Abstract
Background
The underlying reasons for the highly inconsistent clinical outcome data for omega‐3‐polyunsaturated fatty acids (n3‐PUFAs) supplementation in patients with cardiac disease have not been understood yet. The aim of this prospective, randomized, double‐blind, placebo controlled study was to determine the effects of oral treatment with n3‐PUFAs on the anti‐oxidant capacity of HDL in heart failure (HF) patients.
Methods
A total of 40 patients with advanced HF of nonischaemic origin, defined by NT‐proBNP levels of >2000 pg/mL, NYHA class III or IV and a LVEF <35% who were on stable optimized medical therapy for ≥3 months, were consecutively enrolled into this prospective, double‐blind, placebo‐controlled trial and randomized in a 1:1:1 fashion to receive 1 g/day or 4 g/day of n3‐PUFA, or placebo, respectively, for 12 weeks.
Results
After 12 weeks of treatment, the anti‐oxidant function of HDL, measured by the HDL inflammatory index, was found significantly impaired in the treatment group in a dose‐dependent fashion with 0.67 [IQR 0.49‐1.04] for placebo vs 0.71 [IQR 0.55‐1.01] for 1 g/day n3‐PUFA vs 0.98 [IQR 0.73‐1.16] for 4 g/day n3‐PUFA (P for trend = 0.018).
Conclusion
We provide evidence for an adverse effect of n3‐PUFA supplementation on anti‐oxidant function of HDL in nonischaemic heart failure patients, establishing a potential mechanistic link for the controversial outcome data on n3‐PUFA supplementation.
Keywords: heart failure, high‐density lipoprotein, n3‐PUFA
1. BACKGROUND
Omega‐3‐polyunsaturated fatty acids (n3‐PUFAs), essentially found in fish oil, are widely regarded as cardioprotective,1 although intervention trials could not ultimately confirm the beneficial effects of supplementation with n3‐PUFAs on cardiovascular health. Whereas some studies reported a reduction in mortality and cardiovascular (CV) events, others failed to show any CV benefits by n3‐PUFAs supplementation.2 In chronic heart failure (HF), a mild CV risk reduction has been observed with n3‐PUFA treatment.3 These effects have been, among others, attributed to a favourable modification of the lipid profile, including a slight rise in high‐density lipoprotein (HDL) cholesterol levels.4 As HDL cholesterol concentrations were found predictive for HF exacerbations independent of HF aetiology,5 increasing research has focused on HDL. It has become apparent that quality and function of HDL may relate more to outcome in CV diseases than serum levels.6 Particularly, the anti‐oxidant capacity of HDL was found associated with HF and clinical outcome,7 emphasizing the pivotal role of oxidative stress in the pathogenesis of HF. Whether supplementation with n3‐PUFA might modulate the anti‐oxidant function of HDL has not been analysed yet.
1.1. Aims
The aim of this analysis of a prospective, double‐blind, placebo‐controlled, randomized trial was to assess the effects of two different regimens of oral treatment with n3‐PUFA on the anti‐oxidant capacity of HDL in patients with HF of nonischaemic origin.
2. METHODS
2.1. Study design
This is a post hoc analysis of a previously conducted trial. Patients with advanced chronic HF of nonischaemic origin, defined by NT‐proBNP levels of >2000 pg/mL, NYHA class III or IV and a LVEF <35% who were on stable optimized medical therapy for ≥3 months were consecutively enrolled into this prospective, double‐blind, placebo‐controlled, randomized study at the Medical University of Vienna as previously published.8 Patients were randomized in a 1:1:1 fashion to receive 1 g/day or 4 g/day of n3‐PUFA, or placebo, respectively, for 12 weeks using a computer‐generated, permuted block randomization with a block size of four. The study and this post hoc analysis was approved by the local ethics committee and adhered to the Declaration of Helsinki. All patients provided written informed consent.
2.2. Laboratory measurements
Blood was drawn at baseline and after 12 weeks. Plasma samples were collected by immediate centrifugation (2000 g at 4°C for 10 minutes) of EDTA anticoagulated fasting venous blood. Samples were stored at −80°C until required for further analyses. Paired laboratory samples were available for 40 of 43 patients initially recruited.
