Abstract
The photodegradation kinetics of 2‐mercaptobenzothiazole (MBT), a corrosion inhibitor for copper‐based alloys, is studied in high amorphous polyvinyl alcohol coatings subjected to either UV irradiation or indoor light exposure. The photodegradation process proceeds rapidly, thus compromising the anticorrosion ability of the coating. The encapsulation of MBT into layered double hydroxide (LDH) nanocarriers slows down its decomposition kinetics by a factor of three. Besides preserving the corrosion inhibitor, such a strategy allows a controlled release of MBT triggered by corrosion‐related stimuli, for example, presence of chloride species and acid pH. The developed coating guarantees long‐lasting corrosion protection even at low amounts of inhibitor‐loaded LDH nanocarriers (ca. 5 wt %). This also reflects in a high transparency, which makes the protective coating suitable for demanding applications, such as the conservation of high‐value metal works of art.
Keywords: coatings, corrosion, material science, nanoparticles, photodegradation
Corrosion is a natural process that leads to the gradual loss of the structural and functional properties of metal objects due to chemical reactions with the surrounding environment.1 The related financial losses have been already assessed in several studies, which concluded that premature materials degradation costs to industrialized nations up to 3–4 % of their gross domestic product.2 When considering the effects of corrosion on unique metal works of art, the detriment provoked to the whole mankind becomes invaluable.3 This motivates a constant search for effective strategies to arrest, or at least slow down, the deterioration processes related to corrosion phenomena of metal artworks. Nowadays, the application of protective organic coatings is one of the most widespread approaches for the protection of metal surfaces.4 Besides acting as a mere passive barrier that prevents the interaction with aggressive agents in the environment, the new generation of protective coatings also exhibits an active functionality. The latter is typically achieved through the addition of corrosion inhibitors, which form a thin layer of metal–inhibitor complexes covering the underlying surface and protecting it from corrosion.5 From an historical perspective, benzotriazole and related compounds have been widely studied and successfully exploited.6 Owing to concerns about their toxicity, more recently great efforts have been directed towards the identification of alternative less harmful solutions.7 Nonetheless, thanks to their outstanding efficacy, heterocyclic compounds are still among the most used corrosion inhibitors for both industrial applications and heritage conservation purposes, especially in the case of copper and copper‐based alloys.3 A critical limitation of this class of corrosion inhibitors, however, is represented by their light‐sensitivity.8 This leads to the following paradox: metals need protection against corrosion, but the protective coating itself requires to be preserved from light‐induced degradation. Up to now, however, the photochemical degradation of corrosion inhibitors has not received sufficient attention, and typically the problem is technologically overcome by using UV stabilizers. In fact, the few studies on this topic focused on the decomposition of corrosion inhibitors in solution. Hence, pending questions remain about the photodegradation of corrosion inhibitors within polymeric coatings, as well as about the anticorrosion efficacy of the resulting degradation products.8c Herein we report the first targeted study on the detrimental effect of light irradiation on the protective ability of polymeric coating containing 2‐mercaptobenzothiazole (MBT) as representative heterocyclic corrosion inhibitor for copper‐based alloys. We highlight that the decomposition kinetics upon UV or artificial light exposure is a relatively rapid process, and that the degraded molecules quickly lose their anticorrosion ability. More importantly, we also show that loading the MBT molecules into layered double hydroxide (LDH) nanocarriers is an effective way to slow down their photodegradation. To date, the use of nanocarriers has been mainly proposed to control the release and so the availability of the host inhibitor molecules.9 In such systems, the delivery of anticorrosion compounds can be triggered by external stimuli to realize protective coatings with self‐healing features.10 In our study, we exploited LDH nanocarriers since they also allow a corrosion inhibitor release that can be activated by acid pH or presence of aggressive (chloride) species. Indeed, the coupling of the preservation of the corrosion inhibitor and its controlled release ensures a long‐lasting protection of bronze surfaces at relatively low amounts of nanocarriers, making the proposed strategy also suitable for the conservation of high‐value metal works of art in which the transparency and durability of the protective layer are essential requisites.
