Abstract
Background:
Naringenin is a bioactive flavonoid found in grapes and citrus fruits including tangelo, blood orange, lemons, and tangerines. The aims of this study were to investigate the ability of naringenin to scavenge free radicals and determine its ability to protect animals from streptozotocin (STZ) -induced liver damage.
Methods:
The free radical-scavenging activity of naringenin was evaluated by in vitro cell-free assay systems. In animals, the antioxidant potential of orally administered 50 and 100 mg/kg body weight naringenin for 45 days was assessed by measuring TBARS, lipid hydroperoxides, SOD, catalase, GST, GPx, and glutathione levels in liver homogenates prepared from animals injected intraperitoneally with multiple low dose streptozotocin at 50 mg/kg for five consecutive days. The extent of cellular damage caused by STZ administration was analyzed using H & E staining.
Results:
Naringenin showed potent free radical scavenging activity in vitro. Naringenin effectively neutralized (a) hydroxyl radicals, (b) superoxide, (c) hydrogen peroxide, (d) nitric oxide radical, (e) DPPH, and (f) lipid peroxidation. In animals, administration of naringenin reduced lipid peroxidation and increased antioxidant levels. Analysis of liver sections showed the restoration of normal morphology upon treatment with naringenin.
Conclusion:
Naringenin helps to mitigate STZ-induced liver complications by promoting antioxidant defence enzyme activities and increasing glutathione levels.
Key Words: Antioxidants, Flavonoids, Free radicals, Lipid peroxidation, Oxidative stress
Introduction
Free radicals are molecular species with unpaired electrons produced in a variety of biochemical reactions. Due to unpaired electrons, free radicals exhibit high reactivity, thereby helping to maintain normal cell metabolism and defense activities (1). However, when exposed to stimuli such as infectious agents, xenobiotics, drugs, and ionizing radiation, free radical concentrations rise to abnormal levels to combat the deleterious effects of the above exposures (2). Therefore, on-demand free radical production is one defense mechanism adopted by cells. However, excess free radicals damage membrane lipids and nuclear DNA, leading to disorders including diabetes and cancer (3).
To combat the toxic effects of reactive oxygen species (ROS), cells have developed multiple defense strategies that include (a) production of enzymes consisting of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), and (b) synthesis of non-enzymatic reducing agents including glutathione and vitamins E and C (4). Failure to produce these antioxidant defense molecules on time by cells may result in disease development. Hence, dietary supplementation with Downloaded from naturally occurring antioxidants helps in preventing ROS-induced diseases, particularly diabetes and cancer (5).
Streptozotocin (STZ; Zanosar), a widely-used naturally-occurring diabetes-inducing compound, is toxic to pancreatic β-cells, which express high levels of GLUT2 (6). Because of its cytotoxicity, STZ has also been used to treat carcinomas of pancreas and neuro-endocrine tumors (7). However, STZ is toxic and cause damage, which in some instances is detrimental (6). Mechanistically, intracellular STZ decomposes into methylcarbonium ions, which cause DNA alkylation and activation of the nuclear DNA-repair enzyme poly (ADP-ribose) polymerase (PARP) (6). This PARP over-activation depletes cellular NAD+ in both pancreatic β-cells and liver cells. This causes ATP depletion, which reduces protein synthesis and delays insulin release, ultimately leading to necrotic cell death. In addition, STZ is known to damage hepatocytes by promoting apoptosis via the induction of reactive oxygen species (8). Hence, care must be executed while administering STZ for treating pancreatic cancers and neuroendocrine tumors. One strategy is to utilize flavonoids such as naringenin to mitigate the complications of STZ.
Flavonoids are plant-derived phenolic compounds that reduce ROS in cells (9). The ability of flavonoids to reduce ROS is primarily due to the presence of 3-hydroxypyran-4-one, 5-hydroxy, and 4-carbonyl groups in the C ring, rather than the 3,4-dihydroxy group in the B ring (10). Flavonoids exhibit potent anti-inflammatory, antiviral, anti-allergic, antimicrobial, and anticarcinogenic activities (9) insulinomimetic flavonoid found in citrus fruits. Structurally, naringenin has two hydroxyl groups at positions 5 and 7 of the A ring and a carbonyl group at position 4 of the C ring, which helps naringenin to interact with iron and copper ions, thereby quenching free radicals and ROS (11). Prior studies have demonstrated that naringenin could safeguard cells after damage initiated by oxidative stress and inflammatory responses (12). Therefore, naringenin is a potent antioxidant with healthpromoting properties. The aims of this study were to investigate the ability of naringenin to scavenge free radicals and determine its ability to protect animals from STZ-induced liver damage.