HDL anti‐oxidant capacity was measured using the well validated 2′,7′‐ dichlorofluorescein (DCF)‐based cell‐free fluorescent assay that determined the ability of HDL to prevent or enhance the oxidation of low‐density lipoprotein (LDL) in a cell‐free environment.9 This test is based on the characteristics of nonfluorescent DCF diacetate (DCF‐DA) that converts into its fluorescent form (DCF) in the presence of oxidized LDL. In accordance with previous studies, apolipoprotein B‐depleted plasma, which includes HDL, apo‐A1, apo‐A2 and HDL‐associated particles, was used. The analyses were performed in all patients with available plasma samples as previously described.7
Briefly, plasma was depleted of apolipoprotein B containing lipoprotein by precipitation with polyethylene glycol. LDL (100 μg/mL, Merck, Milipore, Darmstadt, Germany) was oxidized for 6 hours in 100 μmol/L CuSO4 (Merck) at 37°C. DCF‐DA (final concentration 2.9 μg/mL), oxidized LDL (final concentration: 1.4 μg/mL) and apolipoprotein B‐depleted plasma (15 μL) were set in individual wells of 96‐well black microplates (Corning, Amsterdam, the Netherlands) and incubated with phosphate‐buffered saline. After one hour of incubation at 37°C, the fluorescence signal was measured at an excitation of 485 nm and emission wavelength of 530 nm using a Synergy H1 Hybrid Microplate Reader (Biotek, Winooski, VT). All samples were plated in duplicate. To calculate the HDL inflammatory index (HII), the fluorescence signal of DCF alone was subtracted from that of DCF incubated with apolipoprotein B depleted patient plasma and log‐transformed before analysis.7 Therefore, a higher HII indicates a poorer anti‐oxidant function.
2.3. Statistical analysis
Discrete data were described by absolute and relative frequencies and compared between groups using chi‐square tests. Continuous data were presented as medians with interquartile ranges and compared between groups using Kruskal‐Wallis tests. Changes after treatment were analysed using the Jonckheere‐Terpstra test for trend. There was no power‐calculation performed for this post hoc analysis. All calculations were carried out using the IBM SPSS statistics package v21.
3. RESULTS
Forty patients, treated with 1 g/day n3‐PUFA (n = 12), 4 g/day n3‐PUFA (n = 12), or placebo (n = 16) were included into the analysis. Baseline characteristics are shown in Table 1. At baseline, no difference in anti‐oxidant function of HDL measured by HII (0.70 [IQR 0.63‐0.97] for placebo vs 0.55 [IQR 0.53‐0.78] for 1 g/day vs 0.63 [IQR 0.55‐0.78] for 4 g/day, P = 0.470) was observed between the groups. After 12 weeks of treatment, we observed a dose‐dependent increase in median HII when compared to placebo (0.67 [IQR 0.49‐1.04] for placebo vs 0.71 [IQR 0.55‐1.01] for 1 g/day n3‐PUFA vs 0.98 [IQR 0.73‐1.16] for 4 g/day n3‐PUFA, P for tend = 0.018). Calculating the per‐patient changes, we found a significant difference in the median change of HII (ΔHII) after 12 weeks (−0.11 [IQR −0.37‐0.17] for placebo vs 0.08 [IQR 0.04‐0.27] for 1 g/day n3‐PUFA vs 0.27 [IQR 0.08‐0.50] for 4 g/day n3‐PUFA, P for trend = 0.008, Figure 1). N3‐PUFA supplementation showed no effect on serum cholesterol levels, including HDL levels. Laboratory parameters are summarized in Table 2.
Table 1.
