Abstract
Background/Aims
Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are common gastrointestinal (GI) disorders and these patients frequently overlap. Trimebutine has been known to be effective in controlling FD co-existing diarrhea-dominant IBS, however its effect on overlap syndrome (OS) patients has not been reported. Therefore, we investigated the effect of trimebutine on the model of OS in guinea pigs.
Methods
Male guinea pigs were used to evaluate the effects of trimebutine in corticotropin-releasing factor (CRF) induced OS model. Different doses (3, 10, and 30 mg/kg) of trimebutine were administered orally and incubated for 1 hour. The next treatment of 10 μg/kg of CRF was intraperitoneally injected and stabilized for 30 minutes. Subsequently, intragastric 3 mL charcoal mix was administered, incubated for 10 minutes and the upper GI transit analyzed. Colonic transits were assessed after the same order and concentrations of trimebutine and CRF treatment by fecal pellet output assay.
Results
Different concentrations (1, 3, and 10 μg/kg) of rat/human CRF peptides was tested to establish the OS model in guinea pigs. CRF 10 μg/kg was the most effective dose in the experimental OS model of guinea pigs. Trimebutine (3, 10, and 30 mg/kg) treatment significantly reversed the upper and lower GI transit of CRF induced OS model. Trimebutine significantly increased upper GI transit while it reduced fecal pellet output in the CRF induced OS model.
Conclusions
Trimebutine has been demonstrated to be effective on both upper and lower GI motor function in peripheral CRF induced OS model. Therefore, trimebutine might be an effective drug for the treatment of OS between FD and IBS patients.
Keywords: Corticotropin-releasing factor, Guinea pigs, Overlap syndrome, Trimebutine
Introduction
Functional dyspepsia (FD) and irritable bowel syndrome (IBS) are common gastrointestinal (GI) diseases comprising 10% to 25% of the general population.1,2 However, there are several reports regarding frequent overlap between FD and IBS patients.3–5 According to the Rome III criteria, subjects with overlapping FD and IBS showed a significant increase in the severity of psychopathology as well as pathophysiological features.6–8 Studies demonstrated that stress is one of the primary factors associated with the onset, exacerbation, and reactivation of many GI disorders.9–11 Strong experimental evidence suggests that the central and peripheral corticotropin-releasing factor (CRF) and its receptors facilitate stress related alterations in GI motility.12–15 Peripheral administration of CRF ligands demonstrated differential action on the upper and lower GI motility.16,17 CRF ligand was found to mimic changes similar to that induced by stress, while its antagonists alleviate stress-induced alteration of GI function.15–18 Therefore, peripheral CRF could be utilized to establish the animal model of the overlap syndrome (OS).18
OS is an abnormal GI condition, where there are frequent overlap between FD and IBS patients.2,3 In other words, both hypomotility and hypermotility GI symptoms exist simultaneously in OS patients.2,3 Since most of the prokinetics are effective in a treating single disease condition, OS patients are therefore difficult to manage. Trimebutine might be an important drug candidate for OS patients, since it has been used for the treatment of various disorders including IBS, gastritis, and dyspepsia.19,20
The mode of actions of trimebutine [3,4,5-trimethoxybenzoic acid 2(dimethylamino)-2-phenylbutylester] on the GI tract is multi-faceted.21–23 It has unique spasmolytic activity and display significant non-selective agonist activity for intestinal opioid receptors μ, κ, and δ subtypes.21–23 It has been reported that trimebutine induces premature phase III of the migrating motor complex (MMC) in the intestine and also been demonstrated to modulate visceral sensitivity.21 Moreover, it is likely to act on interstitial cells of Cajal, a well-known key player in the initiation and regulation of GI motility, as well as smooth muscles and enteric nerves.24 Few studies have reported trimebutine as a multi-ion channel (Ca2+ and K+) regulator in the gut.19,25 Our previous study has confirmed OS model in conscious guinea pigs18 and hence it provides an opportunity to test the efficacy of trimebutine on this model. Therefore, we investigate the effect of trimebutine on a model of OS in guinea pigs to provide an experimental data to apply for the treatment of FD and IBS overlap.
