Abstract
To determine the prevalence of asymptomatic dengue virus-infected blood donors during the largest dengue outbreak in Taiwan history occurred in 2015, we examined the evidence of dengue virus (DENV) infection by the detection of DENV RNA genome using real-time reverse transcription-polymerase chain reaction (real-time RT-PCR), DENV NS1 antigen using rapid diagnosis test (RDT) and anti-dengue antibody using IgM/IgG capture enzyme-linked immunosorbent assay (capture ELISA) and RDT in eight thousand serum samples from blood donations to the blood centers of the Taiwan Blood Services Foundation (TBSF) in Kaohsiung City and Tainan City during the largest dengue outbreak in Taiwan history occurred in 2015. Only one serum sample was positive for DENV RNA detection by using dengue-specific real-time RT-PCR, the virus was DENV-2 determined by serotype-specific real-time RT-PCR and sequencing, and the DENVs in the serum were confirmed as being infectious by a plaque assay. The recipient of this blood did not develop any dengue fever symptom on follow-up. None of the samples was NS1 RDT-reactive. Seventeen IgM-positive samples were identified. There was a low prevalence of asymptomatic confirmed or probable DENV-infected blood donors in our study (0.013% and 0.21%, respectively), and no symptomatic transfusion-transmitted dengue (TT dengue) was developed during the largest dengue outbreak in Taiwan history in highly endemic areas and periods.
Introduction
Dengue is an arthropod-borne viral disease, which produces a wide spectrum of clinical symptoms and outcomes, mainly occurs in tropical and subtropical areas. This disease develops following infection by the dengue virus (DENV), which is an RNA virus with an approximately 11-kilobases positive-sense RNA genome. Dengue viruses are transmitted to humans by the bites of DENV-carrying mosquitoes, especially Aedes aegypti and A. albopictus. DENV infections result in asymptomatic infections, undifferentiated fevers, dengue fever (DF), dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS) [1]; alternatively, they can be classified as dengue with warning signs, dengue without warning signs, and severe dengue [2].
The first dengue outbreak in Taiwan since World War II occurred in 1981 (~8,000 cases, DENV-2) [3]. Over the past three decades, there have been four subsequent large dengue outbreaks (>4000 cases), happened in 1988 (4,389 cases, DENV-1), 2002 (5,388 cases, DENV-1 and DENV-2), 2014 (15,732 cases, DENV-1) and 2015 (43,784 cases, DENV-1 and DENV-2), over the past three decades [4–8](S1 Fig). DF cases in the other 26 years ranged from 10 to 2,179 (mean 573.15, 95% CI 318.30–828.01, median 327, 95% CI 142.29–689.79). Dengue in Taiwan usually starts with imported cases from Southeast Asian countries [9], spreading during the rainy and warm months starting in July, then peaking in October or November, and finally declining when the weather becomes cooler. Accumulated data between 1998 and 2017 revealed DF cases clustered in Kaohsiung City (58.5%), Tainan City (34.0%) and Pingtung County (2.8%) in Taiwan. Among these DF cases, 3.98% were imported cases, while the rest were autochthonous cases.
It is estimated that 50–85% of DENV-infected people have asymptomatic infections, and these asymptomatic people can contribute to DENV transmission since they may be exposed to more mosquitoes through their undisrupted daily routines than sick people [10]. These asymptomatic DENV-infected people might also transmit DENV to other people through blood donation and transfusion [11–13]. Moreover, many studies have reported that DENV was detected in donor blood in blood centers in Honduras, Brazil, Australia [14], Puerto Rico [15] and Saudi Arabia [16] using nucleic acid tests (NAT), such as transcription-mediated amplification (TMA), reverse transcription–polymerase chain reaction (RT-PCR), and antigen/antibody tests. The recipients of DENV-contaminated blood components might contract transfusion-transmitted dengue (TT dengue) [11–13, 17].
Dengue is a notifiable infectious disease in Taiwan determined by the Centers for Disease Control, Taiwan (Taiwan CDC). Human serum samples of suspected dengue cases nationwide must be submitted to the Taiwan CDC or a Taiwan CDC-certificated dengue diagnosis laboratory, Tropical Medicine Center (TMC) of Kaohsiung Medical University Hospital (KMUH) in Kaohsiung City, Taiwan, to confirm DENV infection [6]. Consecutive large dengue outbreaks in Taiwan in 2014–2015 urge us to face the safety of blood transfusion, especially in DF endemic seasons. In 2015, over ninety-seven percent of DF cases were occurred in Tainan City (22,777 cases) and Kaohsiung City (19,784 cases), which are located south of the Tropic of Cancer (E120°~E121°), where A. aegypti is the dominant mosquito species (S2 Fig). There were 106,992 blood donations and 109,511 blood donations to the Kaohsiung City blood center and the Tainan City blood center, respectively, at the time of the dengue outbreak that occurred between September and November in 2015. Additional blood is routinely collected from donors and are stored frozen in a repository of the Taiwan Blood Services Foundation (TBSF) in Hsinchu County located in Northern Taiwan for pathogen surveillance and confirmation testing once transfusion-transmitted infection was suspected. To investigate the prevalence of blood donors with recently asymptomatic DENV infection, we conducted a retrospective study to detect the presence of the DENV RNA genome, NS1 antigen and anti-dengue IgM/IgG in bloods that were donated to the blood centers of the TBSF in Kaohsiung City and Tainan City during the largest dengue outbreak in Taiwan history in 2015.