The active coatings are based on a water‐soluble highly amorphous polyvinyl alcohol (HAVOH), which was selected because of its biodegradability and excellent oxygen barrier properties.11 Poly(allylamine) (PAA) was used as compatibilizer (PAA/HAVOH weight ratio=0.1) to improve the affinity between HAVOH and the metal substrate (Figure 1 a). The resulting coating is transparent, and the amino groups of PAA guarantee a good adhesion to the bronze surface (Figure 1 b). Zn‐Al layered double hydroxide nanoparticles loaded with MBT in anionic form (LDH/MBT) were used as stimuli‐responsive nanocarriers (Figure 1 c). Two kinds of samples were investigated, containing either 2 wt % of MBT freely dispersed in the polymer matrix or 5.5 wt % of MBT‐loaded LDH nanocarriers (corresponding to ca. 2 wt % of inhibitor), hereinafter referred to as HAVOH‐MBT and HAVOH‐LDH/MBT coatings, respectively. Additional details on the raw materials and coating preparation are reported in the Supporting Information, Section S1.
Figure 1.
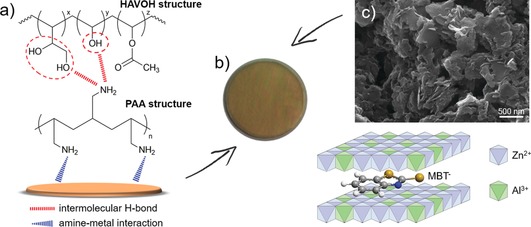
a) HAVOH and PAA chemical structures and their possible interaction with the bronze substrate. b) Picture of a coated bronze disk (PAA/HAVOH weight ratio: 0.1). c) SEM image of LDH/MBT powder and representation of the LDH/MBT structure.
The photodegradation of the corrosion inhibitor molecules was studied by monitoring over time the absorbance value of the MBT peak, A peak, in coatings exposed to UV irradiation. The maximum absorbance wavelength, λ peak, of MBT was found at 317 and 315 nm for HAVOH‐MBT and HAVOH‐LDH/MBT coatings, respectively (inset of Figure 2 a). The normalized absorbance, A N, calculated as A peak(t)/A peak(t=0), is reported in Figure 2 a as a function of the UV irradiation time, t UV, for the two investigated systems. In the investigated time window, the UV/Vis spectra of the neat HAVOH coatings remained unaltered (Supporting Information, Section S3.1), suggesting that the observed trends can be ascribed to phenomena involving the corrosion inhibitor molecules. Mere physical aging can also be excluded, since no variations in A N were noticed for coatings stored in dark conditions (Supporting Information, Section S3.2). Furthermore, possible UV‐induced interactions/reactions with other constituent of the coating were not detected (Supporting Information, Section S3.2). As a result, the decrease of A N exclusively reflects the decomposition of the heterocyclic inhibitor when the coating is subjected to UV irradiation. The MBT photodegradation advances significantly faster when the inhibitor molecules are freely dispersed within the coating than when loaded in the nanocarriers. More specifically, the half‐life time of MBT, t 1/2, in the HAVOH‐LDH/MBT coatings (t 1/2≈360 h) is more than three times longer than that in HAVOH‐MBT coatings (t 1/2≈110 h). The higher photostability of the MBT molecules once encapsulated into LDH nanocarriers is essentially due to the UV‐shielding action of the inorganic layered particles, which thus allow to sensibly slow down the photodegradation process. Besides demonstrating the protective ability of LDH nanocarriers, the data in Figure 2 a also emphasize the photoinstability of MBT molecules even when embedded into a polymeric matrix. The experiments were carried out by using long‐wave UV‐A radiation in the 350–400 nm, that is those of sunlight nearly the Earth's surface. Hence, our results confirm that photosensitive corrosion inhibitors freely dispersed in polymer matrices are not suitable for external applications. Further analyses were performed to inquire into the decomposition of MBT in indoor environments (Figure 2 b). For this purpose, the coatings were stored at ambient conditions in a room only illuminated by fluorescent lamps (Supporting Information, Section S2.2). Notably, the photodegradation kinetics of the corrosion inhibitor is relatively fast also in this condition, and the overall scenario is similar to that observed in the case of UV irradiation. The photodegradation data for UV and artificial light exposure have been satisfactorily fitted by a third‐order and a second‐order reaction model, respectively (Supporting Information, Section S3.4). This difference is related to the different wavelength range used in the two tests, as already found for photolysis of 2‐mercaptobenzothiazole in solution.8a For artificial light irradiation, the analysis of the computed reaction rate constants evidenced that the photodegradation process is slowed down three‐fold thanks to the use of LDH nanocarriers.
Figure 2.
