Summary:
Deficiency of adenosine deaminase 2 is characterized by vasculitis, early-onset strokes, immunodeficiency, and bone marrow failure. We describe a novel pathogenic mutation affecting a consensus N-linked glycosylation sequence and illustrate the essential role of glycosylation in the biology of ADA2.
Keywords: Adenosine deaminase 2, DADA2, vasculitis, hemorrhagic stroke, N-linked glycosylation
To the editors,
Deficiency of adenosine deaminase 2 (DADA2) causes systemic inflammation, immunodeficiency, vasculitis, and early-onset strokes (1, 2). This variable phenotype is caused by recessively inherited loss-of-function mutations of CECR1 (Cat Eye Syndrome Chromosome Region 1), which encodes adenosine deaminase 2 (ADA2). At least 30 pathogenic mutations in the CECR1 gene have described in more than 125 patients (3, 4). While absent plasma ADA2 activity is a hallmark of the disease, how individual mutations affect ADA2 expression and function has been largely unexplored.
We studied a 2 year-old girl with erythematous papules, livedo reticularis, systemic inflammation and intracranial hemorrhage (Fig. 1A, B; see Methods section and Table E1 in Online Repository for clinical summary and laboratory data). The constellation of findings led to high clinical suspicion for DADA2, which was confirmed by near-absent plasma ADA2 activity (Fig. 1C). Maternal plasma ADA2 activity was within the range for DADA2 carriers. Genomic analysis of the patient revealed compound heterozygosity of two novel CECR1 variants: a missense variant (c.385A>C; p.T129P) in exon 3 identified by Sanger sequencing, and a 13.5 kb deletion in the 3’ region detected by multiplex ligation-dependent probe amplification (Fig. 1D). The large deletion was maternally-derived and encompassed exons 8, 9, and 10 (see Fig. E1 in Online Repository). The father was not available for analysis, therefore it remains unknown whether he carries the c.385A>C variant in heterozygous state, or whether this was a de novo mutation in the patient.
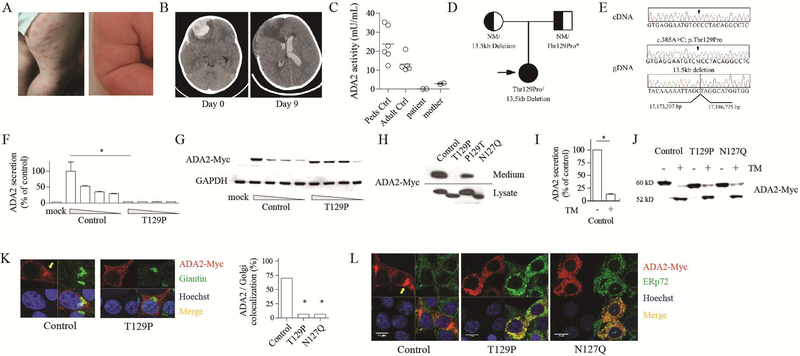
Figure 1.
Deficiency of ADA2 associated with a novel mutation that disrupts N-linked glycosylation. A) Photographs of erythematous papules and livedo reticularis. B) Non-contrast CT images of the brain demonstrating intracranial hemorrhage episodes occurring 9 days apart. C) Quantitation of plasma ADA2 activity using a spectrophotometric assay. D) Pedigree illustration of CECR1 variants in the patient and parents. NM, no mutation. *Paternal genotype is inferred. E) Chromatograms show patient’s complementary DNA (top) and genomic DNA (middle) sequences for the c.385A>C variant. Deletion break-point sequence is shown in the bottom panel. F) ELISA of ADA2 in the medium and G) western blot of intracellular ADA2 expression in 293T cells. Wedge indicates a gradient of plasmid concentration. H) Western blot of wild-type and mutant ADA2. I) ELISA of secreted wild-type ADA2 with or without tunicamycin (TM; 1 μM). J) western blot of intracellular ADA2 expression with or without TM (1 μM). K) Confocal microscopy of ADA2 and giantin staining. Arrow indicates the Golgi apparatus. L) Confocal microscopy of ADA2 and ERp72 staining. Images are representative of 3 independent experiments. *p < 0.05.
Interestingly, sequencing of complementary DNA (cDNA) from the patient revealed exclusive expression of the c.385A>C variant (Fig. 1E), suggesting that the large deletion in the maternally-derived allele impacted RNA expression and/or stability. RNA stability is likely impaired as the deletion encompasses the normal stop codon and 3’untranslated region of the gene. We hypothesized that the T129P substitution must reduce ADA2 expression or function. The variant was not found in the Genome Aggregation Database (gnomAD) (5) and is predicted to be deleterious (SIFT score 0.003; PolyPhen2 score 0.99).
