Abstract
In contrast to their analgesic properties, excessive use of either opioids or alcohol produces a paradoxical emergence of heightened pain sensitivity to noxious stimuli, termed hyperalgesia, which may promote increased use of opioids or alcohol drinking to manage worsening pain symptoms. Hyperalgesia has traditionally been measured in rodents via reflex-based assays, including the von Frey method. To better model the motivational and affective dimensions of pain in a state of opioid/alcohol dependence and withdrawal, this unit describes the use of a non-reflex-based method for measuring pain avoidance-like behavior in dependent rats. In the mechanical conflict-avoidance task, rats run across probes of varying heights to avoid a bright aversive light and to reach a dark goal chamber. A longer latency to exit onto the nociceptive probes reflects increased pain avoidance-like behavior during withdrawal. Mechanical conflict-avoidance testing can be repeated to provide both baseline assessment of pain avoidance behavior and pain avoidance measures after the induction of dependence.
Keywords: Opioids, Alcohol, Dependence, Withdrawal, Pain, Avoidance, Hyperalgesia, Rats
Excessive use of either opioids or alcohol produces the emergence of heightened pain sensitivity to noxious stimuli, termed hyperalgesia. This unit describes the use of the mechanical conflict-avoidance task for measuring alterations in pain sensitivity in opioid- and alcohol-dependent rats during withdrawal. In the mechanical conflict-avoidance task, animals are tested in three connected chambers (brightly lit start chamber, nociceptive probe chamber, dark goal chamber), which are separated by two guillotine doors (Figure 1). In this conflict task, rats run across probes of varying heights (0 mm, 0.5 mm, 1 mm, 2 mm, 3 mm, 4 mm, 5 mm) to avoid a bright aversive light and to reach a dark goal chamber. In this conflict task, a longer latency to exit onto the nociceptive probes reflects increased pain avoidance-like behavior.
Figure 1. The mechanical conflict-avoidance system.
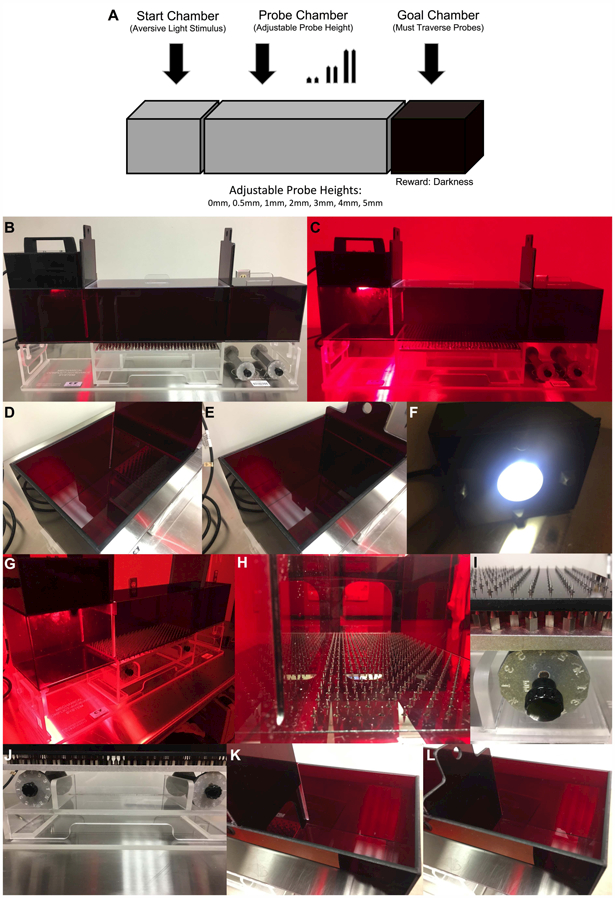
(A) The mechanical conflict-avoidance task is used for measuring alterations in pain avoidance in drug-dependent rats during withdrawal. The mechanical conflict-avoidance apparatus contains three red acrylic chambers: a brightly lit (aversive) start chamber, a probe chamber of adjustable probes heights (0 mm, 0.5 mm, 1 mm, 2 mm, 3 mm, 4 mm, 5 mm), and a dark (rewarding) goal chamber. In this task, rats run across probes of varying heights to avoid a bright aversive light and to reach a dark goal chamber. A longer latency to exit onto the nociceptive probes reflects increased pain avoidance-like behavior. (B) The mechanical conflict-avoidance system. (C) The mechanical conflict-avoidance system in the dark under red light conditions. (D) The start chamber with the guillotine door opened. (E) The start chamber with the guillotine door closed. (F) The lid of the start chamber containing the bright LED light. (G) The mechanical conflict-avoidance system in the dark under red light conditions with the probes extended. (H) View of probe chamber from the start chamber (4 mm probe height). (I) Left adjustable dial for the probe chamber set at 4 mm probe height. (J) Left and right adjustable dials for the probe chamber set at 4 mm probe height. (K) The goal chamber with the guillotine door opened. (L) The goal chamber with the guillotine door closed.
