Abstract
Aims
Dapagliflozin is a selective inhibitor of sodium glucose co‐transporter 2 (SGLT2). This study assessed the efficacy and safety of dapagliflozin 10 mg vs placebo in patients with type 2 diabetes (T2D) and moderate renal impairment (estimated glomerular filtration rate [eGFR], 45–59 mL/min/1.73 m2; chronic kidney disease [CKD] stage 3A).
Materials and methods
In this double‐blind, parallel group, Phase 3 study (NCT02413398, http://clinicaltrials.gov) patients with inadequately controlled T2D (HbA1c 7.0%‐11.0%) were randomized (1:1) to dapagliflozin 10 mg once daily (N = 160) or matching placebo (N = 161) for 24 weeks. Randomization was stratified by pre‐enrolment glucose‐lowering therapy. The primary endpoint was change from baseline in HbA1c at Week 24.
Results
At Week 24, compared with placebo, dapagliflozin significantly decreased HbA1c (difference [95% CI], −0.34% [−0.53, −0.15]; P < 0.001), body weight (difference [95% CI], −1.25 kg [−1.90, −0.59]; P < 0.001), fasting plasma glucose (difference [95% CI], −0.9 mmol/L [−1.5, −0.4]; P = 0.001) and systolic blood pressure (difference [95% CI], −3.1 mm Hg [−6.3, 0.0]; P < 0.05). Decreases from baseline in eGFR were greater with dapagliflozin than placebo at Week 24 (−2.49 mL/min/1.73 m2 [−4.96, −0.02]), however, eGFR returned to baseline levels at Week 27 (3 weeks post‐treatment) (0.61 mL/min/1.73 m2 [−1.59, 2.81]). No increase in adverse events (AEs; 41.9% vs 47.8%) or serious AEs (5.6% vs 8.7%) were reported with dapagliflozin versus placebo. No AEs of bone fractures, amputations or DKA were reported.
Conclusions
The findings of this study (NCT02413398, http://clinicaltrials.gov) support the positive benefit/risk profile of dapagliflozin for the treatment of patients with T2D and CKD 3A.
1. INTRODUCTION
Diabetes is a risk factor for chronic kidney disease (CKD).1 The global rise in diabetes has led to an increase in the incidence of CKD, with over 35% of patients with diabetes aged ≥20 years having a diagnosis of CKD.2 Optimal glycaemic control in patients with diabetes and CKD is crucial, to reduce the risk of further complications, as well as the progression rate of CKD.3 However, glucose‐lowering treatment options in this population are limited, and several drugs have label restrictions concerning CKD.3, 4, 5 Furthermore, patients with CKD are at greater risk of developing severe hypoglycaemia, and medications that carry a high risk of hypoglycaemia3, 4, 5, 6 may be less appropriate for this population. New treatment options for patients with diabetes and CKD are therefore needed to optimize outcomes.
Dapagliflozin is a selective inhibitor of sodium glucose co‐transporter 2 (SGLT2) that causes glycosuria and lowers blood glucose levels regardless of insulin sensitivity and β‐cell secretory function.7, 8 Dapagliflozin is associated with reductions in blood pressure and body weight,9, 10 and carries a low intrinsic risk of hypoglycaemia.11 SGLT2 inhibitors have also demonstrated cardiovascular (CV) and renal benefits.12, 13, 14, 15, 16
Because dapagliflozin's mode of action is dependent on blood glucose levels and glomerular filtration rate (GFR),17 its glucose‐lowering effects are attenuated in patients with moderate renal impairment18 (estimated GFR [eGFR], 30–59 mL/min/1.73 m2; chronic kidney disease [CKD] stage 3) as the result of a reduced filtered glucose load. Kohan et al. reported no significant improvements in glycaemic control with dapagliflozin vs placebo in this population (mean change from baseline in HbA1c [SE], −0.41% [0.17], −0.44% [0.17] and −0.32% [0.17] with dapagliflozin 5 mg, dapagliflozin 10 mg and placebo, respectively).18 However, beneficial effects on body weight (mean change from baseline at Week 24 [SD], −1.34 [0.43] kg and −1.72 [0.44] kg vs 0.68 [0.45] kg for dapagliflozin 5 and 10 mg vs placebo, respectively) and systolic blood pressure (SBP) (mean change from baseline at Week 104 [SD], −0.25 [18.30] mm Hg, −2.51 [16.33] vs 4.14 [14.07], respectively) were observed with dapagliflozin.18 Of note, there was a reduction in HbA1c with dapagliflozin relative to placebo in patients with CKD stage 3A (eGFR, 45–59 mL/min/1.73 m2), but not in those with CKD stage 3B (eGFR, 30–44 mL/min/1.73 m2).
Further understanding regarding the use of dapagliflozin in the management of patients with T2D and CKD is thus required. Here we assess the efficacy and safety of dapagliflozin 10 mg in patients with T2D and CKD stage 3A (eGFR, 45–59 mL/min/1.73 m2).
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized, double‐blind, 2‐arm, parallel group, placebo‐controlled, multinational, Phase 3 study to evaluate the efficacy and safety of dapagliflozin in patients with T2D and CKD stage 3A (eGFR, 45–59 mL/min/1.73 m2; based on the modification of diet in renal disease [MDRD] formula).19 The study was conducted at 88 centres in the USA, Canada, Bulgaria, the Czech Republic, Italy, Poland, Spain and Sweden.
Patients were randomized (1:1) to 24 weeks of treatment with dapagliflozin 10 mg or matching placebo. The study consisted of a 2‐week screening period, a 4‐week single‐blind placebo lead‐in period, a 24‐week double‐blind treatment period and a 3‐week post‐treatment follow‐up period. It was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines as defined by the International Conference on Harmonization. The study was approved by the institutional review boards and independent ethics committees of all participating centres. All participants provided written informed consent prior to inclusion in the study. This study is registered at http://clinicaltrials.gov (NCT02413398) and the study protocol is available online: http://astrazenecagrouptrials.pharmacm.com.