Materials and methods
Human serum samples and ethics statement
Under the entrustment and authority from the Taiwan CDC (please refer to the Financial Disclosure for the funding by Taiwan CDC to JJT and LTL) and the help of coordination with the TBSF, we obtained eight thousand de-identified residual serum samples collected in serum separation tubes (SST, Becton Dickinson, Franklin Lakes, NJ, USA) (4000 from each center), which were randomly selected from the outbreak districts of Kaohsiung City and Tainan City that were collected during the outbreak period, from the staffs of the TBSF and tested for the evidence of DENV infection at the TMC dengue diagnosis laboratory, which is an ISO 15189:2012 certified dengue diagnostic laboratory certificated by the Taiwan CDC, the Taiwan Accreditation Foundation (TAF) and the International Laboratory Accreditation Cooperation (ILAC) Laboratory Combined Mutual Recognition Arrangement (MRA) Mark. The study was reviewed and approved by the Institutional Review Board of Kaohsiung Medical University Hospital (KMUHIRB-EXEMPT (II)-20160009) and waiver of informed consent was obtained because the study play a role in the control and the prevention of dengue fever in Taiwan.
Viral RNA extraction and one-step SYBR green-based real-time RT-PCR for the detection of DENV genome in the pooled samples
For the detection of DENV genome in pooled sera, viral RNA was extracted from 200 μL of the pooled serum (combined ten single serum aliquots into one pool) or the control serum by using the PureLink Viral RNA Mini Kit (Life Technologies; USA) and immediately subjected to real-time RT-PCR analysis. Dengue-specific primers for the DENV RNA detection were DN-F: CAA TAT GCT GAA ACG CGA GAG AAA and DN-R: CCC CAT CTA TTC AGA ATC CCT GCT. Serotype-specific primers for molecular serotyping were DN-F: CAA TAT GCT GAA ACG CGA GAG AAA, D1-R CGC TCC ATA CAT CTT GAA TGA G, D2-R: AAG ACA TTG ATG GCT TTT GA, D3-R: AAG ACG TAA ATA GCC CCC GAC and D4-R: AGG ACT CGC AAA AAC GTG ATG AAT [18]. These primers amplified the genomic region encoding the nucleocapsid or core protein. Real-time RT-PCR was performed in a Mx3000P machine (Agilent/Stratagene, USA) by using the Brilliant II SYBR Green QRT-PCR Low ROX Master Mix system (Agilent/Stratagene, USA). Amplification plots and Tm values were analyzed to verify the specificity of the amplicon.
RNA extraction and one-step SYBR green-based real-time RT-PCR for the detection of DENV genome in serum from single donors
For the detection of DENV RNA genome in single serum from eight thousand blood donors, viral RNA was extracted from 200 μL of the serum by using the TANBead Nucleic Acid Extraction Kit in Smart LabAssist-16 Automated Extraction Instruments (Taiwan Advanced Nanotech Inc., Taoyuan, Taiwan) and immediately subjected to real-time RT-PCR analysis. Dengue-specific primers for the DENV RNA detection were the same as described above. Real-time RT-PCR was performed in a Mx3000P machine (Agilent/Stratagene, USA) by using the One Step RT-QGreen 2X SybrGreen Low ROX Master Mix system (CellSafe, Gyeonggi-do, Republic of Korea). Amplification plots and Tm values were analyzed to verify the specificity of the amplicon.
Preparation of dengue viruses
Four serotypes of DENV, including DENV-1 (US/Hawaii/1994 strain), DENV-2 (New Guinea C strain), DENV-3 (DN8700829A strain) and DENV-4 (DN9000475A strain), were propagated in C6/36 cell line. The stock viruses was diluted in RPMI medium containing 1% fetal calf serum (Gibco-Life Technologies, USA) and added to the C6/36 cells with a final titer of 0.01 multiplicity of infection of. Culture fluids were harvested after incubation for 4–7 days (28°C 5% CO2) until cytopathic effect was observed. Culture supernatants were used as positive control for real-time RT-PCR and as viral antigen in anti-dengue IgM/IgG capture ELISA.
Plaque assay
DENV titer of culture supernatants and serum from blood donor was determined by plaque assay. Briefly, fresh DENV culture supernatants and serum from blood donor were 10-fold serial diluted in the MEM medium (Gibco-Life Technologies) and added to monolayer of Vero cells in 6-well plates at a density of 1 × 106 cells per well. DENV adhesion onto Vero cells was allowed at 37°C, 5% CO2 for 2 h, medium containing DENV was removed before 3 ml overlay medium containing 1.2% methylcellulose was added. Vero cells were incubated for 5–10 days further until plaques became apparently visible by microscopy examination, fixed, and stained with crystal violet. Plaques were counted manually and plaque forming units (PFU) per ml were determined [19].