Materials and Methods
Naringenin, STZ, glibenclamide, butylated hydroxyl toluene, quercetin, tocopherol, vitamin C, and carboxymethyl cellulose were purchased from Sigma-Aldrich (St Louis, MO, USA). All other chemicals used were analytical grade.
A. Determination of radical scavenging activity in cell-free system
To determine whether naringenin could inhibit reactive oxygen and nitrogen species production, the levels of hydroxyl (•OH), superoxide (O2 -), hydrogen peroxide (H2O2) and nitrosyl radicals (NO-) were determined by the methods of Elizabeth and Rao (1990) (13), Sanchez-Moreno (2002) (14), Long et al. (1999) (15), and Garratt (1964) (16), respectively. In addition, the ability of naringenin to scavenge 2,2-diphenyl-1- picrylhydrazyl (DPPH) radicals was determined according to Burits and Bucar (2000) (17). The lipid peroxidation (LPO) activity of naringenin was assessed by the method of by Ohkawa et al. (1979) (18). In brief, Mouse brain homogenates were used as a source of unsaturated lipids in this reaction. First, naringenin was added to the brain homogenates and peroxidation of unsaturated lipids was initiated by adding FeSO4 solution. The percentage inhibition of LPO was quantified by comparing the absorbance of naringenin-treated samples with that of controls containing no antioxidant.
B. STZ-induced liver damage model for assessing the in vivo efficacy of naringenin
Animal experiments were conducted after receiving approval from the JSS Medical College Institutional Animal Ethics Committee (JSSMC/IAEC/18/5675/DEC2013), Mysuru, India. In brief, male albino mice weighing 25-30 g were procured from the Central Animal Facility, JSS Medical College. The mice were maintained under standard laboratory conditions viz. temperature (25 ± 2 °C) and humidity with alternating 12 h light/dark cycles.
Treatment of animals with STZ
Injection of STZ into mice induces oxidative stress and causes liver damage. Streptozotocin was dissolved in 0.1 M citrate buffer, pH 4.5, and injected into mice at a dose of 50 mg/kg body weight/day intraperitoneally for five successive days. Blood (50 µL) was collected from the tail vein and glucose was measured using a glucometer (Accu-Check Active®, Roche). Mice with blood glucose concentrations >250 mg/dL were selected to test the ability of naringenin to mitigate STZinduced liver damage. Twenty-four STZ-treated and 12 normal mice were divided into six groups and treated as shown in Table 1.
Table 1.
Control and experimental mice groups
| Group | No. of mice | Category | Treatment agent | Dose and frequency of the treatment agent (mg/kg) | Route of administr ation | Comment |
|---|---|---|---|---|---|---|
| I | 6 | Control | Vehicle: Carboxy methyl cellulose (CMC) | 0.5%, Every day for 45 days | Oral | Vehicle control |
| II | 6 | Naringenin* (NAR) | 100, every day for 45 days | Oral | Naringenin control | |
| III | 6 | STZ | Streptozotocin (STZ) | 50, 5 consecutive days | Intra peritoneal | STZ alone |
| IV | 6 | STZ, followed by NAR* | STZ 50, 5 consecutive days NAR-50 mg/kg, every day for 45 days | Oral | STZ mice treated with low dose naringenin | |
| V | 6 | STZ, followed by NAR* | STZ - 50, 5 consecutive days NAR- 100 mg/kg, every day for 45 days | Oral | STZ mice treated with high dose naringenin | |
| VI | 6 | STZ, followed by Glibenclamide* (GLC) | STZ - 50, 5 consecutive days GLC- 600 µg/kg, every day for 45 days | Oral | A positive control group |
Naringenin (NAR) and glibenclamide (GLC) were suspended in 0.5% CMC. At the end of the experiment, the animals were deprived of food overnight and their blood collected by cardiac puncture. Serum was isolated and stored at -20 °C. Liver tissue from each animal was minced and homogenized in 100 mM Tris-HCl buffer, pH 7.4, and centrifuged. The supernatant was used to measure of TBARS, lipid hydroperoxides, SOD, CAT, GPx, GST, and GSH.
