Abstract
Background/Aims:
Dendritic cells (DCs) are antigen-presenting cells linking innate and adaptive immunity. DC maturation and migration are governed by alterations of cytosolic Ca2+ concentrations ([Ca2+].). Ca2+ entry is in part accomplished by store-operated Ca2+ (SOC) channels consisting of the membrane pore-forming subunit Orai and the ER Ca2+ sensing subunit STIM. Moreover, DC functions are under powerful regulation of the phosphatidylinositol-3-kinase (PI3K) pathway, which suppresses proinflammatory cytokine production but supports DC migration. Downstream targets of PI3K include serum- and glucocorticoid-inducible kinase isoform SGK3. The present study explored, whether SGK3 participates in the regulation of [Ca2+], and Ca2+-dependent functions of DCs, such as maturation and migration.
Methods/Results:
Experiments were performed with bone marrow derived DCs from gene targeted mice lacking SGK3 (sgk3−/−) and DCs from their wild type littermates (sgk3+/+). Maturation, phagocytosis and cytokine production were similar in sgk3−/− and sgk3+/+ DCs. However, SOC entry triggered by intracellular Ca2+ store depletion with the endosomal Ca2+ ATPase inhibitor thapsigargin (l μM) was significantly reduced in sgk3−/− compared to sgk3+/+ DCs. Similarly, bacterial lipopolysaccharide (LPS, 1 μg/ml)- and chemokine CXCL12 (300 ng/ml)-induced increase in [Ca2+], was impaired in sgk3−/− DCs. Moreover, currents through SOC channels were reduced in sgk3−/− DCs. STIM2 transcript levels and protein abundance were significantly lower in sgk3−/− DCs than in sgk3+/+ DCs, whereas Orai1, Orai2, STIM1 and TRPC1 transcript levels and/or protein abundance were similar in sgk3−/− and sgk3+/+ DCs. Migration of both, immature DCs towards CXCL12 and LPS-matured DCs towards CCL21 was reduced in sgk3−/− as compared to sgk3+/+ DCs. Migration of sgk3+/+ DCs was further sensitive to SOC channel inhibitor 2-APB (50 μM) and to STIM1/STIM2 knock-down.
Conclusion:
SGK3 contributes to the regulation of store-operated Ca2+ entry into and migration of dendritic cells, effects at least partially mediated through SGK3-dependent upregulation of STIM2 expression.
Keywords: CISK, PI3 kinase, Cytosolic Ca2+, Lipopolysaccharides, Orai2, STIM2, Migration
Introduction
Dendritic cells (DCs] are powerful antigen-presenting cells endowed with remarkable plasticity, which allows them to undergo complete genetic reprogramming in response to danger signals generated by harmful non-self [1]. Resting immature DCs are highly phagocytic and continuously internalize soluble and particulate antigens. After taking up an antigen from pathogens, tumors or toxic stimuli, DCs undergo a complex maturation process [1]. The major characteristics of transformation from an immature to a mature DC include decrease in phagocytic activity, upregulation of antigen-presenting and costimulatory molecules, production of certain cytokines and migration to draining lymph nodes [1]. DCs migrate specifically into T cell areas of lymph nodes, where they secrete chemokines that permit the attraction of naive T cells and induce the proliferation and differentiation of antigen-specific T cells [1].
DC functions are governed by Ca2+-signaling, which directs the DC responses to diverse antigens, including toll-like receptor (TLR) ligands, intact bacteria, and microbial toxins [2]. Ca2+ sensitive DC functions include DC activation, maturation, migration, formation of immunological synapses with T cells and finally apoptosis [3, 4]. Moreover, alterations of cytosolic Ca2+ trigger immune suppression [3, 5, 6]. In mouse DCs, activation of inositol trisphosphate receptors (IP3Rs) in the ER and subsequent decline in the level of Ca2+ in intracellular stores induces capacitative Ca2+ entry via Ca2+ release-activated Ca2+ (CRAC) channels [7, 8], a ubiquitous signaling mechanism in non-excitable and excitable cells. In mouse DCs the predominant components of CRAC channels include the pore-forming subunit Orai2 and the Ca2+-sensing subunit STIM2 [9].
Chemotactic response of DCs to several chemokines, such as ligands of the mouse formyl peptide receptor 1, CXCR4 and CCR7 depends on Ca2+ influx [10–13]. Blocking of CRAC channels impairs CCL21-dependent migration [7, 14].
Very little is known about the mechanisms that regulate Ca2+ entry into DCs. A signaling pathway that plays an important role in DC biology is the phosphatidylinositol-3-kinase (PI3K) pathway. In DCs, PI3K belongs to the gate-keeping system, preventing excessive immune responses [15, 16]. PI3K-dependent signaling includes 3-phosphoinositide-dependent kinase (PDK1), which in turn activates further downstream kinases such as PKB/Akt, serum- and glucocorticoid-inducible kinases SGK1 and SGK3, mammalian target of rapamycin (mTOR), and glycogen synthase kinase 3 beta (GSK3β). PI3K-induced suppression of DC maturation and/or inflammatory cytokine production is mediated by PDK1, GSKβ and mTOR [17, 18]. On the other hand, the specific PI3K isoform PI3Kγ is critically important for DC migration as defective DC migration has been observed in PI3Kγ-deficient DCs [19].
SGK3, also known as cytokine-independent survival kinase CISK [20], is expressed in all tissues tested thus far [21] and regulates a wide variety of channels and transporters [22]. In the immune system, SGK3 has been shown to modify Ca2+ flux, Ca2+-activated K+ channels and degranulation of bone marrow-derived mast cells [23]. The aim of the present project was to investigate the role of SGK3 in the regulation of DC ion channels and functions.
Materials and Methods
Mice
All animal experiments were conducted according to the German law for the welfare of animals and were approved by local authorities. Experiments have been performed in SGK3 deficient mice (sgk3−/−] and their wild-type littermates (sgk3+/+). The targeting strategy for disruption of the Sgk3 gene has been described earlier [24].
Mice heterozygous for SGK3 were interbred to yield sgk3−/− and sgk3+/+ mice. Therefore the genetic background of the SGK3 knockout mice is a mixture of C57BL/6J and 129/SvJ. Genotyping was made by PCR on tail DNA using SGK3 and neo-R-specific primers as previously described [24]. Mice had free access to a standard mouse diet (C1310 Altromin, Heidenau, Germany) and tap water.