Detection of DENV NS1 and anti-dengue IgM/IgG by RDT
DENV NS1 and anti-dengue IgM/IgG in serum samples were detected using SD BIOLINE Dengue Duo (STANDARD DIAGNOSTICS, INC., Korea). All RDTs were performed and the results were determined according to the manufacturer's instructions. In brief, for DENV NS1 antigen, approximately 100 μL of serum were added into the sample pad and tests results were interpreted after 15–20 min. Similarly, for anti-dengue IgM/IgG, 10 μL of serum was added into the sample pad and 90–120 μL of assay diluent were then added to the diluent well. Test results were interpreted after 15–20 min.
Detection of anti-dengue IgM and IgG by ELISA
Anti-dengue IgM and IgG of all serum samples were tested at 1:100 dilution by capture ELISA using the detailed experimental procedures described elsewhere [20]. The optical density was determined at 405 nm with a reference at 630 nm in a μQuant Model MQX200 Microplate Spectrophotometer (BioTek Instruments, Inc., USA). To exclude the cross-reaction of the anti-Japanese encephalitis virus (JEV) antibody in the assays, culture supernatant from JEV-infected Vero cells was used as negative control antigen routinely. A positive sample was defined as having a test absorbance≥0.5 and a ratio of the positive control to the negative control≥2.0, and a negative sample was defined as having a test absorbance<0.5.
Results
Sample collection strategy and donor information
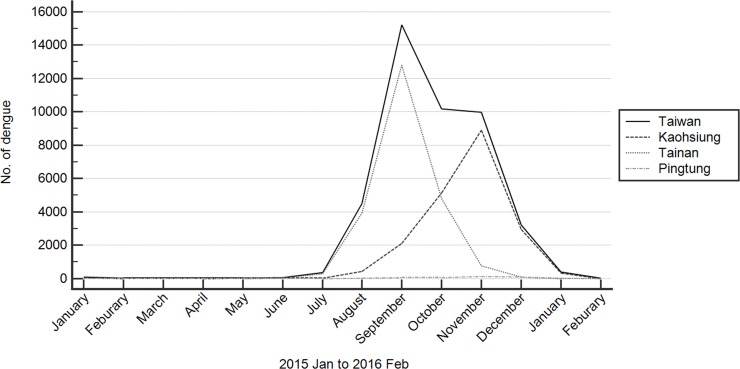
Eight thousand de-identified serum samples that had been collected during the most active dengue outbreak period from 30th August to 26th September in Tainan City and from 4th November to 25th November in Kaohsiung City (Fig 1) were randomly selected for analysis. The information available from the TBSF about the blood donors in this study was shown in Table 1; all donors met the criteria for blood donation suggested by the TBSF at the time they donated blood.
Fig 1. Monthly dengue fever cases in three southeastern cities in 2015.
Monthly confirmed dengue case numbers are shown in three southeastern cities that were the three cities with the highest number of dengue cases in Taiwan’s history. We surveyed the presence of the DENV in the blood collected in the blood centers of Kaohsiung City and Tainan City.
Table 1. Background information of the blood donors.
| Age | Tainan City Blood Center | Kaohsiung City Blood Center | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Subtotal | Male | Female | Subtotal | |||||
| n | % | n | % | n | % | n | % | |||
| <20 | 65 | 2.48 | 112 | 8.15 | 177 | 33 | 1.28 | 75 | 5.29 | 108 |
| 20–29 | 512 | 19.5 | 467 | 33.99 | 979 | 488 | 18.91 | 413 | 29.11 | 901 |
| 30–39 | 783 | 29.82 | 377 | 27.44 | 1,160 | 790 | 30.61 | 385 | 27.13 | 1,175 |
| 40–49 | 647 | 24.64 | 211 | 15.36 | 858 | 676 | 26.19 | 243 | 17.12 | 919 |
| 50–59 | 498 | 18.96 | 169 | 12.3 | 667 | 465 | 18.02 | 237 | 16.7 | 702 |
| >60 | 121 | 4.61 | 38 | 2.77 | 159 | 129 | 5 | 66 | 4.65 | 195 |
| Total | 2,626 | 100 | 1,374 | 100 | 4,000 | 2,581 | 100 | 1,419 | 100 | 4,000 |
Detection of the DENV RNA genome in the pooled serum samples
We performed dengue-specific real-time RT-PCR using Brilliant II SYBR Green QRT-PCR Low ROX Master Mix system to detect the presence of DENV RNA genome in eight hundred pooled serum samples (ten single donations per pool). Only one serum pool was positive according to dengue-specific real-time RT-PCR in the screening. The result was confirmed as being positive, and then the ten single serum samples in the pool were subjected to another round of dengue-specific real-time RT-PCR. One serum sample was positive according to real-time RT-PCR (case no. 1142 in Table 2).