Materials and Methods
Preparation of Animals
Adult male Hartley guinea pigs (250–350 g; Orient Bio, Inc, Seoul, Korea) were acclimatized to their holding room (temperature controlled to 21 ± 1°C, 50 ± 10% humidity. A standard guinea pig diet and drinking water were provided ad libitum. All experiments were conducted in accordance with the guide for the care and use of laboratory animals provided by the animal laboratory ethics committees of the Department of Laboratory Animal Medicine, Medical Research Center, Yonsei University College of Medicine. The study was performed in a manner to confirm with the Helsinki Declaration of 1975, as revised in 2000 and 2008, concerning Human and Animal Rights. This study was approved by the institutional animal care and use committee (IACUC) of Yonsei University College of Medicine with IRB protocol number 2017–0073.
Chemicals and Drugs
The following chemicals and drugs were used: isotonic sodium chloride solution (Dai Han Pharmaceuticals, Seoul, Korea), charcoal (Sigma, Milwaukee, WI, USA), barium sulfate (Taejoon Pharmaceuticals, Seoul, Korea), rat/human (r/h) CRF (Peptide Institute, Inc, Osaka, Japan), and Trimebutine (Samil Pharmaceuticals, Seoul, Korea).
Experimental Design 1: Design of Overlap Syndrome Model of Guinea Pig
The effect of CRF treatment on upper GI transit was tested by charcoal transit assay in fasted guinea pigs. R/h CRF peptides of different concentrations (1, 3, and 10 μg/kg) and vehicle (normal) were injected intraperitoneally in guinea pigs. After 30 minutes of stabilization, the guinea pigs received an intragastric administration of 3 mL of charcoal mixture (consisting of charcoal, barium, and normal saline mixed in a 1:2:6 ratio). Guinea pigs were euthanized after 10 minutes of incubation with the charcoal mix, and the upper GI transit was evaluated by assessing the migration of charcoal mixture from the pylorus to the most distal point of the small intestine. The migration expressed as a percentage (%) of the total length of the small intestine (cm). The effect of CRF treatment on lower GI transit was measured by fecal pellet output (FPO) assay in non-fasted guinea pigs. R/h CRF peptides of different concentrations (1, 3, and 10 μg/kg) and vehicle were injected intraperitoneally in guinea pigs. Each guinea pig was incubated into an individual experimental cage and the cumulative number and weight of fecal pellets produced were measured and recorded for a total of 3 hours.
Experimental Design 2: The Effect of Trimebutine on Upper Gastrointestinal Transit in Overlap Syndrome Model
The effect of trimebutine on upper GI transit was tested by charcoal transit assay in fasted guinea pigs. Guinea pigs were pretreated with different concentrations (3, 10, and 30 mg/kg) of trimebutine and Control (0.9% saline) and incubated for 1 hour. Subsequently, each of these groups were administered 10 μg/kg of CRF or saline intraperitoneally. After 30 minutes of stabilization, 3 mL of charcoal mixture was administered via an orogastric cannula. Guinea pigs were sacrificed after 10 minutes of incubation with charcoal mix, to evaluate the charcoal migration from the pylorus to the most distal point of the small intestine.
Experimental Design 3: The Effect of Trimebutine on Lower Gastrointestinal Transit in Overlap Syndrome Model
The effect of trimebutine on lower GI transit was measured by FPO assay in non-fasted guinea pigs. Guinea pigs were pretreated with different concentrations (3, 10, and 30 mg/kg) of trimebutine and Control (0.9% saline) and incubated for 1 hour. There after each of these groups were administered 10 μg/kg of CRF or saline intraperitoneally. Each guinea pig was incubated into an individual experimental cage and the cumulative number and weight of fecal pellets produced were measured and recorded for total 4 hours.