Cell Culture
Dendritic cells (DCs) were cultured from bone marrow of 7–11 weeks old male and female sgk3+/+ and sgk3−/− mice as described previously [7]. Briefly, bone marrow derived cells were flushed out of the cavities from the femur and tibia with PBS. Cells were then washed twice with RPMI and seeded out at a density of 2 × 106 cells per 60-mm dish. Cells were cultured for 6 days in RPMI 1640 (GIBCO, Carlsbad) containing: 10% FCS, 1% penicillin/streptomycin, 1% glutamine, 1% non-essential amino acids (NEAA) and 0.05% β-mercaptoethanol. Cultures were supplemented with granulocyte macrophage colony-stimulating factor (GM-CSF, 35 ng/mL, Immunotools) and fed with fresh medium containing GM-CSF on days 3 and 6. Experiments were performed on days 7–9. DC maturation was induced by treating the cells with lipopolysaccharides (LPS from E. coli, 1 μg/ml, 24 h, Enzo Life Sciences, Lausen, Switzerland).
Immunostaining and flow cytometry
Cells (106) were incubated in 200 μl PBS, containing 0.1% FCS and fluorochrome-conjugated antibodies at a concentration of 10 μg/ml. A total of 5 × 104 cells were analyzed in each individual experiment. The following antibodies (all from BD Pharmingen, Heidelberg, Germany) were used for staining: APC Hamster Anti-Mouse CD11c (Clone: HL3), PE-conjugated anti-mouse CD86, clone GL1 (Rat IgG2a, κ) and PE-conjugated rat anti-mouse I-A/I-E, clone M5/114.15.2 (IgG2b, κ). Following incubation with the respective antibodies for 60 min at 4°C, cells were washed twice and resuspended in the same buffer and subjected to flow cytometry analysis.
Cytokine Measurement
IL-2, IL-6, IL-12 and TNFα concentrations in DC culture supernatants were determined by using OptEIA ELISA kit (BD Pharmingen) according to the manufacturer’s protocol.
DC phagocytosis assay
DCs (106 cells/ml) were suspended in prewarmed RPMI 1640 medium, pulsed with FITC-conjugated dextran (70 kDa, Sigma) at a final concentration of 1 mg/ml and incubated for 10 min, 30 min, 1h, 2h or 3h at 37°C. Uptake of FITC-conjugated dextran was stopped by adding ice-cold PBS. The cells were then washed three times with ice cold PBS supplemented with 5% FCS and 0.01% sodium azide before FACS analysis.
Alternatively, phagocytosis was measured using pHrodo™ dye (Invitrogen). This assay utilizes E. coli conjugated to the non-quenching pHrodo™ dye, which dramatically increases in fluorescence as the pH becomes more acidic. 3 × 105 cells/well were plated into 96 well plates. The next day the cell culture medium was removed and 100 μl of prepared pHrodo™ BioParticles® (pHrodo™ BioParticles® Conjugates for Phagocytosis, Invitrogen) was added to each well, the plates were covered and incubated for 3 hours at 37°C. The plates were scanned in a fluorescence plate reader using ~550 nm excitation and ~600 nm emission.
Migration
For migration assays transwells inserts (BD Falcon 353097) and BD BioCoat™ Matrigel™ Invasion Chambers (BD Biosciences 354480) were used with a 8 μm pore diameter. The transwells were placed in a 24-well cell culture plate containing cell culture medium (750 μl) with or without either CXC-Motiv-Chemokin 12 (CXCL12, 50 ng/ml, Peprotech, for immature DCs) or Chemokine (C-C motif) ligand 21 (CCL21, 25 ng/ml, Peprotech, for mature DCs) in the absence or in the presence of store-operated Ca2+ channel inhibitor 2-Aminoethoxydiphenyl borate (2-APB, 50 μM, Sigma-Aldrich, Germany) added simultaneously with the chemokine into the upper chamber. The upper chambers were filled with 500 μl cell culture medium containing DCs in a concentration of 50000 cells/ml. The chamber was placed in a 5% CO2 37°C incubator for 4 hours. In the following, the non-migrated cells were gently removed by cotton tipped swab and PBS wash. The transwells were moved to 4% paraformaldehyde (PFA) and incubated overnight. Membranes were removed by scalpel, placed on slides and stained with DAPI. The migrated cells bound to the membrane were then counted.
Measurement of intracellular Ca2+
Fluorescence measurements were carried out with an inverted phase-contrast microscope (Axiovert 100, Zeiss, Oberkochen, Germany). Cells were excited alternatively at λ = 340 (340/26) or 380 (387/11) nm and the light was deflected by a dichroic mirror into either the objective (Fluar 40×/1.30 oil, Zeiss, Oberkochen, Germany) or a camera (Proxitronic, Bensheim, Germany). Emitted fluorescence intensity was recorded at λ = 505 (495/10) nm and data acquisition was accomplished by using specialized computer software (Metafluor, Universal Imaging, Downingtown, USA). As a measure for the increase of cytosolic Ca2+ concentrations, the slope of the changes in the 340/380 nm ratio analyzed from linear regression and the peak were determined for each experiment.
Cells were loaded with Fura-2/AM (2 μM, Molecular Probes, Goettingen, Germany) for 15 min at 37°C, 5% CO2. Intracellular Ca2+ was monitored prior to and following addition of LPS from E. coli (1 μg/ml, Enzo Life Sciences, Lausen, Switzerland) or the chemokine CXCL12 (300 ng/ml) to the Ringer solution (see below). Alternatively, changes in cytosolic Ca2+ were monitored upon depletion of the intracellular Ca2+ stores. Experiments were carried out prior to and during exposure of the cells to Ca2+-free solution. In the absence of Ca2+, the intracellular Ca2+ stores were depleted by inhibition of the vesicular Ca2+ pump by thapsigargin (1 μM, Molecular Probes). Re-addition of Ca2+ allowed assessing the store-operated Ca2+ entry.
Experiments were performed in Ringer solution containing (in mmol/l): 125 NaCl, 5 KCl, 1.2 MgSO4, 32.2 Hepes, 2 Na2HPO4, 2 CaCl2, and 5 glucose at pH 7.4. Ca2+-free solutions contained (in mmol/l): 125 NaCl, 5 KCl, 1.2 MgSO4,2 Na2HPO4, 32.2 Hepes, 0.5 EGTA, 5 glucose, pH 7.4. Ionomycin (10 μM, Calbiochem, Germany) was applied at the end of each experiment as a test for cell viability. The fluorescence ratio was taken as a surrogate of cytosolic Ca2+ concentration [Ca2+]i. No attempt was made to calculate [Ca2+]i.