Table 2. Summary of the analysis of eight thousand blood donations collected during the 2015 dengue outbreak.
| RDT & ELISAb | ||||
|---|---|---|---|---|
| Case No.a | RT-PCR | NS1 | IgM | IgG |
| 695 | - | - | + | + |
| 1142 | + | - | - | - |
| 1368 | - | - | + | + |
| 1830 | - | - | + | - |
| 1946 | - | - | + | - |
| 1981 | - | - | + | + |
| 1986 | - | - | + | + |
| 1993 | - | - | + | + |
| 2204 | - | - | + | + |
| 2232 | - | - | + | - |
| 2434 | - | - | + | - |
| 2510 | - | - | + | + |
| 2642 | - | - | + | + |
| 3659 | - | - | + | + |
| 4975 | - | - | + | + |
| 7371 | - | - | + | + |
| 7520 | - | - | + | + |
| 7528 | - | - | + | + |
| Positive | 1 | 0 | 17 | 13 |
| Negative | 17 | 18 | 1 | 5 |
a Only the cases with a positive result are shown in the table.
b Results of the combination of the RDT and ELISA.
Molecular serotyping, sequencing and infectious titer of dengue virus in donor serum
To understand the serotype of the DENV detected in the donor serum, serotype-specific real-time RT-PCR was performed. The result of the serotype-specific real-time RT-PCR indicated the DENV in this sample was DENV-2 (case no. 1142 in Table 2). The results of the serotype-specific real-time RT-PCR was confirmed by sequencing of the DENV in this serum. To understand the infectious potential of this DENV positive serum, we performed the plaque assay to measure the titer of infectious DENV, which might bring out TT dengue, in this serum. The result of plaque assay indicated that the titer was equivalent to 50 PFU/ml.
Re-evaluation of the presence of the DENV RNA genome using single serum from blood donors
The surveillance of DENV genome using pooled-sample strategy revealed only one donor serum was DENV RNA positive. To investigate if any DENV-positive sample was missed by using pooled-sample strategy, we later re-evaluated the presence of the DENV RNA genome using single serum from eight thousand blood donors. The results of this re-evaluation study indicated there was only one donor serum positive for DENV real-time RT-PCR (case no. 1142 in Table 2).
Dengue NS1 antigen and anti-DENV IgM/IgG detection
The presence of NS1 in the serum is one of the confirmed dengue diagnostic criterion [2]. Thus, we detect the presence of DENV NS1 antigen using SD NS1 RDT. The results indicated that none of the samples were NS1-reactive (Table 2). Next, we analyzed the anti-dengue antibody response of these donors to the recent DENV infection by using IgM/IgG capture ELISA and RDT. We combined the results of ELISA and RDT to maximize the possibility to detect anti-dengue antibody presented in donor’s sera. Seventeen cases were IgM-reactive according to ELISA or RDT (0.21%) (Table 2). In these cases, thirteen cases were IgG-reactive (0.16%).
Discussion
The primers used in this study were based on highly conserved region of the gene encoding the nucleocapsid or core protein of dengue virus genome, and were able to detect all four DENV serotypes. The detection limit of the in-house DENV real-time RT-PCR used in this study was tested using 10-fold serial dilutions of dengue virus culture supernatants (using RPMI medium containing 10% FBS) that had been previously quantitated by plaque assay. The detection limits of the standard serotype-specific primer pairs were 2.5 PFU/ml for DENV-1, 2.1 PFU/ml for DENV-2, 10 PFU/ml for DENV-3, and 19 PFU/ml for DENV-4. In vitro quantification of dengue genomes of supernatants were then performed using Dengue Virus subtypes 1, 2, 3 and 4 genesig Standard Kit (sensitive to < 100 copies of target) (please refer to Supporting methods). The quantification results of dengue virus supernatants suggested that the detection limit of the in-house DENV real-time RT-PCR were approximately equivalent to 1500 copies/ml for DENV-1, 1050 copies/ml for DENV-2, 2000 copies/ml for DENV-3, and 3800 copies/ml for DENV-4.
Our real-time RT-PCR results demonstrated only one DENV viremia case among the eight thousand blood donations collected during the 2015 DF outbreak in southern Taiwan (0.013%). The virus could be cultured from this real-time RT-PCR-positive serum sample, suggesting that a risk of TT dengue existed. The virus was DENV-2 which was confirmed by serotype-specific real-time RT-PCR and sequencing of the RT-PCR products. The virus was confirm to be the cosmopolitan genotype using the phylogenetic analysis of the envelope gene sequence using the methods described in our previous article [21]. The phylogenetic analysis result was similar to the result by Wang et. al.[8]. After reporting this case to the TBSF and the Taiwan CDC in May 2016, the recipient of this blood was contacted for follow-up about seven months later after transfusion. The recipient did not develop any dengue fever symptom after transfusion and thus no symptomatic transfusion-transmitted dengue case was developed in this study. We did not get access to the serum of both the DENV-positive donor and the recipient of the DENV-positive unit on follow-up at that time, one of the major reason was it is out of the scope of the original study approved by the IRB. The donor of this DENV-positive blood was possibly in an early stage of asymptomatic primary DENV infection (Table 2).
