Abstract
Aim
The aim of the present study was to investigate the safety and efficacy of low‐dose mifepristone combined with self‐administered misoprostol for termination of early pregnancy.
Methods
A total of 533 women seeking medical abortion in early pregnancy (≤49 days since the last menstrual period) were divided randomly into hospital‐ (H‐Mis, 250) and self‐ (S‐Mis, 283) administered misoprostol groups. Women in two groups took 100 mg of oral mifepristone in hospital followed by 200 μg of sublingual misoprostol 24 h later in hospital or home. The primary outcome parameter was complete abortion without surgical intervention. Secondary outcomes were uterine bleeding, return of regular menses, side effects and patient acceptability.
Results
High rates of complete abortion were observed for both the H‐Mis group (243/250; 94.8%) and the S‐Mis group (266/283; 94.0%). No significant differences in outcomes (complete abortion/failure rates) or side effects were observed between the two groups. General satisfaction rates were similar for the two groups (H‐Mis, 231/250, 92.4%; S‐Mis, 263/283, 92.9%; P > 0.05). Higher convenience of administration (H‐Mis, 211/250, 84.4%; S‐Mis, 270/283, 95.4%; P < 0.05) and privacy protection (H‐Mis, 214/250, 85.6%; S‐Mis, 267/283, 94.3%; P < 0.05) satisfaction rates were obtained for the S‐Mis group than for the H‐Mis group.
Conclusion
Self‐administered sublingual misoprostol is as safe and effective as hospital‐administered misoprostol following low‐dose mifepristone to terminate early pregnancy (≤49 days of amenorrhoea) with fewer side effects.
Keywords: early medical abortion, low‐dose, mifepristone and misoprostol, self‐administration
Introduction
Since the introduction of mifespristone in the 1980s, its combination with misoprostol for pregnancy termination has been subjected to substantial research internationally.1, 2, 3, 4 The emergence of medical abortion regimens over the past decade has increased attention on very early termination of pregnancy. Mifepristone was found out to influence the human luteal phase endometrium by reducing stromal edema, increasing venular diameter, causing erythrocyte and leukocyte diapedesis, focal hemorrhage and degeneration of the stromal extracellular matrix, thus initiating the eventual degradation of the endometrium.5, 6, 7 The effectiveness of high‐dose mifepristone and misoprostol for abortion is well established, with the earliest medical abortion regimen involving 600 mg of mifepristone, followed 48 h later by 800 μg of misoprostol in women who are less than 49‐day pregnant, counting from the last menstrual period (LMP).8 In systematic review about nine relatively high mifepristone dosage (200 mg) plus misoprostol (400 μg) studies, adverse effects rates, such as pain, nausea and vomiting are reported high, even up to 90%.9
The current standard protocol for medical abortion calls for misoprostol to be administered in a hospital setting under medical staff supervision, in where women can leave if they are not experiencing severe discomfort. Several reports have indicated that the effectiveness and side effects of mifepristone when taken for early pregnancy termination are dose dependent.4, 5, 10, 11 Meckstrot et al. found that reducing the mifepristone dose by two thirds, from 600 to 200 mg, reduced adverse effects without reducing efficacy.12 Furthermore, in large‐scale studies, our research group has found that efficacy for very early pregnancy termination was maintained with even lower doses of mifepristone (50–150 mg) combined with low‐dose misoprostol (200–400 μg), with similar minor side effects being observed across these dose ranges and with the lower dosages resulting in less irregular uterine bleeding.1, 6 More recently, in 2015, we found that with very‐low‐dose mifepristone (50 mg) and misoprostol (200 μg) taken at the time of expected menstruation was efficacious and highly acceptable as a routine (or emergency) contraceptive.3 Women with ultrasound‐dated pregnancies of less than or equal to 6‐week LMP without signs, symptoms or risk factors for ectopic pregnancy can proceed directly to medical termination of pregnancy without the need to delay for further ultrasonography, enabling very early pregnancy termination.13
Self‐administration of an abortifacient represents a radical change for women, especially in regions where abortion is legally restricted.14 In Tunisia, Blum et al. found that medical abortion with the option of home administration of misoprostol is safe and effective; women, particularly young, unmarried women, reported a strong preference for self – rather than hospital – administered misoprostol.15 Cameron and colleagues found that when given clear instructions on how to manage a urine pregnancy test and the symptoms that mandate a medical consult, most women were able to confirm the success of early medical termination of pregnancy themselves.16 Given the burdens of unplanned pregnancies, medical abortion is highly acceptable to women, particularly with home administration.16 In this study, we evaluated the safety, efficacy and acceptability of low‐dose (100 mg) mifepristone combined with hospital versus self‐administered misoprostol (200 μg sublingual) in women undergoing medical termination of very early pregnancy (≤49 days of amenorrhoea).
