Abstract
Background/Aims
Functional dyspepsia (FD) is one of the most common gastrointestinal disorders, and FD imposes social and economic burden worldwide. The aim of this study is to identify the prevalence and risk factors of FD in health check-up population in tertiary centers in Korea.
Methods
A nationwide multicenter prospective study was performed at 9 tertiary healthcare centers in Korea between September 2016 and June 2017. A total of 2525 subjects were investigated based on endoscopic findings and questionnaires with the Rome III criteria, and Helicobacter pylori serology (IgG).
Results
A total of 1714 subjects without organic disease were enrolled. The mean (± SD) age was 51.5 (± 12.7) years, and 917 patients (53.5%) were female. The proportion of H. pylori seropositivity was 51.0% (874/1714). The prevalence of FD was 10.3% (176/1714), and the subtypes of postprandial distress syndrome alone, epigastric pain syndrome alone, and postprandial distress syndrome-epigastric pain syndrome overlap were 4.8%, 3.0%, and 2.5%, respectively. Multivariate analysis showed that female gender (OR, 1.58; 95% CI, 1.14–2.21) and education below college level (OR, 1.45; 95% CI, 1.01–2.07) were related to FD. Multivariate analysis based on age 60 showed female gender as a significant (OR, 2.90; 95% CI, 1.06–7.94) factor in the group ≥60 years.
Conclusions
The prevalence of FD was 10.3% in the health check-up population in Korea. Female sex and education below college level were risk factors for FD. Female sex is a risk factor for FD in old age, underscoring the need for close attention in this age group.
Keywords: Dyspepsia, Female, Gastrointestinal disorders, Risk factors
Introduction
Functional dyspepsia (FD) is one of the most prevalent gastrointestinal disorders, and is defined as a chronic disease with persistent upper gastrointestinal symptoms without any explanatory organic or metabolic causes.1 Large population-based studies revealed that the prevalence of FD ranges from 10% to 30% worldwide.2 In Korea, the prevalence of FD is estimated at 8.1–37.0%.3,4 FD patients have a poor quality of life due to their symptoms and the cost associated with increased social and economic burden in the United States because of FD was nearly $18.4 billion in 2009.5
Clinical awareness of the factors associated with FD contributes to medical care. Moreover, it reduces unnecessary treatment and facilitates positive economic outcomes in FD patients. Several studies investigating the risk factors of FD suggested that old age, female sex, low body mass index, Helicobacter pylori infection, use of aspirin or nonsteroidal anti-inflammatory drugs (NSAIDs), low level of education, and so forth as risk factors for FD.2,6,7 However, the risk factors for FD vary according to the characteristics of cohort, for instance, secondary or tertiary hospitals and urban or rural areas, suggesting the need for nationwide cohorts and health checkups for more general information. Our recent survey based on these conditions showed a decrease in the prevalence of H. pylori and peptic ulcer diseases.8 We hypothesized that the diverse etiology of FD led to differences in risk factors according to sex and age. The aim of this study is to evaluate the prevalence and risk factors of FD according to age and sex-specific differences in a health check-up population in tertiary centers in Korea.
Materials and Methods
Study Subjects and Endoscopic Examination
From September 2016 to June 2017, participants who had health check-up and who underwent upper endoscopy were prospectively enrolled at 9 healthcare centers on a national scale in Korea. The health check-up population was defined as healthy individuals over 18 years old visited the healthcare centers for health screening. The healthcare centers systems are different from a national cancer screening program or outpatient clinic in Korea. Most of the health check-up subjects were examined for health check-up without symptoms. If the participants were not eligible for a health check-up, such as those with alarm features,9 the physicians in the healthcare centers recommended them to visit the outpatient clinic. All subjects were aware that they participate in this study before undergoing endoscopy, and participants with a history of malignancy or gastrointestinal surgery, or severe systemic diseases demanding continuous medication, except hypertension and diabetes were excluded. Patients with underlying diseases diagnosed with endoscopy such as Barrett’s esophagus, reflux esophagitis (except minimal change of the esophagogastric junction), peptic ulcer, or upper gastrointestinal tract dysplasia or cancer confirmed endoscopically with or without biopsy were also excluded. The study protocol was reviewed and approved by the Institutional Review Boards of each participating hospitals in this study and informed consent was acquired from all the participants. The Institutional Review Boards numbers were as follows: Kosin University Gospel Hospital (2016-06-002-010), Seoul National University Bundang Hospital (B-1606/351-303), Keimyung University Dongsan Medical Center (DSMC 2016-06-034-001), Dankook University Hospital (2016-06-006), Jeju National Hospital (2016-09-001), Chonnam National University Hospital (CNUH-2016-109), Healthcare system Gangnam Center Seoul National University Hospital (H-1602-057-740), Gyeongsang National University Hospital (2016-11-002), Hallym University Hospital (2016–59), and Wonkwang University Hospital (201607-HRBR-007).
Endoscopy was performed and evaluated by experts at 9 health care centers participating in the current study. The endoscopic findings were examined by a standardized method. In accordance with typical endoscopic features (such as erythema, erosions, prominent submucosal vascular patterns, discolored nodular mucosal elevations, and so on), superficial gastritis, erosive gastritis, atrophic gastritis (AG), or intestinal metaplasia (IM) were diagnosed. Similarly, these findings were based on the consensus of researchers at the Korean gastrointestinal endoscopy seminars and gastrointestinal endoscopic education programs.
Questionnaire and Demographic Data
Before endoscopy, all participants completed the questionnaire under the supervision of a well-trained interviewer at the 9 participating healthcare centers. The contents of the questionnaire were as follows: history of H. pylori eradication therapy; relatives with gastric cancer; smoking and alcohol habits; residence; education and income levels; history of treatment with aspirin or NSAIDs; and dairy products and salty food intake. Dairy products consumption was graded as low or high, depending on whether participants consumed dairy products for more than 5 days per week. Consumption of salt was evaluated as ‘yes’ or ‘no,’ according to participants’ salt intake after tasting food.
