Abstract
Background/Aims
MicroRNAs (miRNAs) were reported to be responsible for intestinal permeability in diarrhea-predominant irritable bowel syndrome (IBS-D) rats in our previous study. However, whether and how miRNAs regulate visceral hypersensitivity in IBS-D remains largely unknown.
Methods
We established the IBS-D rat model and evaluated it using the nociceptive visceral hypersensitivity test, myeloperoxidase activity assay, restraint stress-induced defecation, and electromyographic (EMG) activity. The distal colon was subjected to miRNA microarray analysis followed by isolation and culture of colonic epithelial cells (CECs). Bioinformatic analysis and further experiments, including dual luciferase assays, quantitative real-time polymerase chain reaction, western blot, and enzyme-linked immunosorbent assay, were used to detect the expression of miRNAs and how it regulates visceral hypersensitivity in IBS-D rats.
Results
The IBS-D rat model was successfully established. A total of 24 miRNAs were differentially expressed in the distal colon of IBS-D rats; 9 were upregulated and 15 were downregulated. Among them, the most significant upregulation was miR-200a, accompanied by downregulation of cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT). MiR-200a mimic markedly inhibited the expression of CNR1/SERT. Bioinformatic analysis and luciferase assay confirmed that CNR1/SERT are direct targets of miR-200a. Rescue experiments that overexpressed CNR1/SERT significantly abolished the inhibitory effect of miR-200a on the IBS-D rats CECs.
Conclusions
This study suggests that miR-200a could induce visceral hyperalgesia by targeting the downregulation of CNR1 and SERT, aggravating or leading to the development and progression of IBS-D. MiR-200a may be a regulator of visceral hypersensitivity, which provides potential targets for the treatment of IBS-D.
Keywords: CNR1, Diarrhea, Hypersensitivity, Irritable bowel syndrome, MiR-200a, SERT
Introduction
Irritable bowel syndrome (IBS) is a chronic gastrointestinal (GI) disorder characterized by recurrent abdominal pain related to a change in bowel habit or defecation. Diagnosis is based on symptom criteria, and the current standards are the Rome IV criteria published in May 2016.1 IBS can be further subtyped using the Bristol stool scale for bowel habits. Diarrhea-predominant irritable bowel syndrome (IBS-D) is generally reported as the most common subtype (28–46% of all IBS),2 which is characterized by altered habit of bowel evacuation, visceral pain, and bloating in the absence of biochemical or anatomical abnormalities.1 Increasing evidence suggests that chronic and acute stress (CAS) play an important role in the pathophysiology of IBS-D.3 Stress is associated with symptom onset, exacerbation, and perpetuation in patients with IBS-D. Thus, stress-induced visceral hypersensitivity was suggested as an important biological feature of IBS-D.3 Serotonin (5-hydroxytryp-tamine [5-HT]) is a neurotransmitter implicated in the process of visceral hypersensitivity, and it was reported that abnormal 5-HT may be the cause of the altered sensation, secretion and motility in GI tract of IBS-D.4 Serotonin transporter (SERT) is a serotonin selective transporter that absorbs 5-HT into GI cells.5 Camilleri5 discovered that SERT was lowly expressed in the local intestinal tract of IBS-D patients, which resulted in elevated intestinal 5-HT levels and correlated with IBS-D susceptibility.5 Cannabinoid receptor 1 (CNR1) is associated with symptom phenotype, pain perception, inhibition of nerve transmission, and colonic transit in IBS-D.6 Inhibition of CNR1 expression may enhance visceral sensitivity and susceptibility to ulcerative colitis.7 Furthermore, CNR1 inhibits GI motility in the enteric nervous system, mainly by inhibiting contractile neurotransmitter release.8 However, the molecular mechanism of visceral hypersensitivity in patients with GI diseases is not fully understood, and it deserves further study.
MicroRNAs (miRNAs) are a family of small non-coding RNAs involved in numerous pathological and physiological processes.9 Previous studies demonstrated that gene expression was regulated by miRNAs, which could bind to the 3′ untranslated region (UTR) of the mRNAs and inhibit their translation or promote the degradation of mRNAs.10–13 Increasing evidence demonstrated that miRNAs were involved in the occurrence and development of GI disorders.14–16 In our previous paper, we reported that the up-expression of miR-144 in the intestinal tissue of the IBS-D rat promotes intestinal permeability.17 However, the role of miRNAs in regulating visceral hypersensitivity of IBS-D remains largely unknow.
For this reason, we constructed CAS IBS-D rat models and used microarray analysis to screen for differentially expressed miR-NAs. We found that miR-200a is highly expressed. Using bioin-formatic analysis and in vitro validation, we identified CNR1 and SERT as potential target genes for miR-200a. Our study provides new ideas into the regulation of miRNAs in IBS-D, which may be a new treatment strategy to relieve symptoms caused by visceral hypersensitivity.
Materials and Methods
Establishment of Diarrhea-predominant Irritable Bowel Syndrome Rat Models18
40 Sprague-Dawley rats (4 weeks, weight 180 ± 10 g) were acquired and rearranged at the Guangzhou University of Chinese Medicine Experimental Animal Center. One week after adaptive feeding, the rats were randomly divided into 2 groups: the control group (10 male and 10 female) and model group (10 male and 10 female). The model group was established by submitting the rats to unpredictable chronic stress for 3 weeks, followed by 7 days of rest and 1 hour of acute restraint stress on day 28 (rat model of CAS). Food (but not water) was removed 10 hours prior to the acute restraint stress exposure. The unpredictable chronic stress included the following 7 stresses given at random: overnight illumination for 12 hours, 45°C environment for 5 minutes, water deprivation for 24 hours, 4°C environment for 3 minutes, tail clamp for 1 minute, level vibration (120/min) for 40 minutes, and food deprivation for 24 hours (Supplementary Material 1).
Nociceptive Visceral Hypersensitivity
The nociceptive visceral hypersensitivity test was performed on day 28 according to the method described previously (Supplementary Material 2).19 EMG activity of the external oblique muscle was used to measure the visceral sensitivity of the IBS-D rat models. The method was as described previously (Supplementary Material 3).20 After completion of the nociceptive visceral hypersensitivity, restraint stress-induced defecation, and EMG activity, the rats were sacrificed to obtain the distal colon.