Measurement of STZ-induced oxidative stress
The LPO levels, in terms of thiobarbituric acid reactive substances (TBARS) formed, and hydroperoxide content, were measured by the methods of Niehaus and Samuelsson (1968) (19), and Jiang et al. (1992) (20), respectively. The TBARS concentration was measured by reading the absorption at 535 nm. To determine the hydroperoxide concentration, 0.2 ml of tissue homogenate was incubated with 1.8 ml of FOX reagent at room temperature for 30 min and the absorbance measured at 560 nm. Antioxidant enzymes in liver tissues and their associated measurement methods were as follows: (a) superoxide dismutase by Kakkar et al. (1984) (21), (b) catalase by Sinha (1972) (22), (c) glutathione peroxidase by Rotruck et al. (1973) (23), glutathione S-transferase by Habig et al. (1974) (24), and antioxidant molecules, including reduced glutathione, by Ellman et al. (1959) (25).
Histological observations of the liver
Liver tissues were fixed in 10% buffered formalin for 24 h and dehydrated using ethanol and xylene for 30 min each. Tissues were then incubated in liquid paraffin at 58 °C two times for 60 min each. Tissue blocks were cut into 5 μm sections and stained using hematoxylin and eosin.
Statistical analysis
Results were expressed as means ± SEMs of six separate experiments (n = 6). Statistical significance was determined using one-way ANOVA, followed by Tukey’s post hoc test, using SPSS statistics 20 (SPSS, Cary, NC, USA); p<0.05 was considered statistically significant.
Results
Naringenin is a potent antioxidant: Hydroxyl radical scavenging activity of naringenin
Naringenin effectively neutralized the generated hydroxyl radicals (Fig. 1a). The IC50 of naringenin was significantly greater than that of tocopherol, which was used as a positive control (Fig. 1b).
Fig. 1.
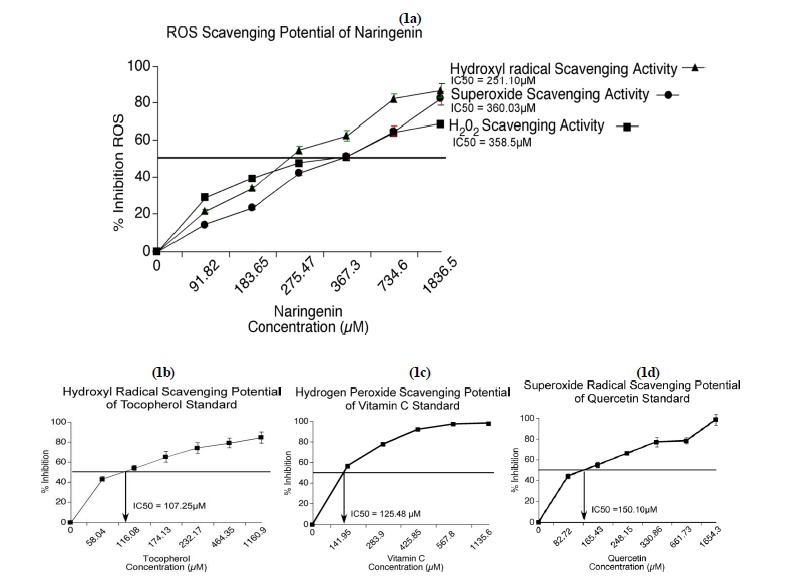
Naringenin destroyed ROS in vitro. Naringenin scavenges ROS with IC50 values of 251.1, 358.5, and 360.03 µM, respectively, for hydroxyl, hydrogen peroxide, and superoxide radicals (1a). The positive controls tocopherol (IC50 = 107.25) for hydroxyl radical scavenging activity (1b), vitamin C (IC50 = 125.48) for hydrogen peroxide radical scavenging (1c) and quercetin (IC50 = 151.10) for superoxide radical scavenging activity (1d) effectively scavenged ROS.
Hydrogen peroxide radical scavenging potential of naringenin
Hydrogen peroxide is a potent oxidant that can damage lipids, proteins, and nucleic acids. The H2O2-destroying ability of naringenin was determined using FOX reagent (15). The efficacy of naringenin was represented as percentage inhibition compared to the negative control containing no antioxidant. Increasing concentration of Vitamin C from 141.95 µM to 1135.6 µM was used as the positive control. The percentage inhibition was significantly greater with vitamin C than with naringenin, and both were greater than the negative control (Fig. 1c).