Patch clamp
Patch clamp experiments were performed at room temperature in voltage-clamp, fast-whole-cell mode with access resistance less than 10 MOhm, which allows accurate voltage clamp of fast whole cell currents. Borosilicate glass pipettes (2–4 MOhm tip resistance; Harvard Apparatus, Kent, UK) manufactured by a microprocessor-driven DMZ puller (Zeitz, Augsburg, Germany) were used in combination with a MS314 electrical micromanipulator (MW, Marzhauser, Wetzlar, Germany). The currents were recorded by an EPC-9 amplifier (Heka, Lambrecht, Germany) using Pulse software (Heka) and an ITC-16 Interface (Instrutech, Port Washington, N.Y., USA). For ICRAC measurements whole-cell currents were elicited by 200 ms square wave voltage pulses from −50 to +50 mV in 10 mV steps delivered from a holding potential of −30 mV. Alternatively, the currents were recorded with 200 ms voltage ramps from −50 to +50 mV. Leak currents determined as the currents at the very beginning of each experiment immediately after reaching the wholecell mode were substracted. The currents were recorded with an acquisition frequency of 10 kHz and 3 kHz low-pass filtered. The liquid junction potential AE between the CsCl-based pipette and the NaCl-based bath solutions estimated according to Barry and Lynch [25] was 1 mV. The data were not corrected for ΔE.
DCs were superfused with a bath solution containing (in mmol/l): 140 NaCl, 5 KCl, 10 CaCl2, 20 glucose, 10 HEPES/NaOH, pH 7.4. In some experiments extracellular Na+ was substituted by NMDG+ and the bath solution contained (in mmol/l): 145 NMDG-Cl, 10 HEPES/NMDG, 20 glucose, 1 MgCl2 and 10 CaCl2. The patch clamp pipettes were filled with an internal solution containing (in mmol/l): 120 CsCl, 35 NaCl, 1 MgATP, 10 EGTA, 10 HEPES/CsOH, 0.04 inositol 1,4,5-trisphosphate (Ins(1,4,5)P3, Enzo Life Sciences), pH 7.4.
Real-time PCR
Total RNA was extracted from mouse dendritic cells in Trizol (Peqlab, Erlangen, Germany) according to the manufacturer’s instructions. After DNAse digestion reverse transcription of total RNA was performed using random hexamers (Roche Diagnostics, Penzberg, Germany) and SuperScriptII reverse transcriptase (Invitrogen, Carlsbad, CA, USA). Polymerase chain reaction (PCR) amplification of the respective genes were set up in a total volume of 20 μl using 40 ng of cDNA, 500 nM forward and reverse primer and 2x GoTaq® qPCR Master Mix SYBR Green (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol. Cycling conditions were as follows: initial denaturation at 95°C for 2 min, followed by 40 cycles of 95°C for 15 sec, 55°C for 15 sec and 68°C for 20 sec. For the amplification the following primers were used (5’->3’orientation):
Orai1, fw CATGGTAGCGATGGTGGAAGTC rev TGCTCATCGTCTTTAGTGCCT;
Orai2, fw ATGGTGGCCATGGTGGAGGT rev ATTGCCTTCAGCGCCTGCA;
STIM1, fw CTTGGCCTGGGATCTCAGAG rev TCAGCCATTGCCTTCTTGCC;
STIM2, fw GCAGGATCTTTAGCCAGAAG rev ACATCTGCTGTCACGGGTGA;
Tbp, fw CAAGCTGGAGGTGATCATCG rev TCCACAGTGCTCTTGAATTCG.
Specificity of PCR products was confirmed by analysis of a melting curve. Real-time PCR amplifications were performed on a CFX96 Real-Time System (Bio-Rad). All experiments were done in duplicate. Amplification of the house-keeping gene Tbp (TATA binding protein) was performed to standardize the amount of sample RNA. Relative quantification of gene expression was achieved using the ΔΔct method as described earlier [26].
Western blot
Cell lysates were separated by 10% SDS-PAGE and blotted on nitrocellulose membranes. The blots were blocked with 10% nonfat-milk in triethanolamine-buffered saline (TBS) and 0.1% Tween-20. Then the blots were probed overnight with one of the following antibodies: rabbit polyclonal ORAI1 (1:200, 50 kDa, Abcam, UK, the specificity of the antibody tested in [27]), goat polyclonal ORAI2 (1:200, 28 kDa, Santa Cruz, USA, the specificity of the antibody tested in [28]), mouse monoclonal STIM1 (1:500, 84 kDa, Abnova, Taiwan, the specificity of the antibody tested in [29]), rabbit polyclonal STIM2 (1:1000, 100 kDa, Cell Signaling, USA, the specificity of the antibody tested in [30]), TRPC1 (1:1000, 91 kDa, Proteintech, Germany) and GAPDH (1:1000, 37 kDa, Cell Signaling, USA). All antibodies were diluted in TBS with 5% BSA and 0.1% Tween-20. The blots were then washed 3 times for 10 min/wash, probed with respective secondary antibodies (anti-rabbit, Cell Signaling; anti-goat, Santa Cruz; anti-mouse, GE Healthcare, UK) diluted 1:3000 for 1 h at room temperature, and washed 3 times for 10 min/wash. Antibody binding was detected with the Western blotting detection reagents (Amersham ECL Western Blotting Detection Reagents, GE Healthcare, UK). Densitometer scans of the blots were performed using Quantity One (BioRad, Munich, Germany).
Silencing of STM
Specific siRNA sequences for STIM2 (Silencer Select Pre-designed (Non-inventoried) siRNA, Ambion, sense UAAUGGUGAGAAAAGCAAAtt, antisense UUUGCUUUUCUCACCAUUAtg, 21 bp], which in our experiments could also silence STIM1, and negative control (Silencer® Select Negative Control No. 1 siRNA, Ambion, Catalog # 4390843) were synthesized and annealed by the manufacturer. siRNA transfection was carried out using the GeneSilencer siRNA transfection reagent (Genlantis, San Diego, CA,USA). 4 × 106 cells were washed and plated in 6-well plates in 1 ml of serum-free RPMI 1640. The STIM2 siRNA and the negative control (1000 ng/well) were incubated with GeneSilencer reagent following the manufacturer’s protocol. Transfection mixture was then added to the wells and incubated for 24 hours. The efficiency of silencing was assessed with RT-PCR and western blotting.
Statistics
Data are provided as means ± SEM, n represents the number of independent experiments. All data were tested for significance using Student’s unpaired two-tailed t-test, one sample t-test or one-way ANOVA (Bonferroni Multiple Comparisons Test) with post test and only results with p < 0.05 were considered statistically significant.