We expressed wild-type and mutant ADA2 in 293T cells using pcDNA3.1-myc/his plasmid (see Methods and Table E2 in Online Repository for experimental details and primer sequences). Wild-type ADA2 was abundantly secreted into the medium and also present intracellularly (Fig. 1F, G). Strikingly, ADA2 with T129P substitution displayed a lower molecular weight (MW) by 2 kD with increased intracellular retention and absent secretion. These characteristics were fully explained by the T129P substitution, as correction of this mutation by site-directed mutagenesis (P129T) reversed these findings and restored ADA2 secretion (Fig. 1H).
The MW reduction due to a missense mutation raised the possibility of disrupted post-translational modifications. Indeed, T129 and the preceding N127 together constitute a consensus N-linked glycosylation sequence (NXS/T) required for the attachment of oligosaccharides (6). N-glycosylation has well-recognized roles in protein folding, trafficking, and function (7). Confirming the presence of N-glycosylation at N127, substituting this residue with glutamine (N127Q) recapitulated the shift in MW and impaired secretion as seen with the T129P mutation (Fig. 1H). Correspondingly, ADA2 activity was significantly impaired by both T129P and N127Q substitutions. Sequence alignment showed that the NXT sequence at this site is highly conserved among eukaryotic species (See Fig. E2A in Online Repository).
Consistent with an important role for glycosylation in ADA2 function, addition of the N-glycosylation inhibitor tunicamycin profoundly inhibited the secretion of wild-type ADA2 (Fig. 1I). The difference in MW between wild-type and mutant ADA2 was abolished by tunicamycin treatment, as unglycosylated protein displayed a lower MW near 52 kD, suggesting the presence of additional N-glycans (Fig. 1J).
To understand how N-linked glycosylation modulates ADA2 trafficking and secretion, we performed confocal microscopy on transfected cells. Wild-type ADA2 displayed a perinuclear Golgi staining pattern in addition to diffuse presence in the cytoplasm (Fig. 1K). In contrast, T129P mutant protein showed intense cytoplasmic foci with minimal Golgi colocalization. T129P and N127Q mutants both showed prominent co-localization with endoplasmic reticulum (ER) (Fig. 1L). These findings suggest that glycosylation at N127 is required for trafficking of ADA2 from the ER to the Golgi apparatus.
In defining the structure of ADA2, Zavialov and colleagues suggested asparagine 127, 185 and 378 as sites for N-glycosylation (8). Our in silico analysis further predicted N174 as a glycosylation target (see Fig. E2B in Online Repository). While the NXS/T consensus sequence is highly conserved for N127, N174 and N378, some species have lost N185 (see Fig. E2C in Online Repository). We searched gnomAD to identify variants that affect the predicted glycosylation sites. Out of 16 total variants, we found 5 missense mutations that disrupt the consensus sequence (see Table E1 in the Online Repository). These mutations are predicted to be deleterious although they have not yet been described in DADA2 patients.
Supporting the presence of glycosylation on asparagine 174, 185 and 378, we found that glutamine substitution of each site resulted in a 2kD reduction in MW similar to the N127Q and T129P mutants (Fig. 2A). Confocal microscopy demonstrated that disruption of N174 and N378 also result in ER retention and defective trafficking of ADA2 to the Golgi apparatus (Fig. 2B). By contrast, substitution of the less evolutionarily-conserved N185 did not alter ADA2 localization.
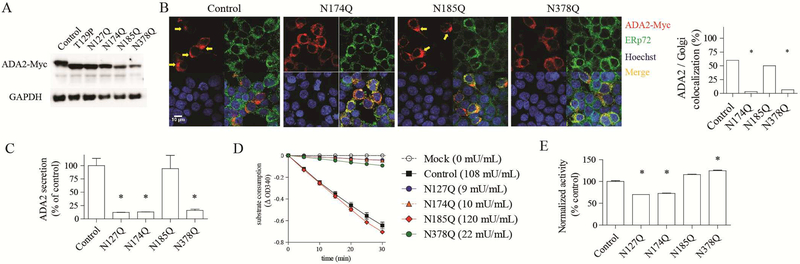
Figure 2.
N-linked glycosylation at three sites is required for ADA2 secretion. A) Western blot of ADA2 mutants with disrupted N-glycosylation sites. B) Confocal microscopy of ADA2 and ERp72 staining. Arrow indicates perinuclear foci consistent with Golgi apparatus. C) ELISA of ADA2 mutants. D) Enzymatic activity of wild-type and mutant ADA2 quantified using a spectrophotometric assay. ADA activity is coupled to consumption of the substrate NADH measured at OD340. E) Enzymatic activity of ADA2 mutants in the medium of transfected cells normalized to the amount of secreted protein. Data are representative of 3 independent experiments. *p < 0.05.
In line with these findings, disruption of N127, N174 or N378 profoundly reduced ADA2 secretion while glycosylation at N185 was dispensable (Fig. 2C). Despite the low levels of secreted protein for three of the glycosylation mutants, ADA2 enzyme activity was detected from all samples (Fig. 2D). When normalized to protein concentration, the enzyme activity of mutant proteins was within 30% of wild-type ADA2 (Fig. 2E), illustrating that N-linked glycosylation is required for ADA2 secretion but less essential for its enzymatic function.
