Abstract
This review provides a comprehensive overview of brain imaging studies of the brain-gut interaction in functional gastrointestinal disorders (FGIDs). Functional neuroimaging studies during gut stimulation have shown enhanced brain responses in regions related to sensory processing of the homeostatic condition of the gut (homeostatic afferent) and responses to salience stimuli (salience network), as well as increased and decreased brain activity in the emotional response areas and reduced activation in areas associated with the top-down modulation of visceral afferent signals. Altered central regulation of the endocrine and autonomic nervous responses, the key mediators of the brain-gut axis, has been demonstrated. Studies using resting-state functional magnetic resonance imaging reported abnormal local and global connectivity in the areas related to pain processing and the default mode network (a physiological baseline of brain activity at rest associated with self-awareness and memory) in FGIDs. Structural imaging with brain morphometry and diffusion imaging demonstrated altered gray- and white-matter structures in areas that also showed changes in functional imaging studies, although this requires replication. Molecular imaging by magnetic resonance spectroscopy and positron emission tomography in FGIDs remains relatively sparse. Progress using analytical methods such as machine learning algorithms may shift neuroimaging studies from brain mapping to predicting clinical outcomes. Because several factors contribute to the pathophysiology of FGIDs and because its population is quite heterogeneous, a new model is needed in future studies to assess the importance of the factors and brain functions that are responsible for an optimal homeostatic state.
Keywords: Dyspepsia, Homeostasis, Irritable bowel syndrome, Neuroimaging, Visceral pain
Introduction
Under stress conditions, gastrointestinal (GI) symptoms such as nausea, diarrhea, and abdominal pain are common, so a close interaction between the brain and the gut has long been assumed.1–4 The availability of brain imaging technology from the 1990s has allowed exploration of the central control of human gut function in health and disease.2 Initial neuroimaging studies using positron emission tomography (PET) reported rectal distention5 and esophageal distention,6 followed by gastric distention.7 Brain imaging research has grown rapidly over the past 2 decades; in particular, various neuroimaging modalities have contributed to our increased understanding of the complex bidirectional interaction between the brain and gut (gut and brain) in functional gastrointestinal disorders (FGIDs). The variety of neuroimaging techniques are summarized in Table. This review provides a comprehensive overview of the brain imaging studies in FGIDs to encourage the understanding of researchers other than brain image experts.
Table.
Brain Imaging Techniques
| Implement | What it measures | Utilities in gastroenterology |
|---|---|---|
| PET | Measures the annihilation photons from positron-electron annihilation. The positron is emitted from the radioactive tracer that is injected intravenously. | |
| [15O]H2O PET | Measures regional cerebral blood flow | Initial studies during gut stimulations. |
| [18F]FDG PET | Measures the regional metabolic rate of glucose | Used mainly for diagnosis and staging of cancers but also for neurological studies |
| Relevant radioligands | Neurotransmitter system (dopamine, serotonin, opioids, cannabinoid etc) | Studies are limited due to the high cost, the limited availability of relevant ligands, and the complexity of the studies. |
| MRI | A non-invasive technique to assess brain function based on endogenous magnetic properties, | |
| fMRI (BOLD signal) | Measures changes in the proportion of oxygenated vs deoxygenated haemoglobin, which is seen in areas of greater neural activity | Most frequently used in particular task based (eg, gut stimulation) studies. More available and better temporal resolution than PET |
| ASL | Measures cerebral blood flow directly, by using arterial blood as an endogenous tracer. | Suitable to measure baseline cerebral blood flow. |
| rsfMRI | Measures spontaneous, low frequency (< 0.1 Hz) fluctuations in the BOLD signal that occur when a subject is not performing an explicit task. rsfMRI investigates synchronous activations between regions that are spatially distinct, occurring in the absence of a task or stimulus, to identify resting state networks. | Several studies used rsfMRI to investigate the resting state networks between healthy controls and patients in a certain disease condition. |
| MR spectroscopy | Quantitative measure of biochemical concentration in the living brain based on the unique MR spectra of different molecules. | Proton MR spectroscopy can reliably detect metabolites such as Glx (Glutamate and Glutamine), γ-aminobutyric acid (GABA), and N-acetylaspartate (NAA). |
| Structural MRI | T1 weighted high-resolution structural MRI used to produce structural imaging. Dynamic alterations in brain structure have been observed even within 5 days. | Assess baseline differences between groups and the central nervous system effects of treatments, aging, and disease. |
| VBM (gray matter) | Whole brain analysis of the density and volume of gray matter in each voxel, which may involve changes in glial number, dendritic spines. | Influence of environmental factors such as early life event on brain structures. |
| Diffusion imaging | Evaluates white matter integrity and anatomy. The tract integrity is expressed commonly as fractional anisotropy and specific fiber tracts between brain regions are identified by tractography. | Diffusion imaging studies in stroke patients are useful as it shows dynamic remodelling of white matter tracts. Longitudinal studies in therapeutic intervention. |
| MEG | Measures the magnetic field generated by the electrical activity of neurons with millisecond temporal resolution. | MEG is used to measure the time courses of brain activity (eg, due to gut stimulation). Not widely available |
| EEG | Measures direct electrical activity of the brain by surface scalp electrodes with millisecond temporal resolution. | EEG is used for evoked potentials to external stimuli in real time or to measure the time courses of brain activity. |
PET, positron emission tomography; fMRI, functional magnetic resonance imaging; BOLD, blood oxygen level-dependent; ASL, arterial spin labelling; rsfMRI, resting-state functional magnetic resonance imaging; MR, magnetic resonance; VBM, voxel-based morphometry; MEG, Magneto-encephalography; EEG, electro-encephalography.
Functional Gastrointestinal Disorders and Brain Imaging (What Are Functional Gastrointestinal Disorders? Why Is Brain Imaging Useful?)
FGIDs are characterized by chronic pain, discomfort, and other general symptoms in various locations within the GI and biliary tracts that occur without an apparent physical, biological, or anatomical etiology.1 They encompass more than 40 different heterogeneous disorders, including functional dyspepsia (FD), functional chest pain, and irritable bowel syndrome (IBS). They are remarkably common diseases, eg, the prevalence of FD and IBS are 10.0–30.0%8 and 11.2%9 worldwide, respectively. It is well known that FGIDs tend to feature comorbid affective disorders such as anxiety, depression, and somatization,10 and their start and development are highly influenced by stress.11 One difficulty in managing symptoms in FGIDs is that there is no identifiable cause or biomarker for diagnosis. To better understand the pathophysiology of FGIDs, authorities such as the Rome committee have proposed a biopsychosocial model with integrated biological, psychological, and social subsystems interacting at multiple levels.1 FGIDs are now defined as gut–brain interaction disorders classified by GI symptoms related to any combination of the following: motility disturbance, visceral hypersensitivity, altered mucosal and immune function, altered gut microbiota, or altered central nervous system processing.11–13 Imaging the brain substantiates the patient’s subjective reports of their symptoms, eg, visceral pain (the key feature of FGIDs), and the initial studies with PET visualized visceral pain associated activated patterns in the brain via esophageal, gastric, and rectal stimulation. These studies evaluated the influence of emotional and cognitive factors on the perception of afferent gut signaling. Conversely, information about the gut environment or chronically enhanced gut signaling may induce transient or long-term neuroplasticity changes in the brain that cause central sensitization as well as affective changes such as anxiety and depression. The most unique aspect of brain imaging studies in neurogastroenterology is the assessment of the correlation between the brain and peripheral functions, such as GI sensation, motility, and immune function, and gut microbiota compositions within the framework of the biopsychosocial model, which takes into account the influences of environmental, psychological, and biological factors on the interaction.
Brain-Gut Interaction: Functional Neuroanatomy of Key Concept of Functional Gastrointestinal Disorders
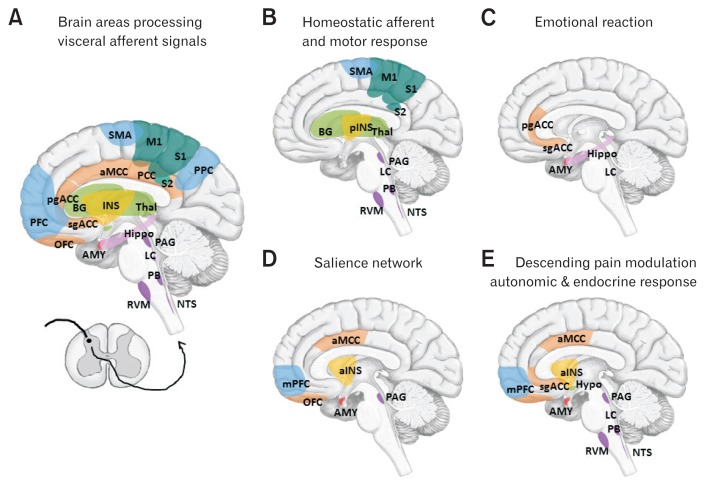
The brain (central nervous system) and gut (enteric nervous system) have bidirectional interactions that regulate physiological functions and maintain an organism’s overall homeostatic state.3 Afferent visceral signals are conveyed from the gut to the brain via the vagal and spinal pathways.14 The vagal and spinal splanchnic and pelvic afferent terminals convey the physiological condition including not only mechanical and chemical signals but also microbiota, immune, or endocrine signals originating from the gastroenterological tract.15 Many afferent signals go unperceived and are used in reflexes that control motility, secretion, blood flow, and other aspects of GI function. The primary vagal and spinal afferents project to the nucleus of the solitary tract in the medulla and lamina I of the dorsal horn, respectively. Signals from both afferent types are integrated in the parabrachial nucleus (PB) within the brainstem, which provides an integrated signal to regulate regions including the forebrain as well as the hypothalamus and amygdala.3 The visceral afferent information is then routed to the thalamus, where 2 parallel streams of information reach the insula and anterior cingulate cortices (ACC). In the brain, visceral afferent signals are processed in regions related to: (1) the perception of the homeostatic condition of the gut (eg, brainstem sensory nuclei, thalamus, and posterior insula); (2) accompanying emotional arousal reactions (eg, locus coeruleus [LC], amygdala, subgenual and pregenual ACC, and hippocampus); (3) the salience network (eg, anterior insula, anterior midcingulate cortex, and amygdala) that is engaged in response to salient stimuli not limited to pain; and (4) the descending pain modulation system (eg, periaqueductal gray [PAG] and rostroventral medulla), which receives direct nociceptive information from the spinoreticular pathway and top-down modulation from the prefrontal executive control and emotional arousal areas of the limbic system and modulates spinal dorsal horn neuron sensitivity.15 Of note, central structures that process visceral sensory signals not only share the structure of the descending modulatory pathway but also have strong connections to the emotional arousal areas and autonomic and endocrine stress response structures.15 Figure 1 shows the brain areas that process visceral afferent signals.