Results
DC maturation, cytokine production and phagocytosis
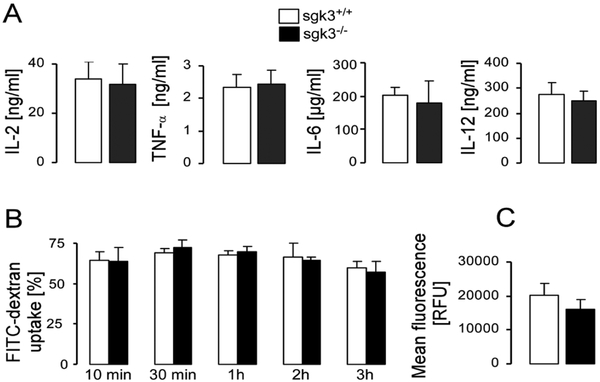
DCs were isolated from the bone marrow of mice lacking functional SGK3 (sgk3−/−) and their wild type littermates (sgk3+/+). DC maturation was induced by the TLR4 ligand LPS (1 μg/ml, 24 h). According to FACS analysis (Fig. 1), maturation markers MHC II and costimulatory molecule CD86 were similarly expressed on CD11c-positive immature and mature cells from sgk3−/− and sgk3+/+ mice. The production of several cytokines including IL-2, IL-12, IL-6 and TNFα by mature DCs was also unaffected by the lack of SGK3 (Fig. 2A). Also the capacity of sgk3+/+ and sgk3−/− DCs for endocytosis assessed by exposure of DCs to FITC-dextran (70 kDa, Fig. 2B) at different time points and to pHrodo-labeled E. coli particles (Fig. 2C) was not different between genotypes.
Fig. 1.
Differentiation and maturation of bone marrow derived dendritic cells (DCs) from sgk3+/+ and sgk3−/− mice. A. Original dot plots of CD11c+CD86+ (above) and CD11c+MHC II+ (below) DCs at the basal level (control, 1st and 2nd panels) and stimulated with LPS (LPS, 1 ng/ml, 24 h, 3rd and 4th panels) from sgk3+/+ (1st and 3rd panels) and sgk3−/− (2nd and 4th panels) mice. Numbers depict the percent of cells in the respective quadrants. B. Arithmetic means ± SEM (n =7) of the percentage of CD11c+CD86+ (left) and CD11c+MHC II+ (right) DCs under control conditions and 24 h after LPS stimulation in primary cultures from sgk3+/+ (open bars) and sgk3−/− (closed bars) mice. *** (p<0.001), ANOVA.
Fig. 2.
Cytokine production and phagocytic capacity are normal in sgk3−/− DCs. A. Arithmetic means ± SEM (n =4–17) of IL-2, TNFα, IL-6 and IL-12 secretion in cultured sgk3−/− DCs (closed barsj and their wild-type littermates sgk3+/+ (open bars) after stimulation with LPS (1 μg/ml, 18 h) and thapsigargin (50 nM, 18 h) for IL-2 production or LPS (1 ng/ml, 24h for IL-6 and IL-12, 4h for TNFα) for all other cytokines. B. Arithmetic means of FITC-dextran (70 kDa) uptake (% ± SEM, n = 8) by sgk3+/+ (open bars) and sgk3−/− (closed bars) DCs measured at different time points (10 min, 30 min, 1h, 2h, 3h). C. Arithmetic means of mean fluorescence (RFU) ± SEM (n =6) of uptake of pHrodo dye-labeled E. coli particles by sgk3+/+ (open bars) and sgk3−/− (closed bars) DCs. Experiments were performed in quintuplicates.
Migration
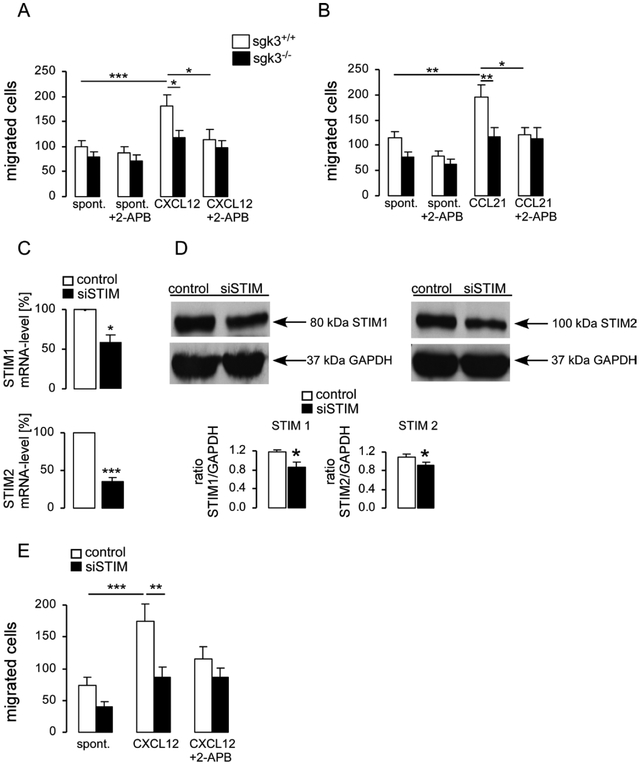
DC migration has been shown to be a PI3K-dependent function [19]. Therefore, the capacity of sgk3+/+ and sgk3−/− immature or LPS-matured DCs to migrate in the absence and presence of CXCL12 (50 ng/ml), a CXCR4 ligand or CCL21 (25 ng/ml), a CCR7 ligand, respectively, was assessed. According to the transwell assay, both CXCL12- and CCL21-dependent migration was less pronounced in sgk3−/− DCs than in sgk3+/+ DCs (Fig. 3A–B). Moreover, CXCL12- and CCL21-dependent migration of sgk3+/+ DCs was sensitive to store-operated Ca2+ (SOC) channel blocker 2-APB (50 nM) (Fig. 3A–B). Since 2-APB has a limited selectivity for SOC channel, we performed additional experiments with siRNA, which decreased both, STIM1 and STIM2 transcript abundance (Fig. 3C–D). Silencing of STIM1 and STIM2 was confirmed by RT-PCR (Fig. 3C) and western blotting (Fig. 3D). CXCL12-induced migration of siSTIM sgk3+/+ DCs was significantly impaired if compared to control sgk3+/+ DCs and was not different from migration of sgk3+/+ DCs treated with 2-APB (Fig. 3E).
Fig. 3.