Restraint Stress-induced Defecation
The rats were housed individually with no restrictions on food intake before testing. At day 28, 10 rats (5 from the control group and 5 from the model group, regardless of the gender) were randomly selected and placed in restraint cages (5 × 5 × 20 cm) for 1 hour at room temperature. The feces excreted during restraint stress were divided into 3 types: hard pellet, soft pellet, and formless stool, and counted separately. Another 10 rats (5 from the control group and 5 from the model group, regardless of the gender) were left unrestrained for 1 hour and served as the unstressed controls. Their feces were also divided into 3 types and counted separately.
Myeloperoxidase Activity Assay
Myeloperoxidase (MPO) activity in colon tissue reflects the level of inflammation. The method was described previously (Supplementary Material 4).21
MicroRNA Microarray Analysis
The distal colons obtained from the model group and control group were analysed using miRNA microarray analysis, which was performed at Guangzhou RiboBio Co, Ltd. The method was as described previously (Supplementary Material 5).17
Isolation and Culture of Colonic Epithelial Cells
Colonic Epithelial Cells (CECs) were isolated and cultured as described previously (Supplementary Material 6).17,22
Bioinformatic Analysis
MiRanda (http://www.microrna.org), PicTar (http://pictar.mdc-berlin.de), and targetScan (http://www.targetscan.org) were used in the bioinformatic analysis of miRNAs. The target genes were verified using in vitro experiments.
RNA Oligoribonucleotides Synthesis and Transfection
The method was described previously.17 Briefly, CECs from the IBS-D rats and control rats were seeded into 6-well culture plates and transfected with RNA oligoribonucleotides (miR-200a mimic, miR-200a inhibitor, mimic control, inhibitor control, silent-CNR1 (si-CNR1), and silent-SERT (si-SERT)) for 6 hours, using Li-pofectamine 2000 (Invitrogen, Carlsbad, CA, USA). CECs from control rats transfected with nothing were treated as the control group, while CECs from IBS-D rats transfected with mimic control were treated as the model group. The CECs were collected and examined using quantitative real-time polymerase chain reaction (qRT-PCR), western blot, enzyme-linked immunosorbent assays (ELISA), and dual luciferase assay.
Dual Luciferase Assay
The method was described previously.17 Briefly, CECs were co-transfected with the RNA oligoribonucleotides (miR-200a mimic, miR-200a inhibitor, mimic control, inhibitor control, si-CNR1, and si-SERT) and reporter vectors (pmirGLO-wt-CNR1, pmirGLO-mut-CNR1, pmirGLO-wt-SERT, and pmirGLO-mut-SERT) using Lipofectamine 2000 (Invitrogen). Luciferase activity was measured using a dual luciferase assay kit (Promega, Madison, WI, USA) according to the manufacturer’s instructions.
Enzyme-linked Immunosorbent Assays
The method was described previously.17 Briefly, ELISA kits (R&D Systems, Minneapolis, MN, USA) was used to determine the CNR1 and SERT levels in the culture medium when CECs were 80–90% confluent. The experiment was repeated in triplicate and averaged. The optical density values indicated the expression levels of CNR1 and SERT.
Quantitative Real-time Polymerase Chain Reaction
The method was described previously.17 Briefly, after total RNA was extracted and complementary DNA (cDNA) was synthesized, qRT-PCR was performed using an ABI Prism 7500 PCR system (Applied Biosystems, Carlsbad, California, USA). Beta-actin was used as an internal reference, and the 2−ΔCt method was used to calculate the mRNA expression level. Each experiment was performed 3 times (Table).
Table.
The Primer Sequences Were as Follows
| CNR1 | Forward | 5′-AGGGTACTTCCCACAGAAATTC-3′ |
| Reverse | 5′-CGTGAAGGTGCCCAGCGTGA-3′ | |
| SERT | Forward | 5′-ATCTCCTAGAACCCTGTAAC-3′ |
| Reverse | 5′-GAAATGGACCTGGAGTATTG-3′ | |
| β-actin | Forward | 5′-GTCGGTGTGAACGGATTTG-3′ |
| Reverse | 5′-TCCCATTCTCAGCCTTGAC-3′ |
CNR1, cannabinoid receptor 1; SERT, serotonin transporter.
Western Blot
The method was described previously.17 Briefly, after the protein was extracted from the CECs, protein concentrations were determined using the BCA protein assay kit (Beyotime, Beijing, China). Equal amounts of proteins were loaded onto sodium do-decyl sulphate-polyacrylamide gels (SDS-PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated with primary antibodies (1:300 dilution) overnight at 4°C, then incubated with a secondary antibody (1:3000 dilution) at 37°C for 1 hour, and examined using a chemiluminescence detection kit. Beta-actin was the internal reference.
Rescue Assay
The method was described previously.17 Briefly, the CECs were transfected with the pcDNA 3.1 plasmid (pcDNA3.1-CNR1, pcDNA3.1-SERT, or miR-200a mimics) respectively, or co-transfected with the overexpression vector and the miR-200a mimic. CECs transfected with no plasmid were treated as the blank group. The expression of CNR1/SERT were detected by qRT-PCR and Western blot.
Statistical Methods
SPSS 17.0 (IBM Corp, Armonk, NY, USA) was used for statistical analysis. Data were expressed as the mean ± standard deviation. Student’s t test was used to compare the differences between the 2 groups. One-way analysis of variance (ANOVA) was used to compare the differences between 3 or more groups, and least significant difference (LSD) was used as a post hoc test to determine which groups were significantly different from each other. The correlation between CNR1, SERT, and miR-200a was determined using Spearman’s correlation coefficients. When P < 0.05, the difference was statistically significant.