Methods
Participants
This trial was conducted at The Third Affiliated Hospital of Guangzhou Medical University and approved by the local Ethics Committee of Guangzhou. Knowing, benefiting, voluntariness, privacy and timely rescueing were strictly followed ethical requirements during the study. Written consent was obtained from all participants before they participated in the study, and all participants were given information about the trial and potential risks of medical abortion using mifepristone and misoprostol. We recruited healthy women who requested legal termination of very early pregnancy in the obstetrics and gynecology department of our hospital between July of 2013 and December of 2016.
The inclusion criteria were as follows: (i) being a pregnant woman in good general health; (ii) being 18–40 years of age; (iii) having regular menstrual cycles of 25‐ to 35‐day duration over the past 6 months; (iv) less than 49‐day interval between the first day of the last menses to the day of mifepristone administration (i.e. pregnancy less than 49‐day LMP); and (v) access to a telephone. Exclusion criteria were as follows: (i) allergy to mifepristone or misoprostol; (ii) history of ectopic pregnancy; (iii) hematological disease (hemolytic illness, coagulation disease or thrombotic disorders) or end‐stage organ (e.g. heart, lung, liver and kidney) failure; (iv) having received hormone replacement therapy; (v) hemoglobin less than 90 g/L; and (vi) having become pregnant while using an intrauterine contraceptive device.
Treatment procedure
Information including the recruitment date, patient age, pregnancy/delivery history, date of LMP, date of misoprostol administration, duration of bleeding, daily blood loss (relative to regular menstrual flow) and urine chorionic gonadotropin level data were collected. In addition, participants were surveyed regarding the convenience of their medication administration. Blood samples were collected for assessment of hemoglobin and β‐human chorionic gonadotropin (β‐hCG) levels by chemiluminescent microparticle immunoassays (Abbott IrelandDiagnostics Division). Transvaginal ultrasonic examination (Voluson S8 Pro; GE Healthcare) was performed.
The participants were divided randomly into two groups: one in which the participants took misoprostol in the hospital (H‐Mis) and another in which the drug was self‐administered (S‐Mis). Women in both groups took oral mifepristone (100 mg in four pills; Zizhu Pharmaceutical) under medical supervision. Twenty‐four hours later, the women took sublingual misoprostol (200 μg in two pills; Zizhu Pharmaceutical) either in the hospital in the presence of their doctors (H‐Mis group) or at home (S‐Mis). They were asked to maintain a diary card for recording days of bleeding and the occurrence of side effects (nausea, vomiting, abdominal pain, headache, etc.). Women in the H‐Mis group were required to remain in the hospital for observed for adverse effects, including bleeding, and then they were discharged home. Food and drinks were prohibited within 2 h before and after taking the medications. Patients in the H‐Mis group were required to come back to have hospital serum β‐hCG test, while patients in the S‐Mis group were provided with home pregnancy tests (urine hCG positivity at 10 days).
To prevent serious complications due to ectopic pregnancy, incomplete abortion or failed abortion, the participants were counseled by a senior clinician to return for medical consult if they experienced continued breast tenderness, continued morning sickness, urine hCG continuous positivity 10 days after taking misoprostol, spotting for more than 10 days, severe abdominal pain or severe bleeding (more than menstrual flow for more than 3 h).1 A final telephone follow‐up was conducted after completion of a post‐treatment menstrual cycle.
The primary outcome was successful medical abortion without a need for follow‐up surgical aspiration. Alleviation of morning sickness, cessation of bleeding, negative urine β‐hCG results and patient satisfaction were secondary outcomes. Medical abortions were considered complete when the following conditions were met: greater than 50% decrease in β‐HCG levels by 10 days after misoprostol administration; ultrasonic confirmation of abortion with no intrauterine remnants; and bleeding not exceeding normal menstrual flow. In cases where patients had intrauterine remnants, bleeding in excess of normal menstrual flow or severe bleeding for more than 3 h, the medical abortion was considered incomplete. If a transvaginal ultrasound examination showed the continued presence of an intrauterine gestational sac, the patient was considered to have an ongoing pregnancy. Patients with incomplete abortions or ongoing pregnancies were treated with uterine aspiration. Patients for whom blood β‐HCG levels changed by less than 50% over 3 days were suspected of having an ectopic pregnancy, and will finally be comfirmed by transvaginal ultrasound. Satisfaction with the procedure was determined by self‐assessment of the patients at the final follow‐up. Reasons for dissatisfaction (e.g. persistent/heavy bleeding, anxiety regarding an ectopic pregnancy and intolerable side effects) were recorded.