Figure 1.
Brain areas processing visceral afferent signals (A) and the areas in which altered brain activity is reported in FGIDs (B–E). (A) Afferent visceral signals conveyed from the gut project to the nucleus of the solitary tract (NTS) and the lamina I of the dorsal horn and are integrated in the parabrachial nucleus (PB) in the brainstem and routed to the thalamus (Thal), where 2 parallel streams of information reach the insula (INS) and anterior cingulate cortices (ACC). (B) Homeostatic afferent: the brain areas related to the sensory processing of the homeostatic condition of the gut are brainstem sensory nuclei (NST and PB), Thal, posterior insula (pINS), and somatosensory cortex (S1 and S2). The basal ganglia (BG), supplementary motor cortex (SMA), primary motor cortex (M1), brainstem nucleus (periaqueductal gray [PAG], locus coeruleus [LC], and rostroventral medulla [RVM]) are associated with preparation of the reaction and motor response to the afferent signals. (C) Emotional reaction: The areas associated with accompanying emotional arousal reactions are the LC, amygdala (AMY), subgenual and pregenual ACC (sgACC and pgACC), medial prefrontal cortex (mPFC), and hippocampus (Hippo). The AMY is a prototypical emotion-related structure with the hippocampal network. The ACC is a multifunctional structure, the sgACC is the principal site of autonomic regulation, and the pgACC is activated in a variety of emotional states. (D) Salience network16,24: this network is engaged in response to salient stimuli but not limited to pain. Core region of salience network is the anterior insula (aINS) and anterior midcingulate cortex (aMCC). The midcingulate cortex, part of the dorsal ACC, is a multifunctional region involved in the executive control of attention. The aINS is essential for the conscious experience (bodily) feelings. (E) Descending pain modulation system: endogenous descending pain modulation structures include the PAG and the RVM, which receives direct nociceptive information from the PB and the spinoreticular pathway and top-down modulation from the prefrontal executive control areas (mPFC and dorsolateral PFC [DLPFC]), aMCC and emotional arousal areas (aINS, sgACC, and AMY) and modulates the sensitivity of spinal dorsal horn neurons. These areas are highly connected with autonomic and endocrine response structures (hypothalamus [Hypo] and brainstem nucleus including NTS).
Important mediators of output signals from the brain to the gut are the autonomic nervous system (ANS) and the hypothalamic-pituitary-adrenal (HPA) axis, which are also the main regulators of the stress response.16,17 Physiological stress such as pain and psychological stress may increase the sympathetic and decrease the parasympathetic tone in the ANS.18,19 It also upregulates the HPA axis and corticotrophin-releasing hormone (CRH) levels,18,20 which activates local inflammatory processes and stimulates circulating cytokines that affect gut function, including motility, secretion, epithelial permeability, immune function, and microbiota composition.15 These changes in peripheral function, in turn, increase peripheral sensitivity to pain15 and alter and amplify visceral afferent signaling to the brain, which may change visceral pain processing, stress regulatory responses, and/or long-term neuroplasticity changes in the brain.3, 21
Functional Neuroimaging: Task Based Studies (During Gut Stimulation and/or Cognitive/ Emotional Task)
Visceral Afferent Signal Processing
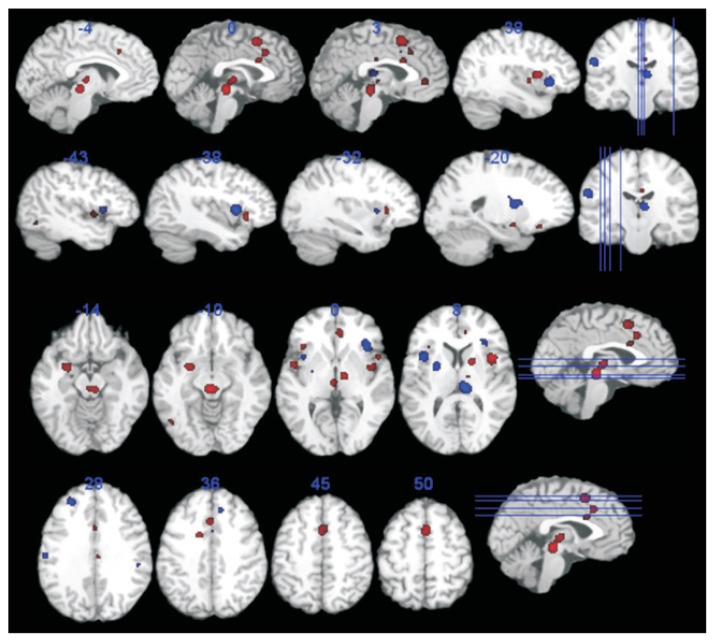
The brain regions consistently activated during rectal, colonic, and gastric distention include the anterior and posterior insula, ACC, primary sensory cortex, prefrontal regions, posterior parietal cortex, and thalamus.21–23 Whereas the insula and ACC were the most commonly reported regions, primary and secondary sensory cortices and the primary motor cortex were more often reported by studies of the upper GI tract.22 These brain areas are similar to the regions activated by somatic pain and the so-called “pain matrix” commonly activated by nociceptive stimuli.24 However, the pain matrix was recently reconceptualized as part of the salient network that is involved in detecting, orienting attention toward, and reacting to salient sensory stimuli, because the majority of brain imaging studies showed activation of the same regions regardless of their stimulus modality.21,24 In health, it may work as alarm signals on physiological conditions of the viscera; however, enhanced (or modified) activation of the salience network may underlie the symptoms of (functional) GI diseases. Meta-analyses of rectal distention confirmed the above results in both healthy controls (HC) and IBS patients.25,26 However, these patients showed stronger brain activation than did HC in regions associated with emotional arousal, including the pregenual ACC and amygdala, as well as in regions involved in endogenous pain inhibition and lower activation in the medial and lateral prefrontal cortex, possibly related to weaker engagement of the cortico-pontine-spinal pain inhibition system (Fig. 2).25 Similarly, the visceral-afferent areas in patients with FD became activated at significantly lower intra-gastric pressures than those in HC.27 These differential brain activities during visceral afferent signaling in FGIDs indicate that patients may have enhanced visceral afferent signal perception, unappropriated accompanying emotional arousal reactions, and weaker top-down modification of visceral signals. Of note, most studies of rectal distention were performed at the same degree of rectal distention in HC and patients. This produced stronger pain sensations in IBS patients. One paper reported that brain responses to distension were similar between normosensitive IBS patients and HC and that hypersensitive IBS patients had greater activation of the insula and smaller deactivation in the pregenual ACC during noxious rectal distension.28 This may mean that the difference in brain responses between HC and IBS with visceral hypersensitivity may not be the specific feature of the intracerebral processing of IBS itself but rather the reflection of differences in the thresholds of the sensory, ie, primary afferent or dorsal horn, neurons.
Figure 2.
Brain areas demonstrating more (red) or less (blue) activation in irritable bowel syndrome compared to healthy control in a meta-analysis of rectal distention. Adapted from Tillisch et al25 with permission.
Affective Factors on the Processing Visceral Afferent Signals
In HC, negative emotional induction by facial or music increased activation of the brain regionh the processing of visceral afferent signals including the ACC and insula, as well as increased ratings of anxiety and discomfort during esophageal sensation/pain.29,30 Public speaking stress enhanced activation in the posterior cingulate cortex (PCC) and somatosensory cortex during a rectal distention paradigm.31 On the other hand, fatty acid infusion into the stomach attenuated activation of the pons, hypothalamus, and hippocampus during sad emotional induction.32 HC with a higher neuroticism score showed higher activity in the salience network and the parahippocampal gyrus during pain expectation and lower activity in the same areas during actual pain as a result of esophageal stimulation.33 In IBS patients compared with HC, anxiety-induced modulation of the neural response to rectal distention was stronger in the insula, ventrolateral prefrontal cortex (VLPFC), and anterior midcingulate cortex (aMCC). In contrast, the modulation of the dorsolateral prefrontal cortex (DLPFC) activation in an anxiety context was reduced, which indicates impaired recruitment of the endogenous mechanism of pain control.31 In association with anxiety, FD patients showed no activation in the pregenual ACC (pgACC), no deactivation in the dorsal pons during distension, and no deactivation in the amygdala during sham treatment.34 This may represent arousal––anxiety-driven failure of pain modulation in FD.34 A history of early adverse life events (EALs) enhances MCC and PCC activation, decreases subgenual ACC (sgACC) activation, and leads to stronger rectal distention-induced pain reporting in IBS patients.35 Activation of the pain modulatory regions (dorsolateral and dorsomedial prefrontal cortices [PFC]) and amygdala is diminished during gastric distention.27 It is well known that EALs are more prevalent in IBS and associated with an increased HPA axis response36 and epigenetic changes.37,38 EALs may influence direct or indirect brain processing of visceral afferent signals. Emotion, personality, and EALs influence the brain’s processing of visceral signals from the gut and vice versa through direct or indirect mechanisms including expectation and genetic or neurohormonal modulation.