FD was evaluated using the translated Korean version of the validated Rome III criteria (Rome III-K questionnaire).10 FD was divided into 2 subtypes: postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS).11 PDS was characterized by symptoms of early satiety, bloating, postprandial fullness and nausea. EPS was defined by the symptoms of epigastric pain and burning.
Helicobacter pylori Test
H. pylori infection was confirmed serologically. Blood samples were promptly collected from each participant who had undergone gastroscopy. Isolated serum samples were stored at −70°C in storage boxes. Using a H. pylori-specific Genedia kit (Green Cross Medical Science, Eumsung, South Korea), which used an H. pylori antigen of Korean strain, enzyme-linked immunosorbent assay was performed for serum H. pylori-specific immunoglobulin G (IgG) to determine H. pylori infection.12 In Korean adults, sensitivity and specificity for the detection of H. pylori were 97.8% and 92.0%, respectively. The cutoff value of optical density at 450 nm (OD450 nm) was 0.406 in serum H. pylori-specific IgG. Each test was carried out twice in accordance with the manufacturer’s guidelines.
Statistical Methods
Statistical analyses were conducted using the SPSS version 18.0 (SPSS Inc, Chicago, IL, USA). Data are shown as means (± SD) or percentages. Comparisons of continuous variables were conducted with Student’s t test or Mann-Whitney U test. Categorical variables were analyzed by using chi-squared test or Fisher’s exact test. Univariate and multivariate logistic regression analyses were conducted to verify the risk factors for FD, which were shown as the adjusted OR and 95% CI. The P-values < 0.05 were considered to manifest statistical significance.
Results
Subject Characteristics
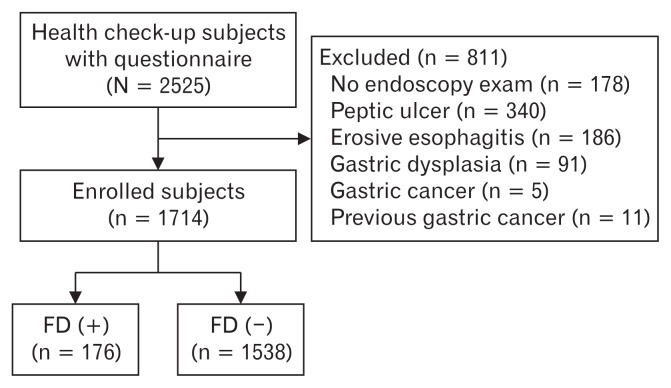
A total of 2525 health check-up participants who answered the questionnaire comprising of the Rome III criteria were included (Fig. 1). Among them, 178 participants did not undergo upper endoscopy. Eleven patients with a history of gastric cancer and 622 participants with organic diseases (eg, peptic ulcer [n = 340], erosive esophagitis [n = 186], gastric dysplasia [n = 91], and gastric cancer [n = 5]) were excluded. With the exception of these patients, 1714 participants were finally enrolled in the analysis (Fig. 1).
Figure 1.
Flow chart of study participants. FD, functional dyspepsia.
The mean (± SD) age of the 1714 participants was 51.5 (± 12.7) years (range, 18 to 90 years), and 917 participants (53.5%) were women (Table 1). The proportion of patients diagnosed with AG and IM via endoscopy was 36.6% (627/1714) and 14.8% (253/1714), respectively. The positive rate of serum H. pylori-specific IgG was 51.0% (874/1714), and the seropositivity of participants with a history of H. pylori eradication therapy was 16.5% (267/1622). The laboratory data and demographic features for enrolled participants are shown in Table 1.
Table 1.
Participants’ Baseline Characteristics (n = 1714)
| Variables | Mean ± SD | |
|---|---|---|
| Age (yr) | 51.5 ± 12.7 | |
| Body mass index (kg/m2)a | 23.8 ± 5.5 | |
| Cholesterol (mg/dL)a | 193.7 ± 43.1 | |
| Triglyceride (mg/dL)a | 120.7 ± 85.3 | |
| Fasting glucose (mg/dL)a | 98.5 ± 32.3 | |
|
| ||
| Variables | n (%) | |
|
| ||
| Gender | Male | 797 (46.5) |
| Female | 917 (53.5) | |
| Age (yr) | < 40 | 336 (19.6) |
| 40–59 | 928 (54.1) | |
| ≥60 | 450 (26.3) | |
| H. pylori IgG | − | 840 (49.0) |
| + | 874 (51.0) | |
| History of H. pylori eradication therapya | − | 1355 (83.5) |
| + | 267 (16.5) | |
| Functional dyspepsia | − | 1538 (89.7) |
| + | 176 (10.3) | |
| Atrophic gastritis | − | 1087 (63.4) |
| + | 627 (36.6) | |
| Intestinal metaplasia | − | 1461 (85.2) |
| + | 253 (14.8) | |
| Relatives with gastric cancera | − | 1488 (86.9) |
| + | 224 (13.1) | |
| Smokinga | − | 1146 (67.0) |
| + | 565 (33.0) | |
| Alcohola | − | 763 (44.6) |
| + | 947 (55.4) | |
| Residencea | Rural | 284 (16.7) |
| Urban | 1415 (83.3) | |
| Educationa | Below college | 790 (46.6) |
| Above college | 905 (53.4) | |
| Income level ($/mo)a | < 3000 | 470 (30.3) |
| 3000–9000 | 865 (55.7) | |
| ≥9000 | 218 (12.7) | |
| NSAIDs use above 1/wka | − | 1546 (90.4) |
| + | 164 (9.6) | |
| Consumption of dairy productsa | − | 1338 (78.3) |
| + | 371 (21.7) | |
| High salt dieta | − | 1500 (87.9) |
| + | 207 (12.1) | |
Some data are missing. Missing values are not included.
SD, standard deviation; H. pylori, Helicobacter pylori; IgG, immunoglobulin G; NSAIDs, non-steroidal anti-inflammatory drugs.