Superoxide scavenging potential of naringenin
Superoxides, which contain a reactive oxygen anion (O2), are oxygen radicals that cause severe damage to cell membranes and enzymes containing iron and manganese. Therefore, we next asked whether naringenin has the ability to down-regulate superoxide production or destroy the produced superoxides. Quercetin was used as a positive control. Quercetin, which has five hydroxyl groups, was significantly more efficient than naringenin at inhibiting superoxide radicals (Figs. 1a and 1d).
Nitric oxide radical scavenging ability of naringenin
Nitric oxide serves diverse biological functions that include (a) a messenger role in neurons, (b) vasodilatation, and (c) antimicrobial activity. However, prolonged exposure of cells to nitric oxide triggers transformation of normal cells into cancerous ones and promotes inflammatory reactions resulting in arthritis and ulcerative colitis. Flavonoids are known to control reactive nitrogen species (RNS) levels in cells. Hence, the effect of naringenin over RNS levels was tested using the Griess Ilosvays reaction (16). Nitric oxide production decreased as the concentration of naringenin or vitamin C increased (Fig. 2a and Fig. 2b).
Fig. 2.
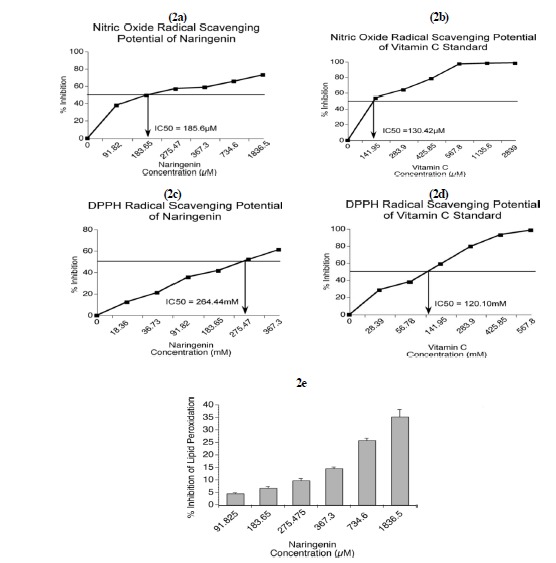
Naringenin prevented the production of reactive nitrogen species, inhibited synthetic DPPH radical, and inhibited the peroxidation of unsaturated lipids present in mice brain homogenates. The ability of naringenin to inhibit RNS production was analyzed by the Griess Ilosvays reaction using sodium nitroprusside. The IC50 of naringenin was 185.6 µM (2a). The nitric acid radical-scavenging potential of vitamin C standard showed an IC50 of 130.42 µM (2b). The ability of naringenin to scavenge synthetic DPPH radical was tested according to Burits and Bucar (2000). The IC50 of naringenin was 264.44 mM (2c). The DPPH radical-scavenging activity of the vitamin C standard showed an IC50 of 120.10 mM (2d). Naringenin inhibited the peroxidation of unsaturated lipids present in mice brain homogenates. The percentage inhibition of lipid peroxidation increased with naringenin concentration (2e).
Potential of naringenin to scavenge synthetic DPPH
Naringenin and vitamin C (used as a standard) have scavenged synthetic free radical DPPH with vitamin C being more potent (Fig. 2c and Fig. 2d).
Naringenin protects unsaturated lipids from undergoing degradation by reactive oxygen species
Lipid peroxidation occurs when unsaturated fatty acids undergo oxidation to yield degraded products. Because lipid oxidation damages cell membranes and bioactive and storage lipids, identification of natural products to prevent their oxidation is important. The percentage inhibition of LPO increased with increased naringenin (Fig. 2e).
Oral administration of naringenin inhibited the formation of TBARS and hydroperoxides in liver tissues of STZ-treated mice
Lipid peroxidation and hydroperoxide formation are key indicators of tissue damage caused by oxidative stress. Streptozotocin induces lipid peroxides and hydroperoxides in liver. The TBARS concentrations were significantly less in livers of control mice and those that received STZ + naringenin than in mice that received STZ alone. The TBARS concentration was less in the mice that received 100 mg/kg of naringenin than in the mice that received 50 mg/kg, but this difference was not significant statistically. The TBARS values for the control mice, the mice that received naringenin alone, and those that received STZ + glibenclamide were all similar and significantly less than those of the mice that received STZ alone (Table 2, TBARS column).
Table 2.