Cognitive Effect on the Processing of Visceral Afferent Signals
Cognition per se is a function of brain activity. Several trends of the psychological states are known to affect the processing of the visceral afferent signals.
Anticipation of visceral pain
Patients with IBS, compared with HC, demonstrated less anticipatory inhibition in the insula, sgACC, amygdala, and dorsal brainstem and showed increases in brain activity in the dorsal ACC and dorsal pons during rectal distention.39 Uncertain anticipation induced stronger MCC activation during the anticipation phase and in the MCC, PCC, and precuneus during subsequent rectal stimulation.40 The anticipation of visceral pain is considered preparatory for cognitive coping strategies that affect the neural processing of the visceral sensation itself. FGID patients may show failure of this preparatory modulation.
Conditioning with visceral stimulation
A Pavlovian conditioning paradigm with visceral stimulation can be used to study the influence of anticipation on visceral pain experience. Brain regions involved in the perception of visceral pain (esophageal and rectal) were activated when pain was conditioned by a cue (visual stimulus) but not actually delivered. This shows that a normal sensory experience may be exaggerated by the conditioning mechanism.41 IBS patients compared to HC showed stronger activation in the PCC and ventrolateral PFC (hypervigilance) and amygdala (pain-related fear) as well as enhanced activation in the ventromedial PFC and lateral orbitofrontal cortex (OFC) to the safety cue during the acquisition phase.42 During reinstatement, they showed enhanced PCC activation (greater attention) during extinction and stronger hippocampal activation (memory reactivation).42 Patients with IBS may be more hypervigilant and apt to engage attention in the associative learning and memory of abdominal pain.
Placebo: expectation of treatment
Placebo designs have demonstrated the effect of negative and positive expectation of treatment on the brain processing of visceral afferent signals. A positive expectation demonstrated greater decreases in activation of the pain modulation areas (DLPFC), pain sensory areas (insula, ACC, sensory cortex, and thalamus), and amygdala.43,44 Negative expectation by nocebo instruction increased activation in sensory regions during anticipation (eg, somatosensory cortex and amygdala) and during painful rectal stimulation (thalamus, insula, and amygdala). IBS patients demonstrated reductions in the placebo effect in pain-processing areas such as the thalamus, somatosensory cortices, insula, and ACC.45–47 IBS might be characterized by impaired cognitive pain modulation. Of note, patients with inflammatory bowel disorder did not show impaired placebo neural response like those with IBS, which suggests the specificity of this finding for IBS.47
Furthermore, a reduction in the effective connectivity of the central executive network circuitry (including the parietal and dorsal lateral PFC) during repeated exposure to a threatening GI stimulus48 and decreased DLPFC in relation to weaker error feedback function during the Wisconsin Card Sorting Test (WCST) in IBS49 support reduced cognitive modulation in patients with FGIDs.
Descending Modulation of Visceral Afferent Signals
Two studies demonstrated that diffuse noxious inhibitory control is dysfunctional in IBS patients compared with HC suggesting dysfunction of spinal and/or supraspinal endogenous pain modulation mechanisms in IBS patients.3,50
Neuroimaging Studies of Mediators of Brain-Gut Interaction
Stress mediators of the brain-gut interaction such as ANS and HPA axis function have been studied repeatedly in FGIDs.19,51 However, studies of the dynamic interaction between the brain and the gut and its circular loop as well as its interaction with other factors as a whole system remain sparse. Farmer et al52 examined the existence of distinct human pain clusters composed of discrete psychobiological and genetic profiles linked with variations in pain perception and processing. Patients in cluster 1 had higher neuroticism, greater baseline sympathetic tone, and higher cortisol release, an over-representation of the short allele of the serotonin transporter-linked polymorphic region (5-HTTLPR); an increased parasympathetic response to pain; and lower pain thresholds compared with patients in cluster 2, who had the opposite profile. Patients in cluster 1 demonstrated greater activity in the left frontal cortex, whereas patients in cluster 2 showed greater activity in the right medial/frontal cortex and anterior insula during esophageal distention.52 The genetic effect of 5-HTTLPR on brain-gut interactions has also been reported. Colorectal distention in individuals with the s/s genotype activates the ACC, hippocampus, and OFC more than that in individuals with the l allele.53 Another study examined the effects of CRH injection on colonic motility, ANS activity, and endocrine responses as well as brain activity alterations between IBS patients and HC. In brain responses to rectal distention, a negative association between the ACTH response to CRH and activity in the pgACC was identified in HC but not in IBS patients. This study indicates impaired top-down inhibitory input to the HPA axis from the pgACC in IBS.54
Sex-based Differences
In HC and patients with FGIDs, greater engagement of the emotional brain circuits was found in females versus greater engagement of the prefrontal circuits in males. During esophageal stimulation, women showed stronger activation in the midcingulate cortex, the affective component of pain.55 During the expectation of rectal pain, stronger coupling was observed among emotional arousal regions in female than in male patients with IBS.56 However, it must be emphasized that the published literature on sex differences in brain activation by visceral stimuli is still sparse and somewhat contradictory. Although many reviews emphasized the importance of considering sex-related differences, no conclusive studies have been performed.
Resting-state Functional Magnetic Resonance Imaging
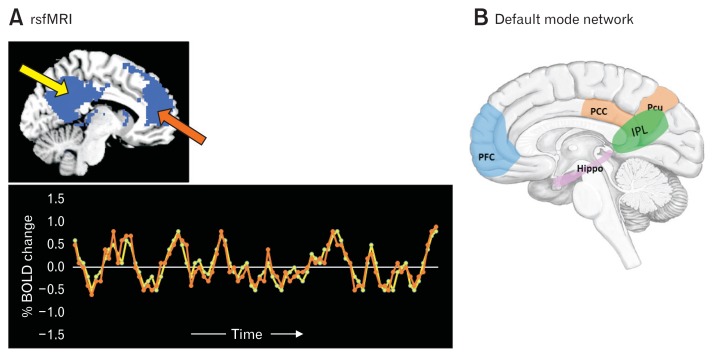
Brain activity is present even in the absence of an externally activated task and causes fluctuations in blood oxygen level-dependent (BOLD) signals. Resting-state functional magnetic resonance imaging (rsfMRI) measures the spontaneous fluctuations during rest and uncovers the intrinsic brain functional architecture, ie, functional connections of specific brain regions and local networks as well as overall organization of functional communication in the brain network (Fig. 3A).57,58 Even at rest, the brain’s functional networks that work together during tasks continue to harmonize along with their own distinguishable frequencies and phases and provide a signature of the functional organization. Alterations of the intrinsic networks may represent endophenotypes of disease vulnerability.
Figure 3.
Resting-state functional magnetic resonance imaging (rsfMRI) and default mode network. (A) rsfMRI is used to investigate synchronous neural activity (as measured with the blood oxygen level-dependent [BOLD] signal) between spatially distinct brain regions and provides the functional architecture of the brain. The lower panel represents the synchronous fMRI BOLD signal activity from the posterior cingulate cortex (yellow arrow in the upper panel) and in the medial prefrontal cortex (orange arrow in the upper panel). Adapted from Raichle.58 (B) Default mode network (DMN): the set of areas that work together at rest and are involved in high-level cognitive processes such as self-awareness and memory. DMN is thought to consist of the medial prefrontal cortex (mPFC), posterior cingulate cortex (PCC), hippocampus (Hippo), superior temporal gyrus, inferior parietal lobule (IPL), and precuneus (Pcu).
Several methods have been proposed to study functional connectivity using rsfMRI. Seed-based analysis correlates the resting-state time series between a seed region of interest and all other voxels in the brain. Independent component analysis (ICA) are model-free data-driven methods that determine spatial component maps of resting-state signals that are maximally independent of each other. In contrast, to detect correlations in fluctuations of the BOLD signals between regions or the components by ICA, the amplitude of low-frequency fluctuations (ALFF) estimates the amount of low-frequency power in each voxel in the brain.59 Regional homogeneity (ReHo) calculates the correlation of a voxel’s time series with that of its local neighboring voxels and focuses on localized short-distance connectivity.59 A graph theoretical analysis was recently proposed to study the topological characteristics of complex networks that can quantify the global organization of the network as well as the local functional characteristics of this brain network.57,60
Use of Resting-state Functional Magnetic Resonance Imaging in Irritable Bowel Syndrome
Studies using rsfMRI in IBS/FD began in the early 2010s; since then, several studies have investigated group differences of resting-state functional connectivity or correlation between parameters of rsfMRI and clinical indices. However, analysis methods of rsfMRI varied across studies and their results demonstrated considerable diversity.