Prevalence and Risk Factors for Functional Dyspepsia According to Subtype of Functional Dyspepsia
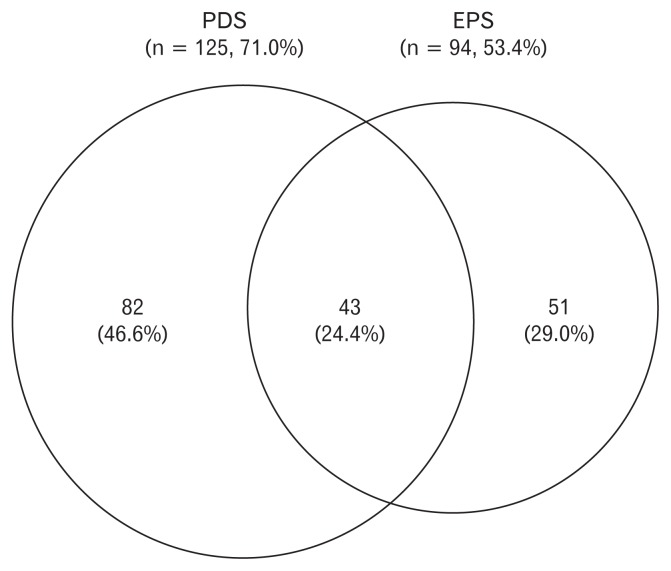
Of the 1714 participants, the prevalence of patients with FD was 10.3% (176/1714), and that of PDS and EPS subtype was 7.3% (125/1714) and 5.5% (94/1714), respectively. By analyzing the patients with overlap of FD (patients with both PDS and EPS) separately, the prevalence of PDS alone, EPS alone, and PDS-EPS overlap was 4.8% (82/1714), 3.0% (51/1714), and 2.5% (43/1714), respectively.
Among the FD patients, the proportion of PDS in 176 FD patients was 71.0% (125/176) and that of EPS was 53.4% (94/176). Therefore, the proportion of PDS-EPS overlap was 24.4% (Fig. 2).
Figure 2.
Proportions of postprandial distress syndrome (PDS) and epigastric pain syndrome (EPS) in 176 patients with functional dyspepsia (FD).
In univariate analysis of risk factors for FD, female sex (P = 0.002), negative serum H. pylori IgG (P = 0.003), and education below college level (P = 0.005) statistically correlated with FD (Table 2). Relatives with gastric cancer (P = 0.076) and NSAIDs usage (P = 0.059) tended to be associated with FD, without statistical significance. In terms of the PDS subtype, female sex (P = 0.032) and negative serum H. pylori IgG (P = 0.009) were statistically correlated. In contrast, EPS subtype showed several risk factors: female sex (P = 0.003), AG (P = 0.027), family history of gastric cancer (P = 0.011), education below college (P = 0.002), and consumption of dairy products (P = 0.038) statistically correlated with EPS (Table 2).
Table 2.
Functional Dyspepsia: Univariate Analysis of Risk Factors
| Variables | FD (−) (%) (n = 1538) (89.7) | FD (+) (%) (n = 176) (10.3) | PDS (+) (%) (n = 125) (7.3) | EPS (+) (%) (n = 94) (5.5) | P-valuea | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| FD (+) vs FD (−) | PDS (+) vs FD (−) | EPS (+) vs FD (−) | |||||
| Age ≥60 (yr) | 399/1538 (25.9) | 51/176 (29.0) | 34/125 (27.2) | 31/94 (33.0) | 0.416 | 0.751 | 0.148 |
| Female | 803/1538 (52.2) | 114/176 (64.8) | 78/125 (62.4) | 64/94 (68.1) | 0.002 | 0.032 | 0.003 |
| BMI (≥25 kg/m2)b | 492/1537 (32.0) | 58/175 (33.1) | 41/124 (33.1) | 30/93 (32.3) | 0.798 | 0.842 | 1.000 |
| Cholesterol (≥240 mg/dL)b | 171/1490 (11.5) | 23/172 (13.4) | 17/121 (14.0) | 15/93 (16.1) | 0.453 | 0.378 | 0.183 |
| Triglyceride (≥150 mg/dL)b | 364/1489 (24.4) | 40/171 (23.4) | 27/120 (22.5) | 22/93 (23.7) | 0.851 | 0.740 | 1.000 |
| Glucose (≥126 mg/dL)b | 117/1490 (7.9) | 13/172 (7.6) | 8/121 (6.6) | 10/93 (10.8) | 1.000 | 0.726 | 0.323 |
| H. pylori IgG positivity | 803/1538 (52.2) | 71/176 (40.3) | 50/125 (40.0) | 39/94 (41.5) | 0.003 | 0.009 | 0.055 |
| History of H. pylori eradicationb | 233/1455 (16.0) | 34/137 (20.4) | 22/117 (18.8) | 18/92 (19.6) | 0.153 | 0.434 | 0.381 |
| Atrophic gastritis | 571/1538 (37.1) | 56/176 (31.8) | 42/125 (33.6) | 24/94 (25.5) | 0.186 | 0.500 | 0.027 |
| Intestinal metaplasia | 233/1538 (15.1) | 20/176 (11.4) | 15/125 (12.0) | 8/94 (8.5) | 0.217 | 0.433 | 0.098 |
| Relatives with gastric cancerb | 193/1536 (12.6) | 31/176 (17.6) | 21/125 (16.8) | 21/94 (22.3) | 0.076 | 0.167 | 0.011 |
| Smokingb | 514/1535 (33.5) | 51/176 (29.0) | 37/125 (29.6) | 23/94 (24.5) | 0.237 | 0.430 | 0.089 |
| Alcoholb | 855/1534 (55.7) | 92/176 (52.3) | 68/125 (54.4) | 47/94 (50.0) | 0.380 | 0.780 | 0.287 |
| Urbanb | 1270/1524 (83.3) | 145/175 (82.9) | 105/125 (84.0) | 75/93 (80.6) | 0.831 | 1.000 | 0.477 |
| Education below collegeb | 691/1521 (45.4) | 99/174 (56.9) | 64/123 (52.0) | 58/93 (62.4) | 0.005 | 0.160 | 0.002 |
| Income level (≥$8500/mo)b | 193/1389 (13.9) | 25/164 (15.2) | 19/115 (16.5) | 11/91 (12.1) | 0.635 | 0.406 | 0.754 |
| NSAIDs useb | 140/1534 (9.1) | 24/176 (13.6) | 13/125 (10.4) | 14/94 (14.9) | 0.059 | 0.629 | 0.070 |
| Consumption of dairy productsb | 339/1533 (22.1) | 32/176 (18.2) | 26/125 (20.8) | 12/94 (12.8) | 0.248 | 0.823 | 0.038 |
| High salt dietb | 182/1531 (11.9) | 25/176 (14.2) | 17/125 (13.6) | 15/94 (16.0) | 0.393 | 0.567 | 0.253 |
Univariate analysis was performed and a P < 0.05 was considered significant.