Sample size calculation and statistical analysis
Sample size calculation indicated that a total sample of 189 subjects, with 95 patients in each group, would achieve 90% power to detect an effect size of 0.10 with χ 2 analysis at a significance level (alpha) of 0.05. Baseline characteristics and outcomes were compared between the two groups with Statistical Package for the Social Sciences 19.0 (IBM). Numerical data were expressed as means ± standard deviations (SD), and categorical data were recorded as proportions. Group results were compared with χ 2 tests; the four‐fold table χ 2 test was used to compare categorical variables, including some proportional data. P‐values less than 0.05 were considered statistically significant.
Results
Of the 600 women who received mifepristone within this study, 67 were excluded (29 H‐Mis and 38 S‐Mis). Twenty‐seven patients (11 H‐Mis and 16 S‐Mis) who decided to proceed pregnancy changed their minds and wished to keep pregnancy due to non‐occurrence of significant symptoms such as bleeding and abdominal pain. Twelve patients did not take their misoprostol (3 H‐Mis and 9 S‐Mis) due to increasing menstrual‐like bleeding. Fourteen patients did not show up to their scheduled follow‐up appointments (7 H‐Mis and 7 S‐Mis), because of abortion occurring on mifepristone alone, spontaneous miscarriage or a decision to proceed with surgical abortion. Ultimately, outcomes were analyzed for 533 women, including 250 women in the H‐Mis group and 283 women in the S‐Mis group (Figure 1).
Figure 1.
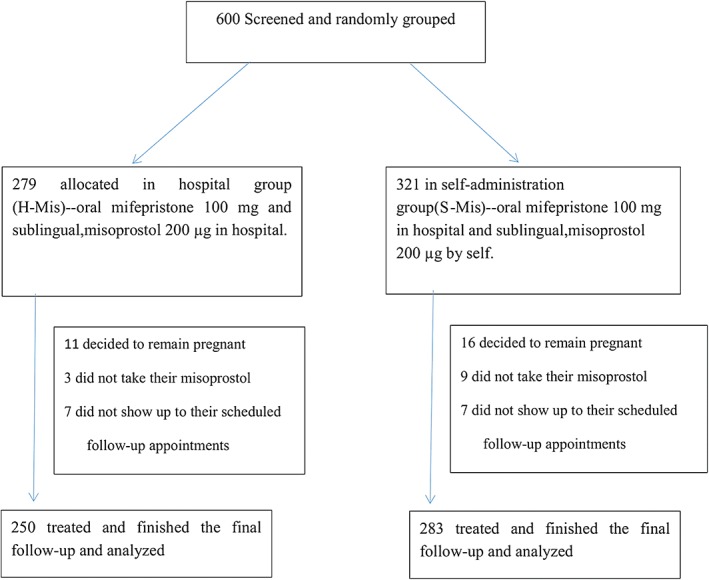
Diagram of study flow (Ms, indicates misoprostol.) Six hundred patients were enrolled in the group and were randomly grouped into the hospital and self‐administration groups and were treated with the same dose of drugs. Finally, data of 250 and 283 patients in the hospital and self‐administration groups were analyzed in our study.
The baseline characteristics of the participants in each group are presented and compared in Table 1. Briefly, patient age, length of menstrual cycle, pregnancy duration relative to LMP, β‐hCG levels and pregnancy/delivery history did not differ between the H‐Mis and S‐Mis groups.
Table 1.