Impaired migration of sgk3−/− DCs. A.-B. Arithmetic means ± SEM (n =9–18) of spontaneous migration and migration in response to either CXCL12 (50 ng/ml, 4h, immature DCs, A) or CCL21 (25 ng/ml, 4h, LPS (1 μg/ml, 24h)-matured DCs, B) sgk3+/+ (open bars) and sgk3−/− (closed bars) DCs in the absence or in the presence of 2-APB (50 μM). * (p<0.05), ** (p<0.01) and *** (p<0.001), ANOVA. C. Arithmetic means (± SEM, n=12) of the abundance of mRNA encoding STIM2 and STIM1 in sgk3+/+ DCs without (control, empty vector) and with (siSTIM) silencing of STIMs as assessed by real-time PCR using TBP mRNA as a reference gene. Relative mRNA expression in siSTIM-DCs was normalized to respective values in control cells. *(p<0.001), one sample t-test. D. Western blot analysis of whole cell lysate protein of STIM1 and STIM2 in control and siSTIM-DCs. Representative experiments showing STIM1 and STIM2 bands and GAPDH as loading control (left). Arithmetic means (± SEM, n=5) of STIM1/GAPDH and STIM2/GAPDH ratio. *(p<0.05), two-tailed unpaired t-test. E. Arithmetic means ± SEM (n =11) of spontaneous migration and migration in response to CXCL12 (50 μg/ml, 4h) of control (open bars) and siSTIM (closed bars) sgk3+/+ DCs in the absence or in the presence of 2-APB (50 nM). ** (p<0.01) and ** (p<0.01), ANOVA.
Store-operated Ca2+ entry
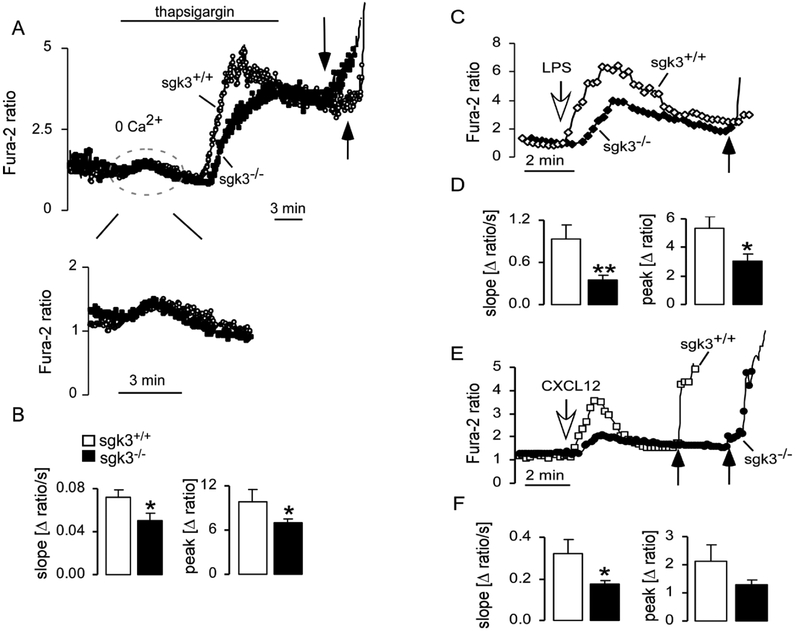
Since DC migration requires SOC entry [3], SOC entry was measured in sgk3−/− and sgk3+/+ DCs. The intracellular stores were emptied by treatment of the cells with the endosomal Ca2+ ATPase inhibitor thapsigargin (1 μM) in the absence of extracellular Ca2+ (Fig. 4A). This resulted in the release of Ca2+ from the cytoplasmic stores which was similar in sgk3−/− and sgk3+/+ DCs (Fig. 4A). The readdition of extracellular Ca2+ was followed by a sharp increase of cytosolic Ca2+ in sgk3+/+ cells, an effect significantly blunted in sgk3−/− cells (Fig. 4A, B). The amplitude of this increase, analyzed as peak (A ratio), and the velocity, analyzed as slope (A ratio/s), were both significantly reduced in sgk3−/− cells (Fig. 4B).
Fig. 4.
Reduced store-operated Ca2+ entry in sgk3DCs. A. Representative tracing showing the Fura-2 fluorescence ratio in Fura-2/AM loaded sgk3+/+ and sgk3−/− DCs. Experiments were carried out prior to and during exposure to nominally Ca2+-free bath solution. Where indicated, thapsigargin (1 μM) was added to the nominally Ca2+-free bath solution and the release of Ca2+ from the stores was assessed (release of Ca2+ is shown in a larger scale). Readdition of extracellular Ca2+ in the presence of thapsigargin reflects the entry of Ca2+ through the SOC channels. At the end of each experiment ionomycin (10 μM; black arrows) was added. For quantification of Ca2+ entry the slope (Δ ratio/s) and the peak (A ratio) of the fluorescence ratio were calculated. B. Arithmetic means (± SEM) of the slope (left) and the peak (right) of the change in Fura-2 fluorescence following addition of thapsigargin (1 μM) in the presence of extracellular Ca2+ to sgk3+/+ (n=32, open bars) and sgk3(n=33, closed bars) DCs. * (p<0.05), two-tailed unpaired t-test. C. Representative original tracings showing the Fura-2 fluorescence ratios (340/380 nm) in sgk3+/+ and sgk3−/−DCs prior to and following acute addition of lipopolysaccharide (LPS, 1 μg/ml; white arrow). At the end of each experiment ionomycin (10 μM; black arrows) was added. D. Arithmetic means (± SEM) of the slope (left) and the peak (right) of the change in Fura-2 fluorescence following addition of LPS to sgk3+/+ (n=59, open bars) and sgk3−/− (n=76, closed bars) DCs. ** (p<0.01), * (p<0.05), two-tailed unpaired t-test. E. Representative original tracings showing the Fura-2 fluorescence ratios in sgk3+/+ and sgk3−/− DCs prior to and following acute addition of CXCL12 (300 ng/ml; white arrow). F. Arithmetic means (± SEM) of the slope (left) and the peak (right) of the change in Fura-2 fluorescence following addition of CXCL12 to sgk3+/+ (n=35, open bars) and sgk3−/− (n=79, closed bars) DCs. * (p<0.05), two-tailed unpaired t-test.
The physiological stimulus inducing SOC entry in DCs is ligation of CD14, the component of TLR4, by LPS or ligation of the chemokine receptor CXCR4 by the chemokine CXCL12 [4, 7]. Accordingly, the LPS- and CXCL12-induced increase of cytosolic Ca2+ concentration was strongly reduced in sgk3−/− DCs as compared to sgk3+/+ DCs (Fig. 4C–F).