Some data are missing. Missing values are not included.
FD, functional dyspepsia; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome; BMI, body mass index; H. pylori, Helicobacter pylori; IgG, Immunoglobulin G; NSAIDs, non-steroidal anti-inflammatory drugs.
In multivariate analysis (Table 3), the risk factors for FD were female sex (OR, 1.58; 95% CI, 1.14–2.21, P = 0.007) and education level below college level (OR, 1.45; 95% CI, 1.01–2.07, P = 0.042). EPS subtype analysis showed that female sex (OR, 1.81; 95% CI, 1.15–2.85, P = 0.011), family history of gastric cancer (OR, 1.97; 95% CI, 1.18–3.30, P = 0.010), and education level below college level (OR, 1.65; 95% CI, 1.01–2.68, P = 0.044) were risk factors. However, there was no statistically significant risk for PDS.
Table 3.
Functional Dyspepsia: Multivariate Analysis of Risk Factors
| Variables | FD (+) vs FD (−) | PDS (+) vs FD (−) | EPS (+) vs FD (−) | |||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| OR (95% CI)a | P-valuea | OR (95% CI)a | P-valuea | OR (95% CI)a | P-valuea | |
| Age ≥60 yr | 1.10 (0.75–1.63) | 0.621 | 1.06 (0.67–1.68) | 0.809 | 1.03 (0.62–1.69) | 0.920 |
| Female | 1.58 (1.14–2.21) | 0.007 | 1.45 (0.99–2.12) | 0.057 | 1.81 (1.15–2.85) | 0.011 |
| Relatives with gastric cancerb | 1.48 (0.97–2.25) | 0.067 | 1.42 (0.87–2.33) | 0.166 | 1.97 (1.18–3.30) | 0.010 |
| Education below collegeb | 1.45 (1.01–2.07) | 0.042 | 1.24 (0.82–1.88) | 0.304 | 1.65 (1.01–2.68) | 0.044 |
| NSAIDs useb | 1.41 (0.87–2.29) | 0.168 | 1.10 (0.59–2.04) | 0.773 | 1.41 (0.76–2.63) | 0.276 |
| Consumption of dairy productsb | 0.78 (0.52–1.18) | 0.242 | 0.90 (0.57–1.43) | 0.663 | 0.54 (0.29–1.00) | 0.053 |
Logistic analysis was performed and a P < 0.05 was considered significant.
Some data are missing. Missing values are not included.
All logistic model including terms of age, gender, relatives with gastric cancer, education, NSAIDs use, and consumption of dairy products.
FD, functional dyspepsia; PDS, postprandial distress syndrome; EPS, epigastric pain syndrome; OR, odds ratio; CI, confidence interval; NSAIDs, non-steroidal anti-inflammatory drugs.
Prevalence and Risk Factors for Functional Dyspepsia According to Sex
When FD was analyzed by sex, the prevalence of FD in female was 12.4% (114/917) and the prevalence of male FD was at 7.8% (62/797). The risk factors varied depending on sex. Education level below college was statistically correlated with FD in male patients (OR, 1.77; 95% CI, 1.01–9.09, P = 0.046). In female patients, negative serum H. pylori IgG (P = 0.007), and education level below college level (P = 0.043) statistically correlated with FD in univariate analysis. However, this significance disappeared after multivariate analysis (Table 4).
Table 4.
Univariate and Multivariate Analyses of Risk Factors for Functional Dyspepsia Based on Sex Differences
| Variables | Male | Multivariate analysis | Female | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| FD (−) (%)(n = 735) (92.2) | FD (+) (%)(n = 62) (7.8) | P-value | OR (95% CI)a | P-valuea | FD (−) (%)(n = 803) (87.6) | FD (+) (%)(n = 114) (12.4) | P-value | OR (95% CI)b | P-valueb | |
| Age ≥60 yr | 180/735 (24.5) | 11/62 (17.7) | 0.279 | 0.52 (0.26–1.07) | 0.077 | 219/803 (27.3) | 40/114 (35.1) | 0.095 | 1.23 (0.76–2.00) | 0.398 |
| BMI (≥25 kg/m2)c | 299/735 (40.7) | 23/62 (37.1) | 0.686 | - | - | 193/802 (24.1) | 35/113 (31.0) | 0.130 | 1.23 (0.78–1.94) | 0.364 |
| Cholesterol (≥240 mg/dL)c | 87/712 (12.2) | 8/62 (12.9) | 0.841 | - | - | 84/778 (10.8) | 15/110 (13.6) | 0.417 | - | - |
| Triglyceride (≥150 mg/dL)c | 249/711 (35.0) | 20/62 (32.3) | 0.781 | - | - | 115/778 (14.8) | 20/109 (18.3) | 0.321 | - | - |
| Glucose (≥126 mg/dL)c | 84/712 (11.8) | 6/62 (9.7) | 0.836 | - | - | 33/778 (4.2) | 7/110 (6.4) | 0.323 | - | - |
| H. pylori IgG positivity | 389/735 (52.9) | 28/62 (45.2) | 0.290 | - | - | 414/803 (51.6) | 43/114 (37.7) | 0.007 | - | - |
| History of H. pylori eradicationc | 122/694 (17.6) | 10/57 (17.5) | 1.000 | - | - | 111/761 (14.6) | 24/110 (21.8) | 0.066 | - | - |
| Atrophic gastritis | 298/735 (40.5) | 24/62 (38.7) | 0.893 | - | - | 273/803 (34.0) | 32/114 (28.1) | 0.243 | - | - |
| Intestinal metaplasia | 144/735 (19.6) | 11/62 (17.7) | 0.867 | - | - | 89/803 (11.1) | 9/114 (7.9) | 0.417 | - | - |
| Relatives with gastric cancerc | 92/735 (12.5) | 12/62 (19.4) | 0.166 | 1.73 (0.88–3.38) | 0.111 | 101/801 (12.6) | 19/114 (16.7) | 0.236 | - | - |
| Smokingc | 476/735 (64.8) | 42/62 (67.7) | 0.680 | - | - | 38/800 (4.8) | 9/114 (7.9) | 0.171 | 1.89 (0.88–4.08) | 0.104 |
| Alcoholc | 567/735 (77.1) | 47/62 (75.8) | 0.875 | - | - | 288/799 (36.0) | 45/114 (39.5) | 0.469 | - | - |
| Urbanc | 630/731 (86.2) | 54/62 (87.1) | 1.000 | - | - | 640/793 (80.7) | 91/113 (80.5) | 1.000 | - | - |