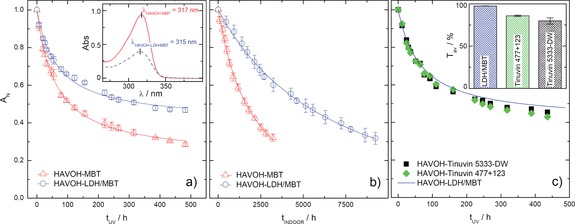
Photodegradation kinetics of MBT in HAVOH‐based coatings under a) UV irradiation and b) artificial lighting. Solid lines are guides for the eye. The inset in (a) shows representative UV/Vis spectra of HAVOH‐MBT (solid line) and HAVOH‐LDH/MBT (dashed line) coatings having the same thickness and total MBT amount. c) Photodegradation kinetics of MBT in HAVOH‐based coatings containing commercial UV absorbers/light stabilizers and HAVOH‐LDH/MBT coatings. The average transmittance of the coatings is reported in the inset.
To fully appreciate the advantages arising from the use of LDH nanocarriers, we compared their protective efficacy to that of commercial UV absorbers/light stabilizers suitable for water‐based coating formulations (Supporting Information, Section S1.3).
Figure 2 c shows that relatively high concentrations of commercial products (>15 wt %) are needed to ensure a protection efficacy comparable to that obtained by using LDH nanocarriers. Consequently, the average transmittance of the coatings, T av, is severely undermined (inset of Figure 2 c), which is often not acceptable especially for cultural heritage conservation purposes. Besides safely storing the corrosion inhibitor by protecting it from photodegradation, LDH nanocarriers also allow the controlled release of MBT molecules triggered by corrosion related stimuli, such as pH changes or presence of aggressive species. MBT release measurements were carried out on LDH dispersions in different media (Figure 3 a). An acid pH determines an increase of the MBT concentration, c MBT, due to the combination of anion exchange processes and partial dissolution of the inorganic layers of the LDH nanoparticles. The presence of chloride species also induces a boosted release of inhibitor molecules, whose amount ultimately depends on the chemical ion‐exchange equilibrium.9b More importantly, the responsive feature of the LDH nanocarriers is also preserved when they are embedded in the organic matrix, as evidenced by the evolution of UV/Vis spectra of HAVOH‐LDH/MBT coatings exposed to hydrochloric acid vapors (Figure 3 b). The value of A peak increases and λ peak shifts to higher wavelengths values (from about 315 nm to about 317 nm after 5 hours of treatment). Since the UV/Vis spectrum of MBT molecules freely dispersed within the HAVOH matrix is characterized by higher optical density and wavelength position of the maximum absorbance peak with respect to MBT encapsulated into LDH nanocarriers (inset of Figure 2 a), the combination of these two events can be regarded as a fingerprint of the inhibitor release process.
Figure 3.
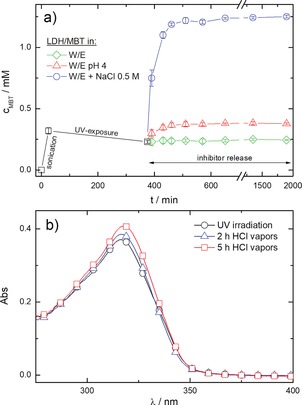
a) MBT concentration after UV irradiation of LDH dispersions and subsequent release in different water/ethanol media. b) UV/Vis spectra of UV‐irradiated HAVOH‐LDH/MBT coatings deposited on glass slides before and after 2 and 5 hours of exposition to hydrochloric acid vapors at 50 °C.
From a practical perspective, the main concern arising from the photodegradation of corrosion inhibitors is related to the eventual loss of their protective properties. We thus evaluated the effect of UV irradiation on the anticorrosion efficacy of HAVOH‐based coatings by accelerated corrosion tests, in which coated bronze disks were exposed to acid vapors. In particular, the samples were kept at 50 °C in a closed glass vessel containing 1 m HCl (additional details in the Supporting Information, Section S2.4). Hydrochloric acid was selected as model aggressive agent because chloride species are responsible for the well‐known bronze disease, that is the irreversible and nearly inexorable corrosion process that characterizes copper‐based alloys.12 In the case of UV‐irradiated HAVOH‐MBT coatings, a significant amount of corrosion products (mainly copper oxide and copper hydroxychlorides) can be recognized on the metal surface after only 30 min of accelerated corrosion treatment (Figure 4 a).
Figure 4.