The infectiosity of DENV had been study extensively in cell models. The infection of DENV exhibits a dose-response manner both in cell model with [22] and without [23] the presence of anti-dengue antibody. In humans, the situation are supposed to be more complicated due to the variation of the immune status and susceptibility in different individuals. According to the statement of Stramer et. al., the infectious dose of DENV by transfusion is largely unknown [13]. No association between donation viral load and transmission to recipients was evident had been reported. It is suggested that the infectious dose required for transfusion transmission may be higher than expected, since 16 RNA-positive units transfused into 16 susceptible recipients only resulted in 5 cases which were considered probable TT cases (the TT rate was 37.5% in their study) [17]. We assumed the reasons why the recipient of the infectious DENV-positive blood in this study did not develop TT dengue can be 1. The virus load is not enough to initiate an effective infection, 2. The low susceptibility to the DENV infection of the recipient, 3. Developed immunity from previous DENV infection against current DENV infection, and 4. It was asymptomatic infection since the recipient did not developed any DF symptoms after transfusion.
The DENV NS1 protein is a 44–49 kilodalton glycosylated protein which actively participates in viral RNA replication. The circulating NS1 level was 10 ng to 50 μg/ml in the blood during the acute phase of DENV infection determined by ELISA and it is suggested that DENV NS1 could be detected even when viral RNA was negative in real-time RT-PCR or in the presence of IgM antibodies [24]. Furthermore, the detection of the presence of NS1 in the blood is one of the dengue diagnostic methods [2]. However, the sensitivity of NS1 RDT was ranging from 44.4% to 87% [25–28] in the detection of DENV when compared to real-time RT-PCR. Besides, the circulating level of NS1 in asymptomatic DENV infections has not been determined. None of the samples tested in this study was NS1 positive. Taken these together, the detection of NS1 might not be useful for DENV screening in bloods from asymptomatic donors at this moment.
In this study, we found seventeen IgM-positive samples (thirteen were IgG positive among these samples) which were real-time RT-PCR negative. The presence of anti-dengue IgM antibody in a single serum sample was recognized as a probable dengue case according to the definition by World Health Organization [2]. So there were seventeen blood donors which were asymptomatic probable DENV-infected volunteers (0.21%). Although the seroprevalence of blood donors had been documented [14–16, 29–35], the impact of the IgM positive- or IgM/IgG dual positivity bloods on the transfusion transmission of DENV is largely unknown today despite it has been proposed that not only DENV but also anti-dengue antibodies may pose a risk to blood transfusion safety [36].
The prevalence of asymptomatic dengue viremia among people aged 20 to 64 years was estimated to be 15.0 per 10,000 (0.15%), by using an established mathematical model using some parameters and based on assumptions, in Tainan during the 2015 epidemic [37]. Conversely, our results showed a relatively low frequency of the presence of DENV RNA genome in the asymptomatic blood donors (0.013%). If their estimation was correct, then there might be some reason the detection rate of DENV RNA genome was relatively low in our study. One limitation of our study is that the detection method of DENV RNA we used was real-time RT-PCR, which is not as sensitive as the TMA or enhanced TMA used in other reports [14, 15, 38]. In addition, the presence of PCR inhibitor in the bloods of donors might confer to the inhibition of real-time RT-PCR in our study. Furthermore, seventeen probable DENV-infected donors might in the antibody response phase of the infection, which the virus might decline to the level below detection limit of real-time RT-PCR used in this study.
To our knowledge, this is the first study to survey the prevalence of asymptomatic confirmed and/or probable DENV-infected blood donors in the dengue endemic time periods in the blood centers in Taiwan. The prevalence of the DENV RNA, NS1 antigen and anti-dengue antibody in blood donors in highly endemic cities in this study was relatively low compared to that in other countries or areas such as Honduras[14], Brazil [14, 38, 39], Puerto Rico [15], Guangzhou [35], Saudi Arabia [33] and India [40](S1 Table).
Conclusions
There was a low prevalence of asymptomatic confirmed or probable DENV-infected blood donors in our study (0.013% and 0.21%, respectively), and no symptomatic TT dengue fever was developed during the largest dengue outbreak in Taiwan history in 2015. Currently, the NATs were performed by the TBSF to screen the presence of HBV, HCV and HIV using a mini-pool of eight samples from eight donors in Taiwan. Our results suggested that the use of a mini-pool (ten sera in a pool) seems acceptable in DENV RNA screening using real-time RT-PCR, since the results was similar to that of the screening using single serum of donors. The cost of the DENV RNA screening will be one-tenth using mini-pool strategy comparing to single serum screening.
Supporting information
Confirmed dengue cases in Taiwan since 1987 based on the data retrieved from the web-based notifiable diseases surveillance system maintained by the Taiwan CDC. Source of data: https://nidss.cdc.gov.tw/en/Default.aspx?op=4.