Statistical Methods
Results for each variable were expressed as percentage of control levels. Statistical analysis was performed using repeated measures ANOVA with post hoc comparison. In all tests analysis, statistical significance was assigned if P < 0.05 based on the mean. Values are means with standard error. All data were analyzed using SPSS version 12.0 for Windows software (SPSS Inc, Chicago, IL, USA).
Results
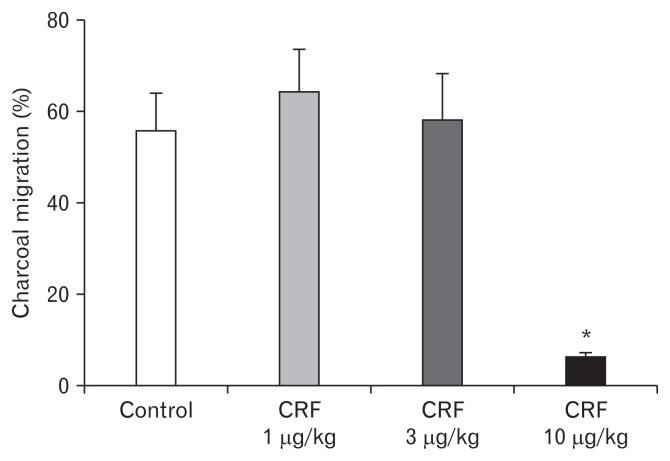
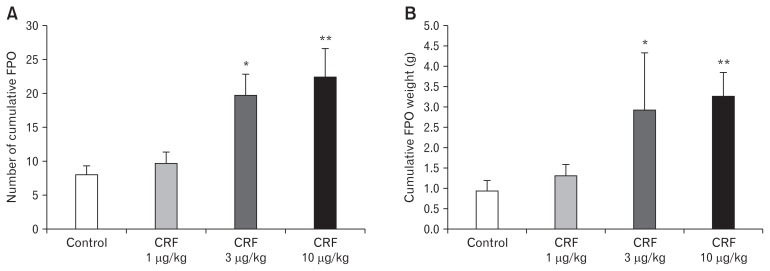
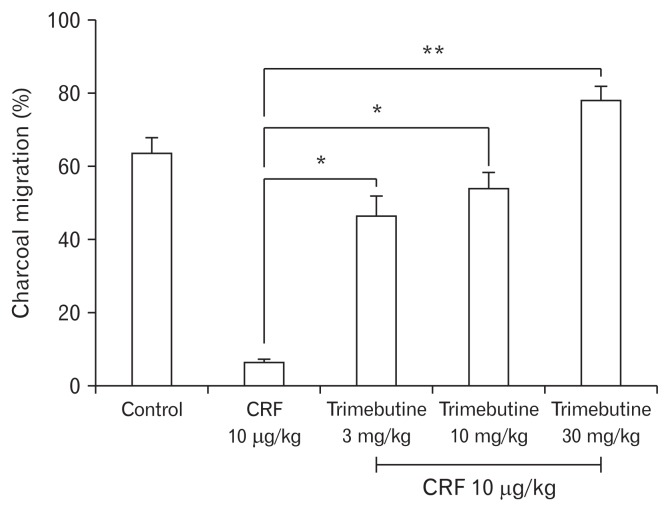
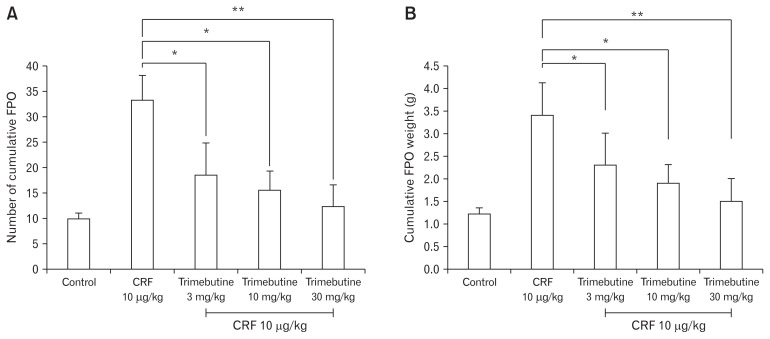
As shown in Figure 1, the percent (%) charcoal migration (cm) in the control group was relatively similar to that of CRF 1 μg/ kg and 3 μg/kg treated groups. The higher dose of intraperitoneal CRF 10 μg/kg demonstrated significant inhibition of upper GI transit, compared to control (6.4 ± 2.2 vs 55.6 ± 19.9) (Fig. 1). In contrast, the effect of different doses of CRF peptide on lower GI transit showed a dose dependent acceleration of the FPO (Fig. 2). As compared to controls, intraperitoneal CRF dose of 3 μg/kg and 10 μg/kg treated guinea pigs significantly increase the cumulative fecal pellets and weight (Fig. 2A and 2B). Intraperitoneal CRF dose of (10 μg/kg) treated guinea pigs significantly increase the cumulative number of FPO (18.8 ± 6.9 vs 33.1 ± 11.9) and weight in grams (2.4 ± 0.8 vs 3.4 ± 2.0) compared to controls. The effect of different doses (3, 10, and 30 mg/kg) of trimebutine was tested independently in OS model of guinea pigs. As shown in Figure 3, trimebutine (3, 10, and 30 mg/kg) treated guinea pigs significantly increased (46.2 ± 14.6; 53.8 ± 11.6; 77.7 ± 10.5 vs 6.4 ± 2.2) the upper GI transit compared to the most effective