Baseline characteristics of patients by group. Reported as mean ± standard deviation or median (range). No significant group differences in baseline characteristics were observed
| Characteristic | Group | P | |
|---|---|---|---|
| H‐Mis (n = 250) | S‐Mis (n = 283) | ||
| Age (years) | 27.6 ± 7.1 | 26.7 ± 8.6 | 0.657 |
| Menstrual cycle length (days) | 29.4 ± 1.6 | 30.9 ± 2.1 | 0.521 |
| β‐human chorionic gonadotropin (mIu/ml) | 13 134.2 ± 1068.6 | 12 339.3 ± 1081.1 | 0.322 |
| Duration of amenorrhea (days) | 45.2 ± 5.1 | 44.6 ± 4.9 | 0.577 |
| Gravidity | 0.5 ± 0.7 | 0.5 ± 0.6 | 0.122 |
| Parity | 0 (0–1) | 0 (0–1) | — |
Outcome data for the groups were recorded when success of abortions was confirmed (success) or further surgical treatments were needed (failed) (Table 2). The success rates for complete abortion were similarly high for the two groups (H‐Mis group 243/250; 94.8% vs S‐Mis group 266/283; 94.0%), with only 17/533 (3.2%) women overall (7/250 in H‐Mis group vs 10/283 in S‐Mis group) requiring curettage for incomplete abortion and 13/533 (2.4%) women overall (6/250 in H‐Mis group vs 7/283 in S‐Mis group) experiencing continued pregnancy post‐treatment (confirmed by transvaginal B ultrasound). There were no cases of suspected ectopic pregnancy in the study cohort. As shown in Table 2, the most common side effects experienced by the patients were abdominal pain, nausea, headache, vomiting and dizziness, with the incidences of each not differing significantly between the two groups.
Table 2.
Summary of outcomes by group. Reported as n (%) or mean ± standard deviation. No significant group differences were observed for any of these outcome parameters
| Outcome parameter | Group | P | |
|---|---|---|---|
| H‐Mis (n = 250) | S‐Mis (n = 283) | ||
| Discharged conceptus | |||
| 1–6 h after misoprostol | 232 (92.8%) | 252 (89.0%) | 0.389 |
| Greater than 6 h after misoprostol | 18 (7.2%) | 31 (11.0%) | 0.292 |
| Discharged gestational tissue | 39 (15.6%) | 46 (16.3%) | 0.733 |
| Days of bleeding | 8.91 ± 2.34 | 9.11 ± 1.89 | 0.507 |
| Efficacy | |||
| Complete abortion (success rate) | 237 (94.8%) | 266 (94.0%) | 0.472 |
| Failure rate (total) | 13 (5.2%) | 17 (6.0%) | 0.878 |
| Incomplete abortion | 7 (2.8%) | 10 (3.5%) | 0.639 |
| Ongoing pregnancy | 6 (2.4%) | 7 (2.5%) | 0.231 |
| Unscheduled reattendance | 55 (22.0%) | 61 (21.6%) | 00.100 |
| Incomplete abortion (suspicious/confirmed) | 18/7 | 21/10 | 0.355 |
| Ongoing pregnancy (suspicious/confirmed) | 19/6 | 17/7 | 0.230 |
| Ectopic pregnancy (suspicious/confirmed) | 3/0 | 2/0 | 0.658 |
| Participant preference | 15 | 21 | 0.453 |
| Adverse effects | |||
| Nausea | 99 (39.6%) | 121 (42.8%) | 0.537 |
| Vomiting | 18 (7.2%) | 21 (7.4%) | 0.768 |
| Abdominal pain | 178 (71.2%) | 212 (74.9%) | 0.255 |
| Dizziness | 37 (14.8%) | 29 (10.2%) | 0.401 |
| Headache | 23 (9.2%) | 19 (6.7%) | 0.298 |
| Satisfaction | |||
| Satisfactory overall | 231 (92.4%) | 263 (92.9%) | 0.376 |
| Convenient administration | 211 (84.4%) | 270 (95.4%) | 0.048 |
| Satisfactory privacy protection | 214 (85.6%) | 267 (94.3%) | 0.057 |
| Concerns about continued pregnancy and safety | 19 (7.6%) | 20 (7.1%) | 0.346 |
The overall patient satisfaction rate for the self‐administration medical abortion procedure in the S‐Mis group was similar to that for the in‐hospital medical abortion procedure in the H‐Mis group (Table 2). However, the satisfaction rate for convenience of administration for the S‐Mis group was significantly higher than that for the H‐Mis group; in addition, a trend of greater privacy protection satisfaction rate for the S‐Mis relative to that for the H‐Mis was observed (Table 2). Those participants in both groups who reported not being satisfied (overall, 7.3% [39/533]) cited concerns about continued pregnancy and safety as the reason for not being satisfied (Table 2).