Results
The Establishment of the Diarrhea-predominant Irritable Bowel Syndrome Rat Model
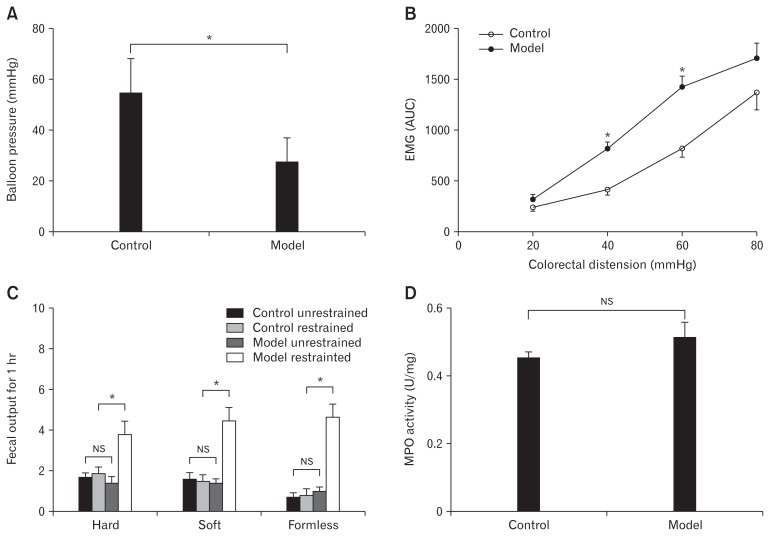
The IBS-D rat model was established using CAS method. Average values of nociceptive thresholds was significant decreased in IBS-D rats in response to graded colorectal distention (Fig. 1A). EMG activity, measured in response to graded colorectal distention, was significantly higher in model rats compared to the control group (Fig. 1B). The fecal output difference for 1 hour was not statistically significant between control group unrestrained rats, control group restrained rats, and model group unrestrained rats. However, fecal output was significantly increased in the model group under restrained stress (Fig. 1C). The level of MPO activity did not differ significantly between the 2 groups after recovery from CAS (Fig. 1D). These results indicated that the CAS-induced IBS-D rat model was successfully established.
Figure 1.
The establishment of the irritable bowel syndrome rat model. (A) The levels of nociceptive visceral hypersensitivity were significantly elevated (*P < 0.05, t test). (B) Electromyographic (EMG) activity was higher in model rats compared to the control group, especially at colorectal distention of 40 mmHg and 60 mmHg (*P < 0.05, t test). (C) The restraint stress-induced defecation test showed that the amount of formless stool in the model restrained group was significantly larger among all groups (*P < 0.05, ANOVA), while the difference between the control unrestrained group, control restrained group and model unrestrained group was not statistically significant (NS, ANOVA and least significant difference). (D) The myeloperoxidase (MPO) activities were no different compared to the control group (NS, t test). AUC, area under the curve.
MicroRNA-200a Was Upregulated and Cannabinoid Receptor 1 and Serotonin Transporter Were Downregulated in Distal Colons of Diarrhea-predominant Irritable Bowel Syndrome Rats
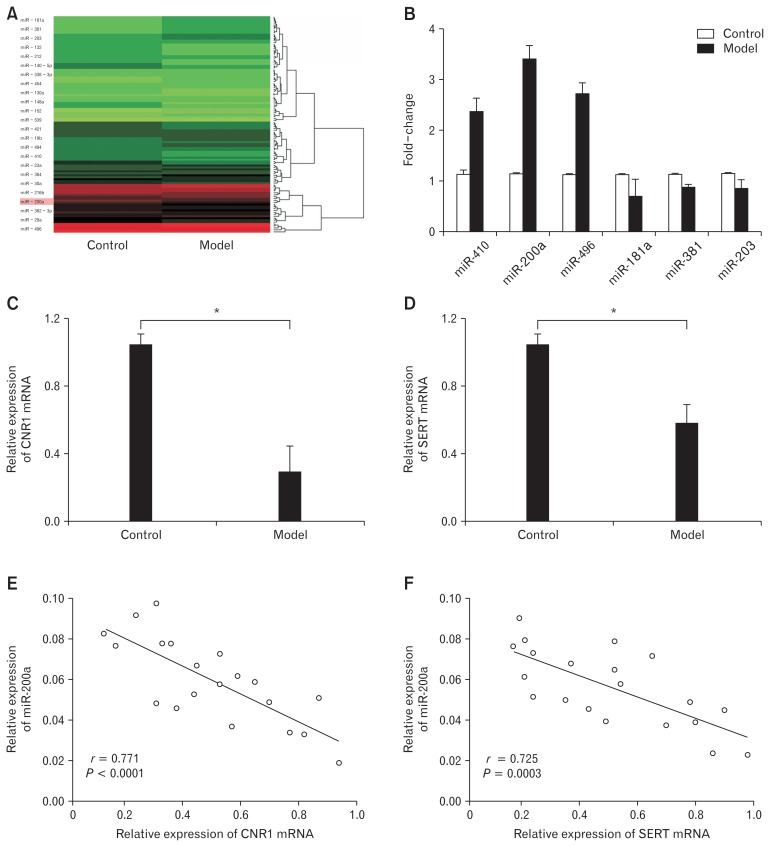
MicroRNA arrays were conducted to identify differentially expressed miRNAs that are associated with IBS-D rats. Expression patterns of miRNAs were compared between IBS-D rats and normal rats. A total of 24 significantly differentially expressed miR-NAs (9 upregulated and 15 downregulated) were confirmed (fold change > 2.0 or < 0.5 and P < 0.05) (Fig. 2A). Quantitative RT-PCR confirmed that miR-200a expression was sharply increased in the distal colons of IBS-D rats (Fig. 2B). Thus, we chose miR-200a as our target in the following experiments. Target mRNAs of miR-200a were determined using the miRGen web tool with the algorithms TargetScanS and PicTar, and CNR1 and SERT genes were identified as possible targets. Compared to the control group, CNR1/SERT were significantly lower expressed in the model group (Fig. 2C and 2D). Further correlation analysis showed that the expressions of CNR1 and SERT were negatively correlated with miR-200a, implying that the expression of CNR1 and SERT was negatively regulated by miR-200a (Fig. 2E and 2F).
Figure 2.
MicroRNA-200a (miR-200a) was upregulated and cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) were downregulated in distal colons of IBS-D rats. (A) There were 24 miRNAs differentially expressed in the model group compared to the control group based on RNA microarray analysis; 9 were upregulated and 15 were downregulated. (B) Quantitative real-time polymerase chain reaction (qRT-PCR) showed that miR-200a was significantly highly expressed in the model group (*P < 0.05, t test). (C, D) Quantitative RT-PCR showed that CNR1 and SERT mRNAs were significantly lowly expressed in the model group (*P < 0.05, t test). (E, F) There was a negative correlation between CNR1/SERT and miR-200a expression (P < 0.05, Spearman’s correlation analysis).