dose (10 μg/ kg) of CRF in the OS model (Fig. 3). Contrary to the effect on upper GI transit, different doses of trimebutine significantly decreased the cumulative number of FPO (18.5 ± 17.8; 15.5 ± 10.7; 12.3 ± 10.4 vs 33.1 ± 11.9) and weight (2.4 ± 2.0; 1.8 ± 1.2; 1.5 ± 1.2 vs 3.4 ± 2.0), compared to only the CRF 10 μg/kg treated OS model (Fig. 4A and 4B).
Figure 1.
The effect of different doses of corticotropin-releasing factor (CRF) peptide on upper gastrointestinal transit in guinea pigs. Data represents mean ± SEM (n = 6/group). *P < 0.05 in comparison to controls.
Figure 2.
Effect of different doses of corticotropin-releasing factor (CRF) peptide on the lower gastrointestinal transit in guinea pigs. (A) The effect of different concentrations of CRF on the number of cumulative fecal pellets. (B) The effect of different concentrations of CRF on the cumulative fecal pellet output (FPO) weight (g). Data represents mean ± SEM (n = 6/group). *P < 0.05, **P < 0.01 in comparison to controls.
Figure 3.
Effect of different doses of trimebutine on the upper gastrointestinal transit in overlap syndrome model of guinea pigs. Data represents mean ± SEM (n = 6/group). *P < 0.05, **P < 0.01 in comparison to corticotropin-releasing factor (CRF) 10 μg/kg only.
Figure 4.
The effect of different doses of trimebutine on the lower gastrointestinal (GI) transit. (A) The effect of different doses of trimebutine on the number of cumulative fecal pellet output (FPO) in OS model of guinea pigs. (B) The effect of different doses of trimebutine on the cumulative fecal weight (g) in OS model of guinea pigs. Data represents mean ± SEM (n = 6/group). *P < 0.05, **P < 0.01 in comparison to corticotropin-releasing factor (CRF) 10 μg/kg only.