Seed-based analysis
A bilateral dorsal anterior insula seed-based analysis reported a negative functional connectivity of the dorsal anterior insula with the medial PFC (mPFC) and precuneus in female IBS patients compared with female controls. Furthermore, a GI-specific anxiety score measured by the visceral sensitivity index was significantly correlated with functional connectivity between the dorsal anterior insula and the dorsal mPFC in male IBS patients and the precuneus in female IBS patients.61 An amygdala seed-based analysis showed a higher functional connectivity between the amygdala and insula, midbrain, and sensorimotor regions in IBS versus HC.62
Independent component analysis
Icenhour et al63 reported that resting-state functional connectivity value in the default mode network (DMN) (Fig. 3B) and the sensorimotor network was associated with rectal perception thresholds, and that the resting-state functional connectivity value in the posterior insula was correlated with the reported symptom severity in IBS.63
Amplitude of low-frequency fluctuations
Hong et al61 reported that female IBS subjects had a frequency power distribution skewed toward higher frequencies in the insula and toward lower frequencies in the sensorimotor cortex to a greater extent than in male IBS subjects. The skew toward higher frequencies in the anterior insula was positively correlated with symptom-related discomfort.61 Another study showed decreased ALFF values in the left superior frontal gyrus, right hippocampus, right middle frontal gyrus, bilateral postcentral, and right superior temporal pole, as well as increased values in the left middle cingulate and left calcarine gyrus in IBS patients.64 Qi et al65 reported that IBS patients showed decreased ALFF in several core DMN regions (mPFC, PCC, and bilateral inferior parietal lobule [IPL]), but increased ALFF in the bilateral posterior insula and cuneus. Additional seed-based analyses demonstrated differences in some inter-regional functional connectivity between IBS and HC.65
Regional homogeneity
IBS patients showed increased ReHo in the postcentral gyrus and thalamus and decreased ReHo in the ACC and PFC.66
Global organizations
Weng et al67 calculated functional connectivity density (FCD) for long- and short-range FCD values. They found that, compared with HC, IBS patients showed decreased long and short FCD in bilateral aMCC and IPL, decreased long FCD in the right anterior insula, and decreased short FCD in the bilateral PFC, sACC, and caudate. The abnormal FCD values in the right anterior insula and left caudate were correlated with IBS severity and disease duration, respectively.67 A graph analysis of the DMN showed decreased global efficiency in IBS patients.68
Use of Resting-state Functional Magnetic Resonance Imaging in Functional Dyspepsia
Seed-based analysis
Compared with HC, FD patients had increased connectivity between PAG and the insula and decreased connectivity between the PAG and the OFC, DLPFC, and hippocampus/parahippocampus. Highly anxious and depressed FD patients had altered PAG connectivity with the ACC, precuneus, DLPFC, and caudate.69 FD patients had higher global interhemispheric functional connectivity than HC, and voxel-wise analysis showed increased interhemispheric functional connectivity in the ACC insula and thalamus.70
Independent component analysis
Liu et al71 used ICA and reported an alteration in the DMN in FD, and dyspepsia symptom severity was positively correlated with the functional connectivity value in pgACC and negatively correlated with the functional connectivity value in OFC.71
A multivariate analysis was used to classify FD patients from controls based on functional connectivity and showed that abnormal functional connections were mainly within or across the limbic/ paralimbic system, PFC, temporoparietal areas, and visual cortex. Furthermore, the classification features were significantly associated with the patients’ dyspepsia symptoms and depression and anxiety scale scores.72
Amplitude of low-frequency fluctuations
Zhou et al73 estimated fractional ALFF (fALFF), the ratio of the power spectrum of low frequency to that of the entire frequency range, and reported group differences in patients with FD who had increased fALFF in multiple regions, including the insula, brainstem, and bilateral cerebellum. The fALFF in the insula was positively correlated with disease severity. Additional seed-based analyses revealed increased functional connectivity between the right cerebellum and multiple brain regions.73
Regional homogeneity
Liu et al74 applied a multivariate analysis and showed highly discriminative brain regions between FD and HC based on ReHo, mainly in the PFC, OFC, supplementary motor cortex, temporal pole, insula, ACC/MCC, thalamus, hippocampus, parahippocampus, and cerebellum. ReHo values were positively correlated with symptom severity in the dorsal mPFC and pgACC, whereas they were correlated with FD disease duration in the MCC, OFC, insula, and temporal pole.74 Nan et al75 found altered ReHo values in multiple brain areas in FD patients. ReHo values in the ACC and thalamus were correlated with symptom severity of dyspepsia.75
Although there is considerable variety in methodology, these results may indicate abnormal functional connectivity in IBS or FD. At the local level, abnormal resting-state functional connectivity was reported, mainly in pain-related areas (anterior insula, ACC, amygdala, and sensory motor cortex) and the DMN (PCC, precuneus, mPFC, and hippocampal complex).
Structural Magnetic Resonance Imaging
Brain Morphometry
Cortical thickness and gray matter density
High-resolution structural MRI measures the brain anatomy and can be used to quantify and systematically compare morphological differences in brain structures.76 The most commonly used method is voxel-based morphometry, images of which provide a measure of gray-matter density or volume. Variations of these measurements may reflect differences in cell types, neuron densities, dendritic harboring, and other causes.
Irritable Bowel Syndrome
IBS patients have increased gray matter density (GMD) in the hypothalamus and increased cortical thickness in the aMCC. A negative correlation between the pain catastrophizing scale and DLPFC thickness and a positive correlation between pain duration and anterior insula thickness is seen in these patients.77 Seminowicz et al78 reported that IBS patients showed increased GMD in the pgACC and OFC and a decreased GMD in the mPFC, DLPFC, PCC, ventral striatum, and thalamus. However, the group differences remained only in the PFC and PCC when anxiety and depression levels were controlled.78 Another study showed that female IBS patients compared with female controls showed increased cortical thickness in the pre- and post-central gyrus and decreased thickness in the bilateral insula and the left subgenual ACC (sgACC).79 Lower gray matter volumes (GMV) in the insula, cingulate, amygdala, hippocampus, putamen, and frontal regions and higher GMV in the left postcentral gyrus were observed in female IBS patients compared with HC. However, many of the differences were accounted for by histories of early trauma.80 Further, they applied the graph theory and found no group differences in global and local network organization.80 In an experiment of the combination of a pain modulation examination using electrical stimulation and heterotopic noxious counter-stimulation, a greater thickness in the right posterior insula was associated with a longer disease duration in IBS, while a thicker right lateral OFC was associated with less pain inhibition in both IBS patients and HC.81 Rectal sensitivity was associated with GMV in the thalamus, insula, PCC, VLPFC, OFC, amygdala, and basal ganglia and negatively associated with GMV in the right thalamus.82 Catecholaminergic gene polymorphisms are associated with morphological alterations in IBS patients.83 Labus et al84 performed a multivariate analysis of morphometric data (volume, mean curvature, surface area, and cortical thickness) to identify potential brain endophenotypes that discriminate IBS patients from HC.84 Although associations between the brain signatures and clinical measures were weak, alterations in sensorimotor regions dominated the morphometry based classifier, which share similar results in other chronic functional pain disorders.84
Functional Dyspepsia
Patients with FD with postprandial pain compared to HC showed a decreased GMD in the bilateral precentral gyrus, mPFC, ACC, MCC, left OFC, and right insula. When considering anxiety and depression, the reduced GM density in the bilateral middle frontal gyrus, left MCC, right precentral gyrus, and insula remained. The GMD decreases in the ACC were significantly associated with symptom scores.85 On the other hand, Liu et al86 found that FD patients had a higher GMV in the bilateral putamen and right caudate, and additional seed-based structural covariance patterns demonstrated that FD-related differences were mainly located in the amygdala, hippocampus/parahippocampus, thalamus, lingual gyrus, and cerebellum. In these patients, significant positive correlations were found between the volumes in the striatum and the FD disease duration.86 Another study demonstrated that FD patients exhibited decreased GMD in the right posterior insula, right inferior frontal cortex, and left MCC.87
White Matter Microstructure
Diffusion imaging detects white matter (WM) bundles and provides measures of WM integrity. Diffusion MRI measures the displacement of water molecules and is much faster along the WM fibers then perpendicular to them. Diffusion imaging quantifies the diffusion properties of WM.88 Fractional anisotropy (FA), a frequently used measure, is related to regional WM features such as axon caliber, fiber density, and myelination. Higher FA values indicate more efficient neuronal conduction through the WM. Probabilistic tractography is used to assess the structural connection between two regions of interest.89
Irritable Bowel Syndrome
Chen et al90 extracted mean FA values from WM regions associated with nociception and provided preliminary evidence for fornix and insular WM alterations in IBS patients. Ellingson et al. found that IBS patients had lower FA values in the basal ganglia and sensory/motor association/integration regions as well as higher FA in the frontal lobe regions and corpus callosum.91 IBS patients had a reduced mean diffusivity within the globus pallidus and a higher mean diffusivity in the thalamus, internal capsule, and coronal radiate projections to the sensorimotor regions. This suggests differential changes in axon/dendritic density in these regions.91 Using multivariate regression analysis, Irimia et al92 reported abnormalities in the mean FA of WM connections innervating the viscerotropic portions of the sensory cortex in IBS patients. Another study including a whole-brain voxel-wise analysis demonstrated that IBS patients had reduced FA in the splenium of the corpus callosum, right retrolenticular area of the internal capsule, and right superior corona radiata.93 Comparing functional constipation, constipation-predominant IBS (IBS-C), and HC, the genu of the corpus callosum showed a negative association with abdominal pain or discomfort intensity in functional connectivity and IBS-C. On the other hand, functional connectivity exhibits more regions of WM changes than IBS-C, such as the corona radiate, which includes projection fibers associated with the corticopontine tract.94
Functional Dyspepsia
One paper reported an increased FA along with reduced mean and radial diffusivity in multiple WM tracts in FD with postprandial distress syndrome (FD-PDS) versus HC and the inclusion of anxiety and depression as covariates abolished the group difference except in the corona radiate. The corona radiate contains commissural and cortico-efferent fibers and the results support cortical modulation of the homeostatic reflex at the central nervous system level in FGID patients.95
These studies may indicate abnormal WM microstructures in the brain associated with visceral afferent signal processing. However, the results are still inconsistent and further studies are required to reach a final conclusion.