Oral administration of naringenin inhibited the formation of TBARS and hydroperoxides, and increased the activities of SOD, CAT, GPx, GST, and GSH in livers of STZ-treated mice
| Groups | TBARS(mM/100 g tissue) | Hydroperoxides (mM/100 g tissue) | SOD | CAT | GPx | GST | GSH (mg/100g tissue) |
|---|---|---|---|---|---|---|---|
| Control | 0.72±0.07# | 67.33±7.6# | 9.49±0.4# | 61.37±1.7# | 10.63±0.4# | 7.15±0.2# | 24.13±1.0# |
| NAR control (100 mg/kg) | 0.70±0.05 | 65.29±5.8 | 9.52±0.4 | 62.36±1.8 | 10.66±0.5 | 7.21±0.4 | 24.66±1.1 |
| STZ alone | 1.63±0.18 | 98.13±11.9 | 3.92±0.2 | 45.81±5.0 | 5.94±0.2 | 3.16±0.2 | 15.24±0.3 |
| STZ+NAR (50 mg/kg) | 1.20±0.10# | 79.83±10.1# | 6.06±0.1# | 53.72±5.8# | 7.73±0.2# | 5.11±0.4# | 19.43±0.9# |
| STZ+NAR (100 mg/kg) | 0.87±0.15# | 66.97±8.8# | 7.39±0.4# | 58.38±5.3# | 8.9±0.6# | 6.01±0.5# | 22.15±1.7# |
| STZ+GLC (600 μg/kg) | 0.78±0.16# | 66.71±7.7# | 8.03±0.3# | 60.49±5.8# | 9.46±0.3# | 6.75±0.3# | 23.71±1.6# |
Values are given as means ± SEMs for groups of six mice in each. One-way ANOVA followed by Tukey’s post hoc test. Control mice, STZ + naringenin (NAR, 50 and 100 mg/kg) and STZ+GLC (600 µg/kg) -treated mice were compared with STZ-treated mice; P<0.05. Activity is expressed as 50% of inhibition of NBT/min for SOD; µM of hydrogen peroxide consumed/min/mg of protein for catalase; µM of glutathione oxidized/min/mg of protein for GPx; and μM of CDNB conjugate formed/min/mg protein for GST.
Similarly, even the hydroperoxide values were significantly less in livers of control mice and those that received STZ + naringenin than in mice that received STZ alone. (Table 2, hydroperoxide column).
Oral administration of naringenin enhanced the activities of SOD, CAT, GPx, GST, and GSH in liver tissues of STZ-treated mice
Superoxide dismutase catalyzes the dismutation of superoxide radicals to H2O2, thus reducing the possibility of superoxide anion reacting with nitric oxide to form peroxynitrite. Superoxide dismutase activities were significantly greater in livers of control mice and in those that received STZ + naringenin than in mice that received STZ alone. The SOD concentration was greater in the mice that received 100 mg/kg of naringenin than in those that received 50 mg/kg, but this difference was not significant statistically. The SOD activity for the control mice, the mice that received naringenin alone, and those that received STZ + glibenclamide were all similar and significantly greater than those of the mice that received STZ alone (Table 2, SOD column). Similarly, the other antioxidant enzymes catalase, glutathione peroxidase, glutathione-s-transferase and the metabolite glutathione levels were high in control animals as well as STZ-animals treated with naringenin or glibenclamide, compared to the animals injected with STZ alone (Table 2).
Oral administration of naringenin restored the altered morphology of liver tissues observed in mice treated with STZ
Histopathology of livers from control mice and mice treated with naringenin alone showed normal histoarchitecture and central vein structure; however, liver sections from mice treated with STZ showed severe histoarchitectural distortion, congested central veins, fibrosis, and vacuole formation (Fig. 3). These changes were mitigated by the addition naringenin. While treatment with 50 mg/kg naringenin showed mild distortion of the histoarchitecture of the liver with moderate fibrosis, the mice receiving 100 mg/kg of naringenin showed almost normal histoarchitecture, with no fibrosis or vacuoles (Fig. 3). Liver sections from mice treated with glibenclamide had normal histoarchitecture with significant enhancement in hepatic cells and no fibrosis (Fig. 3).
Fig. 3.