Discussion
Several studies demonstrated that patients with one of the GI diseases often suffered from a second overlapping disease.26–30 A recent population based study from China based on the Rome III criteria showed 33.0% overlap of patients of IBS in FD patients and 45.9% of FD in IBS patients.27 Again a study from the Japanese general population showed the prevalence of overlaps of FD and/or IBS in GERD, GERD and/or IBS in FD, and GERD and /or FD in IBS was 46.9%, 47.6%, and 34.4% respectively.2 Apart from the high prevalence, our previous study, as well as several other reports have suggested a health related decline in quality of life (HR-QOL) and higher severity symptom scores among patients of FD-IBS overlap.2,26–30 Despite the higher incidence, severity symptom scores and deteriorating HR-QOL among overlap patients, there is no successful animal model with some evidence and construct validity for the human syndrome. In this series, our previous study successfully demonstrated CRF induced OS in the model of guinea pigs that mimic more than one symptom of humans.18 The curative aspect of OS is complex and hence the primary concern has been the development of efficient prokinetic agents. Several studies suggested trimebutine as an effective GI modulator and its common use in the treatment of functional bowel disorders, including IBS and postoperative ileus.20–23 Trimebutine moiety is a non-competitive spasmolytic agent that exhibit substantial agonist action on intestinal opioid receptors including μ and κ.22,23 In animals and humans, trimebutine is metabolized in the liver into an active derivative called N-desmethyl-trimebutine, which blocks the sodium channels activity and also display a potent local anesthetic effect.22 This study focused on the treatment aspects and dichotomic action of trimebutine that provide us an opportunity to test this drug in the experimental OS model. Remarkably, in our study trimebutine modulates the GI motility in CRF induced OS model of guinea pigs. Trimebutine significantly reverses the CRF induced decrease of upper GI transit and optimize the motility similar to that of the healthy control. Similarly, it also reverses the CRF induced increase of lower GI transit and effectively moderate the motility similar to that of healthy controls. Our study corroborates the earlier findings that trimebutine may be a reliable modulator of GI motility, and a potential candidate for the treatment of both hypermotility and hypomotility disorders.20,23 Most of the studies regarding the mechanism of action of trimebutine indicated the involvement of the gastric MMC.21,31 Trimebutine accelerates gastric emptying, it brings premature phase III of the MMC in the intestine and regulates the contractile activity of the colon.21,32 Many naturally occurring peptide modulators control the MMC in the enteric nervous system, and among them the opioid receptor-dependent influences were the most significant.33–35 Trimebutine also acts through the release of GI peptides such as motilin, vasoactive intestinal peptide, gastrin and glucagon.21 Motilin and other compounds involving motilin receptors appeared to play a significant role in the initiation of phase III of the MMC in man and dogs, but not in pigs.32 Reports indicate the pivotal role of serotonin and serotonin receptors in controlling the MMC.36 On the other hand, the effect of trimebutine on stress-induced changes in gut motility presented significant decrease in the duration of phase III of the MMC.37,38
Recently, several improved versions of trimebutine have been developed, such as nitro-arginine based trimebutine salt with the capability to release nitric oxide, a gaseous mediator.39 The nitric oxide released from the nitro-arginine interacts with the opioid agonist to potentiate its analgesic activity.39 Wallace et al40 developed another innovative trimebutine salt capable of releasing in vivo hydrogen sulfide, a gaseous mediator known to reduce nociception as well as being involved in multilevel regulation of physiological and pathological functions in mammalian tissues.41
We do acknowledge that our study has limitations. The main limitation of our method was the indirect evaluation of colonic transit time by measuring the cumulative number and weight of fecal pellets. In conclusion, trimebutine has been demonstrated to be effective on both upper GI and lower GI motor function in peripheral CRF induced OS. Therefore, trimebutine may be an important drug for the treatment of OS between FD and IBS patients. Therefore, further studies are needed to clarify the mechanistic action of trimebutine in OS model.
Footnotes
Financial support: This study was supported by Samil Pharmaceuticals, Seoul, Korea.
Conflicts of interest: None.