Molecular Imaging
Molecular imaging modalities such as magnetic resonance spectroscopy and PET characterize biological processes at the molecular and cellular levels. Magnetic resonance spectroscopy assesses the levels of biochemical metabolites in the brain such as glutamate/glutamine (Gly) and γ-aminobutyric acid (GABA). PET uses radioligands injected into the bloodstream that have high binding affinity for target molecules. There are few molecular imaging studies in the field of gastroenterology.
Irritable Bowel Syndrome
Niddam et al96 demonstrated a reduction in hippocampal Gly in IBS patients and that Gly concentrations were inversely related to emotional stress indicators in IBS patients. These findings indicated abnormal functioning of hippocampal glutamatergic neurotransmission and dysfunction of the inhibitory role of the hippocampus in IBS.96
A PET study demonstrated that serotonin synthesis was greater in the right medial temporal gyrus in female IBS patients than in female controls.97 A follow-up study investigated the effect after a 14-day oral treatment with alosetron, a 5-HT3 receptor antagonist, and found that 5-HT synthesis was greater in male IBS patients than in female IBS patients.98
Functional Dyspepsia
Mak et al99 found enhanced glutamate transmission in the somatosensory cortex in FD-PDS linked to post-prandial distress chronicity and severity as well as anxiety. A PET study on FD revealed that the level of the serotonin transporter in the midbrain and thalamus was higher in patients with FD than in HC. This indicates upregulation of the serotonin transporter level in gut afferent signal-related areas in patients with FD.100 Another PET study of a cannabinoid-1 receptor radioligand demonstrated that FD patients had higher cannabinoid-1 receptor availability in areas related to visceral nociception as well as homeostatic and hedonic regulation of food intake, which may indicate sustained endocannabinoid dysfunction in FD.101
Other Studies
Repetitive transcranial magnetic stimulation targeting the right secondary somatosensory cortex in patients with chronic pancreatitis and severe visceral pain revealed a significant analgesic effect that was correlated with an increase in excitatory neurotransmitter levels such as glutamate and N-acetyl aspartate.102
Microbiota-Brain Interaction in Functional Gastrointestinal Disorders
Findings in rodents indicate that the gut microbiota may contribute to the pathophysiology of FGIDs103 as well as brain disorders including autism spectrum disorders,104 Parkinson’s disease,105 and affective disorders.106 The burgeoning number of preclinical studies demonstrated that the gut microbiota plays a significant role in brain development,107 the stress response system,108,109 and the brain neurotransmitter system.110,111 However, there is very limited evidence that replicates these animal data in the brains of patients with gut-brain disorders.110
Healthy women who consumed dairy products containing probiotic bacteria had a reduced response in a network including the primary interoceptive and somatosensory regions as well as the midbrain regions during an emotional face recognition task.112 In addition, activity in the interoceptive, affective, and prefrontal regions has changed during resting-state measurements.112 In healthy women, bacterial genus-based clusters were identified: a cluster with a greater abundance of Bacteroides and one with a greater abundance of Prevotella. The Prevotella group showed less hippocampal activity on viewing negative valence images.112 Patterns of white and gray matter imaging discriminated between the 2 clusters.113 In a placebo-controlled trial of IBS patients taking probiotics, Bifidobacterium longum reduced responses to negative emotional stimuli in the amygdala and frontolimbic regions and reduced depression scores.114 Labus et al115 clustered subgroups of IBS patients based on distinct microbial clusters, but this grouping was rarely correlated with the clinical symptoms of IBS. They also found structural brain alterations, primarily in the sensory integration areas and in the salience network in correlation with the microbial taxa. This correlation was different between IBS patients and HC.115 Short-chain fatty acids, which are gut microbiota-driven metabolites produced from fermentable carbohydrates such as acetate, propionate, and butyrate, modulated feeding behavior via central mechanisms in rodents.116 A diet-associated increase in colonic propionate production and the level of increased propionate were associated with reduced brain activity during high-energy food picture evaluation in the caudate and nucleus accumbens. This finding suggests attenuating reward-based appetite behavior and was independent of changes in plasma peptide tyrosine-tyrosine and glucagon-like peptide 1, glucose, or insulin concentrations.117 The levels of gut hormones, such as ghrelin, that promote appetite are associated with increased activation in the PFC, amygdala, and insula and decreased activation in the hypothalamus. In contrast, glucose, insulin, leptin, peptide tyrosine-tyrosine, and glucagon-like peptide 1 affect the same brain regions conversely.118 Although there is some preliminary evidence, neuroimaging studies aiming to prove the preclinical gut microbiota findings have just started and further well-designed translational and clinical studies are required to study the role of microbiota in human health and disease.
Progress of Analysis of Neuroimaging
Shift From Brain Mapping to Predicting Clinical Outcome
Traditional brain analyses focused on brain mapping to identify brain regions responding to a task or showing a different response between HC and patients in a disease condition. In contrast, recent machine learning algorithms or multivariate analyses are used as a data-driven approach to identify brain activity patterns that have specific profiles to predict outcomes. For example, using these techniques, pain levels can be predicted from brain activity patterns or a classifier can be constructed to distinguish IBS from HC based on their brain activity. Using structural MRI data, Labus et al84 developed a classification model to identify the regions that contribute most to the classification. The model discriminated patients from HC with 70% accuracy and provided a morphological brain signature for IBS patients.84 Liu and Nan adapted this approach to distinguish patients with FD from HC using rsfMRI.72,74 Such generalized brain patterns can be used as a brain-based biomarker of the specific disease to assess the effect of pharmacological or therapeutic interventions.
Multicenter Collaborations and Big Data in Neuroimaging
To date, most neuroimaging studies have had a modest sample size (< 50 subjects). However, studies with big neuroimaging data (> 1000 subjects) are ongoing such as the human connectome project, UK Biobank, and ENIGMA consortium.119 Although the big data approach aims to increase the power of neuroimaging studies, it will also lead to the detection of subtle effects with a small effect size. Machine learning techniques or ICA can be used to determine the most important factors from these data sets. Multiple-center collaborations may contribute to these big data projects; at the same time, they will also increase the variability due to differences in design, scanner, and acquisition settings as well as differences related to the sample population including age, sex, social status, and other population variables.
Connectivity and Modeling of Complex Systems
With regard to brain imaging analyses, initial research methods aimed to estimate regional brain activity that is associated with some contrast, eg, rectal distention versus non-rectal distention. However, brain function changes dynamically in space and time within brain networks and are influenced by visceral information. Some connectivity analyses can assess the causal relationship between regions. Using a dynamic causal model, Aizawa et al49 revealed that the input from the PFC to the insula was significantly weaker during WCST in IBS patients. Labus et al120 examined the effect of tryptophan depletion, which decreased brain serotonin levels, on differential connectivity between the ACC, mPFC, amygdala, and pons during rectal distention between HC and IBS patients. Using graph theory, Qi et al68 demonstrated that IBS patients showed a topological reorganization of the DMN to a non-optimized regularity configuration.
Conclusion and Future Directions
The current paper reviewed neuroimaging studies in the field of FGIDs. Neuroimaging is particularly useful to increase our understanding of the brain-gut/gut-brain interaction dysfunction in FGIDs. Functional neuroimaging studies during GI stimulation have revealed that regional brain activity in regions associated with sensory processing including the thalamus, sensory cortex, and posterior insula and in regions in the salience network such as the anterior insula and dorsal ACC is related to visceral sensations during stimulation in patients with FGIDs at physiologically similar levels. In addition, altered brain activity is found in areas associated with emotional response including the amygdala and ACC as well as top-down regulation from the PFC. Descending pain inhibitory system failure was also demonstrated. Some studies also showed the dysfunction of brain regulation on the brain-gut interaction by investigating the association between brain activity and HPA and ANS parameters. These studies suggested that in FGIDs, dysfunctional top-down regulation from the PFC and dysfunctional interactions in homeostatic areas like the hypothalamus, amygdala, and nuclei of the brainstem were associated with autonomic responses and pain regulation.
Studies using rsfMR demonstrated that the intrinsic connectivity during rest may differ between HC and patients, whereas gray matter and WM morphometry findings indicated some alterations. However, these results require replication in larger samples.