Baseline characteristics according to treatment group (n = 40)
| Placebo n = 16 | 1 g n3‐PUFA n = 12 | 4 g n3‐PUFA n = 12 | |
|---|---|---|---|
| Age, years | 56 (46‐63) | 59 (54‐63) | 64 (58‐66) |
| Female, %(n) | 25 (4) | 17 (2) | / |
| Aetiology %(n) | |||
| Hypertension | 69 (11) | 67 (8) | 50 (6) |
| Myocarditis | 6 (1) | 8 (1) | 25 (3) |
| Valvular disease | / | / | 8 (1) |
| Alcohol | 13 (2) | / | 8 (1) |
| Other | 13 (2) | 25 (3) | 8 (1) |
| NYHA III, %(n) | 94 (15) | 83 (10) | 92 (11) |
| LVEF, % | 25 (19‐30) | 25 (22‐33) | 27 (19‐29) |
| NT‐proBNP, pg/mL | 2950 (2187‐4525) | 3017 (2269‐5306) | 3149 (2586‐4891) |
| BMI, kg/m2 | 27 (24‐31) | 26 (25‐28) | 28 (26‐30) |
| CRT, %(n) | 25 (4) | 33 (4) | 25 (3) |
| Beta blocker, %(n) | 100 (16) | 100 (12) | 100 (12) |
| ACEi/ARB, %(n) | 100 (16) | 100 (12) | 100 (12) |
| MRA, %(n) | 63 (10) | 75 (9) | 91 (11) |
| Statin, %(n) | 25 (4) | 25 (3) | 25 (3) |
| Hyperlipidemia, %(n) | 38 (6) | 33 (4) | 42 (5) |
| Diabetes, %(n) | 13 (2) | 17 (2) | 33 (4) |
| Systolic BP, mm Hg | 105 (102‐115) | 104 (97‐113) | 112 (104‐120) |
| Haemoglobin, g/dL | 12.9 (12.2‐13.6) | 13.4 (12.4‐13.8) | 14.1 (12.6‐14.7) |
| eGFR, mL/min/1.73 m2 | 70 (59‐87) | 62 (40‐82) | 77 (54‐97) |
| Triglycerides, mg/dL | 130 (96‐168) | 157 (99‐190) | 103 (78‐156) |
| Total cholesterol, mg/dL | 167 (153‐196) | 160 (141‐182) | 161 (156‐214) |
| LDL, mg/dL | 103 (93‐111) | 83 (74‐113) | 95 (88‐117) |
| HDL, mg/dL | 42 (32‐55) | 45 (32 ‐51) | 37 (33‐49) |
| AST, U/L | 25 (22‐34) | 24 (19‐29) | 27 (24‐31) |
| ALT, U/L | 27 (23‐33) | 19 (18‐22) | 25 (19‐34) |
| Fibrinogen, mg/dL | 424 (359‐502) | 416 (367‐493) | 372 (337‐441) |
| Leukocytes, G/L | 6.1 (5.5‐6.5) | 6.6 (5.6‐8) | 7.6 (6.7‐9.3) |
| CRP, mg/dL | 0.46 (0.18‐1.32) | 0.49 (0.27‐0.83) | 0.62 (0.27‐1.58) |
| HDL Inflammatory Index | 0.70 (0.63‐0.97) | 0.55 (0.53‐0.77) | 0.63 (0.55‐0.78) |
Counts are given as numbers and percentages. Continuous variables are given as median (interquartile range (IQR)).
ACEi, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BP, blood pressure; CRP, c‐reactive protein; CRT, cardiac resynchronization therapy; eGFR, estimated glomerular filtration rate calculated using Cockcroft‐Gault formula; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NYHA, New York heart association class; PUFA, polyunsaturated fatty acids.
Figure 1.
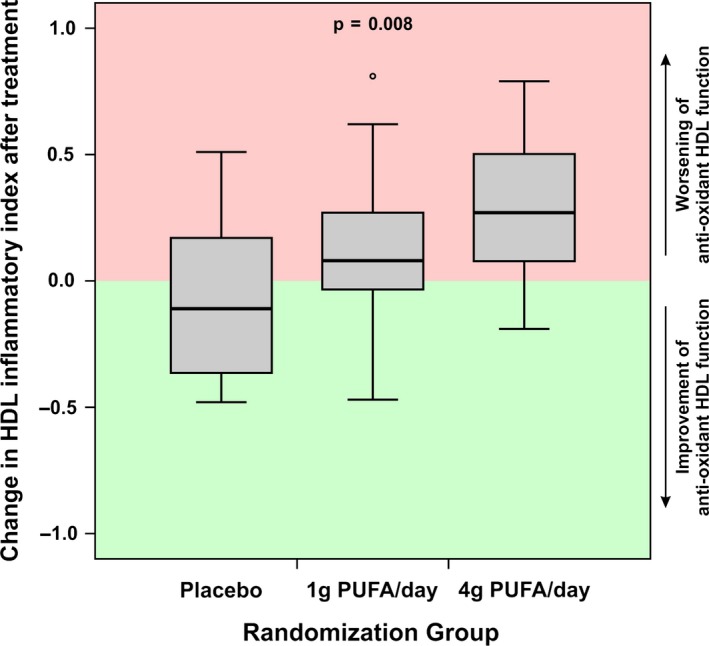
Boxplot showing the median changes of HDL Inflammatory index (ΔHII) after 12 weeks with either placebo, 1 g/day or 4 g/day n3‐PUFA
Table 2.