Discussion
The present results demonstrated that self‐administration of low‐dose misoprostol following mifepristone is as safe and effective as an equivalent dose regimen with hospital‐administered misoprostol, with the H‐Mis and S‐Mis groups in this study experiencing similar success rates and occurrence rates for side effects. These findings extend our earlier findings showing that low‐dose mifepristone combined with misoprostol could is a safe and effective method of very early pregnancy termination (≤35 days of amenorrhoea) or emergency contraception.3, 6, 17
Low‐dose mifepristone combined with misoprostol
The low rates of side effects and short bleeding period observed in our study is likely attributable to our use of a low dosage, and perhaps also to the sublingual administration route employed. Sublingual administration of misoprostol is advantageous in that it precludes the inconvenience of vaginal administration and also avoids the first‐pass liver effects that are associated with oral administration, while enabling rapid pregnancy termination.18, 19
Heikinheimo et al. reported that a single low‐dosage administration of mifepristone (100 mg) yielded serum concentrations similar to those achieved with higher doses.20 Subsequently, Creinin et al.11 and Kapoor et al.21 demonstrated that 100‐mg mifepristone had effectiveness similar to that of 200‐mg mifepristone for terminating pregnancies up to 56‐day LMP. Combined mifepristone‐misoprostol regimens have been demonstrated to terminate both intrauterine and ectopic pregnancies.22, 23, 24 Furthermore, we demonstrated recently that very‐low‐dosage mifepristone (50 mg)‐misoprostol (200 μg) was useful and acceptable for not only very early pregnancy termination, but also for menstrual regulation if taken at the time of expected menstruation, with this lower dose regimen causing less irregular vaginal bleeding than higher dose regimens.3, 6 Our results are consistent with the work of other researchers recommending low doses of mifepristone for medical abortion11, 25 as well as with earlier studies indicating that some irregular bleeding is generally acceptable for abortion induction.5, 10, 11
Self‐administration advantages
Many medical abortion studies, dating as far back as 1997, have shown that abortifacients can be self‐administered safely.26, 27, 28 Nowadays, women in the USA who choose medical abortion take misoprostol at home according to a standard protocol. In a study in Vietnam and Tunisia, misoprostol efficacy and acceptability were found to be higher among home users than among patients in whom it was administered in the hospital.29 In a prospective, comparative, non‐randomized, open‐label study in Nepal, Conkling et al. found that self‐administration of vaginal misoprostol was safe and effective for termination within 63‐day LMP.30, 31
In this study, we found that home administration of low‐dose misoprostol was highly acceptable. Although the satisfaction rates for the H‐Mis and S‐Mis groups were both high, participants indicated that they preferred self‐administration owing to convenience and privacy protection. Our findings that women preferred self‐administration of misoprostol with the support and company of their partners or friends over hospital‐administered misoprostol are consistent with a prior European study reporting very high patient satisfaction with self‐administered medical abortion.32 Given the mounting evidence in favor of self‐administered mifepristone being safe and effective, some researchers have argued that this option should be offered to all women as a routine medical abortion service.31 Although the very high patient acceptability of and physician willingness to recommend self‐administered misoprostol have been linked to privacy concerns, it is also noteworthy that self‐administration can reduce treatment costs and give patients control over the timing of abortion induction.31, 33
Our study had some limitations. First, our cohort consisted only of women who had no prior experience with medical abortion. Consequently, the S‐Mis group may have been more apt to worry about the discomfort associated with the abortion (e.g. abdominal pain and bleeding) and therefore perhaps more likely to seek unnecessary follow‐up medical consultation. Given that unscheduled consults were similar between the H‐Mis and S‐Mis groups in this study, it appears that this concern was largely avoided, perhaps owing to the low‐dose regimen producing relatively low incidence rates of side effects. Second, this study was conducted at a single center; our results should be confirmed in larger multicenter studies. Third, because our secondary outcomes relied in part on patient self‐reporting, it is possible that our feedback from patients was incomplete. Finally, this study does not include long‐term outcomes, such as recurrent ectopic pregnancy or subsequent fertility.
In conclusion, the results of this study confirm the safety, efficacy, feasibility and patient acceptability of low‐dose (100 mg) mifepristone with low‐dose (200 μg), self‐administered sublingual misoprostol. These findings support the suggestion that the medical abortion regimen examined here would be appropriate to offer as an option for women seeking medical abortions.