Current through store-operated Ca2+ channels
We employed whole-cell patch clamp to analyze the current through SOC channels in sgk3−/− and sgk3+/+ DCs. As shown in Fig. 5A–B the conductance of the inward current activated by Ca2+ store depletion with inositoltrisphosphate (IP3) in the pipette solution was strongly impaired in sgk3−/− DCs. This IP3 –activated current in mouse DCs has been shown to have all properties typical for SOC current (or ICRAC) [8]. To confirm the Ca2+ selectivity of the measured current we replaced extracellular Na+ by NMDG+. Neither reversal potential of the current/voltage relationship nor current amplitude were altered in NMDG+ containing bath (Fig. 5C).
Fig. 5.
Reduced currents through store-operated Ca2+ channels in sgk3−/− DCs. A. Mean current-voltage (I/V) relationships of currents activated by 40 μM IP3 normalized to cell capacitance in sgk3+/+ (n=14) and sgk3−/− (n=11) DCs. * (p<0.05), two-tailed unpaired t-test. B. Mean whole-cell conductance of inward currents (± SEM) calculated by linear regression of I/V curves shown in (A) between −50 and −10 mV in sgk3+/+ (n = 14, open bars) and sgk3−/− (n=11, closed bars) DCs. * (p<0.05), two-tailed unpaired t-test. C. Original ramp currents activated by 40 μM IP3 recorded in Na+-containing bath and thereafter upon substitution of Na+ by NMDG+.
Transcript levels and protein abundance of store-operated Ca2+ channel subunits
In view of the differences in SOC activity as well as LPS- and CXCL12-induced Ca2+ entry between sgk3−/− and sgk3+/+ DCs, we analyzed whether the expression of the components of SOC channels, STIM and Orai are influenced by SGK3. As illustrated in Fig. 6, both, transcript levels (Fig. 6A) and protein abundance (Fig. 6B–C) of STIM2 were significantly (P<0.04) lower in sgk3−/− DCs than in sgk3+/+ DCs (Fig. 6A). No significant differences were observed in transcript levels and protein abundance of Orail, Orai2 or STIM1 (Fig. 6A–C). Protein abundance of transient receptor potential channel 1 (TRPC1), which at least theoretically could also be involved in SOC entry in DCs, was also not different between sgk3+/+ and sgk3−/− cells (Fig. 6B–C).
Fig. 6.
Transcript and protein abundance of store-operated Ca2+ channel subunits in sgk3+/+ and sgk3−/− DCs. A. Arithmetic means (± SEM, n=4–16) of the abundance of mRNA encoding Orail, Orai2, STIM1 and STIM2 in sgk3+/+ and sgk3−/− DCs as assessed by real-time PCR using TBP mRNA as a reference gene. Relative mRNA expression in sgk3−/− DCs was normalized to respective values in sgk3+/+ cells. *(p<0.05), one sample t-test. B. Western blot analysis of whole cell lysate protein of Orail, Orai2, STIM1, STIM2 and TRPC1. Representative experiments showing Orai1–2, STIM1–2 and TRPC1 bands and GAPDH as loading control. For Orai1 and STIM1 the results from both immature and LPS (1 μg/ml, 24 h)-matured DCs are shown. C. Arithmetic means ± SEM of Orai1/GAPDH ratio (n =5), Orai2/GAPDH ratio (n =7), STIM1/GAPDH ratio (n =5), STIM2/GAPDH ratio (n =15) and TRPC1/GAPDH (n=8). Protein abundance in sgk3−/− DCs was normalized to respective values in sgk3+/+ cells. *(p<0.05), one sample t-test.
Discussion
The present study reveals that lack of SGK3 decreases the activity of store-operated Ca2+ channels and migration of murine dendritic cells (DCs). In contrast, DC maturation, induced by the engagement of TLR4 by LPS was not affected in sgk3−/− cells. It has been shown that maturation of DCs derived from PDK1 hypomorphic mice was enhanced with increased levels of costimulatory molecules compared to wild type cells [17]. Our data show that SGK3 is not responsible for this effect downstream from PDK1. Similarly, SGK3 does apparently not play a critical role in LPS-induced production of cytokines, which is known to increase through genetic and/or pharmacological inhibition of PI3K [15], PDK1 [17] or mTOR [18]. LPS-induced production of cytokines is further decreased upon pharmacological inhibition of GSK3 [18].
DCs phagocytic capability was also unaffected by genetic knockout of SGK3. In contrast, chemokine-induced migration of both, immature and mature sgk3−/− DCs was impaired if compared to sgk3+/+ cells. It is worth noting, however, that our results do not rule out that SGK3 affects DC chemotaxis rather than (or in addition to) migration. The result is consistent with a study showing impaired migration of bone marrow derived DCs from PI3Kγ−/− mice [19]. DCs derived from PI3Kγ−/− mice have a reduced ability to migrate in response to chemokines and the number of DCs in lymph nodes is also decreased in PI3Kγ−/− mice [19]. PI3Kγ (class IB), a unique isoform, which, in contrast to tyrosine kinase sensitive a, p and 5 isoforms of PI3K (class IA) [31], is associated with seven-transmembrane spanning receptors including chemotactic receptors [31]. Similar to SGK3-deficient DCs, PI3Kγ−/− DCs have normal cytokine production, and hence a complex scenario may be proposed in which class IA PI3K kinase - PDK1-mTOR-GSK3 axis may restrain the production of proinflammatory cytokines but class IB PI3K-SGK3 regulate DC migration. It should be kept in mind though, that in vivo migration does not only depend on the migratory machinery of the DC but as well on the function of other cell types. Thus, the present observations do not allow safe conclusions on migration of DCs in vivo.
One of the possible mechanisms underlying impaired in vitro migration of sgk3−/− DCs could be defective Ca2+ entry through store-operated Ca2+ (SOC) channels since migration of sgk3+/+ DCs could be inhibited with SOC channel blocker 2-APB. In view of the limited selectivity of 2-APB, however, it cannot be entirely excluded that 2-APB is partially effective by interfering with other targets [32]. In several studies SOC entry has been shown to be essential for DC migration [3, 7, 14]. Indeed, SOC entry and SOC currents were strongly reduced in sgk3−/− DCs. Therefore, SGK3 could stimulate DC migration by upregulation of CRAC channels. Our study does not exclude, however, that in addition to regulating CRAC channels, SGK3 influences the activity of other Ca2+ channels or of channels that set the membrane potential and thus contribute to the regulation of SOC entry.