Laboratory values after 12 weeks of treatment with different dosages of n3‐PUFA
| Placebo n = 16 | 1 g n3‐PUFA n = 12 | 4 g n3‐PUFA n = 12 | P‐value | |
|---|---|---|---|---|
| HDL inflammatory index | 0.67 (0.49‐1.04) | 0.71 (0.55‐1.01) | 0.98 (0.73‐1.16) | 0.018 |
| Haemoglobin, g/dL | 13.1 (12‐14.5) | 13.6 (12.3‐14) | 14.5 (12.4‐15.4) | 0.125 |
| NT‐proBNP, pg/mL | 2168 (1428‐2764) | 2301 (1268‐4825) | 3603 (903‐7111) | 0.158 |
| Total cholesterol, mg/dL | 169.5 (162.3‐190.00) | 175.5 (142‐201.8) | 166 (147.5‐189.3) | 0.686 |
| HDL, mg/dL | 45.5 (37.5 ‐54) | 48 (33.5‐56) | 39.5 (32.5‐43.8) | 0.741 |
| LDL, mg/dL | 99.2 (80.3‐114.9) | 96.5 (72.8‐125.4) | 107.7 (91.3‐124.4) | 0.304 |
| Triglycerides, mg/dL | 126 (106‐154) | 145 (118‐162) | 90 (67‐124) | 0.065 |
| CRP, mg/dL | 0.54 (0.23‐0.77) | 0.40 (0.21‐0.7) | 0.38 (0.28‐0.93) | 0.505 |
| Leukocytes, G/L | 6.4 (5.8‐7.8) | 5.7 (5.4‐8.3) | 7 (6.4‐8.2) | 0.437 |
| eGFR, mL/min/1.73 m2 | 63 (47‐71) | 49 (37‐61) | 54 (46‐67) | 0.339 |
| AST, U/L | 27 (24.00‐33) | 24 (21‐29) | 32 (27‐36) | 0.358 |
| ALT, U/L | 27 (21‐32) | 23. (17‐30) | 29 (24‐45) | 0.238 |
| Fibrinogen, mg/dL | 412 (372‐475) | 415 (357‐458) | 421 (387‐443) | 0.593 |
Numbers are median (interquartile range). ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, c reactive protein; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; yGT, gamma‐glutamyl transpeptidase.
4. DISCUSSION
In this study, we evaluated the effects of n3‐PUFA treatment on anti‐oxidant HDL function in patients with heart failure on top of guideline‐directed medical therapy. We found a dose‐dependent worsening of anti‐oxidant HDL function after 12 weeks of treatment with 1 g/day or 4 g/day n3‐PUFA when compared to placebo. No effect of n3‐PUFA supplementation was observed on HDL cholesterol levels.
This is the first study evaluating the impact of n3‐PUFA supplements on HDL function in HF patients. We focused on their effect on anti‐oxidant capacities of HDL as oxidative stress is considered to play a crucial role in the pathogenesis of HF.10 Recent studies identified HDL as an important and significant prognostic factor of the anti‐oxidant defence system in HF patients.7 As n3‐PUFA supplementation was found to increase HDL cholesterol levels4 and to reduce the CV risk in HF patients,3, 11 we speculated that n3‐PUFAs may improve anti‐oxidant capacities of HDL. However, in the current cohort of HF patients, n3‐PUFA supplementation did not improve but actually weakened the anti‐oxidant capacity of HDL. The difference in HII of 0.31 between placebo and 4 g/day of PUFA is substantial. A similar difference in case–control study was shown to be predictive of the occurrence of acute myocardial infarction.12 Importantly, cardiac events appear to decrease the anti‐oxidative capacity of HDL/increase the HII,9 highlighting that the dose‐dependent increase in HII for the individual patient seen in the treatment groups in this trial is indicative of a detrimental process.