(TIF)
Confirmed dengue case numbers are shown in only the three southeastern cities that were the second-level administrative divisions with the highest levels of endemic dengue in Taiwan’s history. The gray line represents the Northern Tropic. This figure was generated using the map data described above and Quantum GIS v2.18.15 (QGIS Development Team, 2018. QGIS Geographic Information System. Open Source Geospatial Foundation. URL http://www.qgis.org/en/site/) using WGS84 (EPSG: 4326) as the default Coordinate Reference System (CRS) for datum transformations. Taiwan map data were retrieved from the Taiwan Geospatial One-Stop Portal developed by the Information Center of the Taiwan Ministry of The Interior and used under the Open Government Data License.
(TIF)
(DOCX)
(DOCX)
Acknowledgments
We thank the Taiwan Blood Services Foundation for providing de-identified residual serum samples from blood donors.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The funding was supported by the Ministry of Health and Welfare, Taiwan (https://www.mohw.gov.tw/mp-2.html, MOHW104-CDC-C-114-114901 to JJT and LTL, and MOHW107-TDU-B-212-123006 to JJT), and the National Health Research Institutes, Taiwan (grant no. MR-107-PP-40, http://english.nhri.org.tw/NHRI_WEB/nhriw001Action.do, to JJT). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.World Health Organization. Dengue Haemorrhagic fever: diagnosis, treatment, prevention and control 2nd ed. Geneva: World Health Organization; 1997. [Google Scholar]
- 2.World Health Organization. Dengue: guidelines for diagnosis, treatment, prevention and control. 2009. [PubMed]
- 3.Wu YC. [Epidemic dengue 2 on Liouchyou Shiang, Pingtung County in 1981]. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1986;19(3):203–11. . [PubMed] [Google Scholar]
- 4.Huang JH, Liao TL, Chang SF, Su CL, Chien LJ, Kuo YC, et al. Laboratory-based dengue surveillance in Taiwan, 2005: a molecular epidemiologic study. Am J Trop Med Hyg. 2007;77(5):903–9. . [PubMed] [Google Scholar]
- 5.Lin CC, Huang YH, Shu PY, Wu HS, Lin YS, Yeh TM, et al. Characteristic of dengue disease in Taiwan: 2002–2007. Am J Trop Med Hyg. 2010;82(4):731–9. 10.4269/ajtmh.2010.09-0549 ; PubMed Central PMCID: PMCPMC2844571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang SF, Yang CF, Hsu TC, Su CL, Lin CC, Shu PY. Laboratory-Based Surveillance and Molecular Characterization of Dengue Viruses in Taiwan, 2014. Am J Trop Med Hyg. 2016;94(4):804–11. 10.4269/ajtmh.15-0534 ; PubMed Central PMCID: PMCPMC4824222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen HL, Lin SR, Liu HF, King CC, Hsieh SC, Wang WK. Evolution of dengue virus type 2 during two consecutive outbreaks with an increase in severity in southern Taiwan in 2001–2002. Am J Trop Med Hyg. 2008;79(4):495–505. . [PubMed] [Google Scholar]
- 8.Wang SF, Chang K, Loh EW, Wang WH, Tseng SP, Lu PL, et al. Consecutive large dengue outbreaks in Taiwan in 2014–2015. Emerg Microbes Infect. 2016;5(12):e123 10.1038/emi.2016.124 ; PubMed Central PMCID: PMCPMC5180368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shu PY, Su CL, Liao TL, Yang CF, Chang SF, Lin CC, et al. Molecular characterization of dengue viruses imported into Taiwan during 2003–2007: geographic distribution and genotype shift. Am J Trop Med Hyg. 2009;80(6):1039–46. Epub 2009/05/30. . [PubMed] [Google Scholar]
- 10.Duong V, Lambrechts L, Paul RE, Ly S, Lay RS, Long KC, et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc Natl Acad Sci U S A. 2015;112(47):14688–93. 10.1073/pnas.1508114112 ; PubMed Central PMCID: PMCPMC4664300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chuang V, Wong TY, Leung YH, Ma E, Law YL, Tsang O, et al. Review of dengue fever cases in Hong Kong during 1998 to 2005. Hong Kong Med J. 2008;14(3):170–7. . [PubMed] [Google Scholar]
- 12.Tambyah PA, Koay ES, Poon ML, Lin RV, Ong BK, Transfusion-Transmitted Dengue Infection Study G. Dengue hemorrhagic fever transmitted by blood transfusion. N Engl J Med. 2008;359(14):1526–7. 10.1056/NEJMc0708673 . [DOI] [PubMed] [Google Scholar]
- 13.Stramer SL, Linnen JM, Carrick JM, Foster GA, Krysztof DE, Zou S, et al. Dengue viremia in blood donors identified by RNA and detection of dengue transfusion transmission during the 2007 dengue outbreak in Puerto Rico. Transfusion. 2012;52(8):1657–66. 10.1111/j.1537-2995.2012.03566.x . [DOI] [PubMed] [Google Scholar]