2.2. Participants
The study included male and female patients (≥18 to <75 years) who have had T2D for >12 months, inadequate glycaemic control (HbA1c ≥7.0% and ≤11.0% at screening) and a body mass index (BMI) of 18–45 kg/m2 at Visit 1, who are undergoing a stable glucose‐lowering treatment regimen (stable diet and exercise alone or in combination with any approved oral glucose‐lowering medication, except SGLT2‐inhibitors, and/or long/intermediate‐acting insulin or mixed insulin), and who had CKD 3A (eGFR, 40–65 mL/min/1.73 m2 at Visit 1 to enter the lead‐in period and eGFR, 45–59 mL/min/1.73 m2 at Visits 1, 2 or 3 to be randomized).
Patients were excluded if they had a history of severe uncontrolled hypertension, certain CV/vascular diseases within 3 months prior to enrolment (myocardial infarction, cardiac surgery or revascularization, unstable angina, unstable heart failure, heart failure Class IV according to the New York Heart Association [NYHA], transient ischaemic attack or significant cerebrovascular disease, unstable or previously undiagnosed arrhythmia), or certain renal diseases (rapid worsening of renal function from Visit 1 to Visit 3, intercurrent kidney disease other than diabetic nephropathy, renal transplant, dialysis or ultrafiltration). The use of metformin was restricted to doses for moderate renal impairment (eGFR, 30–59 mL/min/1.73 m2) according to local guidelines or the investigator's judgement. Patients were excluded if they had received treatment with an SGLT2 inhibitor, a glucagon‐like peptide 1 (GLP‐1) receptor agonist or a rapid/short‐acting insulin at screening. In addition, patients who had a serum potassium level of >5.5 mmol/L, a serum calcium level of <1.99 mmol/L or > ULN, or a haemoglobin level of ≤90 g/L were also excluded. The full list of inclusion/exclusion criteria can be found in Table S1.
2.3. Interventions
Patients with T2D were randomized to dapagliflozin 10 mg once daily or matching placebo, taken orally in the morning, in addition to their usual care. Randomization was stratified by pre‐enrolment glucose‐lowering therapy (long/intermediate‐acting and mixed insulin regimen, metformin, sulphonylurea, thiazolidinedione or other regimen). Oral glucose‐lowering drugs (apart from SGLT2 inhibitors), insulin (apart from rapid/short‐acting insulins), antihypertensive drugs, lipid‐lowering drugs and anti‐platelet drugs were permitted as long as the dose remained constant throughout the 24‐week treatment period. Patients who developed a loss of glycaemic control during the 24‐week treatment period, defined as fasting plasma glucose (FPG) of >13.3 mmol/L during Weeks 4–12 or FPG of >11.1 mmol/L during Weeks 12–24, were eligible for open‐label rescue medication in addition to the blinded treatment. Rescue medication could comprise any appropriate glucose‐lowering agent, with the exception of SGLT2 inhibitors.
Study visits took place during enrolment (Week −6), lead‐in (Weeks −4, −1), randomization (Week 0), double‐blind treatment (Weeks 1, 4, 12, 24) and the follow‐up period (Week 27). HbA1c was recorded at Weeks −6, 0, 4, 12, 24, 27. Body weight and seated SBP were recorded at Weeks −6, −4, −1, 0, 1, 4, 12, 24, 27. FPG was recorded at Weeks 0, 4, 12, 24, 27.
2.4. Endpoints
The primary efficacy outcome was mean change from baseline in HbA1c at Week 24. Secondary efficacy outcomes comprised mean changes from baseline in body weight, FPG and seated SBP at Week 24.
Exploratory endpoints included the proportion of patients achieving HbA1c <7% at 24 weeks, change from baseline in urine albumin:creatinine ratio (UACR) at Week 24 (all patients and according to albuminuria status), change from baseline in fasting serum uric acid at Week 24 and the number of patients receiving rescue medication after failing to maintain adequate glycaemic control over 24 weeks.
Safety objectives included adverse events (AEs), serious AEs and AEs of interest, based on a predefined list of preferred terms from the Medical Dictionary for Regulatory Activities (MedDRA) (Table S2), including genital and urinary tract infections, volume depletion, renal impairment/failure, bone fractures and diabetic ketoacidosis (DKA); mean change from baseline in heart rate at 24 weeks; mean change in eGFR from baseline to Week 24 and at the 3‐weeks post‐treatment follow‐up period; the proportion of patients discontinuing study medication because of worsening renal insufficiency, defined as confirmed eGFR of 30 mL/min/1.73 m2, over 24 weeks; mean change from baseline in haematocrit at Week 24; and mean change from baseline in bicarbonate at Week 24.
The proportion of patients who experienced hypoglycaemia events and the frequency of such events were also evaluated. Major hypoglycaemia was defined as a symptomatic episode requiring external assistance because of severe impairment in consciousness or behaviour, with a capillary or plasma glucose value <3.0 mmol/L and prompt recovery after glucose or glucagon administration. Minor hypoglycaemia was defined as either a symptomatic episode with a capillary or plasma glucose value <3.5 mmol/L or a capillary or plasma glucose value <3.5 mmol/L, without symptoms, that does not qualify as a major episode. Other episodes of hypoglycaemia were defined as an episode reported by an investigator that did not meet the criteria for a major or minor episode.
2.5. Randomization and masking
Eligible patients were assigned a unique randomization code using an interactive voice response system (IVRS) or interactive web response system (IWRS). For each randomized patient the IVRS/IWRS provided the investigator with a unique Kit ID number matching the treatment arm to which the patient was assigned.
2.6. Statistical analysis
2.6.1. Sample size
Assuming a common standard deviation (SD) of 0.9% in the primary endpoint, 143 patients per treatment group for whom both baseline and at least 1 post‐baseline HbA1c measurements were available would provide 80% power to detect a predicted treatment difference of 0.3% in the primary endpoint at a 2‐sided significance level of 0.05, using a 2‐sample t‐test. Assuming that 5% of randomized patients failed to qualify for inclusion in the full analysis set because of missing baseline and/or all post‐randomization values for this primary endpoint, a total of 302 randomized patients, 151 per treatment group, were needed for the study.