Cannabinoid Receptor 1 and Serotonin Transporter Are Direct Targets of MicroRNA-200a
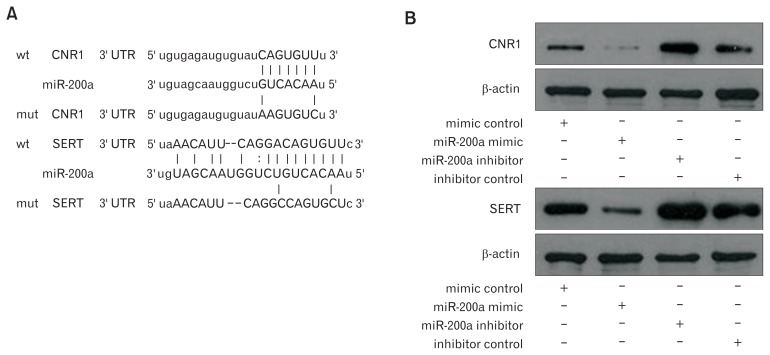
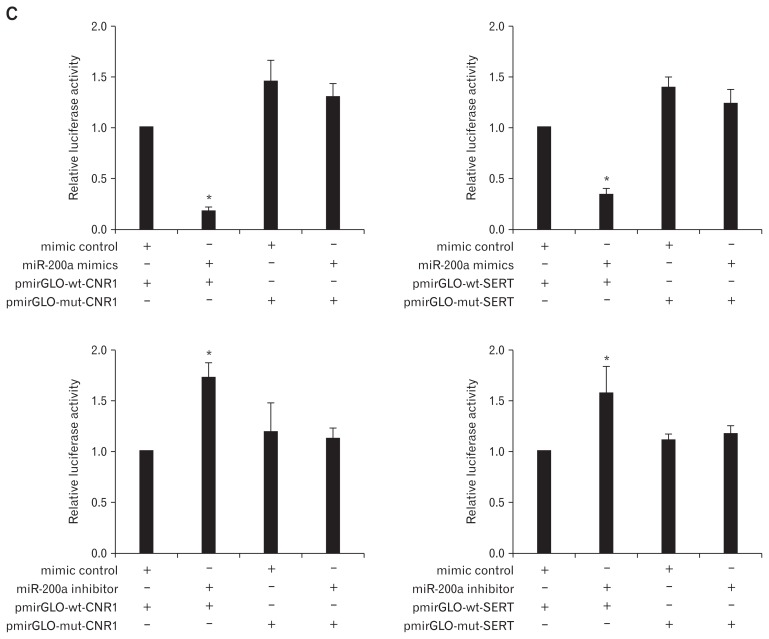
Next, the underlying target genes of miR-200a in IBS-D rat CECs were further investigated. Bioinformatic analyses found that the CNR1 and SERT mRNAs contain 3′ UTR sequences matching the seed region of miR-200a (Fig. 3A), indicating that CNR1/ SERT are potential target genes for miR-200a. Western blot analysis showed that CNR1 and SERT were lowly expressed in the miR-200a mimic group and highly expressed in the miR-200a inhibitor group compared to expression in the relevant control groups (Fig. 3B). Furthermore, dual luciferase assays showed that luciferase activity was significantly reduced in CECs co-transfected with the miR-200a mimic and pmirGLO-wt-CNR1/SERT compared to CECs co-transfected with the miR-200a mimic and pmirGLO-mt-CNR1/SERT. However, compared to CECs co-transfected with the miR-200a inhibitor and pmirGLO-mt-CNR1/SERT, lu-ciferase activity was significantly enhanced in CECs co-transfected with the miR-200a inhibitor and pmirGLO-wt-CNR1/SERT (Fig. 3C). These results indicated that CNR1/SERT are potential targets for miR-200a (group A: CECs co-transfected with mimic control and pmirGLO-wt-CNR1/SERT, group B: CECs co-transfected with the miR-200a mimic and pmirGLO-wt-CNR1/ SERT).
Figure 3.
Cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) are direct targets of microRNA-200a (miR-200a). (A) Bioinformatic analyses show the predicted miR-200a binding site at the 3′ untranslated region (UTR) of CNR1 and SERT. (B) Western blot showed that CNR1 and SERT were lower expressed in the miR-200a mimic group and highly expressed in the miR-200a inhibitor group compared to the relevant control groups. (C) Dual luciferase assays showed that luciferase activity was significantly reduced in colonic epithelial cells (CECs) co-transfected with the miR-200a mimic and pmirGLO-wt-CNR1/SERT compared to CECs co-transfected with the miR-200a mimic and pmirGLO-mt-CNR1/SERT. However, luciferase activity was significantly enhanced in CECs co-transfected with the miR-200a inhibitor and pmirGLO-wt-CNR1/SERT compared to CECs co-transfected with the miR-200a inhibitor and pmirGLO-mt-CNR1/SERT (*P < 0.05, ANOVA).
Cannabinoid Receptor 1 and Serotonin Transporter Were Lowly Expressed and MicroRNA-200a Was Highly Expressed in Diarrhea-predominant Irritable Bowel Syndrome Colonic Epithelial Cells
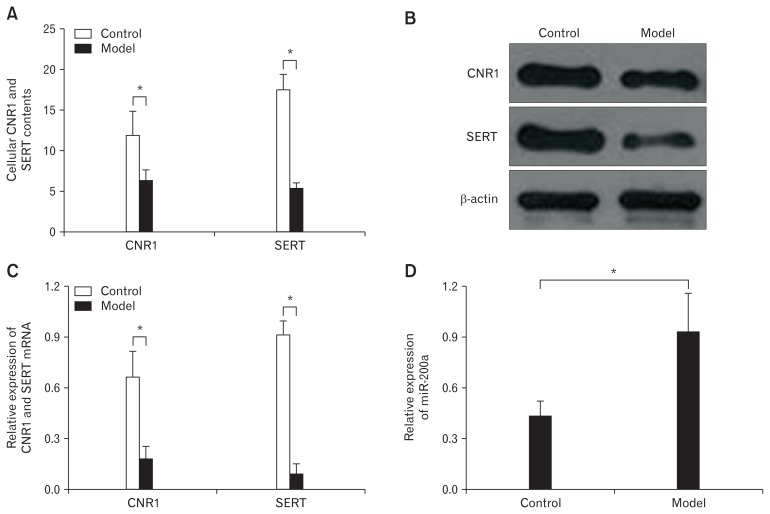
ELISA results revealed a significant decrease in CNR1 and SERT expression in IBS-D rat CECs compared to expression in the control group (Fig. 4A). The protein (Fig. 4B) and mRNA (Fig. 4C) expression levels of CNR1 and SERT were similar to ELISA results. In addition, qRT-PCR results showed that miR-200a was significantly highly expressed in the model group compared to the control group at day 7 (Fig. 4D). These results are consistent with the miRNA microarray analysis.
