizzi, A. , Minenna, P. , Minenna, L. , & Farina, R. (2012). Minimally invasie transcrestal sinus floor elevation with graft biomaterials. A randomized clinical trial. Clinical Oral Implants Research, 23, 424–432. 10.1111/j.1600-0501.2011.02318.x [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Franceschetti, G. , Stacchi, C. , Minenna, L. , Riccardi, O. L. , Di Raimondo, R. , … Farina, R. (2014). Minimally‐invasive transcrestal sinus floor elevation with a deproteinized bovine bone or ß‐tricalcium phosphate: A multicenter, randomized, controlled clinical trial. Journal of Clinical Periodontology, 41, 311–319. 10.1111/jcpe.12210 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Franceschetti, G. , Trisi, P. , & Farina, R. (2015). Incremental, transcrestal sinus floor elevation with a minimally invasive technique in the rehabilitation of severe maxillary atrophy. Clinical and histological findings from a proof‐of‐concept case series. Journal of Oral and Maxillofacial Surgery, 73, 861–888. 10.1016/j.joms.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Minenna, P. , Franceschetti, G. , Farina, R. , & Minenna, L. (2008). Smart Lift: Una nuova procedura minimamente invasiva per la elevazione del pavimento del seno mascellare. Dental Cadmos, 76, 71–83. [Google Scholar]
- Trombelli, L. , Minenna, P. , Franceschetti, G. , Minenna, L. , & Farina, R. (2010). Transcrestal sinus floor elevation with a minimally invasive technique. Journal of Periodontology, 81, 158–166. 10.1902/jop.2009.090275 [DOI] [PubMed] [Google Scholar]
- Trombelli, L. , Minenna, P. , Franceschetti, G. , Minenna, L. , Itro, A. , & Farina, R. (2010). Minimally invasive technique for transcrestal sinus floor elevation: A case report. Quintessence International, 41, 363–369. [PubMed] [Google Scholar]
- Yu, H. , & Qiu, L. (2017). A prospective randomized controlled trial of two‐window versus solo‐window technique by lateral sinus floor elevation in atrophic posterior maxilla: Results from a 1‐year observational phase. Clinical Implant Dentistry and Related Research, 19, 783–792. 10.1111/cid.12505 [DOI] [PubMed] [Google Scholar]
- Zitzmann, N. U. , & Schärer, P. (1998). Sinus elevation procedures in the resorbed posterior maxilla. Comparison of the crestal and lateral approaches. Oral Surgery Oral Medicine Oral Pathology Oral Radiology and Endodontics, 85, 8–17. 10.1016/S1079-2104(98)90391-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