Ethics approval and consent to participate
This trial was conducted at The Third Affiliated Hospital of Guangzhou Medical University and approved by the local Ethics Committee of Guangzhou. Written consent was obtained from all participants before they participated in the study, and all the participants were given information about the trial and potential risks of medical abortion using mifepristone and misoprostol.
Availability of data and materials
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Disclosure
This manuscript has not been published elsewhere in whole or in part and all the data is original. All authors have read and approved the content, and agree to submit it for consideration for publication in your journal. There are no ethical/legal conflicts involved in the article. The terms of this arrangement have been reviewed and approved by the Guangzhou Medical University in accordance with its policy on objectivity in research. This manuscript has been edited and proofread by Medjaden Bioscience Limited and Oxford English Edition.
Acknowledgments
Our clinical research would not have been possible without the help of all the doctors who helped to collect the original data, along with permission from the participants for ongoing research. The authors would like to express the deepest appreciation to participants who participated in this study by providing their valuable information. The authors would like to thank the staffs of the Department of Obstetrics and Gynecology in the third affiliated hospital of Guangzhou Medical University. Without their persistent help, this study would not have been possible.
References
- 1. Gardner DK, Kelley RL. Impact of the IVF laboratory environment on human preimplantation embryo phenotype. J Dev Orig Health Dis 2017; 8: 418–435. [DOI] [PubMed] [Google Scholar]
- 2. Khurana NK, Niemann H. Energy metabolism in preimplantation bovine embryos derived in vitro or in vivo . Biol Reprod 2000; 62: 847–856. [DOI] [PubMed] [Google Scholar]
- 3. Li C, Chen D, Song L et al Effectiveness and safety of lower doses of mifepristone combined with misoprostol for the termination of ultra‐early pregnancy: A dose‐ranging randomized controlled trial. Reprod Sci 2015; 22: 706–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heikinheimo O, Leminen R, Suhonen S. Termination of early pregnancy using flexible, low‐dose mifepristone‐misoprostol regimens. Contraception 2007; 76: 456–460. [DOI] [PubMed] [Google Scholar]
- 5. Anonymous . Lowering the doses of mifepristone and gameprost for early abortion: A randomised controlled trial World Health Organization Task Force on Post‐Ovulatory Methods for Fertility Regulation. BJOG 2001; 108: 738–742. [PubMed] [Google Scholar]
- 6. Li C, Chen D, Sheng X et al The lowest dosages of mifepristone and misoprostol to terminate ultra‐early pregnancy. Zhonghua Fu Chan Ke Za Zhi 2012; 47: 764–768. [PubMed] [Google Scholar]
- 7. Papp C, Schatz F, Krikun G, Hausknecht V, Lockwood CJ. Biological mechanisms underlying the clinical effects of mifepristone (RU 486) on the endometrium. Early Pregnancy 2000; 4: 230–239. [PubMed] [Google Scholar]
- 8. Grimes DA, Creinin MD. Induced abortion: An overview for internists. Ann Intern Med 2004; 140: 620–626. [DOI] [PubMed] [Google Scholar]
- 9. Ngo TD, Park MH, Shakur H, Free C. Comparative effectiveness, safety and acceptability of medical abortion at home and in a clinic: A systematic review. Bull World Health Organ 2011; 89: 360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jerbi M, Hidar S, Sahraoui W et al Mifepristone 100 mg for early medical abortion. J Gynecol Obstet Biol Reprod 2005; 34: 257–261. [DOI] [PubMed] [Google Scholar]
- 11. Creinin MD, Pymar HC, Schwartz JL. Mifepristone 100 mg in abortion regimens. Obstet Gynecol 2001; 98: 434–439. [DOI] [PubMed] [Google Scholar]
- 12. Meckstroth KR, Darney PD. Prostaglandins for first‐trimester termination. Best Pract Res Clin Obstet Gynaecol 2003; 17: 745–763. [DOI] [PubMed] [Google Scholar]