| Education below collegec | 280/728 (38.5) | 29/62 (46.8) | 0.223 | 1.77 (1.01–9.09) | 0.046 | 411/793 (51.8) | 70/112 (62.5) | 0.043 | 1.27 (0.79–2.04) | 0.322 |
| Income level (≥$8500/mo)c | 93/651 (14.3) | 10/57 (17.5) | 0.555 | - | - | 100/738 (13.6) | 15/107 (14.0) | 0.880 | - | - |
| NSAIDs usec | 61/734 (8.3) | 6/62 (9.7) | 0.637 | - | - | 79/800 (9.9) | 18/114 (15.8) | 0.072 | 1.34 (0.74–2.45) | 0.336 |
| Consumption of dairy productsc | 145/734 (19.8) | 9/62 (14.5) | 0.403 | - | - | 194/799 (24.3) | 23/114 (20.2) | 0.410 | - | - |
| High-salt dietc | 102/734 (13.9) | 9/62 (14.5) | 0.849 | - | - | 80/797 (10.0) | 16/114 (14.0) | 0.193 | - | - |
Logistic analysis was performed and a P < 0.05 was considered significant. Logistic model including terms of age, relatives with gastric cancer, and education.
Logistic analysis was performed and a P < 0.05 was considered significant. Logistic model including terms of age, body mass index (BMI), smoking, education, and non-steroidal anti-inflammatory drugs (NSAIDs) use.
Some data are missing. Missing values are not included.
FD, functional dyspepsia; OR, odds ratio; CI, confidence interval; H. pylori, Helicobacter pylori; IgG, Immunoglobulin G.
Prevalence and Risk Factors for Functional Dyspepsia According to the Age
When analysis was performed depending on age, the prevalence of FD at age ≥60 years was 11.3% (51/450) compared with 9.9% (125/1264) in patients aged below 60. There was no statistically significant risk factor for FD in patients below the age of 60. By contrast, female sex (P = 0.001), body mass index (≥25 kg/m2) (P = 0.039), negative serum H. pylori IgG (P = 0.018), and alcohol (P = 0.020) statistically correlated with FD in patients ≥60 years in univariate analysis. Among them, female sex (OR, 2.90; 95% CI, 1.06–7.94, P = 0.039) remained statistically significant after multivariate analysis (Table 5).
Table 5.
Univariate and Multivariate Analysis of Risk Factors for Functional Dyspepsia Based on Age
| Variables | Age < 60 | Multivariate analysis | Age ≥60 | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||
| FD (−) (%)(n = 1139) (90.1) | FD (+) (%)(n = 125) (9.9) | P-value | OR (95% CI)a | P-valuea | FD (−) (%) (n = 399) (88.7) | FD (+) (%) (n = 51) (11.3) | P-value | OR (95% CI)b | P-valueb | |
| Female | 584/1139 (51.3) | 74/125 (59.2) | 0.109 | 1.32 (0.90–1.94) | 0.150 | 219/399 (54.9) | 40/51 (78.4) | 0.001 | 2.90 (1.06–7.94) | 0.039 |
| BMI (≥25 kg/m2)c | 368/1139 (32.3) | 35/125 (28.0) | 0.363 | - | - | 124/398 (31.2) | 23/50 (46.0) | 0.039 | 1.70 (0.92–3.15) | 0.093 |
| Cholesterol (≥240 mg/dL)c | 131/1104 (11.9) | 16/123 (13.0) | 0.663 | - | - | 40/386(10.4) | 7/49 (14.3) | 0.461 | - | - |
| Triglyceride (≥150 mg/dL)c | 276/1104 (25.0) | 28/123 (22.8) | 0.660 | - | - | 88/385 (22.9) | 12/48 (25.0) | 0.719 | - | - |
| Glucose (≥126 mg/dL)c | 59/1104 (5.3) | 9/123 (7.3) | 0.402 | - | - | 58/386 (15.0) | 4/49 (8.2) | 0.277 | - | - |
| H. pylori IgG positivity | 591/1139 (51.9) | 53/125 (42.4) | 0.048 | - | - | 212/399 (53.1) | 18/51 (35.3) | 0.018 | - | - |
| History of H. pylori eradicationc | 146/1081 (13.5) | 19/118 (16.1) | 0.481 | - | - | 87/374 (23.3) | 15/49 (30.6) | 0.287 | - | - |
| Atrophic gastritis | 346/1139 (30.4) | 32/125 (25.6) | 0.304 | - | - | 225/399 (56.4) | 24/51 (47.1) | 0.233 | - | - |
| Intestinal metaplasia | 119/1139 (10.4) | 12/125 (9.6) | 0.878 | - | - | 114/399 (28.6) | 8/51 (15.7) | 0.065 | - | - |
| Relatives with gastric cancerc | 140/1137 (12.3) | 22/125 (17.6) | 0.120 | 1.52 (0.92–2.49) | 0.101 | 53/399 (13.3) | 9/51 (17.6) | 0.390 | - | - |
| Smokingc | 405/1137 (35.6) | 43/125 (34.4) | 0.844 | - | - | 109/398 (27.4) | 8/51 (15.7) | 0.090 | 0.63 (0.20–2.04) | 0.443 |
| Alcoholc | 703/1136 (61.9) | 81/125 (64.8) | 0.561 | - | - | 152/398 (38.2) | 11/51 (21.6) | 0.020 | 0.66 (0.28–1.56) | 0.345 |
| Urbanc | 996/1131 (88.1) | 107/125 (85.6) | 0.391 | - | - | 274/393 (69.7) | 38/50 (76.0) | 0.413 | - | - |
| Education below collegec | 368/1126 (32.7) | 53/123 (43.1) | 0.027 | 1.46 (0.99–2.14) | 0.053 | 323/395 (81.8) | 46/51 (90.2) | 0.168 | 1.28 (0.47–3.49) | 0.627 |
| Income level (≥$8500/mo)c | 173/1012 (17.1) | 22/114 (19.3) | 0.601 | - | - | 20/377 (5.3) | 3/50 (6.0) | 0.742 | - | - |
| NSAIDs usec | 64/1135 (5.6) | 8/125 (6.4) | 0.685 | - | - | 76/399 (19.0) | 16/51 (31.4) | 0.063 | 1.54 (0.78–3.05) | 0.212 |
| Consumption of dairy productsc | 266/1136 (23.4) | 22/125 (17.6) | 0.177 | 0.69 (0.42–1.13) | 0.142 | 73/397 (18.4) | 10/51 (19.6) | 0.848 | - | - |
| High salt dietc | 130/1134 (11.5) | 16/125 (12.8) | 0.659 | - | - | 52/397 (13.1) | 9/51 (17.6) | 0.386 | - | - |
Logistic analysis was performed and a P-value < 0.05 was considered significant. Logistic model including terms of gender, relatives with gastric cancer, education, and consumption of dairy products.