- 14.Linnen JM, Vinelli E, Sabino EC, Tobler LH, Hyland C, Lee TH, et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion. 2008;48(7):1355–62. 10.1111/j.1537-2995.2008.01772.x . [DOI] [PubMed] [Google Scholar]
- 15.Mohammed H, Linnen JM, Munoz-Jordan JL, Tomashek K, Foster G, Broulik AS, et al. Dengue virus in blood donations, Puerto Rico, 2005. Transfusion. 2008;48(7):1348–54. 10.1111/j.1537-2995.2008.01771.x . [DOI] [PubMed] [Google Scholar]
- 16.Ashshi AM. Serodetection of Dengue virus and its antibodies among blood donors in the western region of Saudi Arabia: a preliminary study. Blood Transfus. 2015;13(1):135–8. 10.2450/2014.0134-14 ; PubMed Central PMCID: PMCPMC4317098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sabino EC, Loureiro P, Lopes ME, Capuani L, McClure C, Chowdhury D, et al. Transfusion-Transmitted Dengue and Associated Clinical Symptoms During the 2012 Epidemic in Brazil. J Infect Dis. 2016;213(5):694–702. 10.1093/infdis/jiv326 ; PubMed Central PMCID: PMCPMC4747611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shu PY, Chang SF, Kuo YC, Yueh YY, Chien LJ, Sue CL, et al. Development of Group- and Serotype-Specific One-Step SYBR Green I-Based Real-Time Reverse Transcription-PCR Assay for Dengue Virus. Journal of Clinical Microbiology. 2003;41(6):2408–16. 10.1128/JCM.41.6.2408-2416.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai JJ, Liu LT, Lin PC, Tsai CY, Chou PH, Tsai YL, et al. Validation of the Pockit Dengue Virus Reagent Set for Rapid Detection of Dengue Virus in Human Serum on a Field-Deployable PCR System. J Clin Microbiol. 2018;56(5). doi: 10.1128/JCM.01865-17 PMID: 29436418; PubMed Central PMCID: PMCPMC5925719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shu PY, Chen LK, Chang SF, Yueh YY, Chow L, Chien LJ, et al. Comparison of Capture Immunoglobulin M (IgM) and IgG Enzyme-Linked Immunosorbent Assay (ELISA) and Nonstructural Protein NS1 Serotype-Specific IgG ELISA for Differentiation of Primary and Secondary Dengue Virus Infections. Clinical and Vaccine Immunology. 2003;10(4):622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu PY, Ke GM, Chen PC, Liu LT, Tsai YC, Tsai JJ. Spatiotemporal dynamics and epistatic interaction sites in dengue virus type 1: a comprehensive sequence-based analysis. PloS one. 2013;8(9):e74165 10.1371/journal.pone.0074165 ; PubMed Central PMCID: PMC3767619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flipse J, Diosa-Toro MA, Hoornweg TE, van de Pol DP, Urcuqui-Inchima S, Smit JM. Antibody-Dependent Enhancement of Dengue Virus Infection in Primary Human Macrophages; Balancing Higher Fusion against Antiviral Responses. Sci Rep. 2016;6:29201 10.1038/srep29201 ; PubMed Central PMCID: PMCPMC4933910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diamond MS, Edgil D, Roberts TG, Lu B, Harris E. Infection of human cells by dengue virus is modulated by different cell types and viral strains. J Virol. 2000;74(17):7814–23. ; PubMed Central PMCID: PMCPMC112311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alcon S, Talarmin A, Debruyne M, Falconar A, Deubel V, Flamand M. Enzyme-linked immunosorbent assay specific to Dengue virus type 1 nonstructural protein NS1 reveals circulation of the antigen in the blood during the acute phase of disease in patients experiencing primary or secondary infections. J Clin Microbiol. 2002;40(2):376–81. 10.1128/JCM.40.2.376-381.2002 ; PubMed Central PMCID: PMCPMC153354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cam BV, Fonsmark L, Hue NB, Phuong NT, Poulsen A, Heegaard ED. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am J Trop Med Hyg. 2001;65(6):848–51. . [DOI] [PubMed] [Google Scholar]
- 26.Anga G, Barnabas R, Kaminiel O, Tefuarani N, Vince J, Ripa P, et al. The aetiology, clinical presentations and outcome of febrile encephalopathy in children in Papua New Guinea. Ann Trop Paediatr. 2010;30(2):109–18. 10.1179/146532810X12703902243818 . [DOI] [PubMed] [Google Scholar]