We have recently identified two mechanisms of CRAC channel regulation through another isoform of SGK, SGK1. In HEK293 cells SGK1 upregulates CRAC channel abundance through phosphorylation of ubiquitin ligase Nedd4–2 (neural precursor cell expressed, developmentally down-regulated 4–2), which disrupts the interaction of Nedd4–2 with Orai1, a pore forming subunit of SOC channels [33]. As a result, SGK1 increases the Orai1 protein abundance in the plasma membrane thus enhancing SOC entry [33]. Additionally, SGK1 leads to NF-κB dependent transcriptional upregulation of Orai1 and STIM1, as shown in HEK293 cells, mast cells and platelets [34, 35]. Here we show that STIM2, which together with Orai2, is considered to be a major contributor to the SOC entry machinery in DCs [9], was less expressed on both transcript and protein level in SGK3-deficient DCs. Therefore, the mechanism of SGK3-dependent regulation of STIM2 may also involve upregulation of STIM2 transcription. At least in theory, SGK3 may, similar to SGK1, participate in the regulation of NF-κB, which in turn may influence the expression not only of STIM1, but as well of STIM2. However, expression of STIM1, which is upregulated by NFκB in other cell types [34, 35], is seemingly unaffected by lack of SGK3. Thus, other SGK3-sensitive mechanisms may participate in the regulation of STIM2 protein abundance or function. It is worth noting that even though there is considerable overlap of targets and regulation of SGK1 and SGK3, the kinases are apparently not serving identical functions [22, 36].
We have recently demonstrated that both SGK1 and SGK3 isoforms regulate SOC entry in murine mast cells. Degranulation, a process that has been shown to depend on Ca2+ influx through SOC, was impaired in both SGK3-deficient and SGK1-deficient mast cells [23, 37]. SGK1-dependent regulation of Orai1 and STIM1, main components of SOC entry in mast cells [38, 39], could underlie the phenotype of sgk1−/− mast cells, whereas SGK3 could additionally regulate other subunits of SOC channels, such as STIM2.
In conclusion, SGK3 is a new important regulator of STIM2 expression. SGK3 thus upregulates store-operated Ca2+ entry and migration in dendritic cell.
Acknowledgements
The authors gratefully acknowledge the expert support of Dr. Frank Essmann with pHrodo assay.
References
- 1.Banchereau J, Briere F, Caux C, Davoust J, Lebecque S, Liu YJ, Pulendran B, Palucka K: Immunobiology of dendritic cells. Annu Rev Immunol 2000;18:767–811. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SF, Kusner DJ: The regulation of dendritic cell function by calcium-signaling and its inhibition by microbial pathogens. Immunol Res 2007;39:115–127. [DOI] [PubMed] [Google Scholar]
- 3.Shumilina E, Huber SM, Lang F: Ca2+ signaling in the regulation of dendritic cell functions. Am J Physiol Cell Physiol 2011;300:C1205–C1214. [DOI] [PubMed] [Google Scholar]
- 4.Zanoni I, Ostuni R, Capuano G, Collini M, Caccia M, Ronchi AE, Rocchetti M, Mingozzi F, Foti M, Chirico G, Costa B, Zaza A, Ricciardi-Castagnoli P, Granucci F: CD14 regulates the dendritic cell life cycle after LPS exposure through NFAT activation. Nature 2009;460:264–268. [DOI] [PubMed] [Google Scholar]
- 5.Heise N, Shumilina E, Nurbaeva MK, Schmid E, Szteyn K, Yang W, Xuan NT, Wang K, Zemtsova IM, Duszenko M, Lang F: Effect of dexamethasone on Na+/Ca2+ exchanger in dendritic cells. Am J Physiol Cell Physiol 2011;300:C1306–C1313. [DOI] [PubMed] [Google Scholar]
- 6.Shumilina E, Xuan NT, Matzner N, Bhandaru M, Zemtsova IM, Lang F: Regulation of calcium signaling in dendritic cells by 1,25-dihydroxyvitamin D3. FASEB J 2010;24:1989–1996. [DOI] [PubMed] [Google Scholar]
- 7.Matzner N, Zemtsova IM, Nguyen TX, Duszenko M, Shumilina E, Lang F: Ion channels modulating mouse dendritic cell functions. J Immunol 2008;181:6803–6809. [DOI] [PubMed] [Google Scholar]
- 8.Hsu S, O’Connell PJ, Klyachko VA, Badminton MN, Thomson AW, Jackson MB, Clapham DE, Ahern GP: Fundamental Ca2+ signaling mechanisms in mouse dendritic cells: CRAC is the major Ca2+ entry pathway. J Immunol 2001;166:6126–6133. [DOI] [PubMed] [Google Scholar]
- 9.Bandyopadhyay BC, Pingle SC, Ahern GP: Store-operated Ca2+ signaling in dendritic cells occurs independently of STIM1. J Leukoc Biol 2011;89:57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Partida-Sanchez S, Gasser A, Fliegert R, Siebrands CC, Dammermann W, Shi G, Mousseau BJ, Sumoza-Toledo A, Bhagat H, Walseth TF, Guse AH, Lund FE: Chemotaxis of mouse bone marrow neutrophils and dendritic cells is controlled by adp-ribose, the major product generated by the CD38 enzyme reaction. J Immunol 2007;179:7827–7839. [DOI] [PubMed] [Google Scholar]
- 11.Barbet G, Demion M, Moura IC, Serafini N, Leger T, Vrtovsnik F, Monteiro RC, Guinamard R, Kinet JP, Launay P: The calcium-activated nonselective cation channel TRPM4 is essential for the migration but not the maturation of dendritic cells. Nat Immunol 2008;9:1148–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koski GK, Schwartz GN, Weng DE, Czerniecki BJ, Carter C, Gress RE, Cohen PA: Calcium mobilization in human myeloid cells results in acquisition of individual dendritic cell-like characteristics through discrete signaling pathways. J Immunol 1999;163:82–92. [PubMed] [Google Scholar]
- 13.Rubartelli A, Poggi A, Zocchi MR: The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol 1997;27:1893–1900. [DOI] [PubMed] [Google Scholar]
- 14.Xuan NT, Shumilina E, Matzner N, Zemtsova IM, Biedermann T, Goetz F, Lang F: Ca2+-dependent functions in peptidoglycan-stimulated mouse dendritic cells. Cell Physiol Biochem 2009;24:167–176. [DOI] [PubMed] [Google Scholar]
- 15.Fukao T, Tanabe M, Terauchi Y, Ota T, Matsuda S, Asano T, Kadowaki T, Takeuchi T, Koyasu S: PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol 2002;3:875–881. [DOI] [PubMed] [Google Scholar]
- 16.Fukao T, Koyasu S: PI3K and negative regulation of TLR signaling. Trends Immunol 2003;24:358–363. [DOI] [PubMed] [Google Scholar]