Recent developments of analytical methods to assess the pathophysiology of FGIDs are promising but the results show complex inter-study variability. Because FGIDs are complex, we must understand their features in several dimensions: within the gut association; within the brain association; brain-gut interactions with its mediators including ANS, HPA, and immune parameters; within a longitudinal time span from genomic predisposition and/ or epigenomic change influenced by the early life environment; to alterations accompanying age; and associations among modalities (functional, structural, neurochemical, etc). However, we also need to understand the variability across laboratories, scanners, study designs, methods, and regions and races worldwide. To solve these problems, multicenter studies with multimodal neuroimaging techniques that consider the interaction with peripheral physiological changes using new methods such as deep learning and graph theory may be helpful. However, because so many factors are interacting in FGIDs, the population will be quite heterogeneous and a new model is needed to understand such complex interactions. In future studies, it may be possible to determine whether FGIDs represent a shift from the optimal standard homeostatic state which contributes to the symptoms.
Footnotes
Financial support: This research was supported by a Grant -in-Aid for Scientific Research from the Japanese Ministry of Education, Science and Culture to Michiko Kano (Grant No. 26460898).
Conflicts of interest: None.
Author contributions: Michiko Kano: searching manuscripts for this review, drafting of the manuscript, and critical revision of the manuscript; and Patrick Dupont, Qasim Aziz, and Shin Fu-kudo: critical revision of the manuscript for important intellectual content.
References
- 1.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology. 2016;150:1262–1279. e2. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 2.Aziz Q, Thompson DG. Brain-gut axis in health and disease. Gastroenterology. 1998;114:559–578. doi: 10.1016/S0016-5085(98)70540-2. [DOI] [PubMed] [Google Scholar]
- 3.Mayer EA. Gut feelings: the emerging biology of gut-brain communication. Nat Rev Neurosci. 2011;12:453–466. doi: 10.1038/nrn3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukudo S, Nomura T, Muranaka M, Taguchi F. Brain-gut response to stress and cholinergic stimulation in irritable bowel syndrome. A preliminary study. J Clin Gastroenterol. 1993;17:133–141. doi: 10.1097/00004836-199309000-00009. [DOI] [PubMed] [Google Scholar]
- 5.Silverman DH, Munakata JA, Ennes H, Mandelkern MA, Hoh CK, Mayer EA. Regional cerebral activity in normal and pathological perception of visceral pain. Gastroenterology. 1997;112:64–72. doi: 10.1016/S0016-5085(97)70220-8. [DOI] [PubMed] [Google Scholar]
- 6.Aziz Q, Andersson JL, Valind S, et al. Identification of human brain loci processing esophageal sensation using positron emission tomography. Gastroenterology. 1997;113:50–59. doi: 10.1016/S0016-5085(97)70079-9. [DOI] [PubMed] [Google Scholar]
- 7.Vandenberghe J, Dupont P, Van Oudenhove L, et al. Regional cerebral blood flow during gastric balloon distention in functional dyspepsia. Gastroenterology. 2007;132:1684–1693. doi: 10.1053/j.gastro.2007.03.037. [DOI] [PubMed] [Google Scholar]
- 8.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lovell RM, Ford AC. Global prevalence of and risk factors for irritable bowel syndrome: a meta-analysis. Clin Gastroenterol Hepatol. 2012;10:712–721. e4. doi: 10.1016/j.cgh.2012.02.029. [DOI] [PubMed] [Google Scholar]
- 10.Van Oudenhove L, Crowell MD, Drossman DA, et al. Biopsychosocial aspects of functional gastrointestinal disorders: how central and environmental processes contribute to the development and expression of functional gastrointerinal disorders. Gastroenterology. 2016;150:1355–1367. e2. doi: 10.1053/j.gastro.2016.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Enck P, Aziz Q, Barbara G, et al. Irritable bowel syndrome. Nat Rev Dis Primers. 2016;2:16014. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukudo S. IBS: Autonomic dysregulation in IBS. Nat Rev Gastroenterol Hepatol. 2013;10:569–571. doi: 10.1038/nrgastro.2013.166. [DOI] [PubMed] [Google Scholar]
- 14.Cawthon CR, de La Serre CB. Gut bacteria interaction with vagal afferents. Brain Res. 2018 doi: 10.1016/j.brainres.2018.01.012. pii:S0006-8993(18)30020-9. [DOI] [PubMed] [Google Scholar]
- 15.Vanner S, Greenwood-Van Meerveld B, Mawe G, et al. Fundamentals of neurogastroenterology: basic science. Gastroenterology. 2016;150:1280–1291. doi: 10.1053/j.gastro.2016.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol. 2015;12:592–605. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fukudo S. Role of corticotropin-releasing hormone in irritable bowel syndrome and intestinal inflammation. J Gastroenterol. 2007;42(suppl 17):48–51. doi: 10.1007/s00535-006-1942-7. [DOI] [PubMed] [Google Scholar]
- 18.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stengel A, Taché Y. CRF and urocortin peptides as modulators of energy balance and feeding behavior during stress. Front Neurosci. 2014;8:52. doi: 10.3389/fnins.2014.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayer EA, Gupta A, Kilpatrick LA, Hong JY. Imaging brain mechanisms in chronic visceral pain. Pain. 2015;156(suppl 1):S50–S63. doi: 10.1097/j.pain.0000000000000106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mayer EA, Aziz Q, Coen S, et al. Brain imaging approaches to the study of functional GI disorders: a rome working team report. Neurogastroenterol Motil. 2009;21:579–596. doi: 10.1111/j.1365-2982.2009.01304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Al Omran Y, Aziz Q. Functional brain imaging in gastroenterology: to new beginnings. Nat Rev Gastroenterol Hepatol. 2014;11:565–576. doi: 10.1038/nrgastro.2014.89. [DOI] [PubMed] [Google Scholar]
- 24.Legrain V, Iannetti GD, Plaghki L, Mouraux A. The pain matrix reloaded: a salience detection system for the body. Prog Neurobiol. 2011;93:111–124. doi: 10.1016/j.pneurobio.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Tillisch K, Mayer EA, Labus JS. Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology. 2011;140:91–100. doi: 10.1053/j.gastro.2010.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheehan J, Gaman A, Vangel M, Kuo B. Pooled analysis of brain activity in irritable bowel syndrome and controls during rectal balloon distension. Neurogastroenterol Motil. 2011;23:336–346. e158. doi: 10.1111/j.1365-2982.2010.01635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Oudenhove L, Vandenberghe J, Dupont P, et al. Regional brain activity in functional dyspepsia: a H215O-PET study on the role of gastric sensitivity and abuse history. Gastroenterology. 2010;139:36–47. doi: 10.1053/j.gastro.2010.04.015. [DOI] [PubMed] [Google Scholar]
- 28.Larsson MB, Tillisch K, Craig AD, et al. Brain responses to visceral stimuli reflect visceral sensitivity thresholds in patients with irritable bowel syndrome. Gastroenterology. 2012;142:463–472. e3. doi: 10.1053/j.gastro.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips ML, Gregory LJ, Cullen S, et al. The effect of negative emotional context on neural and behavioural responses to oesophageal stimulation. Brain. 2003;126:669–684. doi: 10.1093/brain/awg065. [DOI] [PubMed] [Google Scholar]
- 30.Coen SJ, Yágüez L, Aziz Q, et al. Negative mood affects brain processing of visceral sensation. Gastroenterology. 2009;137:253–261. e1–e2. doi: 10.1053/j.gastro.2009.02.052. [DOI] [PubMed] [Google Scholar]
- 31.Elsenbruch S, Rosenberger C, Bingel U, Forsting M, Schedlowski M, Gizewski ER. Patients with irritable bowel syndrome have altered emotional modulation of neural responses to visceral stimuli. Gastroenterology. 2010;139:1310–1319. doi: 10.1053/j.gastro.2010.06.054. [DOI] [PubMed] [Google Scholar]
- 32.Van Oudenhove L, McKie S, Lassman D, et al. Fatty acid-induced gut-brain signaling attenuates neural and behavioral effects of sad emotion in humans. J Clin Invest. 2011;121:3094–3099. doi: 10.1172/JCI46380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coen SJ, Kano M, Farmer AD, et al. Neuroticism influences brain activity during the experience of visceral pain. Gastroenterology. 2011;141:909–917. e1. doi: 10.1053/j.gastro.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 34.Van Oudenhove L, Vandenberghe J, Dupont P, et al. Abnormal regional brain activity during rest and (anticipated) gastric distension in functional dyspepsia and the role of anxiety: a H215O-PET study. Am J Gastroenterol. 2010;105:913–924. doi: 10.1038/ajg.2010.39. [DOI] [PubMed] [Google Scholar]
- 35.Ringel Y, Drossman DA, Leserman JL, et al. Effect of abuse history on pain reports and brain responses to aversive visceral stimulation: an FMRI study. Gastroenterology. 2008;134:396–404. doi: 10.1053/j.gastro.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 36.Videlock EJ, Adeyemo M, Licudine A, et al. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Provencal N, Suderman MJ, Guillemin C, et al. The signature of maternal rearing in the methylome in rhesus macaque prefrontal cortex and T cells. J Neurosci. 2012;32:15626–15642. doi: 10.1523/JNEUROSCI.1470-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provencal N, Binder EB. The effects of early life stress on the epigenome: from the womb to adulthood and even before. Exp Neurol. 2015;268:10–20. doi: 10.1016/j.expneurol.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28:349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kano M, Muratsubaki T, Morishita J, et al. Influence of uncertain anticipation on brain responses to aversive rectal distension in patients with irritable bowel syndrome. Psychosom Med. 2017;79:988–999. doi: 10.1097/PSY.0000000000000484. [DOI] [PubMed] [Google Scholar]