Author contributions: Zahid Hussain and Da Hyun Jung were responsible for data analysis and drafting of the manuscript; Young Ju Lee was in charge of guinea pig handling and experimentation; and Hyojin Park was the corresponding author, and was responsible for study concept and design, critical revision, and study supervision.
References
- 1.Ford AC, Marwaha A, Lim A, Moayyedi P. Systematic review and meta-analysis of the prevalence of irritable bowel syndrome in individuals with dyspepsia. Clin Gastroenterol Hepatol. 2010;8:401–409. doi: 10.1016/j.cgh.2009.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Kaji M, Fujiwara Y, Shiba M, et al. Prevalence of overlaps between GERD, FD and IBS and impact on health-related quality of life. J Gastroenterol Hepatol. 2010;25:1151–1156. doi: 10.1111/j.1440-1746.2010.06249.x. [DOI] [PubMed] [Google Scholar]
- 3.Talley NJ, Dennis EH, Schettler-Duncan VA, Lacy BE, Olden KW, Crowell MD. Overlapping upper and lower gastrointestinal symptoms in irritable bowel syndrome patients with constipation or diarrhea. Am J Gastroenterol. 2003;98:2454–2459. doi: 10.1111/j.1572-0241.2003.07699.x. [DOI] [PubMed] [Google Scholar]
- 4.Agréus L, Svärdsudd K, Nyrén O, Tibblin G. Irritable bowel syndrome and dyspepsia in the general population: overlap and lack of stability over time. Gastroenterology. 1995;109:671–680. doi: 10.1016/0016-5085(95)90373-9. [DOI] [PubMed] [Google Scholar]
- 5.Caballero-Plasencia AM, Sofos-Kontoyannis S, Valenzuela-Barraco M, Martín-Ruiz JL, Casado-Caballero FJ, López-Mañas JG. Irritable bowel syndrome in patients with dyspepsia: a community-based study in southern Europe. Eur J Gastroenterol Hepatol. 1999;11:517–522. doi: 10.1097/00042737-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;8(suppl 8):28–36. discussion 37. [PubMed] [Google Scholar]
- 7.Levy RL, Olden KW, Naliboff BD, et al. Psychosocial aspects of the functional gastrointestinal disorders. Gastroenterology. 2006;130:1447–1458. doi: 10.1053/j.gastro.2005.11.057. [DOI] [PubMed] [Google Scholar]
- 8.Corsetti M, Caenepeel P, Fischler B, Janseens J, Tack J. Impact of coexisting irritable bowel syndrome on symptoms and pathophysiological mechanisms in functional dyspepsia. Am J Gastroenterol. 2004;99:1152–1159. doi: 10.1111/j.1572-0241.2004.30040.x. [DOI] [PubMed] [Google Scholar]
- 9.Tack J, Demedts I, Dehondts G, et al. Clinical and pathophysiological characteristics of acute-onset functional dyspepsia. Gastroenterology. 2002;122:1738–1747. doi: 10.1053/gast.2002.33663. [DOI] [PubMed] [Google Scholar]
- 10.Chang L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology. 2011;140:761–765. doi: 10.1053/j.gastro.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caso JR, Leza JC, Menchen L. The effects of physical and psychological stress on the gastrointestinal tract: lessons from animal models. Curr Mol Med. 2008;8:299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- 12.Taché Y, Martinez V, Million M, Wang L. Stress and the gastrointestinal tract. III. Stress-related alterations of gut motor function: role of brain corticotropin-releasing factor receptors. Am J Physiol Gastrointest Liver Physiol. 2001;280:G173–G177. doi: 10.1152/ajpgi.2001.280.2.G173. [DOI] [PubMed] [Google Scholar]