Figure 4.
Cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) were lower expressed and microRNA-200a (miR-200a) was highly expressed in diarrhea-predominant irritable bowel syndrome colonic epithelial cells (CECs). (A) The enzyme-linked immunosorbent assay shows a significant decrease in CNR1 and SERT expression in CECs from the model group compared to expression in these same cells from the control group on day 7 (*P < 0.05, t test). (B) The western blot shows that CNR1 and SERT were significantly downregulated in the model group compared to the control group. (C) Quantitative real-time polymerase chain reaction shows similar results with western blot (*P < 0.05, t test). (D) MiR-200a was significantly upregulated in the model group compared to expression in the control group on day 7 (*P < 0.05, t test).
MicroRNA-200a Inhibited the Expression of Cannabinoid Receptor 1 and Serotonin Transporter in Diarrhea-predominant Irritable Bowel Syndrome Colonic Epithelial Cells
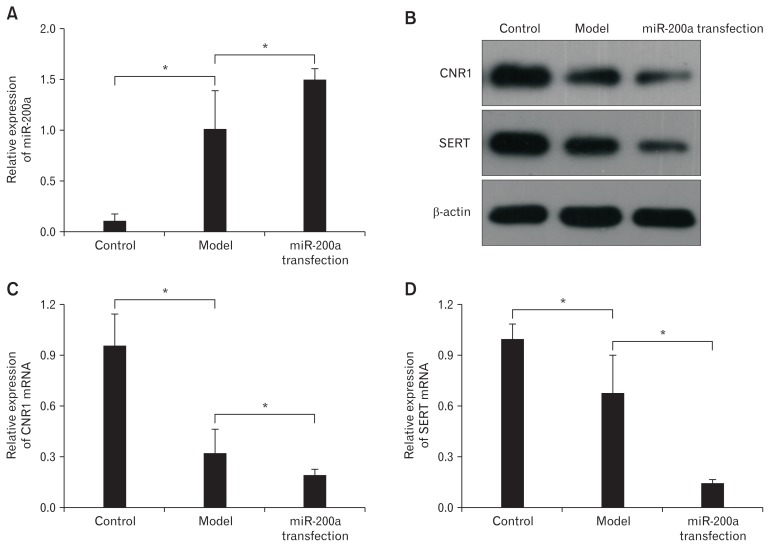
Quantitative RT-PCR showed that miR-200a expression was significantly higher in the miR-200a transfection group than the model group and the control group, indicating successful transfection (Fig. 5A). Correspondingly, the western blot (Fig. 5B) and qRT-PCR (Fig. 5C and 5D) results showed that CNR1/SERT were downregulated significantly. The above results indicated that miR-200a effectively inhibited the expression of CNR1 and SERT, which were closely connected with visceral hypersensitivity.
Figure 5.
MicroRNA-200a (miR-200a) inhibited the expression of cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) in diarrhea-predominant irritable bowel syndrome colonic epithelial cells (CECs). (A) Quantitative real-time polymerase chain reaction (qRT-PCR) showed that miR-200a expression was significantly higher in the miR-200a transfection group than in the model group and the control group, indicating successful transfection (*P < 0.05, ANOVA and least significant difference [LSD]). (B) The western blot showed a significant reduction in the expression of CNR1 and SERT in the miR-200a group. (C, D) Quantitative RT-PCR shows similar results with western blot (*P < 0.05, ANOVA and LSD).
MicroRNA-200a More Strongly inhibited the Expression of Cannabinoid Receptor 1 and Serotonin Transporter in Diarrhea-predominant Irritable Bowel Syndrome Colonic Epithelial Cells Than si-CNR1 and si-SERT
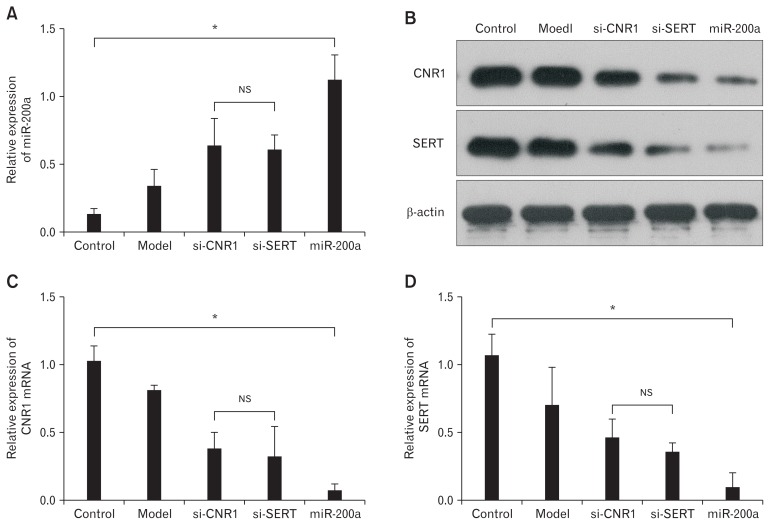
IBS-D CECs were transfected with miR-200a, si-CNR1, and si-SERT to compare their efficacy in relation to visceral hypersensitivity. Quantitative RT-PCR showed successful transfection of miR-200a, si-CNR1, and si-SERT (Fig. 6A). Western blot (Fig. 6B) and qRT-PCR (Fig. 6C and 6D) showed that CNR1 and SERT were significantly reduced in the miR-200a, si-CNR1, and si-SERT groups compared to the model group, and miR-200a group was most reduced. Overall, miR-200a upregulation has a stronger effect on inhibiting the expression of CNR1 and SERT than si-CNR1 and si-SERT in IBS-D CECs.
Figure 6.
MicroRNA-200a (miR-200a) more strongly inhibited the expression of cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) in diarrhea-predominant irritable bowel syndrome colonic epithelial cells (CECs) than si-CNR1 and si-SERT. (A) Quantitative real-time polymerase chain reaction (qRT-PCR) showed successful transfection of miR-200a, si-CNR1, and si-SERT (*P < 0.05, ANOVA), and there were no significant differences between si-CNR1 group and the si-SERT group (NS, least significant difference [LSD]). (B) Western blot showed that CNR1 and SERT were lowly expressed in the miR-200a and si-CNR1/si-SERT groups compared to the model group. Furthermore, CNR1 and SERT were significantly downregulated in the miR-200a group compared to the si-CNR1 and si-SERT groups. (C, D) Quantitative RT-PCR shows similar results to western blot (*P < 0.05, ANOVA; NS, LSD).