- 41.Yágüez L, Coen S, Gregory LJ, et al. Brain response to visceral aversive conditioning: a functional magnetic resonance imaging study. Gastroenterology. 2005;128:1819–1829. doi: 10.1053/j.gastro.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 42.Icenhour A, Langhorst J, Benson S, et al. Neural circuitry of abdominal pain-related fear learning and reinstatement in irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:114–127. doi: 10.1111/nmo.12489. [DOI] [PubMed] [Google Scholar]
- 43.Lu HC, Hsieh JC, Lu CL, et al. Neuronal correlates in the modulation of placebo analgesia in experimentally-induced esophageal pain: a 3T-fMRI study. Pain. 2010;148:75–83. doi: 10.1016/j.pain.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 44.Kotsis V, Benson S, Bingel U, et al. Perceived treatment group affects behavioral and neural responses to visceral pain in a deceptive placebo study. Neurogastroenterol Motil. 2012;24:935–e462. doi: 10.1111/j.1365-2982.2012.01968.x. [DOI] [PubMed] [Google Scholar]
- 45.Price DD, Craggs J, Verne GN, Perlstein WM, Robinson ME. Placebo analgesia is accompanied by large reductions in pain-related brain activity in irritable bowel syndrome patients. Pain. 2007;127:63–72. doi: 10.1016/j.pain.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 46.Lee HF, Hsieh JC, Lu CL, et al. Enhanced affect/cognition-related brain responses during visceral placebo analgesia in irritable bowel syndrome patients. Pain. 2012;153:1301–1310. doi: 10.1016/j.pain.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 47.Schmid J, Langhorst J, Gaß F, et al. Placebo analgesia in patients with functional and organic abdominal pain: a fMRI study in IBS, UC and healthy volunteers. Gut. 2015;64:418–427. doi: 10.1136/gutjnl-2013-306648. [DOI] [PubMed] [Google Scholar]
- 48.Labus JS, Naliboff BD, Berman SM, et al. Brain networks underlying perceptual habituation to repeated aversive visceral stimuli in patients with irritable bowel syndrome. Neuroimage. 2009;47:952–960. doi: 10.1016/j.neuroimage.2009.05.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aizawa E, Sato Y, Kochiyama T, et al. Altered cognitive function of prefrontal cortex during error feedback in patients with irritable bowel syndrome, based on FMRI and dynamic causal modeling. Gastroenterology. 2012;143:1188–1198. doi: 10.1053/j.gastro.2012.07.104. [DOI] [PubMed] [Google Scholar]
- 50.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut. 2011;60:1589–1599. doi: 10.1136/gutjnl-2011-300253. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka Y, Kanazawa M, Kano M, et al. Differential activation in amygdala and plasma noradrenaline during colorectal distention by administration of corticotropin-releasing hormone between healthy individuals and patients with irritable bowel syndrome. PLoS One. 2016;11:e0157347. doi: 10.1371/journal.pone.0157347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Farmer AD, Coen SJ, Kano M, et al. Psychophysiological responses to pain identify reproducible human clusters. Pain. 2013;154:2266–2276. doi: 10.1016/j.pain.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 53.Fukudo S, Kanazawa M, Mizuno T, et al. Impact of serotonin transporter gene polymorphism on brain activation by colorectal distention. Neuroimage. 2009;47:946–951. doi: 10.1016/j.neuroimage.2009.04.083. [DOI] [PubMed] [Google Scholar]
- 54.Kano M, Muratsubaki T, Van Oudenhove L, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7:12425. doi: 10.1038/s41598-017-09635-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kano M, Farmer AD, Aziz Q, et al. Sex differences in brain response to anticipated and experienced visceral pain in healthy subjects. Am J Physiol Gastrointest Liver Physiol. 2013;304:G687–G699. doi: 10.1152/ajpgi.00385.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Labus JS, Naliboff BN, Fallon J, et al. Sex differences in brain activity during aversive visceral stimulation and its expectation in patients with chronic abdominal pain: a network analysis. Neuroimage. 2008;41:1032–1043. doi: 10.1016/j.neuroimage.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van den Heuvel MP, Hulshoff Pol HE. Exploring the brain network: a review on resting-state fMRI functional connectivity. Eur Neuropsychopharmacol. 2010;20:519–534. doi: 10.1016/j.euroneuro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 58.Raichle ME. The restless brain; how intrinsic activity organizes brain function. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140172. doi: 10.1098/rstb.2014.0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhan X, Yu R. A window into the brain: advances in psychiatric fMRI. Biomed Research International. 2015;2015 doi: 10.1155/2015/542467. 542467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bijsterbosch J, Harrison S, Duff E, Alfaro-Almagro F, Woolrich M, Smith S. Investigations into within- and between-subject resting-state amplitude variations. Neuroimage. 2017;159:57–69. doi: 10.1016/j.neuroimage.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hong JY, Kilpatrick LA, Labus J, et al. Patients with chronic visceral pain show sex-related alterations in intrinsic oscillations of the resting brain. J Neurosci. 2013;33:11994–12002. doi: 10.1523/JNEUROSCI.5733-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Qi R, Liu C, Ke J, et al. Abnormal amygdala resting-state functional connectivity in irritable bowel syndrome. AJNR Am J Neuroradiol. 2016;37:1139–1145. doi: 10.3174/ajnr.A4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Icenhour A, Witt ST, Elsenbruch S, et al. Brain functional connectivity is associated with visceral sensitivity in women with irritable bowel syndrome. Neuroimage Clin. 2017;15:449–457. doi: 10.1016/j.nicl.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ma X, Li S, Tian J, et al. Altered brain spontaneous activity and connectivity network in irritable bowel syndrome patients: a resting-state fMRI study. Clin Neurophysiol. 2015;126:1190–1197. doi: 10.1016/j.clinph.2014.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Qi R, Liu C, Ke J, et al. Intrinsic brain abnormalities in irritable bowel syndrome and effect of anxiety and depression. Brain Imaging Behav. 2016;10:1127–1134. doi: 10.1007/s11682-015-9478-1. [DOI] [PubMed] [Google Scholar]
- 66.Ke J, Qi R, Liu C, et al. Abnormal regional homogeneity in patients with irritable bowel syndrome: a resting-state functional MRI study. Neurogastroenterol Motil. 2015;27:1796–1803. doi: 10.1111/nmo.12692. [DOI] [PubMed] [Google Scholar]
- 67.Weng Y, Qi R, Liu C, et al. Disrupted functional connectivity density in irritable bowel syndrome patients. Brain Imaging Behav. 2017;11:1812–1822. doi: 10.1007/s11682-016-9653-z. [DOI] [PubMed] [Google Scholar]
- 68.Qi R, Ke J, Schoepf UJ, et al. Topological reorganization of the default mode network in irritable bowel syndrome. Mol Neurobiol. 2016;53:6585–6593. doi: 10.1007/s12035-015-9558-7. [DOI] [PubMed] [Google Scholar]
- 69.Liu P, Wang G, Liu Y, et al. Disrupted intrinsic connectivity of the periaqueductal gray in patients with functional dyspepsia: a resting-state fMRI study. Neurogastroenterol Motil. doi: 10.1111/nmo.13060. Published Online First: 24 Mar 2017. [DOI] [PubMed] [Google Scholar]
- 70.Zhou G, Liu P, Zeng F, et al. Increased interhemispheric resting-state functional connectivity in functional dyspepsia: a pilot study. NMR Biomed. 2013;26:410–415. doi: 10.1002/nbm.2878. [DOI] [PubMed] [Google Scholar]
- 71.Liu P, Zeng F, Zhou G, et al. Alterations of the default mode network in functional dyspepsia patients: a resting-state fmri study. Neurogastroenterol Motil. 2013;25:e382–e388. doi: 10.1111/nmo.12131. [DOI] [PubMed] [Google Scholar]
- 72.Nan J, Liu J, Li G, et al. Whole-brain functional connectivity identification of functional dyspepsia. PLoS One. 2013;8:e65870. doi: 10.1371/journal.pone.0065870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zhou G, Liu P, Wang J, et al. Fractional amplitude of low-frequency fluctuation changes in functional dyspepsia: a resting-state fMRI study. Magn Reson Imaging. 2013;31:996–1000. doi: 10.1016/j.mri.2013.03.019. [DOI] [PubMed] [Google Scholar]
- 74.Liu P, Qin W, Wang J, et al. Identifying neural patterns of functional dyspepsia using multivariate pattern analysis: a resting-state FMRI study. PLoS One. 2013;8:e68205. doi: 10.1371/journal.pone.0068205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nan J, Liu J, Zhang D, et al. Altered intrinsic regional activity and corresponding brain pathways reflect the symptom severity of functional dyspepsia. Neurogastroenterol Motil. 2014;26:660–669. doi: 10.1111/nmo.12311. [DOI] [PubMed] [Google Scholar]
- 76.Kanai R, Rees G. The structural basis of inter-individual differences in human behaviour and cognition. Nat Rev Neurosci. 2011;12:231–242. doi: 10.1038/nrn3000. [DOI] [PubMed] [Google Scholar]
- 77.Blankstein U, Chen J, Diamant NE, Davis KD. Altered brain structure in irritable bowel syndrome: potential contributions of pre-existing and disease-driven factors. Gastroenterology. 2010;138:1783–1789. doi: 10.1053/j.gastro.2009.12.043. [DOI] [PubMed] [Google Scholar]