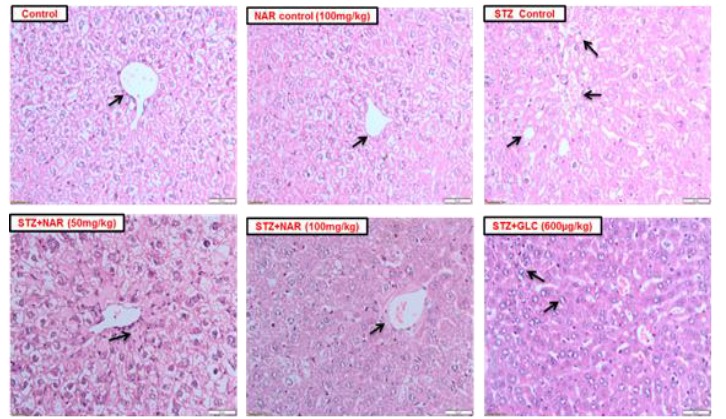
Naringenin restored normal hepatocyte morphology and architecture. H & E staining of liver (Mag 20x, Scale 50 µm) (a) control and naringenin only: normal histoarchitecture with normal central vein (arrow), (b) STZ only: severe distortion in tissue architecture, very small central vein (arrow), fibrosis, and vacuole formation. STZ + naringenin: normal histoarchitecture with a central vein (arrow) and absence of fibrosis and vacuoles. Livers of glibenclamide (600 µg/kg) treated mice: normal histoarchitecture with enhancement of hepatocytes and no fibrosis.
Discussion
Oxidative stress produces oxidized lipids, DNA breaks, and protein and carbohydrate modifications that (a) transform normal cells into cancer cells, (b) cause cells to cells undergo apoptosis or autophagic death, and (c) negatively affect cells’ abilities to execute repair processes (26). These effects cause several diseases including diabetes; hence, controlling oxidative stress is vital for good health. To explore the antioxidant potential of naringenin, we first assessed the capacity of naringenin to scavenge free radicals using in vitro assay systems. We showed that naringenin is a potent antioxidant that can scavenge free radicals. This may be due to (1) the ability of naringenin to efficiently chelate trace metals such as iron and copper, which are potential enhancers of ROS generation, and (2) the ability of naringenin to oxidize superoxide and hydroxyl radicals by donating the hydrogen atom.
Administration of STZ initiates free radical generation and lipid oxidation (27). The LPO level was determined by quantifying TBARS and hydroperoxides. An association between LPO and STZ was well documented in earlier studies. We determined the oxidative state of liver tissue after naringenin administration in mice. Naringenin administration reduced the TBARS and hydroperoxide levels in STZ-treated mice livers, implying reduced oxidative stress. These findings suggest that naringenin exerts its antioxidant potential by scavenging free radicals and protecting liver tissue from LPO.
Antioxidants can scavenge free radicals and protect cells from oxidative stress-induced damage (28). It is well understood that STZ induces alterations in tissue antioxidant levels (29). The significant reduction in liver antioxidants observed in this study could be due to excess generation of free radicals. With naringenin treatment, liver antioxidant activities in STZ-treated mice were similar to those of controls. These results support earlier findings reporting the use of the flavonoids resveratrol and quercitin to reverse STZ-induced tissue damage (30). Naringenin is beneficial not only for its antioxidant properties, but also for its anti-hyperglycemic function, which offers protection against diseases related to elevated glucose levels. In conclusion, naringenin is a potent antioxidant to be considered for further development as a therapeutic for mitigating oxidative stress-mediated diseases.
Acknowledgements
The author, Rajappa Rashmi gratefully acknowledges the Backward Classes Welfare Department, Government of Karnataka, India for Ph.D. fellowship. Additionally, Rajappa Rashmi and Salai Bojan Magesh acknowledge JSS Academy of Higher Education and Research, Mysuru, Karnataka, India for the research fellowship and JSS Mahavidyapeetha, Mysuru for their constant support and encouragement.