2.6.2. Efficacy analyses
Efficacy analyses were performed on the full analysis set, comprising all randomized patients who received at least 1 dose of double‐blind study medication and for whom a baseline value and at least 1 post‐baseline efficacy value were available. The primary efficacy analysis, change from baseline in HbA1c at Week 24, was based on a mixed effects model with repeated measures (MMRM) using “direct likelihood” which assumed that missing data were missing at random. The primary analysis included measurements before rescue medication or discontinuation of the double‐blind study medication. The model included the fixed categorical effects of treatment, week, randomization stratification factor (glucose‐lowering treatment strata) and treatment‐by‐week interaction, as well as the continuous fixed covariate effects of baseline measurement and baseline measurement‐by‐week interaction. Point estimates and 95% confidence intervals for mean change within each treatment group, as well as the difference in mean change estimates between the dapagliflozin and placebo groups, were calculated. P values for the differences in estimates at Week 24 between the dapagliflozin and placebo groups were also calculated and reported at the nominal level. A sequential testing procedure was employed to accommodate multiple comparisons, whereby tests for secondary efficacy endpoints (changes from baseline in body weight, FPG and seated SBP) were performed only if the primary endpoint comparison, and all previous ordered secondary comparisons, were significant. Secondary and exploratory endpoints were analysed using an MMRM, similar to the model used in the primary analysis. Analysis of covariance (ANCOVA) was used in exploratory analyses for endpoints measured at baseline and at the end of treatment using the last observation carried forward (LOCF). ANCOVA models contained fixed categorical effects for randomization stratification factor and treatment, and a fixed covariate effect for baseline measurement.
2.6.3. Sensitivity analyses
Sensitivity analyses were conducted to assess the robustness of the primary analysis (Appendix S1).
2.6.4. Safety analyses
Safety analyses were performed on the safety analysis set, comprising all patients who received at least 1 dose of double‐blind study medication during the double‐blind treatment period. Safety analyses were performed using descriptive statistics. Comparisons between dapagliflozin 10 mg and matching placebo were not made for safety variables and inferential testing was not performed.
For mean changes from baseline in eGFR, data over 24 weeks were analysed with missing data assumptions specific to MMRM. Data over the full 27‐week period were analysed similarly through Week 27.
3. RESULTS
3.1. Patients
The study was conducted between June 15, 2015 and November 7, 2017. A total of 321 patients were randomized: 160 to the dapagliflozin 10 mg group and 161 to the placebo group (Figure S1). Most patients completed the study, regardless of discontinuation of double‐blind treatment (156 patients [97.5%] in the dapagliflozin group and 154 patients [95.7%] in the placebo group) and most also completed the 24‐week double‐blind treatment period (149 patients [93.1%] in the dapagliflozin group and 146 patients [90.7%] in the placebo group). Demographics and baseline characteristics were balanced between treatment groups (Table 1).
Table 1.
Demographic and baseline characteristics (all randomized patients)
| Dapagliflozin 10 mg (N = 160) | Placebo (N = 161) | |
|---|---|---|
| Age, years, mean (median) | 65.3 (66.0) | 66.2 (68.0) |
| Age categories, n (%) | ||
| <65 years | 64 (40.0) | 46 (28.6) |
| ≥65 years | 96 (60.0) | 115 (71.4) |
| Sex, n (%) | ||
| Male | 91 (56.9) | 91 (56.5) |
| Female | 69 (43.1) | 70 (43.5) |
| Race, n (%) | ||
| White | 141 (88.1) | 140 (87.0) |
| Black/African American | 11 (6.9) | 12 (7.5) |
| Asian | 5 (3.1) | 8 (5.0) |
| American Indian/Alaska native | 2 (1.3) | 0 |
| Other | 1 (0.6) | 1 (0.6) |
| Ethnic group, n (%) | ||
| Hispanic or Latino | 33 (20.6) | 44 (27.3) |
| Not Hispanic or Latino | 127 (79.4) | 117 (72.7) |
| Geographic region, n (%) | ||
| North America | 64 (40.0) | 76 (47.2) |
| Europe | 96 (60.0) | 85 (52.8) |
| Weight, kg, mean (SD) | 92.4 (16.8) | 88.3 (16.2) |
| BMI, kg/m2, mean (SD) | 32.6 (4.7) | 31.6 (5.0) |
| eGFR, mL/min/1·73 m2,mean (SD) | 53.3 (8.7) | 53.6 (10.6) |
| UACR mg/g, median (range) | 23.5 (2.7–5852.0) | 29.0 (3.8‐8474.0) |
| Duration since T2D diagnosis, mean, years (SD) | 14.3 (8.1) | 14.5 (8.3) |
| HbA1c, %, mean (SD) | 8.33 (1.08) | 8.03 (1.08) |
| HbA1c by category, n (%) | ||
| <8 | 61 (38.1) | 93 (57.8) |
| ≥8 to <9 | 56 (35.0) | 40 (24.8) |
| ≥9 to <10 | 29 (18.1) | 19 (11.8) |
| ≥10 | 14 (8.8) | 9 (5.6) |
| FPG, mmol/L, mean (SD) | 10.1 (3.7) | 9.6 (3.0) |
| SBPa, mm Hg, mean (SD) | 135.7 (14.6) | 135.0 (15.6) |
| Glucose‐lowering treatment, n (%) | ||
| Insulin | 80 (50.0) | 80 (49.7) |
| Metformin | 111 (69.4) | 103 (64.0) |
| Sulphonylurea | 64 (40.0) | 67 (41.6) |
| Antihypertensive treatment, n (%) | ||
| ACE inhibitor/ARB | 137 (85.6) | 132 (82.0) |
| Diuretics | 67 (41.9) | 68 (42.2) |
| Beta blockers | 59 (36.9) | 77 (47.8) |
| Other antihypertensive | 21 (13.1) | 20 (12.4) |
Abbreviations: ACE, angiotensin‐converting enzyme; ARB, angiotensin II receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; SBP, systolic blood pressure; SD, standard deviation; T2D, type 2 diabetes; UACR, urine albumin‐to‐creatinine ratio.