Logistic analysis was performed and a P-value < 0.05 was considered significant. Logistic model including terms of gender, body mass index (BMI), smoking, education, and non-steroidal anti-inflammatory drugs (NSAIDs) use.
Some data are missing. Missing values are not included.
FD, functional dyspepsia; OR, odds ratio; CI, confidence interval; H. pylori, Helicobacter pylori; IgG, Immunoglobulin G.
Discussion
The current study demonstrated that the prevalence of FD was 10.3%. The prevalence of PDS subtype was rather higher at 7.3% compared with EPS subtype at 5.5%. In addition, the prevalence of FD in females (12.4%) was higher than in males (7.8%), and was 11.3% in individuals aged ≥60 years, slightly higher than those aged below 60 (9.9%). Risk factors varied slightly according to these groups in that female gender and education below college level were risk factors for FD. In particular, education below college level was associated with FD in male patients, and female sex correlated with elderly FD patients (age ≥60 years). Although some results are consistent with those of previous studies, these results provide recent information for the prevalence and risk factors of FD in a Korean health check-up population. In addition, there has been no study which analyzed the prevalence and risk factors comprehensively according to FD subtype, sex, and age as in the present study. Therefore, our results could arouse interest in researchers who had investigated FD researches and gender studies.
The prevalence of FD varied depending on where the studies were conducted. The FD prevalence in patients who visited tertiary centers was 45.8% in Korea using the Rome III criteria.13 However, another study of FD in Korea reported that the prevalence in patients who underwent health check-ups was 8.1% according to the Rome III criteria.14 The differences in FD prevalence may be due to the severity of dyspeptic symptoms of patients visiting tertiary hospitals compared with patients undergoing health check-ups. Interestingly, our study group conducted a similar study about 5 years ago,4 and the prevalence rate of FD was 20.4%, which was higher than in the present study. In the previous study, we used the upper gastrointestinal symptom questionnaire instead of the Rome criteria. The Rome III criteria were more stringent than the symptom questionnaire, and may have influenced the difference of FD prevalence rates.
Our study revealed that the proportion of PDS and EPS in patients with FD was 71.0% and 53.4%, respectively. Therefore, the proportion of PDS alone, EPS alone and PDS-EPS overlap was 46.6%, 29.0%, and 24.4%, respectively. According to previous studies, the proportion of PDS was higher than that of EPS. In a population-based endoscopic study in Italy, the prevalence of FD was 11.0%, and the proportion of PDS and EPS was 67.5% and 48.2%, respectively.15 Thus, 15.8% of patients had PDS-EPS overlap. Our previous study also showed that the proportion of PDS, EPS, and PDS-EPS overlap in patients with health checkups was 68.2%, 46.4%, and 14.6%, respectively.4 However, another population study reported that the proportion of PDS-EPS overlap was 64.0% in India using the Rome III criteria.16 The prevalence of FD was 14.9%, which was similar to the current study, but the proportion of PDS was 91.0% and that of EPS was 73.0%.16 The participants in the Indian study were mostly (97.0%) vegetarian and the socioeconomic classes were lower than in the current study.16 Therefore, dietary habits and socioeconomic differences affect the results.15,16
Regarding the risk factors for FD, several studies reported2,17 that the female sex was significantly connected with FD as showed by the results of our study. In a population-based study in Australia, the preponderance of female was statistically higher than in males in most of the functional gastrointestinal disorders including FD,17 and a meta-analysis also revealed that the female sex (OR, 1.24; 95% CI, 1.13–1.36) was one of the independent risk factors for uninvestigated dyspepsia.18 Regarding the relationship between females and FD, the differences in sex hormones affect gastric motility and visceral sensitivity. The female hormones, such as estrogen and progesterone, altered gastric emptying.19 Therefore, gastric emptying in the luteal phase, when the sex hormones levels are increased, is delayed compared to that in the follicular phase,20 and the gastric emptying time in premenopausal females was longer than in males.20–22 In addition, other studies revealed that visceral pain perception of females can be affected by cyclical changes in female sex hormones.23,24 Based on these factors, it is presumed that female sex hormones might have a partial effect on gastric motility and visceral pain, implicating female sex as a risk factor for FD.
However, it is difficult to interpret the association of older FD patients with female sex hormones, and appeared to be connected with psychological problems. It is widely known that psychological comorbidity is strongly related with female sex and high prevalence rates of psychological disorders in the elderly. A recent study in Korea reported higher levels of anxiety and depression scores in female FD patients compared to male FD patients.19 Another recent study presented that the prevalence of anxiety disorders was about 20% in the elderly.25 Therefore, old age and female sex might be affected by psychological factors, which are associated with FD.