Overexpression of Cannabinoid Receptor 1 and Serotonin Transporter Partially Rescued the Inhibitory Effects of MicroRNA-200a on Diarrhea-predominant Irritable Bowel Syndrome Rat Colonic Epithelial Cells
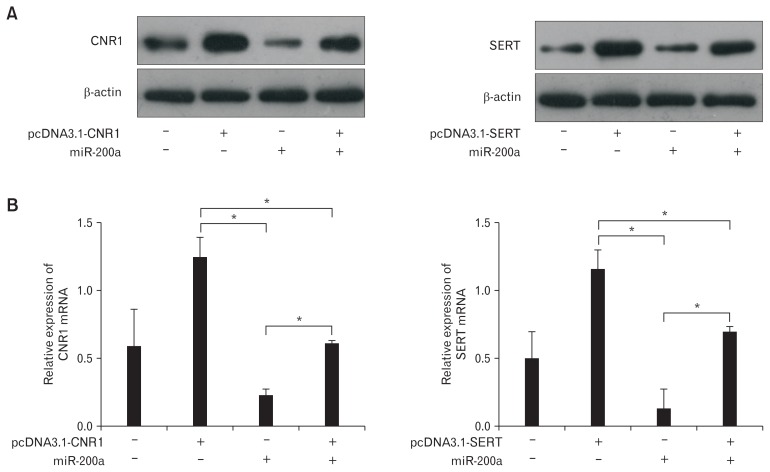
IBS-D rat CECs were co-transfected with CNR1 or SERT overexpression vectors lacking the 3′ UTR to confirm the inhibitory effects of miR-200a on IBS-D rat CECs. Western blot (Fig. 7A) and qRT-PCR (Fig. 7B) both showed that the expression of CNR1/SERT was significantly upregulated in CECs transfected with pcDNA3.1-CNR1/SERT, but it was significantly downregulated in CECs transfected with miR-200a. In addition, a significant increase in CNR1/SERT was detected in CECs transfected with pcDNA3.1-CNR1/SERT alone compared to CECs co-transfect-ed with pcDNA3.1-CNR1/SERT and miR-200a. These results indicated that overexpressed CNR1/SERT could partially rescue the inhibitory effect of miR-200a on CNR1/SERT.
Figure 7.
Overexpression of cannabinoid receptor 1 (CNR1) and serotonin transporter (SERT) partially rescued the inhibitory effects of microR-NA-200a (miR-200a) on diarrhea-predominant irritable bowel syndrome rat colonic epithelial cells (CECs). (A) Western blots showed that the expression of CNR1/SERT was significantly upregulated in CECs transfected with pcDNA3.1-CNR1/SERT but significantly downregulated in CECs transfected with miR-200a. In addition, a significant increase in CNR1/SERT was detected in CECs transfected with pcDNA3.1-CNR1/ SERT alone compared to CECs co-transfected with pcDNA3.1-CNR1/SERT and miR-200a. (B) Quantitative RT-PCR shows similar results to western blot (P < 0.05, ANOVA and least significant difference).
Discussion
MiRNAs were largely reported to be involved in various biological activities in eukaryotes, regulating gene expression required for critical biological processes.23–25 However, it remains largely unclear how miRNAs act in the development and progression of IBS. A study by Koukos et al26 found that lacking miRNAs in the intestinal epithelium could lead to barrier function and abnormal intestinal epithelial differentiation and architecture. Zhou et al27 reported that miR-29 could target nuclear factor-κB-repressing factor and Claudin 1 to increase intestinal permeability. Gut miR-199a/ b expression in IBS-D was significantly decreased, suggesting that it might drive increased transient receptor potential vanilloid type 1 expression, leading to increased GI symptoms and abdominal pain.28 At present, visceral hypersensitivity is considered the main pathogenesis of IBS-D, which can increase the sensitivity of the distal colon to stimuli and lead to abdominal pain. Recently, it has been increasingly accepted that abnormal miRNA expression correlates with the pathological features of increased visceral hypersensitivity.29,30 Therefore, it makes sense to identify which specific miRNAs and their target genes are involved in regulating visceral hypersensitivity to elucidate the pathogenesis of the disease and develop novel effective treatments. In this paper, we established a CAS IBS-D rat model and identified 24 differentially expressed miRNAs in the distal colon. Among them, miR-200a was upregulated the most. But in our previous study,15 IBS-D rat model was established with acetic acid and miR-144 was up-regulated prominently. According to our observation, both of acetic acid model vs CAS mode could successfully established the IBS-D rat model. Ecetic acid model was predominant with formless stool while CAS mode was predominant with increased defecate frequency and abdominal pain, which is closely related to visceral hypersensitivity. MiR-144 was also upregulated in CAS mode (data not shown), but the difference was less than 2 fold and hence not shown on the heatmap. Subsequent bioinformatics indicated that miR-200a maybe bind into CNR1/ SERT, triggering us to further investigate miR-200a and its role in visceral hypersensitivity. CNR1 is a G-protein coupled receptor that acts within the endocannabinoid system, which is involved in modulating neurophysiological processes controlling visceral hypersensitivity, GI motility, intolerance, intestinal infection, and psychological disturbances.31 Hong et al32 also reported that CNR1 receptors are downregulated in dorsal root ganglia neurons innervating the colon under chronic stress induction. Treatment with CNR1 agonists can prevent stress-induced visceral hyperalgesia. Further research showed that elevated endocannabinoid levels, possibly induced by enhanced glucocorticoids under chronic stress, reduced the CNR1 expression level, suggesting that CNR1 is a potential therapeutic target for chronic stress-related visceral pain.33
Another target gene identified was SERT, also known as solute carrier family 6 member 4 (SLC6A4). It is a serotonin-selective transport protein that transports the neurotransmitter 5-HT from synaptic spaces into presynaptic neurons and removes 5-HT from the interstitial space.34 5-HT is synthesized in enterochromaffin cells and modulates visceral sensitivity of the GI tract. It was reported that SERT could uptake 5-HT into enterocytes to ensure the recapture of serotonin.4 The low-grade inflammation in IBS patients may be contributed by alterations in 5-HT.4 Indeed, studies showed SERT changes in depression,35 obsessive-compulsive disorder36 anxiety,37 and visceral hypersensitivity.38,39 Moreover, SERT transgenic mice could reverse key nociceptive signalling and may be a potential approach to treating patients with increased visceral hypersensitivity.40
In the CAS induced IBS-D rat model, we observed that the level of MPO activity did not differ compared to the control group. However, the nociceptive visceral hypersensitivity levels were elevated in the model group. In addition, the amount of formless stool in the model restrained group was significantly larger and EMG activity was higher in model rats, suggesting the IBS-D animal model was successfully established. Evidence from this animal model also indicated that miR-200a may modulate CNR1- and SERT-associated visceral hypersensitivity. Further in vivo experiments showed that miR-200a upregulation enhanced visceral hypersensitivity by inhibiting CNR1/SERT expressed in CECs of IBS-D rats. In summary, miR-200a may promote the progress of IBS-D, and miR-200a negatively correlated with CNR1/SERT. Though the exact mechanism of miR-200a in IBS-D is not yet understood, we hypothesize that it may be mediated by the endogenous interaction between miR-200a and CNR1/SERT. The detailed regulatory mechanism between miR-200a and CNR1/SERT requires further investigation.