SBP data are based on the full analysis set (n = 158 for dapagliflozin, n = 161 for placebo); all other data are based on all randomized patients.
3.2. Primary endpoint
Dapagliflozin significantly improved HbA1c over 24 weeks, with no significant change with placebo (Figure 1A). Adjusted mean changes from baseline (95% CI) at Week 24 were −0.37% (−0.56, −0.18) with dapagliflozin and −0.03% (−0.22, 0.16) with placebo. The difference between dapagliflozin and placebo (95% CI) was −0.34% (−0.53, −0.15); P < 0.001.
Figure 1.
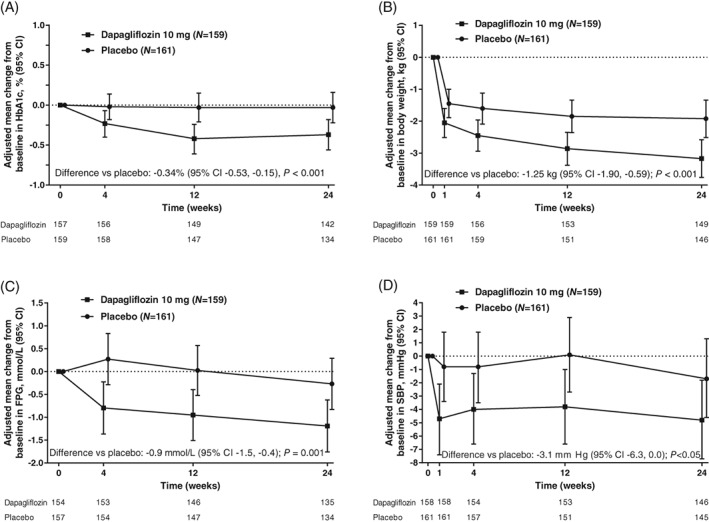
Adjusted mean change from baseline (95% CI) in A, HbA1c; B, body weight; C, FPG; and D, Seated SBP, over 24 weeks (full analysis set). A, Mean baseline HbA1c (SD), 8.35% (1.06) with dapagliflozin and 8.03% (1.09) with placebo; adjusted mean change from baseline in HbA1c at Week 24 (95% CI), −0.37% (−0.56, −0.18) with dapagliflozin and −0.03% (−0.22, 0.16) with placebo. B, Mean baseline body weight (SD), 92.51 (16.73) kg with dapagliflozin and 88.30 (16.23) kg with placebo; adjusted mean change from baseline at Week 24 (95% CI), −3.17 kg (−3.76, −2.58) with dapagliflozin and −1.92 kg (−2.51, −1.34) with placebo. C, Mean baseline FPG (SD), 10.2 (3.7) mmol/L with dapagliflozin and 9.6 (3.0) mmol/L with placebo; adjusted mean change from baseline at Week 24 (95% CI), −1.2 mmol/L (−1.8, −0.6) and −0.3 mmol/L (−0.8, 0.3) with placebo. (D) Mean baseline seated SBP (SD), 135.7 (14.6) mm Hg with dapagliflozin and 135.0 (15.6) mm Hg with placebo; adjusted mean change from baseline at Week 24 (95% CI), −4.8 mm Hg (−7.7, −1.8) and −1.7 mm Hg (−4.6, 1.3) with placebo. CI, confidence interval; FPG, fasting plasma glucose; SBP, systolic blood pressure; SD, standard deviation
3.3. Secondary endpoints
Dapagliflozin was associated with significant reductions in body weight over 24 weeks. The adjusted mean change from baseline (95% CI) at Week 24 was −3.17 kg (−3.76, −2.58) with dapagliflozin and −1.92 kg (−2.51, −1.34) with placebo (Figure 1B). The difference between dapagliflozin and placebo (95% CI) was −1.25 kg (−1.90, −0.59); P < 0.001. The adjusted mean percent change from baseline (95% CI) in body weight at Week 24 was −3.42% (−4.05, −2.78) with dapagliflozin and −2.02% (−2.66, −1.38) with placebo. The difference between dapagliflozin and placebo (95% CI) was −1.43% (−2.15, −0.69); P < 0.001.
Dapagliflozin significantly improved FPG compared with placebo over 24 weeks. The adjusted mean change from baseline at Week 24 (95% CI) was −1.2 mmol/L (−1.8, −0.6) with dapagliflozin and −0.3 mmol/L (−0.8, 0.3) with placebo (Figure 1C). The difference between dapagliflozin and placebo (95% CI) was −0.9 mmol/L (−1.5, −0.4); P = 0.001.
Dapagliflozin significantly reduced seated SBP compared with placebo over 24 weeks. The adjusted mean change from baseline at Week 24 (95% CI) was −4.8 mm Hg (−7.7, −1.8) with dapagliflozin and −1.7 mmHg (−4.6, 1.3) with placebo (Figure 1D). The difference between dapagliflozin and placebo (95% CI) was −3.1 mm Hg (−6.3, 0.0); P < 0.05.
3.4. Exploratory endpoints
The adjusted proportion of patients achieving HbA1c <7% (95% CI) at 24 weeks (LOCF) was similar between the dapagliflozin and placebo groups (12.1% [7.4, 19.1] vs 8.3% [4.7, 14.2], respectively; P = 0.209).