- 13. Heller R, Cameron S. Termination of pregnancy at very early gestation without visible yolk sac on ultrasound. J Fam Plann Reprod Health Care 2015; 41: 90–95. [DOI] [PubMed] [Google Scholar]
- 14. Tanuwijaya F. Abortion on law and moral perspective in Indonesia. J Law Policy Glob 2014; 28: 21. [Google Scholar]
- 15. Blum J, Hajri S, Chélli H, Mansour FB, Gueddana N, Winikoff B. The medical abortion experiences of married and unmarried women in Tunis, Tunisia. Contraception 2004; 69: 63–69. [DOI] [PubMed] [Google Scholar]
- 16. Cameron ST, Glasier A, Johnstone A, Dewart H, Campbell A. Can women determine the success of early medical termination of pregnancy themselves? Contraception 2015; 91: 6–11. [DOI] [PubMed] [Google Scholar]
- 17. Takahama K, Hoshiai H, Yajima A. Words and definition of early abortion. Nihon Funin Gakkai Zasshi 1989; 34: 297–301. [PubMed] [Google Scholar]
- 18. Tang OS, Schweer H, Lee SWH, Ho PC. Pharmacokinetics of repeated doses of misoprostol. Hum Reprod 2009; 24: 1862–1869. [DOI] [PubMed] [Google Scholar]
- 19. Dickinson JE, Jennings BG, Doherty DA. Mifepristone and oral, vaginal, or sublingual misoprostol for second‐trimester abortion: A randomized controlled trial. Obstet Gynecol 2014; 123: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 20. Heikinheimo O. Pharmacokinetics of the antiprogesterone RU 486 in women during multiple dose administration. J Steroid Biochem 1989; 32: 21–25. [DOI] [PubMed] [Google Scholar]
- 21. Kapoor G, Salhan S, Sarda N, Aggarwal D. Minimal effective dose of mifepristone for medical abortion. J Indian Med Assoc 2014; 112: 96–99. [PubMed] [Google Scholar]
- 22. Li C, Wei M, Fu M, Li M. Clinical study of terminating biochemical pregnancy and early clinical pregnancy with mifepristone and misoprostol. Zhonghua Fu Chan Ke Za Zhi 2007; 42: 542–545. [PubMed] [Google Scholar]
- 23. Xiao B, von Hertzen H, Zhao H, Piaggio G. Menstrual induction with mifepristone and misoprostol. Contraception 2003; 68: 489–494. [DOI] [PubMed] [Google Scholar]
- 24. Bygdeman M. The possibility of using mifepristone for menstrual induction. Contraception 2003; 68: 495–498. [DOI] [PubMed] [Google Scholar]
- 25. Kapp N, Borgatta L, Ellis SC, Stubblefield P. Simultaneous very low dose mifepristone and vaginal misoprostol for medical abortion. Contraception 2006; 73: 525–527. [DOI] [PubMed] [Google Scholar]
- 26. Schaff EA, Fielding SL, Westhoff C et al Vaginal misoprostol administered 1, 2, or 3 days after mifepristone for early medical abortion: A randomized trial. JAMA 2000; 284: 1948–1953. [DOI] [PubMed] [Google Scholar]
- 27. Schaff EA. Mifepristone: ten years later. Contraception 2010; 81: 1–7. [DOI] [PubMed] [Google Scholar]
- 28. Schaff EA, Stadalius LS, Eisinger SH, Franks P. Vaginal misoprostol administered at home after mifepristone (RU486) for abortion. J Fam Pract 1997; 44: 353–360. [PubMed] [Google Scholar]
- 29. Elul B, Hajri S, Ngoc NN et al Can women in less‐developed countries use a simplified medical abortion regimen? Lancet 2001; 357: 1402–1405. [DOI] [PubMed] [Google Scholar]
- 30. Shrestha A, Sedhai LB. A randomized trial of hospital vs home self administration of vaginal misoprostol for medical abortion. Kathmandu Univ Med J 2014; 12: 185–189. [DOI] [PubMed] [Google Scholar]
- 31. Conkling K, Karki C, Tuladhar H, Bracken H, Winikoff B. A prospective open‐label study of home use of mifepristone for medical abortion in Nepal. Int J Gynaecol Obstet 2015; 128: 220–223. [DOI] [PubMed] [Google Scholar]
- 32. Kopp Kallner H, Fiala C, Stephansson O, Gemzell‐Danielsson K. Home self‐administration of vaginal misoprostol for medical abortion at 50‐63 days compared with gestation of below 50 days. Hum Reprod 2010; 25: 1153–1157. [DOI] [PubMed] [Google Scholar]
- 33. Fiala C, Winikoff B, Helström L, Hellborg M, Gemzell‐Danielsson K. Acceptability of home‐use of misoprostol in medical abortion. Contraception 2004; 70: 387–392. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.