- 27.Tricou V, Vu HT, Quynh NV, Nguyen CV, Tran HT, Farrar J, et al. Comparison of two dengue NS1 rapid tests for sensitivity, specificity and relationship to viraemia and antibody responses. BMC infectious diseases. 2010;10:142 10.1186/1471-2334-10-142 ; PubMed Central PMCID: PMC2895602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pipattanaboon C, Sasaki T, Nishimura M, Setthapramote C, Pitaksajjakul P, Leaungwutiwong P, et al. Cross-reactivity of human monoclonal antibodies generated with peripheral blood lymphocytes from dengue patients with Japanese encephalitis virus. Biologics. 2013;7:175–87. 10.2147/BTT.S47438 ; PubMed Central PMCID: PMC3747787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Faddy HM, Seed CR, Fryk JJ, Hyland CA, Ritchie SA, Taylor CT, et al. Implications of dengue outbreaks for blood supply, Australia. Emerg Infect Dis. 2013;19(5):787–9. 10.3201/eid1905.121664 ; PubMed Central PMCID: PMCPMC3647514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harif NF, Kader ZS, Joshi SR, Yusoff NM. Seropositive status of dengue virus infection among blood donors in North Malaysia. Asian J Transfus Sci. 2014;8(1):64 10.4103/0973-6247.126702 ; PubMed Central PMCID: PMCPMC3943155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Low SL, Lam S, Wong WY, Teo D, Ng LC, Tan LK. Dengue seroprevalence of healthy adults in Singapore: serosurvey among blood donors, 2009. Am J Trop Med Hyg. 2015;93(1):40–5. 10.4269/ajtmh.14-0671 ; PubMed Central PMCID: PMCPMC4497902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ranjan P, Natarajan V, Bajpai M, Gupta E. High Seroprevalence of Dengue Virus Infection in Blood Donors From Delhi: A Single Centre Study. J Clin Diagn Res. 2016;10(10):DC08–DC10. 10.7860/JCDR/2016/21262.8711 ; PubMed Central PMCID: PMCPMC5121675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashshi AM. The prevalence of dengue virus serotypes in asymptomatic blood donors reveals the emergence of serotype 4 in Saudi Arabia. Virol J. 2017;14(1):107 10.1186/s12985-017-0768-7 ; PubMed Central PMCID: PMCPMC5466713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gao Z, Zhang Y, Yang Y, Xu M, Liao P, He W, et al. Dengue virus infections among blood donors in Guangxi of China, 2013–2014. Transfus Med. 2017. 10.1111/tme.12448 . [DOI] [PubMed] [Google Scholar]
- 35.Liao Q, Shan Z, Wang M, Huang J, Xu R, Huang K, et al. An evaluation of asymptomatic Dengue infections among blood donors during the 2014 Dengue outbreak in Guangzhou, China. J Med Virol. 2017;89(11):2037–40. 10.1002/jmv.24883 . [DOI] [PubMed] [Google Scholar]
- 36.Ribas-Silva RC, Eid AA. Dengue antibodies in blood donors. Rev Bras Hematol Hemoter. 2012;34(3):193–5. 10.5581/1516-8484.20120048 ; PubMed Central PMCID: PMCPMC3459621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chien YW, Shu YC, Chuang KT, Yeh CY, Ko WC, Ko NY, et al. High estimated prevalence of asymptomatic dengue viremia in blood donors during a dengue epidemic in southern Taiwan, 2015. Transfusion. 2017;57(11):2649–56. 10.1111/trf.14281 . [DOI] [PubMed] [Google Scholar]
- 38.Busch MP, Sabino EC, Brambilla D, Lopes ME, Capuani L, Chowdhury D, et al. Duration of Dengue Viremia in Blood Donors and Relationships Between Donor Viremia, Infection Incidence and Clinical Case Reports During a Large Epidemic. J Infect Dis. 2016;214(1):49–54. 10.1093/infdis/jiw122 ; PubMed Central PMCID: PMCPMC4907419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dias LL, Amarilla AA, Poloni TR, Covas DT, Aquino VH, Figueiredo LT. Detection of dengue virus in sera of Brazilian blood donors. Transfusion. 2012;52(8):1667–71. 10.1111/j.1537-2995.2012.03729.x . [DOI] [PubMed] [Google Scholar]
- 40.Mangwana S. Dengue viremia in blood donors in Northern India: Challenges of emerging dengue outbreaks to blood transfusion safety. Asian J Transfus Sci. 2015;9(2):177–80. 10.4103/0973-6247.154253 ; PubMed Central PMCID: PMCPMC4562141. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Confirmed dengue cases in Taiwan since 1987 based on the data retrieved from the web-based notifiable diseases surveillance system maintained by the Taiwan CDC. Source of data: https://nidss.cdc.gov.tw/en/Default.aspx?op=4.
(TIF)
Confirmed dengue case numbers are shown in only the three southeastern cities that were the second-level administrative divisions with the highest levels of endemic dengue in Taiwan’s history. The gray line represents the Northern Tropic. This figure was generated using the map data described above and Quantum GIS v2.18.15 (QGIS Development Team, 2018. QGIS Geographic Information System. Open Source Geospatial Foundation. URL http://www.qgis.org/en/site/) using WGS84 (EPSG: 4326) as the default Coordinate Reference System (CRS) for datum transformations. Taiwan map data were retrieved from the Taiwan Geospatial One-Stop Portal developed by the Information Center of the Taiwan Ministry of The Interior and used under the Open Government Data License.
(TIF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.