Dapagliflozin did not reduce mean percent changes from baseline in UACR at Week 24 in the overall population (difference vs placebo [95% CI], 8.0% [−14.4, 36.3]; P = 0.513) (Figure S2A). However, in patients with baseline UACR ≥30 mg/g, mean percent reductions from baseline in UACR were observed with dapagliflozin relative to placebo at Week 4 (difference [95% CI], −30.7% [−47.3, −8.9]; P = 0.009) and at Week 12 (difference [95% CI], −41.7% [−57.1, −21.0]; P < 0.001). Although the mean percent reductions from baseline in UACR were maintained from Week 12 to Week 24 with dapagliflozin, the difference vs placebo at Week 24 was not significant (−14.0% [−42.3, 28.0]; P = 0.454) (Figure S2B), because of a decrease in UACR from Week 12 to Week 24 in the placebo group. Dapagliflozin was associated with significant reductions in fasting serum uric acid compared with placebo at Week 24 (LOCF; adjusted mean changes from baseline [95% CI], −11.9 μmol/L [−32.1, 8.3] vs 13.0 μmol/L [−6.8, 32.8]; difference [95% CI], −24.9 [−40.7, −9.0]; P = 0.002).
The number of patients receiving rescue medication after failing to maintain adequate glycaemic control during the 24‐week treatment period was similar between the dapagliflozin and placebo groups (8 [5.0%] vs 10 [6.2%], respectively; difference [95% CI], −1.2 [−6.2, 3.9]; P = 0.809).
3.5. Safety outcomes
Dapagliflozin was well‐tolerated and AEs were balanced between treatment arms, with numerically fewer AEs (41.9% vs 47.8%) and serious AEs (5.6% vs 8.7%) reported with dapagliflozin than with placebo, respectively (Table 2). The proportion of AEs leading to discontinuation of study medication was also balanced between the dapagliflozin and placebo groups (1.9% in both). One patient in the dapagliflozin group had an eGFR value of 30.3 mL/min/1.73 m2 that was registered by the investigator as an AE; however, eGFR returned to baseline following discontinuation of study medication. Patients in the dapagliflozin and placebo groups had similar percentages of urinary tract infections (2.5% vs 3.7%) and genital infections (1.9% vs 1.2%) (Table 2).
Table 2.
Safety summary (safety analysis set)
| Dapagliflozin 10 mg(N = 160) | Placebo(N = 161) | |
|---|---|---|
| AEs | ||
| Any AE | 67 (41.9) | 77 (47.8) |
| Any related AE | 17 (10.6) | 10 (6.2) |
| Any AE leading to discontinuation | 3 (1.9) | 3 (1.9) |
| Death | 0 | 0 |
| Serious AEs | ||
| Any serious AE | 9 (5.6) | 14 (8.7) |
| Any related serious AE | 1 (0.6) | 0 |
| Any serious AE leading to discontinuation | 2 (1.3) | 2 (1.2) |
| AEs of interest | ||
| Genital infection | 3 (1.9) | 2 (1.2) |
| Urinary tract infection | 4 (2.5) | 6 (3.7) |
| Hypotension/dehydration/hypovolaemia | 3 (1.9) | 0 |
| Renal impairment/failure | 1 (0.6) | 2 (1.2) |
| Bone fractures | 0 | 0 |
| DKA | 0 | 0 |
Non‐serious AEs were included up to the last day of double‐blind treatment plus 4 days. Serious AEs were included up to the last day of double‐blind treatment plus 30 days. Includes data after rescue.
Abbreviations: AE, adverse event; DKA, diabetic ketoacidosis.
Few AEs of hypotension/dehydration/hypovolaemia (1.9% vs 0.0%) or renal impairment/failure (0.6% vs 1.2%) were reported. No AEs of bone fractures, amputations or DKA were reported and no patients had elevated liver enzymes or experienced liver‐related AEs. There were no meaningful changes in seated heart rate at Week 24 (mean changes from baseline [SD], −0.2 [8.9] bpm with dapagliflozin [baseline value, 73.1 bpm] vs −0.2 [8.8] bpm with placebo [baseline value, 73.7 bpm]).
The proportion of patients with hypoglycaemia was balanced between the dapagliflozin and placebo groups (12.5% vs 13.7%, respectively), with the majority of these patients receiving insulin (8.8% vs 11.8%, respectively) (Table 3). No patients discontinued study medication because of hypoglycaemia and no episodes of major hypoglycaemia were reported.
Table 3.
Summary of hypoglycaemia events (safety analysis set)
| Dapagliflozin 10 mg (N = 160) | Placebo (N = 161) | |||
|---|---|---|---|---|
| Patients, n (%) | Events, n | Patients, n (%) | Events, n | |
| Total | 20 (12.5) | 44 | 22 (13.7) | 62 |
| Major | 0 | 0 | 0 | 0 |
| Minor | 12 (7.5) | 34 | 16 (9.9) | 53 |
| Other | 8 (5.0) | 10 | 6 (3.7) | 9 |
| Insulin‐based total | 14 (8.8) | 27 | 19 (11.8) | 53 |
| Major | 0 | 0 | 0 | 0 |
| Minor | 9 (5.6) | 21 | 13 (8.1) | 44 |
| Other | 5 (3.1) | 6 | 6 (3.7) | 9 |
| Metformin‐based total | 6 (3.8) | 17 | 3 (1.9) | 9 |
| Major | 0 | 0 | 0 | 0 |
| Minor | 3 (1.9) | 13 | 3 (1.9) | 9 |
| Other | 3 (1.9) | 4 | 0 | 0 |
| Sulphonylurea‐based total | 0 | 0 | 0 | 0 |
| Thiazolidinediones‐based total | 0 | 0 | 0 | 0 |
| Other total | 0 | 0 | 0 | 0 |
Hypoglycaemia events were included up to the last day of double‐blind treatment plus 4 days. Includes data after rescue.
Decreases from baseline in eGFR were larger with dapagliflozin compared with placebo after 4 weeks (difference vs placebo [95% CI], −4.90 mL/min/1.73 m2 [−6.73, −3.07]), after 12 weeks (difference vs placebo [95% CI], −4.75 mL/min/1.73 m2 [−6.98, −2.52]) and after 24 weeks (difference vs placebo [95% CI], −2.49 mL/min/1.73 m2 [−4.96, −0.02]) (Figure 2). With the inclusion of 3 weeks of off‐treatment data, eGFR returned to baseline at Week 27 (difference vs placebo [95% CI], 0.61 mL/min/1.73 m2 [−1.59, 2.81]) (Figure 2). One patient in the dapagliflozin group met a pre‐defined safety objective of worsening renal insufficiency, defined as a confirmed eGFR level < 30 mL/min/1.73 m2, but eGFR returned to baseline following study drug interruption. This episode was not reported as an AE.