In conclusion, we determined that miR-200a plays a role in visceral hypersensitivity and directly targets CNR1 and SERT. Using miRNA to reverse key nociceptive signalling processes that lead to visceral pain requires further investigation. In general, this study offers a potential promising new therapeutic method for reversing visceral hypersensitivity. However, it is necessary to study the detailed mechanism and feasibility of this new therapy before conducting clinical trials.
Supplementary Materials
Footnotes
Note: To access the supplementary materials mentioned in this article, visit the online version of Journal of Neurogastroenterology and Motility at http://www.jnmjournal.org/, and at https://doi.org/10.5056/jnm18037.
Financial support: This study was supported by the China Postdoctoral Science Foundation (No. 2017M612641) and the Chinese Medicine Science Research Project of Guangdong Province (No. 20181095).
Conflicts of interest: None.
Author contributions: Qiuke Hou and Yongquan Huang contributed equally to this work; Qiuke Hou, Yongquan Huang, and Fengbin Liu designed the research; Qiuke Hou, Yongquan Huang, Shuilian Zhu, Changrong Zhang, Peiwu Li, and Zheng-kun Hou performed the research; Yongquan Huang, Fengbin Liu, and Xinlin Chen analysed the data; and Qiuke Hou and Yongquan Huang wrote the paper.
References
- 1.Drossman DA, Hasler WL. Rome IV-functional GI disorders: disorders of gut-brain interaction. Gastroenterology. 2016;150:1257–1261. doi: 10.1053/j.gastro.2016.03.035. [DOI] [PubMed] [Google Scholar]
- 2.Oshima T, Miwa H. Epidemiology of functional gastrointestinal disorders in Japan and in the world. J Neurogastroenterol Motil. 2015;21:320–329. doi: 10.5056/jnm14165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asano T, Tanaka KI, Tada A, et al. Aminophylline suppresses stress-induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci Rep. 2017;7:40214. doi: 10.1038/srep40214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen MX, Chen Y, Fu R, Liu SY, Yang QQ, Shen TB. Activation of 5-HT and NR2B contributes to visceral hypersensitivity in irritable bowel syndrome in rats. Am J Transl Res. 2016;8:5580–5590. [PMC free article] [PubMed] [Google Scholar]
- 5.Camilleri M. Is there a SERT-ain association with IBS? Gut. 2004;53:1396–1399. doi: 10.1136/gut.2004.039826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang Y, Nie Y, Li Y, Zhang L. Association of cannabinoid type 1 receptor and fatty acid amide hydrolase genetic polymorphisms in Chinese patients with irritable bowel syndrome. J Gastroenterol Hepatol. 2014;29:1186–1191. doi: 10.1111/jgh.12513. [DOI] [PubMed] [Google Scholar]
- 7.Storr M, Emmerdinger D, Diegelmann J, et al. The cannabinoid 1 receptor (CNR1) 1359 G/A polymorphism modulates susceptibility to ulcerative colitis and the phenotype in Crohn’s disease. PLoS One. 2010;5:e9453. doi: 10.1371/journal.pone.0009453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abalo R, Chen C, Vera G, et al. In vitro and non-invasive in vivo effects of the cannabinoid-1 receptor agonist AM841 on gastrointestinal motor function in the rat. Neurogastroenterol Motil. 2015;27:1721–1735. doi: 10.1111/nmo.12668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McKenna LB, Schug J, Vourekas A, et al. MicroRNAs control intestinal epithelial differentiation, architecture, and barrier function. Gastroenterology. 2010;139:1654–1664. 1664, e1. doi: 10.1053/j.gastro.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Hara SP, Mott JL, Splinter PL, Gores GJ, LaRusso NF. MicroR-NAs: key modulators of posttranscriptional gene expression. Gastroenterology. 2009;136:17–25. doi: 10.1053/j.gastro.2008.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalla R, Ventham NT, Kennedy NA. MicroRNAs: new players in inflammatory bowel disease. Gut. 2015;64:1008. doi: 10.1136/gutjnl-2014-307891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Cheng J, Fan XM. MicroRNAs: new therapeutic targets for intestinal barrier dysfunction. World J Gastroenterol. 2014;20:5818–5825. doi: 10.3748/wjg.v20.i19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu F, Zikusoka M, Trindade A, et al. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135:1624–1635. e24. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- 14.Neesse A, Gress TM. Emerging role of microRNAs to tackle drug resistance in pancreatic cancer. Gut. 2015;64:1842–1843. doi: 10.1136/gutjnl-2015-309503. [DOI] [PubMed] [Google Scholar]
- 15.Kalla R, Ventham NT, Kennedy NA, et al. MicroRNAs: new players in IBD. Gut. 2015;64:504–517. doi: 10.1136/gutjnl-2014-307891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen HT, Dalmasso G, Müller S, Carrière J, Seibold F, Darfeuille-Michaud A. Crohn’s disease-associated adherent invasive Escherichia coli modulate levels of microRNAs in intestinal epithelial cells to reduce autophagy. Gastroenterology. 2014;146:508–519. doi: 10.1053/j.gastro.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Hou Q, Huang Y, Zhu S, et al. MiR-144 increases intestinal permeability in IBS-D rats by targeting OCLN and ZO1. Cell Physiol Biochem. 2017;44:2256–2268. doi: 10.1159/000486059. [DOI] [PubMed] [Google Scholar]