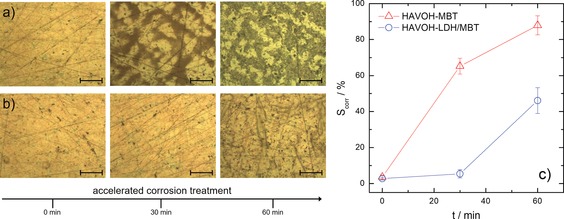
Representative optical micrographs (scale bar=20 μm) of the coating/metal interface in UV‐irradiated (two weeks exposure) bronze disks covered with a) HAVOH‐MBT and b) HAVOH‐LDH/MBT coatings before and after accelerated corrosion treatments of different duration. c) Percentage of corroded surface after accelerated corrosion treatment.
Evidently, the photodegradation of the inhibitor molecules brings about a dramatic reduction of their anticorrosion ability. More specifically, the protective efficacy against corrosion of the HAVOH‐MBT coatings extinguished after two weeks of UV irradiation or equivalently after only 5–6 months of indoor exposition, according to correlation between data in Figure 2 a and b. It thus emerges that the use of active coatings containing photosensitive corrosion inhibitors freely dispersed in the polymer matrix in real‐life applications is questionable, owing to the relatively fast loss of protective properties over time. In fact, this holds true not only for cultural heritage preservation purposes, but also for any application for which a long‐lasting anticorrosion protection is aimed. On the other hand, despite the extremely aggressive conditions adopted for the tests, the HAVOH‐LDH/MBT coating perfectly protects the bronze substrate during 30 minutes of accelerated corrosion treatment (Figure 4 b). The nanocarriers are able to safely store the MBT molecules, thus keeping the coating able to protect the underlying metal substrate. Indeed, the appearance of the disk surface is identical to that of the non‐treated system, and the percentage of corroded surface, S corr, is negligible (Figure 4 c). After one hour of accelerated treatment, S corr increases to about 50 %, which is still lower than that reached with HAVOH‐MBT coating after only 30 minutes (S corr≈65 %). The computed data in terms of percentage of corroded surface highlight the effective photodegradation resistance and durable anticorrosion efficacy of MBT molecules encapsulated into LDH nanocarriers.
In conclusion, we present the first experimental evidence of MBT photodegradation in polymeric coatings subjected to either UV exposure or artificial light irradiation. We show that the loss of the anticorrosion efficacy of the inhibitor molecules after photodegradation compromises the use of active coatings for any external or internal application in which durable performances are needed. Most importantly, we highlight that the photodegradation process is remarkably hindered by encapsulating the corrosion inhibitor into inorganic LDH nanocarriers. The MBT molecules can be then released in the coating only in the presence of corrosion‐related external stimuli. As a result, active coatings containing inhibitor‐loaded LDH nanocarriers are suitable for a long‐lasting protection of metal surfaces from corrosion. Notably, the reliable and durable anticorrosion efficacy of the developed HAVOH‐LDH/MBT coatings is coupled with high transparency. This aspect represents a crucial requisite for cultural heritage conservation purposes, especially in the case of metals with mirror‐like finishing such as those here investigated. More in general, the obtained results can motivate the development of novel approaches for the protection from photodegradation of corrosion inhibitors based on light‐sensitive heterocyclic compounds.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The work has been carried out within the NANORESTART project funded by the European Union's Horizon 2020 Research and Innovation programme under the grant agreement No 646063. The authors thank A. Aldi and F. Docimo for the technical support in the setup for the UV/Vis measurements. Dr. E. Migliore is acknowledged for graphical assistance. Dr. C. Riccucci and Dr. M. Pascucci are kindly acknowledged for helpful discussions and for optical analyses.
M. Salzano de Luna, G. G. Buonocore, C. Giuliani, E. Messina, G. Di Carlo, M. Lavorgna, L. Ambrosio, G. M. Ingo, Angew. Chem. Int. Ed. 2018, 57, 7380.
References
- 1. Revie R. W., Corrosion and Corrosion Control, Wiley, Hoboken, 2008. [Google Scholar]
- 2.
- 2a.G. H. Koch, M. P. Brongers, N. G. Thompson, Y. P. Virmani, J. H. Payer, Report No. FHWA-RD-01-156, Federal Highway Administration, Washington DC, 2002;
- 2b. Wang D., Bierwagen G. P., Prog. Org. Coat. 2009, 64, 327–338. [Google Scholar]
- 3. Dillmann P., Watkinson D., Angelini E., Adriaens A., Corrosion and conservation of cultural heritage metallic artefacts, Woodhead Publishing, 2013. [Google Scholar]
- 4. Lyon S. B., Bingham R., Mills D. J., Prog. Org. Coat. 2017, 102, 2–7. [Google Scholar]
- 5.