Socioeconomic status has been suggested as one of the risk factors for FD. Unfortunately, in a majority of population-based surveys, there was no significant association between FD and socioeconomic status including education levels.2 A large cross-sectional study revealed that low educational attainment was significantly connected with dyspepsia in British adults.26 A Canadian population study also showed that upper gastrointestinal symptoms were more common in participants with lower educational levels.27 By contrast, a population study in Malaysia reported that higher levels of education were independent risk factors for dyspepsia.28 The relationship is still unclear, and further well-designed studies are needed.
H. pylori is considered to affect the pathogenesis of FD. Several epidemiologic studies reported that H. pylori infection rates in FD patients were higher than in matched control participants,29 and a meta-analysis also presented that the summary OR for H. pylori infection in non-ulcer dyspepsia was 1.6 (95% CI, 1.4–1.8).30 However, H. pylori seropositivity was not related to FD in the current study, and H. pylori seronegativity was associated with FD patients. In 2015, the Kyoto global consensus proposed a new category of H. pylori-associated dyspepsia, which was separated from the FD.31 This guideline has been widely promulgated suggesting that H. pylori may affect dyspeptic symptoms, resulting in a more active H. pylori eradication therapy in patients with FD. FD patients in our study also received additional H. pylori eradication therapy compared to those without FD, which seems to have influenced the results.
There are several limitations in this study. First, H. pylori infection was confirmed only by serologic testing. The specificity of invasive tests, such as rapid urease test and histology, is higher than noninvasive tests.32 In addition, the results of serologic tests may be influenced by gastric atrophy or recent use of antibiotics, proton pump inhibitors, or eradication therapy.33 However, serologic tests are relatively inexpensive and safe and used to investigate the prevalence of H. pylori in the general population compared to urea breath test, histology, or rapid urease test.34 Second, the number of FD patients was rather small and multivariate analysis failed to reveal additional risk factors in each condition. To overcome this limitation, the national survey lasted more than 10 months and most of the health centers expected early completion. Nevertheless, the advantages of the current study are its national scope in Korea, and survey of all the enrolled subjects with questionnaires using the Rome III criteria and endoscopy.
In conclusion, FD is a relatively prevalent gastrointestinal disorder in a health check-up population in tertiary centers in Korea, with a higher prevalence in females and older groups. The risk factors for FD are female sex and low education level. Female sex is a risk factor for FD in old age, warranting close monitoring of older female in the population.
Footnotes
Financial support: This research was funded by Support Program for Women in Science, Engineering and Technology through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (No. 2016H1C3A1903202).
Conflicts of interest: None.
Author contributions: Sung Eun Kim collected and analyzed the data, and wrote a manuscript; Nayoung Kim designed and reviewed the manuscript; Ju Yup Lee, Kyung Sik Park, Jeong Eun Shin, Kwangwoo Nam, Hyeon Ju Kim, Hyun Joo Song, Young-Eun Joo, Dae-Seong Myung, Ji-Hyun Seo, Hyun Jin Jo, Seon Mie Kim, Hyun Jin Kim, Gwang Ho Baik, Sang Hyeon Choi, and Suck Chei Choi collected the data; and Seon Hee Lim reviewed the manuscript. All authors have contributed significantly, and that all authors are in agreement with the content of the manuscript.
References
- 1.Stanghellini V, Chan FK, Hasler WL, et al. Gastroduodenal disorders. Gastroenterology. 2016;150:1380–1392. doi: 10.1053/j.gastro.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 2.Mahadeva S, Goh KL. Epidemiology of functional dyspepsia: a global perspective. World J Gastroenterol. 2006;12:2661–2666. doi: 10.3748/wjg.v12.i17.2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung HK, Keum BR, Jo YJ, et al. Diagnosis of functional dyspepsia: a systematic review. Korean J Gastroenterol. 2010;55:296–307. doi: 10.4166/kjg.2010.55.5.296. [Korean] [DOI] [PubMed] [Google Scholar]
- 4.Kim SE, Park HK, Kim N, et al. Prevalence and risk factors of functional dyspepsia: a nationwide multicenter prospective study in Korea. J Clin Gastroenterol. 2014;48:e12–e18. doi: 10.1097/MCG.0b013e31828f4bc9. [DOI] [PubMed] [Google Scholar]
- 5.Lacy BE, Weiser KT, Kennedy AT, Crowell MD, Talley NJ. Functional dyspepsia: the economic impact to patients. Aliment Pharmacol Ther. 2013;38:170–177. doi: 10.1111/apt.12355. [DOI] [PubMed] [Google Scholar]
- 6.Kim SE, Park YS, Kim N, et al. Effect of Helicobacter pylori eradication on functional dyspepsia. J Neurogastroenterol Motil. 2013;19:233–243. doi: 10.5056/jnm.2013.19.2.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaib Y, El-Serag HB. The prevalence and risk factors of functional dyspepsia in a multiethnic population in the United States. Am J Gastroenterol. 2004;99:2210–2216. doi: 10.1111/j.1572-0241.2004.40052.x. [DOI] [PubMed] [Google Scholar]
- 8.Nam K, Shin JE, Kim SE, et al. Prevalence and risk factors of upper gastrointestinal diseases in health check-up subjects and analysis of recent trends: a nationwide multicenter cross-sectional study in Korea. Scand J Gastroenterol. doi: 10.1080/00365521.2018.1487992. Published Online First: 31 Aug 2018. [DOI] [PubMed] [Google Scholar]
- 9.Miwa H, Ghoshal UC, Fock KM, et al. Asian consensus report on functional dyspepsia. J Gastroenterol Hepatol. 2012;27:626–641. doi: 10.1111/j.1440-1746.2011.07037.x. [DOI] [PubMed] [Google Scholar]