- 18.Sun J, Wu X, Meng Y, et al. Electro-acupuncture decreases 5-HT, CGRP and increases NPY in the brain-gut axis in two rat models of diarrhea-predominant irritable bowel syndrome (D-IBS) BMC Complement Altern Med. 2015;15:340. doi: 10.1186/s12906-015-0863-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q, Price DD, Dreher KL, et al. Localized colonic stem cell transplantation enhances tissue regeneration in murine colitis. J Cell Mol Med. 2012;16:1900–1915. doi: 10.1111/j.1582-4934.2011.01485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winston J, Shenoy M, Medley D, Naniwadekar A, Pasricha PJ. The vanilloid receptor initiates and maintains colonic hypersensitivity induced by neonatal colon irritation in rats. Gastroenterology. 2007;132:615–627. doi: 10.1053/j.gastro.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 21.Krawisz JE, Sharon P, Stenson WF. Quantitative assay for acute intestinal inflammation based on myeloperoxidase activity. Assessment of inflammation in rat and hamster models. Gastroenterology. 1984;87:1344–1350. [PubMed] [Google Scholar]
- 22.Booth C, Patel S, Bennion GR, Potten CS. The isolation and culture of adult mouse colonic epithelium. Epithelial Cell Biol. 1995;4:76–86. [PubMed] [Google Scholar]
- 23.Ren HX, Zhang FC, Luo HS, Zhang G, Liang LX. Role of mast cell-miR-490-5p in irritable bowel syndrome. World J Gastroenterol. 2017;23:93–102. doi: 10.3748/wjg.v23.i1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q, Verne GN. miRNA-based therapies for the irritable bowel syndrome. Expert Opin Biol Ther. 2011;11:991–995. doi: 10.1517/14712598.2011.577060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fourie NH, Peace RM, Abey SK, et al. Elevated circulating miR-150 and miR-342-3p in patients with irritable bowel syndrome. Exp Mol Pathol. 2014;96:422–425. doi: 10.1016/j.yexmp.2014.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koukos G, Polytarchou C, Kaplan JL, et al. A microRNA signature in pediatric ulcerative colitis: deregulation of the miR-4284/CXCL5 pathway in the intestinal epithelium. Inflamm Bowel Dis. 2015;21:996–1005. doi: 10.1097/MIB.0000000000000339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou Q, Costinean S, Croce CM, et al. MicroRNA 29 targets nuclear factor-κB-repressing factor and claudin 1 to increase intestinal permeability. Gastroenterology. 2015;148:158–169. e8. doi: 10.1053/j.gastro.2014.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou Q, Yang L, Larson S, et al. Decreased miR-199 augments visceral pain in patients with IBS through translational upregulation of TRPV1. Gut. 2016;65:797–805. doi: 10.1136/gutjnl-2013-306464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao XJ, Mao WM, Wang Q, Yang GG, Wu WJ, Shao SX. MicroRNA-24 inhibits serotonin reuptake transporter expression and aggravates irritable bowel syndrome. Biochem Biophys Res Commun. 2016;469:288–293. doi: 10.1016/j.bbrc.2015.11.102. [DOI] [PubMed] [Google Scholar]
- 30.Baudry A, Mouillet-Richard S, Schneider B, Launay JM, Kellermann O. miR-16 targets the serotonin transporter: a new facet for adaptive responses to antidepressants. Science. 2010;329:1537–1541. doi: 10.1126/science.1193692. [DOI] [PubMed] [Google Scholar]
- 31.Vianna CR, Donato J, Jr, Rossi J, et al. Cannabinoid receptor 1 in the vagus nerve is dispensable for body weight homeostasis but required for normal gastrointestinal motility. J Neurosci. 2012;30:10331–10337. doi: 10.1523/JNEUROSCI.4507-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong S, Fan J, Kemmerer ES, Evans S, Li Y, Wiley JW. Reciprocal changes in vanilloid (TRPV1) and endocannabinoid (CB1) receptors contribute to visceral hyperalgesia in the water avoidance stressed rat. Gut. 2009;58:202–210. doi: 10.1136/gut.2008.157594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hong S, Zheng G, Wiley JW. Epigenetic regulation of genes that modulate chronic stress-induced visceral pain in the peripheral nervous system. Gastroenterology. 2015;148:148–157. e7. doi: 10.1053/j.gastro.2014.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reigstad CS, Linden DR, Szurszewski JH, Sonnenburg JL, Farrugia G, Kashyap PC. Correlated gene expression encoding serotonin (5-HT) receptor 4 and 5-HT transporter in proximal colonic segments of mice across different colonization states and sexes. Neurogastroenterol Motil. 2016;28:1443–1448. doi: 10.1111/nmo.12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutiérrez B, Bellón JÁ, Rivera M, et al. The risk for major depression conferred by childhood maltreatment is multiplied by BDNF and SERT genetic vulnerability: a replication study. J Psychiatry Neurosci. 2015;40:187–196. doi: 10.1503/jpn.140097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolmarans de W, Brand L, Stein DJ, Harvey BH. Reappraisal of spontaneous stereotypy in the deer mouse as an animal model of obsessive-compulsive disorder (OCD): response to escitalopram treatment and basal serotonin transporter (SERT) density. Behav Brain Res. 2013;256:545–553. doi: 10.1016/j.bbr.2013.08.049. [DOI] [PubMed] [Google Scholar]
- 37.Charoenphandhu J, Teerapornpuntakit J, Nuntapornsak A, Krishnamra N, Charoenphandhu N. Anxiety-like behaviors and expression of SERT and TPH in the dorsal raphé of estrogen- and fluoxetine-treated ovariectomized rats. Pharmacol Biochem Behav. 2011;98:503–510. doi: 10.1016/j.pbb.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 38.Cui XF, Zhou WM, Yang Y, et al. Epidermal growth factor upregulates serotonin transporter and its association with visceral hypersensitivity in irritable bowel syndrome. World J Gastroenterol. 2014;37:13521–13529. doi: 10.3748/wjg.v20.i37.13521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikander A, Rana SV, Sinha SK, et al. Serotonin transporter promoter variant: analysis in Indian IBS patients and control population. J Clin Gastroenterol. 2009;43:957–961. doi: 10.1097/MCG.0b013e3181b37e8c. [DOI] [PubMed] [Google Scholar]
- 40.Galligan JJ, Patel BA, Schneider SP, et al. Visceral hypersensitivity in female but not in male serotonin transporter knockout rats. Neurogastroenterol Motil. 2013;25:e373–e381. doi: 10.1111/nmo.12133. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.