- 5a. Antonijevic M. M., Petrovic M. B., Int. J. Electrochem. Sci. 2008, 3, 1–28; [Google Scholar]
- 5b. Finšgar M., Milošev I., Corr. Sci. 2010, 52, 2737–2749; [Google Scholar]
- 5c. Montemor M. F., Surf. Coat. Technol. 2014, 258, 17–37. [Google Scholar]
- 6.
- 6a. Cotton J. B., Scholes I. R., Br. Corr. J. 1967, 2, 1–5; [Google Scholar]
- 6b. Finšgar M., Milošev I., Corr. Sci. 2010, 52, 2737–2749. [Google Scholar]
- 7.
- 7a. Raja P. B., Sethuraman M. G., Mater. Lett. 2008, 62, 113–116; [Google Scholar]
- 7b. Sastri V. S., Green corrosion inhibitors: theory and practice, Wiley, Hoboken, 2012; [Google Scholar]
- 7c. Verma C., Ebenso E. E., Quraishi M. A., J. Mol. Liq. 2017, 233, 403–414. [Google Scholar]
- 8.
- 8a. Malouki M. A., Richard C., Zertal A., J. Photochem. Photobiol. A 2004, 167, 121–126; [Google Scholar]
- 8b. Allaoui A., Malouki M. A., Wong-Wah-Chung P., J. Photochem. Photobiol. A 2010, 212, 153–160; [Google Scholar]
- 8c. Serdechnova M., Ivanov V. L., Domingues M. R. M., Evtuguin D. V., Ferreira M. G., Zheludkevich M. L., Phys. Chem. Chem. Phys. 2014, 16, 25152–25160. [DOI] [PubMed] [Google Scholar]
- 9.
- 9a. Abdullayev E., Abbasov V., Tursunbayeva A., Portnov V., Ibrahimov H., Mukhtarova G., Lvov Y., ACS Appl. Mater. Interfaces 2013, 5, 4464–4471; [DOI] [PubMed] [Google Scholar]
- 9b. Poznyak S. K., Tedim J., Rodrigues L. M., Salak A. N., Zheludkevich M. L., Dick L. F. P., Ferreira M. G. S., ACS Appl. Mater. Interfaces 2009, 1, 2353–2362; [DOI] [PubMed] [Google Scholar]
- 9c. Tedim J., Poznyak S. K., Kuznetsova A., Raps D., Hack T., Zheludkevich M. L., Ferreira M. G. S., ACS Appl. Mater. Interfaces 2010, 2, 1528–1535; [DOI] [PubMed] [Google Scholar]
- 9d. Shchukin D. G., Sukhorukov G. B., Möhwald H., Angew. Chem. Int. Ed. 2003, 42, 4472–4475; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 4610–4613; [Google Scholar]
- 9e. Fu J., Chen T., Wang M., Yang N., Li S., Wang Y., Liu X., ACS Nano 2013, 7, 11397–11408; [DOI] [PubMed] [Google Scholar]
- 9f. Chen T., Chen R., Jin Z., Liu J., J. Mater. Chem. A 2015, 3, 9510–9516. [Google Scholar]
- 10.
- 10a. Zheludkevich M. L., Shchukin D. G., Yasakau K. A., Möhwald H., Ferreira M. G., Chem. Mater. 2007, 19, 402–411; [Google Scholar]
- 10b. Shchukin D. G., Polym. Chem. 2013, 4, 4871–4877; [Google Scholar]
- 10c. Herrmann S., Kostrzewa M., Wierschem A., Streb C., Angew. Chem. Int. Ed. 2014, 53, 13596–13599; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 13814–13817; [Google Scholar]
- 10d. Wei H., Wang Y., Guo J., Shen N. Z., Jiang D., Zhang X., Zhu J., Wang Q., Shao L., Lin H., Wei S., Guo Z., J. Mater. Chem. A 2015, 3, 469–480. [Google Scholar]
- 11.
- 11a. Yan N., Capezzuto F., Buonocore G. G., Lavorgna M., Xia H., Ambrosio L., ACS Appl. Mater. Interfaces 2015, 7, 22678–22685; [DOI] [PubMed] [Google Scholar]
- 11b. Donato K. Z., Lavorgna M., Donato R. K., Raucci M. G., Buonocore G. G., Ambrosio L., Schrekker H. S., Mauler R. S., ACS Sustainable Chem. Eng. 2017, 5, 1094–1105. [Google Scholar]
- 12. Scott D. A., J. Am. Inst. Conserv. 1990, 29, 193–206. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