- 17.Zaru R, Mollahan P, Watts C: 3-phosphoinositide-dependent kinase 1 deficiency perturbs Toll-like receptor signaling events and actin cytoskeleton dynamics in dendritic cells. J Biol Chem 2008;283:929–939. [DOI] [PubMed] [Google Scholar]
- 18.Ohtani M, Nagai S, Kondo S, Mizuno S, Nakamura K, Tanabe M, Takeuchi T, Matsuda S, Koyasu S: Mammalian target of rapamycin and glycogen synthase kinase 3 differentially regulate lipopolysaccharide-induced interleukin-12 production in dendritic cells. Blood 2008;112:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Prete A, Vermi W, Dander E, Otero K, Barberis L, Luini W, Bernasconi S, Sironi M, Santoro A, Garlanda C, Facchetti F, Wymann MP, Vecchi A, Hirsch E, Mantovani A, Sozzani S: Defective dendritic cell migration and activation of adaptive immunity in PI3Kgamma-deficient mice. EMBO J 2004;23:3505–3515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu D, Yang X, Songyang Z: Identification of CISK, a new member of the SGK kinase family that promotes IL-3-dependent survival. Curr Biol 2000;10:1233–1236. [DOI] [PubMed] [Google Scholar]
- 21.Kobayashi T, Deak M, Morrice N, Cohen P: Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J 1999;344 Pt 1:189–197. [PMC free article] [PubMed] [Google Scholar]
- 22.Lang F, Bohmer C, Palmada M, Seebohm G, Strutz-Seebohm N, Vallon V: (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol Rev 2006;86:1151–1178. [DOI] [PubMed] [Google Scholar]
- 23.Zemtsova IM, Heise N, Frohlich H, Qadri SM, Kucherenko Y, Boini KM, Pearce D, Shumilina E, Lang F: Blunted IgE-mediated activation of mast cells in mice lacking the serum- and glucocorticoid-inducible kinase SGK3. Am J Physiol Cell Physiol 2010;299:C1007–C1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, Pearce D: Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell 2004;15:4278–4288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barry PH, Lynch JW: Liquid junction potentials and small cell effects in patch-clamp analysis. J Membr Biol 1991;121:101–117. [DOI] [PubMed] [Google Scholar]
- 26.Pfaffl MW: A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 2001;29:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Woo JS, Cho CH, Lee KJ, Kim dH, Ma J, Lee EH: Hypertrophy in skeletal myotubes induced by junctophilin-2 mutant, Y141H, involves an increase in store-operated Ca2+ entry via Orai1. J Biol Chem 2012;287:14336–14348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundivakkam PC, Freichel M, Singh V, Yuan JP, Vogel SM, Flockerzi V, Malik AB, Tiruppathi C: The Ca2+ Sensor Stromal Interaction Molecule 1 (STIM1) Is Necessary and Sufficient for the Store-Operated Ca2+ Entry Function of Transient Receptor Potential Canonical (TRPC) 1 and 4 Channels in Endothelial Cells. Mol Pharmacol 2012;81:510–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Varga-Szabo D, Braun A, Kleinschnitz C, Bender M, Pleines I, Pham M, Renne T, Stoll G, Nieswandt B: The calcium sensor STIM1 is an essential mediator of arterial thrombosis and ischemic brain infarction. J Exp Med 2008;205:1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berna-Erro A, Braun A, Kraft R, Kleinschnitz C, Schuhmann MK, Stegner D, Wultsch T, Eilers J, Meuth SG, Stoll G, Nieswandt B: STIM2 regulates capacitive Ca2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci Signal 2009;2:ra67. [DOI] [PubMed] [Google Scholar]
- 31.Wymann MP, Sozzani S, Altruda F, Mantovani A, Hirsch E: Lipids on the move: phosphoinositide 3-kinases in leukocyte function. Immunol Today 2000;21:260–264. [DOI] [PubMed] [Google Scholar]
- 32.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM: 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J 2002;16:1145–1150. [DOI] [PubMed] [Google Scholar]
- 33.Eylenstein A, Gehring EM, Heise N, Shumilina E, Schmidt S, Szteyn K, Munzer P, Nurbaeva MK, Eichenmuller M, Tyan L, Regel I, Foller M, Kuhl D, Soboloff J, Penner R, Lang F: Stimulation of Ca2+-channel Orai1/STIM1 by serum- and glucocorticoid-inducible kinase 1 (SGK1). Faseb J 2011;25:2012–2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borst O, Schmidt EM, Munzer P, Schonberger T, Towhid ST, Elvers M, Leibrock C, Schmid E, Eylenstein A, Kuhl D, May AE, Gawaz M, Lang F: The serum- and glucocorticoid-inducible kinase 1 (SGK1) influences platelet calcium signaling and function by regulation of Orai1 expression in megakaryocytes. Blood 2012;119:251–261. [DOI] [PubMed] [Google Scholar]
- 35.Eylenstein A, Schmidt S, Gu S, Yang W, Schmid E, Schmidt EM, Alesutan I, Szteyn K, Regel I, Shumilina E, Lang F: Transcription factor NF-kappaB regulates expression of pore-forming Ca2+ channel unit, Orai1, and its activator, STIM1, to control Ca2+ entry and affect cellular functions. J Biol Chem 2012;287:2719–2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grahammer F, Artunc F, Sandulache D, Rexhepaj R, Friedrich B, Risler T, McCormick JA, Dawson K, Wang J, Pearce D, Wulff P, Kuhl D, Lang F: Renal function of gene-targeted mice lacking both SGK1 and SGK3. Am J Physiol Regul Integr Comp Physiol 2006;290:R945–R950. [DOI] [PubMed] [Google Scholar]
- 37.Sobiesiak M, Shumilina E, Lam RS, Wolbing F, Matzner N, Kaesler S, Zemtsova IM, Lupescu A, Zahir N, Kuhl D, Schaller M, Biedermann T, Lang F: Impaired mast cell activation in gene-targeted mice lacking the serum- and glucocorticoid-inducible kinase SGK1. J Immunol 2009;183:4395–4402. [DOI] [PubMed] [Google Scholar]
- 38.Vig M, DeHaven WI, Bird GS, Billingsley JM, Wang H, Rao PE, Hutchings AB, Jouvin MH, Putney JW, Kinet JP: Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat Immunol 2008;9:89–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baba Y, Nishida K, Fujii Y, Hirano T, Hikida M, Kurosaki T: Essential function for the calcium sensor STIM1 in mast cell activation and anaphylactic responses. Nat Immunol 2008;9:81–88. [DOI] [PubMed] [Google Scholar]