Figure 2.
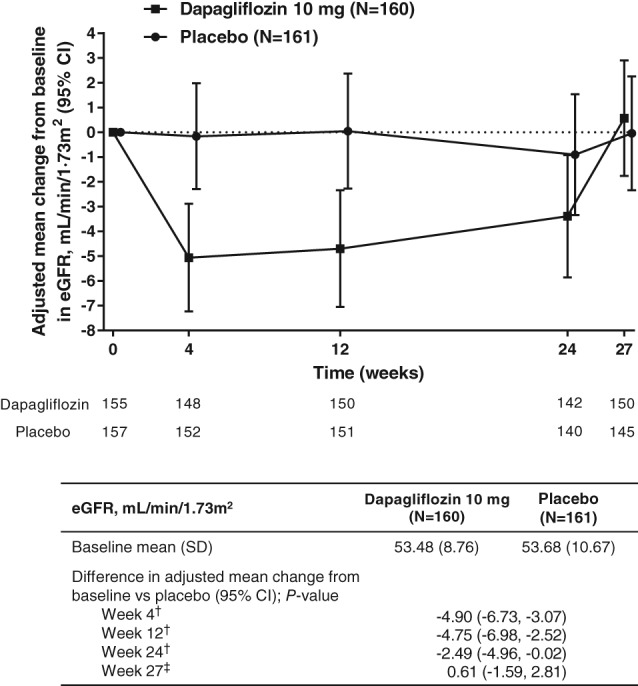
Adjusted mean change from baseline in eGFR (95% CI) during 24‐week treatment period and 3‐week follow‐up period (safety analysis set). †Data analysed with missing data assumptions specific to the repeated measures model, with missing data considered to be missing at random. ‡Data analysed separately using an extension of the analysis model to include Week 27, enabling the pattern in missing data to change with the inclusion of post‐treatment follow‐up. CI, confidence interval; eGFR, estimated glomerular filtration rate; SD, standard deviation
Mean changes from baseline in haematocrit (SD) at Week 24 were 2.5% (2.9) and 0.3% (3.1) with dapagliflozin and placebo, respectively, and mean changes from baseline in bicarbonate (SD) at Week 24 were −0.40 (2.52) mmol/L and 0.43 (2.51) mmol/L, respectively.
4. DISCUSSION
In this study, dapagliflozin significantly improved glycaemic control, body weight and SBP over 24 weeks in patients with T2D and CKD stage 3A, with a safety profile consistent with established parameters for this treatment.
Placebo‐corrected reductions in HbA1c of −0.34% were reported at Week 24, which, while lower than those in patients with normal renal function,20, 21, 22 were almost identical to a post hoc analysis of the Kohan et al. study in patients with T2D and CKD stage 3A18 and with a pooled analysis of 11 randomized controlled trials by Petrykiv et al.23 The extent of HbA1c lowering in the current study was also similar to that observed with other SGLT2 inhibitors in patients with CKD stage 3.24, 25
The benefits concerning body weight and SBP that were independent of renal function in this study are in line with previous reports of benefits with the use of dapagliflozin.18, 23 Petrykiv et al. reported reductions in body weight and SBP with dapagliflozin 10 mg regardless of baseline renal function, which were accompanied by increases in haematocrit and decreases in UACR, eGFR, bicarbonate and uric acid.23 It is noteworthy that the reductions in SBP seen in our study with use of dapagliflozin were not accompanied by changes in heart rate. Previous studies indicate that higher resting heart rate and lower heart rate variability are both risk factors for end‐stage renal disease (ESRD) and CKD‐related hospitalizations.26 Glucose‐lowering treatments that reduce body weight and SBP without affecting heart rate may be particularly valuable in this population.
SGLT2 treatment is associated with a transient drop in eGFR that is reversible after treatment discontinuation.11, 25, 27 Findings from the current trial were consistent with findings concerning patients with normal or near‐normal renal function, for whom dapagliflozin treatment is associated with reversible decreases in eGFR and long‐term eGFR stabilization.11 Interestingly, in the present study, there was a trend to recovery of eGFR towards baseline values at Week 24, similar to what is seen in patients with normal renal function. Dapagliflozin was also associated with a decrease in UACR over 24 weeks in patients with baseline UACR ≥30 mg/g and with a significant difference relative to placebo at Weeks 4 and 12. However, improvements in the placebo arm at Week 24 resulted in a non‐significant difference in UACR between the dapagliflozin and placebo groups at this specific time‐point. This decrease in UACR is consistent with previous observations concerning dapagliflozin.28
AEs were generally balanced between treatment groups in this study. The frequency of hypoglycaemia, urinary tract infections and genital infections was similar between treatment groups and no DKA events, elevated liver enzymes or liver‐related AEs were reported. In addition, no AEs of bone fractures or amputations were reported during the study, consistent with previous studies.29, 30
Limitations to this study include the timeframe, which was too short to assess long‐term effects of dapagliflozin. Furthermore, the vast majority of patients were Caucasian (>87%), restricting generalizability of the results. Another potential limitation is that patients with recent CV events and a rapid decline in eGFR during the pre‐randomization visit were excluded, as were patients receiving certain glucose‐lowering medications (ie, GLP‐1 receptor agonists and rapid/short‐acting insulin).
As expected, glycaemic efficacy with dapagliflozin was lower than that reported in patients with normal renal function, consistent with previous studies on SGLT2 inhibitors18, 24, 25 and in line with their mode of action, which is dependent on GFR and, subsequently, the filtered glucose load.17 However, the clinical value of dapagliflozin was demonstrated within this population, including significant improvements in HbA1c and FPG, in addition to reductions in body weight, SBP, serum uric acid and UACR.