In the light of previous reports showing that treatment with n3‐PUFAs improves left ventricular function in HF by reducing ventricular remodelling and myocardial fibrosis,8, 11 our finding is rather surprising at first. However, upon a closer look, this result may provide preliminary evidence for a mechanistic link for previous conflicting study results.13 Whereas a reduction in the inflammatory burden by n3‐PUFA treatment has been confirmed by several trials, clinical outcome data are highly inconsistent. Particularly, recent meta‐analyses challenged the suggested favourable effects of n3‐PUFA on cardiovascular outcomes among high‐risk patients.14 One may speculate that the adverse effects of n3‐PUFAs on anti‐oxidant HDL function may mitigate their positive influence on ventricular remodelling and inflammatory burden, resulting in a neutral net effect on outcome. Additionally, one might speculate that the effect of n3‐PUFA on HDL function is related to the overall inflammatory burden and supplementation might be in fact beneficial, that is in the presence of coronary artery disease. However, caution needs to be exerted when interpreting the data of this trial as only patients with nonischaemic HF and NYHA classes III or IV were included and it is unknown what the effects of n3‐PUFA on HDL function in other HF populations might be. Further studies investigating this in broader populations appear warranted to test if this is indeed related to the controversial trial results.
The underlying pathophysiological mechanisms remain unclear but may include pro‐oxidant effects of n3‐PUFA. It has become evident that inflammatory and oxidative stress can critically alter HDL protein composition and quality.15 These structural changes make HDL lose its protective capacity and might even result in an adverse cardiovascular phenotype.16 There is rising evidence that n3‐PUFA supplements are highly vulnerable to oxidation leading to the generation of genotoxic and cytotoxic compounds.17 Animal studies revealed that the intestinal absorption of those pro‐oxidant molecules may trigger oxidative stress within the upper intestine in a dose‐dependent manner.17 As apolipoprotein A1, the major constituent of HDL is significantly synthesized by the upper intestine, and one may speculate that the pro‐oxidant intestinal environment could impair the protein quality. Particularly, oxidative post‐translational modifications of apolipoprotein A1 were found to crucially affect HDL functions.18 A causal relationship of n3‐PUFA supplementation and worsening of anti‐oxidant HDL function is supported by a dose‐dependent effect in the present study. This observation is in line with animal studies showing an increase in oxidative stress by n3‐PUFA supplements in a dose‐dependent fashion.17 It is worth noting that this dose dependency was not observed in most randomized clinical trials and was not associated with effect in a large meta‐analysis,14 emphasizing that the results found in our population should be interpreted carefully and seen as hypothesis generating.
4.1. Limitations
This trial is limited by its small sample size. Therefore, any conclusions should be drawn with care and regarded as primarily hypothesis generating or used as an explanation for available larger trial date.
Another limitation is that our study reflects the experience of a single tertiary centre. However, this ensures the inclusion of a homogenous patient population, a consistent quality of follow‐up procedures as well as a coherent adherence to clinical routine. Furthermore, we enrolled only patients with heart failure of nonischaemic origin. Strict inclusion criteria were applied to focus mainly on the effects of n3‐PUFA on the lipid profile in the setting of heart failure and ventricular remodelling that should not be affected by vascular inflammatory processes.
5. CONCLUSIONS
In conclusion, this double‐blind, placebo‐controlled, randomized trial reveals evidence for an adverse effect of n3‐PUFA supplementation on anti‐oxidant capacities of HDL in nonischaemic heart failure patients. This finding might provide a mechanistic link for the controversial outcome data on n3‐PUFA supplementation.
CONFLICT OF INTEREST
The authors declare no competing interests.
AUTHOR CONTRIBUTION
R. Wurm, M. Huelsmann, K. Distelmaier, D. Moertl and R. Berger were involved in designing the study. L. Schrutka and A. Hammer acquired/collected the data. RW, MH and KD analysed and interpreted the data. RW and KD drafted the manuscript. N. Pavo, I. Lang and G. Goliasch provided critical revisions to the manuscript draft. All authors have read and approved the manuscript as is.
ACKNOWLEDGEMENTS
This project has been funded by the Medical Scientific Fund of the Mayor of the City of Vienna (2015; to KD).