References
- 1.Lobo V, Patil A, Phatak A, Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn Rev. 2010;4:118–26. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahman K. Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging. 2007;2:219–36. [PMC free article] [PubMed] [Google Scholar]
- 3.Moskovitz J, Yim MB, Chock PB. Free radicals and disease. Arch Biochem Biophys. 2002;397:354–9. doi: 10.1006/abbi.2001.2692. [DOI] [PubMed] [Google Scholar]
- 4.Sies H. Strategies of antioxidant defense. Eur J Biochem. 1993;215:213–9. doi: 10.1111/j.1432-1033.1993.tb18025.x. [DOI] [PubMed] [Google Scholar]
- 5.Scalbert A, Manach C, Morand C, Remesy C, Jimenez L. Dietary polyphenols and the prevention of diseases. Crit Rev Food Sci Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 6.Eleazu CO, Eleazu KC, Chukwuma S, Essien UN. Review of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humans. J Diabetes Metab Disord. 2013;12:60–6. doi: 10.1186/2251-6581-12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Krug S, Boch M, Daniel H, Nimphius W, Muller D, Michl P, et al. Streptozocin-based chemotherapy in patients with advanced neuroendocrine neoplasms–predictive and prognostic markers for treatment stratification. PLoS One. 2015;10(12):e0143822. doi: 10.1371/journal.pone.0143822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kohl T, Gehrke N, Schad A, Nagel M, Worns MA, Sprinzl MF, et al. Diabetic liver injury from streptozotocin is regulated through the caspase-8 homolog cFLIP involving activation of JNK2 and intrahepatic immunocompetent cells. Cell death & disease. 2013;4(7):e712. doi: 10.1038/cddis.2013.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paganga G, Al-Hashim H, Khodr H, Scott BC, Aruoma OI, Hider RC, Halliwell B, Rice-Evans CA. Mechanisms of antioxidant activities of quercetin and catechin. Redox Rep. 1996;2:359–64. doi: 10.1080/13510002.1996.11747075. [DOI] [PubMed] [Google Scholar]
- 11.Alam MA, Subhan N, Rahman MM, Uddin SJ, Reza HM, Sarker SD. Effect of citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms of action. Adv Nutr. 2014;5:404–17. doi: 10.3945/an.113.005603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayaraman J, Jesudoss VA, Menon VP, Namasivayam N. Anti-inflammatory role of naringenin in rats with ethanol induced liver injury. Toxicol Mech Methods. 2012;22:568–76. doi: 10.3109/15376516.2012.707255. [DOI] [PubMed] [Google Scholar]
- 13.Elizabeth K, Rao MNA. Oxygen radical scavenging activity of curcumin. Int J Pharm. 1990;58:237–40. [Google Scholar]
- 14.Sanchez-Moreno C. Methods used to evaluate the free radical scavenging activity in foods and biological systems. Food Sci Technol Int. 2002;8(121):37. [Google Scholar]
- 15.Long LH, Evans PJ, Halliwell B. Hydrogen peroxide in human urine: implications for antioxidant defense and redox regulation. Biochem Biophys Res Commun. 1999;262:605–09. doi: 10.1006/bbrc.1999.1263. [DOI] [PubMed] [Google Scholar]
- 16.Garrat DC. The Quantitative analysis of drugs. Chapman and Hall Ltd. 1964:533–37. [Google Scholar]
- 17.Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14:323–28. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 18.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–58. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 19.Niehaus WG, Samuelsson B. Formation of malonaldehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 20.Jiang ZY, Hunt JV, Wolff SP. Ferrous ion oxidation in the presence of xylenol orange for detection of lipid hydroperoxide in low density lipoprotein. Anal Biochem. 1992;202:384–89. doi: 10.1016/0003-2697(92)90122-n. [DOI] [PubMed] [Google Scholar]
- 21.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–32. [PubMed] [Google Scholar]
- 22.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 23.Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 24.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–39. [PubMed] [Google Scholar]
- 25.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 26.Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ. J. 2012;5:9–19. doi: 10.1097/WOX.0b013e3182439613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bukan N, Sancak B, Yavuz O, Koca C, Tutkun F, Ozcelikay AT, Altan N. Lipid peroxidation and scavenging enzyme levels in the liver of streptozotocin-induced diabetic rats. Indian J Biochem Biophys. 2003;40:447–50. [PubMed] [Google Scholar]
- 28.Kurutas EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J. 2016;15:71–92. doi: 10.1186/s12937-016-0186-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adeyemi DO, Ukwenya VO, Obuotor EM, Adewole SO. Anti-hepatotoxic activities of Hibiscus sabdariffa L. in animal model of streptozotocin diabetes-induced liver damage. BMC Complement Altern Med. 2014;14:277–87. doi: 10.1186/1472-6882-14-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Alam MM, Meerza D, Naseem I. Protective effect of quercetin on hyperglycemia, oxidative stress and DNA damage in alloxan induced type 2 diabetic mice. Life Sci. 2014;109:8–14. doi: 10.1016/j.lfs.2014.06.005. [DOI] [PubMed] [Google Scholar]