The long‐term renal benefits of dapagliflozin in CKD are currently being explored in an ongoing renal outcomes study (NCT03036150; DAPA‐CKD). In addition, 2 CV outcome trials are currently in progress; these involve patients with T2D and either established CV disease or multiple CV risk factors (NCT01730534; DECLARE‐TIMI 58)31 or chronic heart failure (NCT03036124; DAPA‐HF). The efficacy and safety of dapagliflozin in patients with T2D, albuminuria and moderate renal impairment is also being investigated in an ongoing study (NCT02547935).
In conclusion, significant improvements in HbA1c, body weight and SBP over 24 weeks in patients with T2D and stage 3A CKD were demonstrated with use of dapagliflozin, with no increase in AEs or serious AEs. The findings of this study support a positive benefit/risk profile of dapagliflozin in patients with T2D and CKD stage 3A.
Supporting information
Supplementary Appendix.
Table S1 Full inclusion and exclusion criteria.
Table S2. Predefined list of medical dictionary for regulatory activities (MedDRA) preferred terms.
Figure S1 Patient disposition.
Figure S2 Adjusted mean change from baseline in UACR (95% CI) over 24 weeks in (A) all patients and (B) patients with a baseline UACR of ≥30 mg/g (full analysis set).
ACKNOWLEDGMENTS
The authors would like to thank the patients for their participation in this study, and the study investigators and contributors from each site. Data in this manuscript were presented as an oral presentation at the Endocrine Society 2018 annual conference (ENDO 2018), Chicago, March 17–20, 2018. This study was funded by AstraZeneca. Writing support was provided by Helen Brereton, inScience Communications, Springer Healthcare, and was funded by AstraZeneca.
Conflict of interest
P. F. has served as an advisory board member and speaker for AstraZeneca, Eli Lilly and Boehringer Ingelheim. S. D. P. has served on advisory boards for AstraZeneca, Boehringer Ingelheim, Eli Lilly, GlaxoSmithKline, Intarcia, Janssen, Merck, Novartis, Novo Nordisk A/S, Laboratoires Servier, Sanofi and Takeda; has been a research investigator for Merck, Novartis and Takeda; and has been a speaker for Boehringer Ingelheim, Novartis and Takeda. J. B. B. has received contracted consulting fees, paid to his institution, and travel support from Adocia, AstraZeneca, Dexcom, Elcelyx Therapeutics, Eli Lilly, Intarcia Therapeutics, Lexicon, Metavention, NovaTarg, Novo Nordisk, Sanofi, and vTv Therapeutics; has received grant support from AstraZeneca, Boehringer Ingelheim, Johnson & Johnson, Lexicon, Novo Nordisk, Sanofi, Theracos and vTv Therapeutics; has served on the board of the AstraZeneca HealthCare Foundation; and holds stock options in Mellitus Health and PhaseBio. R. G. has served on advisory boards for Amgen, Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi, Takeda and Valeant; has served as a research investigator for AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Novo Nordisk, Sanofi and Takeda; and has been a speaker for Amgen, Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck, Mylan, Novo Nordisk, Sanofi, Laboratoires Servier and Valeant. F. G. has served as an advisory board member for AstraZeneca; has served as a research investigator for Eli Lilly; has served as a speaker for AstraZeneca and Eli Lilly; has received consulting fees from AstraZeneca, Sanofi, Abbott, Boehringer Ingelheim, Eli Lilly, MedImmune, Merck Sharp & Dohme and Roche Diabetes Care; and has received grants from Lifescan, Eli Lilly and Takeda. D. R. is an employee of AstraZeneca. A. M. L., C. D. S. and P. S. are employees of and shareholders in AstraZeneca.
Fioretto P, Del Prato S, Buse JB, et al. Efficacy and safety of dapagliflozin in patients with type 2 diabetes and moderate renal impairment (chronic kidney disease stage 3A): The DERIVE Study. Diabetes Obes Metab. 2018;20:2532–2540. 10.1111/dom.13413
Funding information AstraZeneca; National Institutes of Health, Grant/Award Number: UL1TR001111; This study was funded by AstraZeneca. J. B. B. is supported by a grant from the National Institutes of Health (UL1TR001111).
REFERENCES
- 1. Kramer H, Molitch ME. Screening for kidney disease in adults with diabetes. Diabetes Care. 2005;28:1813‐1816. [DOI] [PubMed] [Google Scholar]
- 2. Foundation NK . Diabetes and chronic kidney disease. 2016. https://www.kidney.org/news/newsroom/factsheets/Diabetes-And-CKD. Accessed March 13, 2018.