- 13.Taché Y, Martinez V, Wang L, Million M. CRF1 receptor signaling pathways are involved in stress-related alterations of colonic function and viscerosensitivity: implications for irritable bowel syndrome. Br J Pharmacol. 2004;141:1321–1330. doi: 10.1038/sj.bjp.0705760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taché Y, Perdue MH. Role of peripheral CRF signalling pathways in stress-related alterations of gut motility and mucosal function. Neurogastroenterol Motil. 2004;16(suppl 1):137–142. doi: 10.1111/j.1743-3150.2004.00490.x. [DOI] [PubMed] [Google Scholar]
- 15.Williams CL, Peterson JM, Villar RG, Burks TF. Corticotropin-releasing factor directly mediates colonic responses to stress. Am J Physiol. 1987;253(4 Pt 1):G582–G586. doi: 10.1152/ajpgi.1987.253.4.G582. [DOI] [PubMed] [Google Scholar]
- 16.Martínez V, Wang L, Rivier JE, Vale W, Taché Y. Differential actions of peripheral corticotropin-releasing factor (CRF), urocortin II, and urocortin III on gastric emptying and colonic transit in mice: role of CRF receptor subtypes 1 and 2. J Pharmacol Exp Ther. 2002;301:611–617. doi: 10.1124/jpet.301.2.611. [DOI] [PubMed] [Google Scholar]
- 17.Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119:1569–1579. doi: 10.1053/gast.2000.20251. [DOI] [PubMed] [Google Scholar]
- 18.Hussain Z, Kim HW, Huh CW, Lee YJ, Park H. The effect of peripheral CRF peptide and water avoidance stress on colonic and gastric transit in guinea pigs. Yonsei Med J. 2017;58:872–877. doi: 10.3349/ymj.2017.58.4.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagasaki M, Komori S, Ohashi H. Effect of trimebutine on voltage-activated calcium current in rabbit ileal smooth muscle cells. Br J Pharmacol. 1993;110:399–403. doi: 10.1111/j.1476-5381.1993.tb13823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee HT, Kim BJ. Trimebutine as a modulator of gastrointestinal motility. Arch Pharm Res. 2011;34:861–864. doi: 10.1007/s12272-011-0600-7. [DOI] [PubMed] [Google Scholar]
- 21.Delvaux M, Wingate D. Trimebutine: mechanism of action, effects on gastrointestinal function and clinical results. J Int Med Res. 1997;25:225–246. doi: 10.1177/030006059702500501. [DOI] [PubMed] [Google Scholar]
- 22.Roman FJ, Lanet S, Hamon J, et al. Pharmacological properties of trimebutine and N-monodesmethyltrimebutine. J Pharmacol Exp Ther. 1999;289:1391–1397. [PubMed] [Google Scholar]
- 23.Cenac N, Castro M, Desormeaux C, et al. A novel orally administered trimebutine compound (GIC-1001) is anti-nociceptive and features peripheral opioid agonistic activity and hydrogen sulphide-releasing capacity in mice. Eur J Pain. 2016;20:723–730. doi: 10.1002/ejp.798. [DOI] [PubMed] [Google Scholar]
- 24.Won KJ, Sanders KM, Ward SM. Interstitial cells of Cajal mediate mechanosensitive responses in the stomach. Proc Natl Acad Sci USA. 2005;102:14913–14918. doi: 10.1073/pnas.0503628102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morisawa T, Hasegawa J, Tanabe K, Watanabe A, Kitano M, Kishimoto Y. Effects of trimebutine maleate on delayed rectifier K+ currents in guineapig ventricular myocytes. J Pharm Pharmacol. 2000;52:403–408. doi: 10.1211/0022357001774156. [DOI] [PubMed] [Google Scholar]
- 26.Nastaskin I, Mehdikhani E, Conklin J, Park S, Pimentel M. Studying the overlap between IBS and GERD: a systematic review of the literature. Dig Dis Sci. 2006;51:2113–2120. doi: 10.1007/s10620-006-9306-y. [DOI] [PubMed] [Google Scholar]