Gain-of-glycosylation mutations leading to attachment of oligosaccharides to unexpected regions of a protein have been implicated in immunodeficiency syndromes (9). We explored gnomAD and identified two predicted gain-of-glycosylation CECR1 variants, I480T and K481N. Both variants are extremely rare in the general population and they create an additional NXS/T sequence at the C-terminus. Interestingly, only I480T was predicted to be glycosylated in silico (see Fig. E3A in Online Repository). This was verified experimentally as expression of I480T mutant resulted in an increased MW by 2 kD while K481N substitution displayed minimal impact (see Fig. E3B in Online Repository; note blurring of upper border of band). Functionally, the secretion of I480T mutant was severely compromised compared to wild-type ADA2, while the K481N mutant had moderate effects on ADA2 secretion (see Fig. E3C in Online Repository). ADA2 enzyme activity in the medium of cells expressing I480T and K481N mutants was directly proportional to the amount of secreted ADA2 protein. These data highlight that precise glycosylation is necessary for trafficking of ADA2 to the secretory pathway.
DADA2 is an intriguing disease with wide-spectrum of clinical findings spanning autoinflammation and immunodeficiency. Our work identifies not only a new set of causal mutations but more generally demonstrates the importance of N-glycosylation for normal ADA2 function. To our knowledge this is the first report of an autoinflammatory disease caused by a defect in glycosylation. Interestingly, patients with congenital defects of glycosylation (CDG) can show features of skin inflammation, stroke-like episodes and immunodeficiency. The etiology of these manifestations is unclear, and whether ADA2 glycosylation and secretion is affected in CDG warrants investigation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Drs. Raif Geha, Janet Chou and Craig Platt (BCH) for helpful discussions and critical reading of the manuscript. We thank Jacob Sundel (BWH) for technical assistance and Susan Kelly (Duke University) for assistance in the development of the spectrophotometric assay for ADA2 quantification. We thank the patient and family members for their participation and contribution to our studies.
Funding: This work was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01AR065538 and P30AR070253 and the Fundación Bechara (PAN), National Institutes of Health (NIH) T32AI007512, the Rheumatology Research Foundation Investigator Award and Boston Children’s Hospital Faculty Career Development Fellowship (PYL), the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, NIH (LDN), the Division of Intramural Research, National Human Genome Research Institute, NIH (IA). All authors declare no conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Navon Elkan P, Pierce SB, Segel R, Walsh T, Barash J, Padeh S, et al. Mutant adenosine deaminase 2 in a polyarteritis nodosa vasculopathy. The New England journal of medicine. 2014;370(10):921–31. Epub 2014/02/21. [DOI] [PubMed] [Google Scholar]
- 2.Zhou Q, Yang D, Ombrello AK, Zavialov AV, Toro C, Stone DL, et al. Early-onset stroke and vasculopathy associated with mutations in ADA2. The New England journal of medicine. 2014;370(10):911–20. Epub 2014/02/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caorsi R, Penco F, Schena F, Gattorno M. Monogenic polyarteritis: the lesson of ADA2 deficiency. Pediatric rheumatology online journal. 2016;14(1):51 Epub 2016/09/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hashem H, Kelly SJ, Ganson NJ, Hershfield MS. Deficiency of Adenosine Deaminase 2 (DADA2), an Inherited Cause of Polyarteritis Nodosa and a Mimic of Other Systemic Rheumatologic Disorders. Current rheumatology reports. 2017;19(11):70 Epub 2017/10/07. [DOI] [PubMed] [Google Scholar]
- 5.Lek M, Karczewski KJ, Minikel EV, Samocha KE, Banks E, Fennell T, et al. Analysis of protein-coding genetic variation in 60,706 humans. Nature. 2016;536(7616):285–91. Epub 2016/08/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kasturi L, Eshleman JR, Wunner WH, Shakin-Eshleman SH. The hydroxy amino acid in an Asn-X-Ser/Thr sequon can influence N-linked core glycosylation efficiency and the level of expression of a cell surface glycoprotein. The Journal of biological chemistry. 1995;270(24):14756–61. Epub 1995/06/16. [DOI] [PubMed] [Google Scholar]
- 7.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–67. Epub 2006/09/09. [DOI] [PubMed] [Google Scholar]
- 8.Zavialov AV, Yu X, Spillmann D, Lauvau G. Structural basis for the growth factor activity of human adenosine deaminase ADA2. The Journal of biological chemistry. 2010;285(16):12367–77. Epub 2010/02/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vogt G, Chapgier A, Yang K, Chuzhanova N, Feinberg J, Fieschi C, et al. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nature genetics. 2005;37(7):692–700. Epub 2005/06/01. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.