- 10.Song KH, Jung HK, Min BH, et al. Development and validation of the Korean Rome III questionnaire for diagnosis of functional gastrointestinal disorders. J Neurogastroenterol Motil. 2013;19:509–515. doi: 10.5056/jnm.2013.19.4.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tack J, Talley NJ, Camilleri M, et al. Functional gastroduodenal disorders. Gastroenterology. 2006;130:1466–1479. doi: 10.1053/j.gastro.2005.11.059. [DOI] [PubMed] [Google Scholar]
- 12.Kim SY, Ahn JS, Ha YJ, et al. Serodiagnosis of Helicobacter pylori infection in Korean patients using enzyme-linked immunosorbent assay. J Immunoassay. 1998;19:251–270. doi: 10.1080/01971529808005485. [DOI] [PubMed] [Google Scholar]
- 13.Park JM, Choi MG, Cho YK, et al. Functional gastrointestinal disorders diagnosed by Rome III questionnaire in Korea. J Neurogastroenterol Motil. 2011;17:279–286. doi: 10.5056/jnm.2011.17.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noh YW, Jung HK, Kim SE, Jung SA. Overlap of erosive and non-erosive reflux diseases with functional gastrointestinal disorders according to Rome III criteria. J Neurogastroenterol Motil. 2010;16:148–156. doi: 10.5056/jnm.2010.16.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zagari RM, Law GR, Fuccio L, et al. Epidemiology of functional dyspepsia and subgroups in the Italian general population: an endoscopic study. Gastroenterology. 2010;138:1302–1311. doi: 10.1053/j.gastro.2009.12.057. [DOI] [PubMed] [Google Scholar]
- 16.Ghoshal UC, Singh R. Frequency and risk factors of functional gastrointestinal disorders in a rural Indian population. J Gastroenterol Hepatol. 2017;32:378–387. doi: 10.1111/jgh.13465. [DOI] [PubMed] [Google Scholar]
- 17.Koloski NA, Talley NJ, Boyce PM. Epidemiology and health care seeking in the functional GI disorders: a population-based study. Am J Gastroenterol. 2002;97:2290–2299. doi: 10.1111/j.1572-0241.2002.05783.x. [DOI] [PubMed] [Google Scholar]
- 18.Ford AC, Marwaha A, Sood R, Moayyedi P. Global prevalence of, and risk factors for, uninvestigated dyspepsia: a meta-analysis. Gut. 2015;64:1049–1057. doi: 10.1136/gutjnl-2014-307843. [DOI] [PubMed] [Google Scholar]
- 19.Choi YJ, Park YS, Kim N, et al. Gender differences in ghrelin, nociception genes, psychological factors and quality of life in functional dyspepsia. World J Gastroenterol. 2017;23:8053–8061. doi: 10.3748/wjg.v23.i45.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gill RC, Murphy PD, Hooper HR, Bowes KL, Kingma YJ. Effect of the menstrual cycle on gastric emptying. Digestion. 1987;36:168–174. doi: 10.1159/000199414. [DOI] [PubMed] [Google Scholar]
- 21.Datz FL, Christian PE, Moore J. Gender-related differences in gastric emptying. J Nucl Med. 1987;28:1204–1207. [PubMed] [Google Scholar]
- 22.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology. 1989;96:11–17. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 23.Meerveld BG, Johnson AC. Mechanisms of stress-induced visceral pain. J Neurogastroenterol Motil. 2018;24:7–18. doi: 10.5056/jnm17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hellström B, Anderberg UM. Pain perception across the menstrual cycle phases in women with chronic pain. Percept Mot Skills. 2003;96:201–211. doi: 10.2466/pms.2003.96.1.201. [DOI] [PubMed] [Google Scholar]
- 25.Canuto A, Weber K, Baertschi M, et al. Anxiety disorders in old age: psychiatric comorbidities, quality of life, and prevalence according to age, gender, and country. Am J Geriatr Psychiatry. 2018;26:174–185. doi: 10.1016/j.jagp.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 26.Moayyedi P, Forman D, Braunholtz D, et al. The proportion of upper gastrointestinal symptoms in the community associated with Helicobacter pylori, lifestyle factors, and nonsteroidal anti-inflammatory drugs. Leeds HELP study group. Am J Gastroenterol. 2000;95:1448–1455. doi: 10.1111/j.1572-0241.2000.2126_1.x. [DOI] [PubMed] [Google Scholar]
- 27.Tougas G, Chen Y, Hwang P, Liu MM, Eggleston A. Prevalence and impact of upper gastrointestinal symptoms in the Canadian population: findings from the DIGEST study. Domestic/international gastroenterology surveillance study. Am J Gastroenterol. 1999;94:2845–2854. doi: 10.1111/j.1572-0241.1999.01427.x. [DOI] [PubMed] [Google Scholar]
- 28.Mahadeva S, Yadav H, Rampal S, Goh KL. Risk factors associated with dyspepsia in a rural Asian population and its impact on quality of life. Am J Gastroenterol. 2010;105:904–912. doi: 10.1038/ajg.2010.26. [DOI] [PubMed] [Google Scholar]
- 29.Miwa H, Ghoshal UC, Gonlachanvit S, et al. Asian consensus report on functional dyspepsia. J Neurogastroenterol Motil. 2012;18:150–168. doi: 10.5056/jnm.2012.18.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jaakkimainen RL, Boyle E, Tudiver F. Is Helicobacter pylori associated with non-ulcer dyspepsia and will eradication improve symptoms? A meta-analysis. BMJ. 1999;319:1040–1044. doi: 10.1136/bmj.319.7216.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sugano K, Tack J, Kuipers EJ, et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut. 2015;64:1353–1367. doi: 10.1136/gutjnl-2015-309252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monteiro L, de Mascarel A, Sarrasqueta AM, et al. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am J Gastroenterol. 2001;96:353–358. doi: 10.1111/j.1572-0241.2001.03518.x. [DOI] [PubMed] [Google Scholar]
- 33.Malfertheiner P, Megraud F, O’Morain C, et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III consensus report. Gut. 2007;56:772–781. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garza-González E, Perez-Perez GI, Maldonado-Garza HJ, Bosques-Padilla FJ. A review of Helicobacter pylori diagnosis, treatment, and methods to detect eradication. World J Gastroenterol. 2014;20:1438–1449. doi: 10.3748/wjg.v20.i6.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]