- 78.Seminowicz DA, Labus JS, Bueller JA, et al. Regional gray matter density changes in brains of patients with irritable bowel syndrome. Gastroenterology. 2010;139:48–57. e2. doi: 10.1053/j.gastro.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jiang Z, Dinov ID, Labus J, et al. Sex-related differences of cortical thickness in patients with chronic abdominal pain. PLoS One. 2013;8:e73932. doi: 10.1371/journal.pone.0073932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Labus JS, Dinov ID, Jiang Z, et al. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain. 2014;155:137–149. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Piche M, Chen JI, Roy M, Poitras P, Bouin M, Rainville P. Thicker posterior insula is associated with disease duration in women with irritable bowel syndrome (IBS) whereas thicker orbitofrontal cortex predicts reduced pain inhibition in both IBS patients and controls. J Pain. 2013;14:1217–1226. doi: 10.1016/j.jpain.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 82.Elsenbruch S, Schmid J, Kullmann JS, et al. Visceral sensitivity correlates with decreased regional gray matter volume in healthy volunteers: a voxel-based morphometry study. Pain. 2014;155:244–249. doi: 10.1016/j.pain.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 83.Orand A, Gupta A, Shih W, et al. Catecholaminergic gene polymorphisms are associated with GI symptoms and morphological brain changes in irritable bowel syndrome. PLoS One. 2015;10:e0135910. doi: 10.1371/journal.pone.0135910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Labus JS, Van Horn JD, Gupta A, et al. Multivariate morphological brain signatures predict patients with chronic abdominal pain from healthy control subjects. Pain. 2015;156:1545–1554. doi: 10.1097/j.pain.0000000000000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zeng F, Qin W, Yang Y, et al. Regional brain structural abnormality in meal-related functional dyspepsia patients: a voxel-based morphometry study. PLoS One. 2013;8:e68383. doi: 10.1371/journal.pone.0068383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu P, Zeng F, Yang F, et al. Altered structural covariance of the striatum in functional dyspepsia patients. Neurogastroenterol Motil. 2014;26:1144–1154. doi: 10.1111/nmo.12372. [DOI] [PubMed] [Google Scholar]
- 87.Nan J, Liu J, Mu J, et al. Anatomically related gray and white matter alterations in the brains of functional dyspepsia patients. Neurogastroenterol Motil. 2015;27:856–864. doi: 10.1111/nmo.12560. [DOI] [PubMed] [Google Scholar]
- 88.Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- 89.Behrens TE, Johansen-Berg H, Woolrich MW, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 90.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 91.Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154:1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Irimia A, Labus JS, Torgerson CM, Van Horn JD, Mayer EA. Altered viscerotopic cortical innervation in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2015;27:1075–1081. doi: 10.1111/nmo.12586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fang J, Li S, Li M, et al. Altered white matter microstructure identified with tract-based spatial statistics in irritable bowel syndrome: a diffusion tensor imaging study. Brain Imaging Behav. 2017;11:1110–1116. doi: 10.1007/s11682-016-9573-y. [DOI] [PubMed] [Google Scholar]
- 94.Nan J, Zhang L, Chen Q, et al. White matter microstructural similarity and diversity of functional constipation and constipation-predominant irritable bowel syndrome. J Neurogastroenterol Motil. 2018;24:107–118. doi: 10.5056/jnm17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou G, Qin W, Zeng F, et al. White-matter microstructural changes in functional dyspepsia: a diffusion tensor imaging study. Am J Gastroenterol. 2013;108:260–269. doi: 10.1038/ajg.2012.405. [DOI] [PubMed] [Google Scholar]
- 96.Niddam DM, Tsai SY, Lu CL, Ko CW, Hsieh JC. Reduced hippocampal glutamate-glutamine levels in irritable bowel syndrome: preliminary findings using magnetic resonance spectroscopy. Am J Gastroenterol. 2011;106:1503–1511. doi: 10.1038/ajg.2011.120. [DOI] [PubMed] [Google Scholar]
- 97.Nakai A, Kumakura Y, Boivin M, et al. Sex differences of brain serotonin synthesis in patients with irritable bowel syndrome using alpha-[C11]methyl-L-tryptophan, positron emission tomography and statistical parametric mapping. Can J Gastroenterol. 2003;17:191–196. doi: 10.1155/2003/572127. [DOI] [PubMed] [Google Scholar]
- 98.Nakai A, Diksic M, Kumakura Y, D’Souza D, Kersey K. The effects of the 5-HT3 antagonist, alosetron, on brain serotonin synthesis in patients with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17:212–221. doi: 10.1111/j.1365-2982.2004.00615.x. [DOI] [PubMed] [Google Scholar]
- 99.Mak ADP, Northoff G, Yeung DKW, et al. Increased glutamate in somatosensory cortex in functional dyspepsia. Sci Rep. 2017;7:3926. doi: 10.1038/s41598-017-04405-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tominaga K, Tsumoto C, Ataka S, et al. Regional brain disorders of serotonin neurotransmission are associated with functional dyspepsia. Life Sci. 2015;137:150–157. doi: 10.1016/j.lfs.2015.07.023. [DOI] [PubMed] [Google Scholar]
- 101.Ly HG, Ceccarini J, Weltens N, et al. Increased cerebral cannabinoid-1 receptor availability is a stable feature of functional dyspepsia: a [18F] MK-9470 PET study. Psychother Psychosom. 2015;84:149–158. doi: 10.1159/000375454. [DOI] [PubMed] [Google Scholar]
- 102.Fregni F, Potvin K, DaSilva D, et al. Clinical effects and brain metabolic correlates in non-invasive cortical neuromodulation for visceral pain. Eur J Pain. 2011;15:53–60. doi: 10.1016/j.ejpain.2010.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eisenstein M. Microbiome: bacterial broadband. Nature. 2016;533:S104–S106. doi: 10.1038/533S104a. [DOI] [PubMed] [Google Scholar]
- 104.Hsiao EY, McBride SW, Hsien S, et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell. 2013;155:1451–1463. doi: 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sampson TR, Debelius JW, Thron T, et al. Gut microbiota regulate motor deficits and neuroinflammation in a model of parkinson’s disease. Cell. 2016;167:1469–1480. e12. doi: 10.1016/j.cell.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Park AJ, Collins J, Blennerhassett PA, et al. Altered colonic function and microbiota profile in a mouse model of chronic depression. Neurogastroenterol Motil. 2013;25:733–e575. doi: 10.1111/nmo.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Goyal MS, Venkatesh S, Milbrandt J, Gordon JI, Raichle ME. Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci USA. 2015;112:14105–14112. doi: 10.1073/pnas.1511465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sudo N, Chida Y, Aiba Y, et al. Postnatal microbial colonization programs the hypothalamic-pituitary-adrenal system for stress response in mice. J Physiol. 2004;558(Pt 1):263–275. doi: 10.1113/jphysiol.2004.063388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Dinan TG, Cryan JF, Stanton C. Gut microbes and brain development have black box connectivity. Biol Psychiatry. 2018;83:97–99. doi: 10.1016/j.biopsych.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 110.Mayer EA, Tillisch K, Gupta A. Gut/brain axis and the microbiota. J Clin Invest. 2015;125:926–938. doi: 10.1172/JCI76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Greenwood-Van Meerveld B, Cryan JF. Stress and the microbiota-gut-brain axis in visceral pain: relevance to irritable bowel syndrome. CNS Neurosci Ther. 2016;22:102–117. doi: 10.1111/cns.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tillisch K, Labus J, Kilpatrick L, et al. Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology. 2013;144:1394–1401. e1–e4. doi: 10.1053/j.gastro.2013.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tillisch K, Mayer EA, Gupta A, et al. Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosom Med. 2017;79:905–913. doi: 10.1097/PSY.0000000000000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pinto-Sanchez MI, Hall GB, Ghajar K, et al. Probiotic bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: a pilot study in patients with irritable bowel syndrome. Gastroenterology. 2017;153:448–459. e8. doi: 10.1053/j.gastro.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 115.Labus JS, Hollister EB, Jacobs J, et al. Differences in gut microbial composition correlate with regional brain volumes in irritable bowel syndrome. Microbiome. 2017;5:49. doi: 10.1186/s40168-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Frost G, Sleeth ML, Sahuri-Arisoylu M, et al. The short-chain fatty acid acetate reduces appetite via a central homeostatic mechanism. Nat Commun. 2014;5:3611. doi: 10.1038/ncomms4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Byrne CS, Chambers ES, Alhabeeb H, et al. Increased colonic propionate reduces anticipatory reward responses in the human striatum to high-energy foods. Am J Clin Nutr. 2016;104:5–14. doi: 10.3945/ajcn.115.126706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zanchi D, Meyer-Gerspach AC, Suenderhauf C, et al. Differential effects of L-tryptophan and L-leucine administration on brain resting state functional networks and plasma hormone levels. Sci Rep. 2016;6:35727. doi: 10.1038/srep35727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Smith SM, Nichols TE. Statistical challenges in “big data” human neuroimaging. Neuron. 2018;97:263–268. doi: 10.1016/j.neuron.2017.12.018. [DOI] [PubMed] [Google Scholar]
- 120.Labus JS, Mayer EA, Jarcho J, et al. Acute tryptophan depletion alters the effective connectivity of emotional arousal circuitry during visceral stimuli in healthy women. Gut. 2011;60:1196–1203. doi: 10.1136/gut.2010.213447. [DOI] [PMC free article] [PubMed] [Google Scholar]