- 3. KDOQI clinical practice guideline for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850‐886. [DOI] [PubMed] [Google Scholar]
- 4. Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycaemia in type 2 diabetes, 2015: a patient‐centred approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of diabetes. Diabetologia. 2015;58:429‐442. [DOI] [PubMed] [Google Scholar]
- 5. Cavanaugh KL. Diabetes management issues for patients with chronic kidney disease. Clin Diabetes. 2007;25:90‐97. [Google Scholar]
- 6. Schernthaner G, Ritz E, Schernthaner GH. Strict glycaemic control in diabetic patients with CKD or ESRD: beneficial or deadly? Nephrol Dial Transplant. 2010;25:2044‐2047. [DOI] [PubMed] [Google Scholar]
- 7. Maranghi M, Carnovale A, Durante C, Tarquini G, Tiseo G, Filetti S. Pharmacokinetics, pharmacodynamics and clinical efficacy of dapagliflozin for the treatment of type 2 diabetes. Expert Opin Drug Metab Toxicol. 2015;11:125‐137. [DOI] [PubMed] [Google Scholar]
- 8. List JF, Woo V, Morales E, Tang W, Fiedorek FT. Sodium‐glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care. 2009;32:650‐657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Weber MA, Mansfield TA, Cain VA, Iqbal N, Parikh S, Ptaszynska A. Blood pressure and glycaemic effects of dapagliflozin versus placebo in patients with type 2 diabetes on combination antihypertensive therapy: a randomised, double‐blind, placebo‐controlled, phase 3 study. Lancet Diabetes Endocrinol. 2016;4:211‐220. [DOI] [PubMed] [Google Scholar]
- 10. Ptaszynska A, Hardy E, Johnsson E, Parikh S, List J. Effects of dapagliflozin on cardiovascular risk factors. Postgrad Med. 2013;125:181‐189. [DOI] [PubMed] [Google Scholar]
- 11. Ptaszynska A, Johnsson KM, Parikh SJ, de Bruin TWA, Apanovitch AM, List JF. Safety profile of dapagliflozin for type 2 diabetes: pooled analysis of clinical studies for overall safety and rare events. Drug Saf. 2014;37:815‐829. [DOI] [PubMed] [Google Scholar]
- 12. Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644‐657. [DOI] [PubMed] [Google Scholar]
- 13. Kosiborod M, Cavender MA, Fu AZ, et al. Lower risk of heart failure and death in patients initiated on sodium‐glucose Cotransporter‐2 inhibitors versus other glucose‐lowering drugs: the CVD‐REAL study (comparative effectiveness of cardiovascular outcomes in new users of sodium‐glucose Cotransporter‐2 inhibitors). Circulation. 2017;136:249‐259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Seidu S, Kunutsor SK, Cos X, Gillani S, Khunti K, For and on behalf of Primary Care Diabetes Europe . SGLT2 inhibitors and renal outcomes in type 2 diabetes with or without renal impairment: a systematic review and meta‐analysis. Prim Care Diabetes. 2018;12:265‐283. [DOI] [PubMed] [Google Scholar]
- 15. Skrtic M, Cherney DZ. Sodium‐glucose cotransporter‐2 inhibition and the potential for renal protection in diabetic nephropathy. Curr Opin Nephrol Hypertens. 2015;24:96‐103. [DOI] [PubMed] [Google Scholar]
- 16. Zinman B, Wanner C, Lachin JM, et al. EMPA‐REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117‐2128. [DOI] [PubMed] [Google Scholar]
- 17. Fioretto P, Zambon A, Rossato M, Busetto L, Vettor R. SGLT2 inhibitors and the diabetic kidney. Diabetes Care. 2016;39(suppl 2):S165‐S171. [DOI] [PubMed] [Google Scholar]
- 18. Kohan DE, Fioretto P, Tang W, List JF. Long‐term study of patients with type 2 diabetes and moderate renal impairment shows that dapagliflozin reduces weight and blood pressure but does not improve glycemic control. Kidney Int. 2014;85:962‐971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Levey AS, Coresh J, Greene T, et al. for Chronic Kidney Disease Epidemiology Collaboration. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 2007;53:766‐772. [DOI] [PubMed] [Google Scholar]
- 20. Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double‐blind, placebo‐controlled trial. Lancet. 2010;375:2223‐2233. [DOI] [PubMed] [Google Scholar]
- 21. Strojek K, Yoon KH, Hruba V, Elze M, Langkilde AM, Parikh S. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with glimepiride: a randomized, 24‐week, double‐blind, placebo‐controlled trial. Diabetes Obes Metab. 2011;13:928‐938. [DOI] [PubMed] [Google Scholar]
- 22. Wilding JP, Norwood P, T'Joen C, Bastien A, List JF, Fiedorek FT. A study of dapagliflozin in patients with type 2 diabetes receiving high doses of insulin plus insulin sensitizers: applicability of a novel insulin‐independent treatment. Diabetes Care. 2009;32:1656‐1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Petrykiv S, Sjostrom CD, Greasley PJ, Xu J, Persson F, HJL H. Differential effects of dapagliflozin on cardiovascular risk factors at varying degrees of renal function. Clin J Am Soc Nephrol. 2017;12:751‐759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barnett AH, Mithal A, Manassie J, et al. EMPAREG RENAL Trial Investigators. Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2014;2:369‐384. [DOI] [PubMed] [Google Scholar]
- 25. Yale JF, Bakris G, Cariou B, et al. Efficacy and safety of canagliflozin in subjects with type 2 diabetes and chronic kidney disease. Diabetes Obes Metab. 2013;15:463‐473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Brotman DJ, Bash LD, Qayyum R, et al. Heart rate variability predicts ESRD and CKD‐related hospitalization. Clin J Am Soc Nephrol. 2010;21:1560‐1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wanner C, Inzucchi SE, Lachin JM, et al. EMPA‐REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375:323‐334. [DOI] [PubMed] [Google Scholar]
- 28. Fioretto P, Stefansson BV, Johnsson E, Cain VA, Sjöström CD. Dapagliflozin reduces albuminuria over 2 years in patients with type 2 diabetes mellitus and renal impairment. Diabetologia. 2016;59:2036‐2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jabbour S, Seufert J, Scheen A, Bailey CJ, Karup C, Langkilde AM. Dapagliflozin in patients with type 2 diabetes mellitus: a pooled analysis of safety data from phase IIb/III clinical trials. Diabetes Obes Metab. 2017;20:620‐628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dziuba J, Alperin P, Racketa J, et al. Modeling effects of SGLT‐2 inhibitor dapagliflozin treatment versus standard diabetes therapy on cardiovascular and microvascular outcomes. Diabetes Obes Metab. 2014;16:628‐635. [DOI] [PubMed] [Google Scholar]
- 31. Raz I, Mosenzon O, Bonaca MP, et al. Declare‐Timi 58: participants' baseline characteristics. Diabetes Obes Metab. 2018;20:1102‐1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Appendix.
Table S1 Full inclusion and exclusion criteria.
Table S2. Predefined list of medical dictionary for regulatory activities (MedDRA) preferred terms.
Figure S1 Patient disposition.
Figure S2 Adjusted mean change from baseline in UACR (95% CI) over 24 weeks in (A) all patients and (B) patients with a baseline UACR of ≥30 mg/g (full analysis set).