Wurm R, Schrutka L, Hammer A, et al. Polyunsaturated fatty acids supplementation impairs anti‐oxidant high‐density lipoprotein function in heart failure. Eur J Clin Invest. 2018;48:e12998 10.1111/eci.12998
REFERENCES
- 1. Catapano AL, Graham I, De Backer G, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. European Heart Journal. 2016;37:2999‐3058. [DOI] [PubMed] [Google Scholar]
- 2. Kimmig LM, Karalis DG. Do omega‐3 polyunsaturated fatty acids prevent cardiovascular disease? A review of the randomized clinical trials. Lipid Insights. 2013;6:13‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tavazzi L, Maggioni AP, Marchioli R, et al. Effect of n‐3 polyunsaturated fatty acids in patients with chronic heart failure (the gissi‐hf trial): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2008;372:1223‐1230. [DOI] [PubMed] [Google Scholar]
- 4. Eslick GD, Howe PR, Smith C, Priest R, Bensoussan A. Benefits of fish oil supplementation in hyperlipidemia: a systematic review and meta‐analysis. Int J Cardiol. 2009;136:4‐16. [DOI] [PubMed] [Google Scholar]
- 5. Christ M, Klima T, Grimm W, Mueller HH, Maisch B. Prognostic significance of serum cholesterol levels in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2006;27:691‐699. [DOI] [PubMed] [Google Scholar]
- 6. Khera AV, Cuchel M, de la Llera‐Moya M, et al. Cholesterol efflux capacity, high‐density lipoprotein function, and atherosclerosis. N Engl J Med. 2011;364:127‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schrutka L, Distelmaier K, Hohensinner P, et al. Impaired high‐density lipoprotein anti‐oxidative function is associated with outcome in patients with chronic heart failure. J Am Heart Assoc 2016;5:e004169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moertl D, Hammer A, Steiner S, Hutuleac R, Vonbank K, Berger R. Dose‐dependent effects of omega‐3‐polyunsaturated fatty acids on systolic left ventricular function, endothelial function, and markers of inflammation in chronic heart failure of nonischemic origin: a double‐blind, placebo‐controlled, 3‐arm study. Am Heart J. 2011;161:915. e911–919 [DOI] [PubMed] [Google Scholar]
- 9. Patel PJ, Khera AV, Jafri K, Wilensky RL, Rader DJ. The anti‐oxidative capacity of high‐density lipoprotein is reduced in acute coronary syndrome but not in stable coronary artery disease. J Am Coll Cardiol. 2011;58:2068‐2075. [DOI] [PubMed] [Google Scholar]
- 10. Okonko DO, Shah AM. Heart failure: mitochondrial dysfunction and oxidative stress in chf. Nat Rev Cardiol. 2015;12:6‐8. [DOI] [PubMed] [Google Scholar]
- 11. Heydari B, Abdullah S, Pottala JV, et al. Effect of omega‐3 acid ethyl esters on left ventricular remodeling after acute myocardial infarction: the omega‐remodel randomized clinical trial. Circulation. 2016;134:378‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Distelmaier K, Wiesbauer F, Blessberger H, et al. Impaired antioxidant hdl function is associated with premature myocardial infarction. Eur J Clin Invest. 2015;45:731‐738. [DOI] [PubMed] [Google Scholar]
- 13. Saravanan P, Davidson NC, Schmidt EB, Calder PC. Cardiovascular effects of marine omega‐3 fatty acids. Lancet. 2010;376:540‐550. [DOI] [PubMed] [Google Scholar]
- 14. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events: a systematic review and meta‐analysis. JAMA. 2012;308:1024‐1033. [DOI] [PubMed] [Google Scholar]
- 15. Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013;54:2575‐2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riwanto M, Rohrer L, Roschitzki B, et al. Altered activation of endothelial anti‐ and proapoptotic pathways by high‐density lipoprotein from patients with coronary artery disease: role of high‐density lipoprotein‐proteome remodeling. Circulation. 2013;127:891‐904. [DOI] [PubMed] [Google Scholar]
- 17. Awada M, Soulage CO, Meynier A, et al. Dietary oxidized n‐3 pufa induce oxidative stress and inflammation: role of intestinal absorption of 4‐hhe and reactivity in intestinal cells. J Lipid Res. 2012;53:2069‐2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Undurti A, Huang Y, Lupica JA, Smith JD, DiDonato JA, Hazen SL. Modification of high density lipoprotein by myeloperoxidase generates a pro‐inflammatory particle. J Biol Chem. 2009;284:30825‐30835. [DOI] [PMC free article] [PubMed] [Google Scholar]