- 27.Wang A, Liao X, Xiong L, et al. The clinical overlap between functional dyspepsia and irritable bowel syndrome based on Rome III criteria. BMC Gastroenterol. 2008;8:43. doi: 10.1186/1471-230X-8-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee HJ, Lee SY, Kim JH, et al. Depressive mood and quality of life in functional gastrointestinal disorders: differences between functional dyspepsia, irritable bowel syndrome and overlap syndrome. Gen Hosp Psychiatry. 2010;32:499–502. doi: 10.1016/j.genhosppsych.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Park JM, Choi MG, Cho YK, et al. Functional gastrointestinal disorders diagnosed by Rome III questionnaire in Korea. J Neurogastroenterol Motil. 2011;17:279–286. doi: 10.5056/jnm.2011.17.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park H. Functional gastrointestinal disorders and overlap syndrome in Korea. J Gastroenterol Hepatol. 2011;26(suppl 3):12–14. doi: 10.1111/j.1440-1746.2011.06644.x. [DOI] [PubMed] [Google Scholar]
- 31.Chaussade S, Grandjouan S, Couturier D, Thierman-Duffaud D, Henry JF. Induction of phase III of the migrating motor complex in human small intestine by trimebutine. Eur J Clin Pharmacol. 1987;32:615–618. doi: 10.1007/BF02455998. [DOI] [PubMed] [Google Scholar]
- 32.Vantrappen G, Peeters T. Motilin. In: Schultz SG, editor. Handbook of physiology. The gastrointestinal system. II. Bethesda MD: American Physiological Society; 1989. pp. 545–558. [Google Scholar]
- 33.Romański KW. Importance of the enteric nervous system in the control of the migrating motility complex. Physiol Int. 2017;104:97–129. doi: 10.1556/2060.104.2017.2.4. [DOI] [PubMed] [Google Scholar]
- 34.Raehal KM, Bohn LM. Mu opioid receptor regulation and opiate responsiveness. AAPS J. 2005;7:E587–E591. doi: 10.1208/aapsj070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valori R, Shannon S, Reddy N, Deniel EE, Sollins SM. The action of trimebutine maleate on gastrointestinal motility is mediated by opiate receptors in human subjects. Gastroenterol Clin BioI. 1987;11(3 Pt 2):102B–104B. [PubMed] [Google Scholar]
- 36.Lördal M, Hellström PM. Serotonin stimulates migrating myoelectric complex via 5-HT3 receptors dependent on cholinergic pathways in rat small intestine. Neurogastroenterol Motil. 1999;11:1–10. doi: 10.1046/j.1365-2982.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- 37.Diop L, Pascaud X, Le Gallou B, Junien JL. Travel stress alters the intestinal myoelectric complex in rats: antagonistic effect of trimebutine. Life Sci. 1992;50:263–271. doi: 10.1016/0024-3205(92)90333-K. [DOI] [PubMed] [Google Scholar]
- 38.Valori RM, Kumar D, Wingate DL. Effects of different types of stress and “prokinetic” drugs on the control of the fasting motor complex in humans. Gastroenterology. 1986;90:1890–1900. doi: 10.1016/0016-5085(86)90258-1. [DOI] [PubMed] [Google Scholar]
- 39.Distrutti E, Mencarelli A, Renga B, et al. A nitro-arginine derivative of trimebutine (NO2-Arg-Trim) attenuates pain induced by colorectal distension in conscious rats. Pharmacol Res. 2009;59:319–329. doi: 10.1016/j.phrs.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Wallace J, Cirino G, Santagada V, Caliendo G. Salts of trimebutine and n-desmethyl trimebutine. Washington DC: US Patent and Trademark Office; 2010. [Google Scholar]
- 41.Wei HJ, Xu JH, Li MH, et al. Hydrogen sulfide inhibits homocysteine-induced endoplasmic reticulum stress and neuronal apoptosis in rat hippocampus via upregulation of the BDNF-TrkB pathway. Acta Pharmacol Sin. 2014;35:707–715. doi: 10.1038/aps.2013.197. [DOI] [PMC free article] [PubMed] [Google Scholar]