The mechanical conflict-avoidance system was first used for assessing nociceptive behavior in rats with a chronic constriction nerve injury and testing the acute analgesic properties of drugs (e.g. morphine, pregabalin) on pain avoidance behavior (Harte et al., 2016). More recently, the mechanical conflict-avoidance system was used to measure the motivation to avoid noxious stimuli during withdrawal from chronic morphine administration (Pahng et al., 2017). Compared to traditional reflex-based pain measures (e.g. von Frey, see Edwards et al., 2012b, Hargreaves, see Roltsch Hellard et al., 2016) where an animal elicits a withdrawal response when a stimulus reaches a noxious threshold, the mechanical conflict-avoidance system tests the interaction of pain and goal-directed behavior as the animal chooses whether or not to exit onto the nociceptive probes to avoid a bright aversive light. This conflict task provides an excellent method for modeling the motivational and affective dimensions of pain that are beyond the sensory component of pain (for review, see Shurman et al., 2010; LeBlanc et al., 2015).
The protocol described in this unit is used to measure pain avoidance-like behavior in rats in a state of opioid/alcohol dependence and withdrawal using the mechanical conflict-avoidance system (Basic Protocol 1). During the habituation period, rats are allowed to freely explore the three chambers of the mechanical conflict-avoidance system. During the training period, rats are conditioned to run across the probe chamber in the absence of probes (0 mm) and enter dark goal chamber to avoid the bright aversive light in the start chamber. During the baseline stimulus-response testing period, animals must run across probes of varying heights each day to avoid the bright aversive light in the start chamber. Stimulus-response curves are created based on the latency to exit onto the probes (measure of pain avoidance-like behavior) at different probe heights (0mm–5mm).
Based on the baseline stimulus-response curves, animals are split into equivalent groups to maintain non-significant differences at each probe height and similar stimulus-response curves between groups (Support Protocol 1). The two groups are then randomly assigned to either dependence or control conditions. During induction of opioid or alcohol dependence, rats go through daily patterns of intoxication followed by spontaneous withdrawal (natural occurring withdrawal) for an extended period of time (Support Protocol 2). After successful induction of dependence, animals undergo an additional stimulus-response testing period to measure the latency to exit onto the probes (measure of pain avoidance) at varying heights over the course of 7 days. During dependence and withdrawal stimulus-response testing, all testing takes place when dependent animals are in acute or “short-term” withdrawal (e.g. 8 hours or 24 hours) after intoxication from long-term and intermittent alcohol or opioid exposure. The effects of group and probe height on the latency to exit onto the probes are quantified to determine alterations in pain avoidance-like behavior in dependent animals during withdrawal.
NOTE: All animal use protocols must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and be in accordance with governmental guidelines regarding the care and use of laboratory animals.
BASIC PROTOCOL 1:
MEASURING PAIN AVOIDANCE-LIKE BEHAVIOR IN DRUG-DEPENDENT RATS
Materials
Male Wistar rats (200 to 300 g at the beginning of the experiment)
This basic protocol describes testing mechanical pain avoidance-like behavior in male Wistar rats. However, the task can easily be tested in other male rat strains and in female rats. This is described in more detail in the Experimental Conditions section of the Commentary. There are a few options for testing pain avoidance-like behavior in mice, which are also discussed in the Experimental Conditions section.
Mechanical conflict-avoidance system (Figure 1) (Manufacturer: Coy Lab Products)
The apparatus (87.3 cm wide × 21 cm deep × 43.2 cm high, Stoelting, Item #57925) contains three red acrylic chambers: a brightly lit start chamber (21.5 cm wide × 16.5 cm deep × 16.5 cm high) with an intense overhead LED light (wavelength ~490 nm-690 nm, foot-candles ~500–600), a nociceptive probe chamber with adjustable probe heights (39.5 cm wide × 16.5 cm deep × 16.5 cm high), and a dark goal chamber (21.5 cm wide × 16.5 cm deep × 16.5 cm high). The three chambers contain individual acrylic lids and the lid of the start chamber contains the LED light (15 cm above start chamber). The lid of the start chamber with LED light contains a switch to turn the light off and on as well as a cord to plug the LED light into a nearby wall outlet. The three chambers are separated by two acrylic guillotine doors that can be raised and secured by magnets to allow movement through the different chambers. The nociceptive probe chamber contains a perforated floor with stainless steel probes (spread evenly within 1 cm in any direction) that can be adjusted to different heights from the surface of the floor (0 mm, 0.5 mm, 1 mm, 2 mm, 3 mm, 4 mm, 5 mm). The tips of the probes are sharp enough to confer a noxious stimulus without causing injury to the animals.
Diluted quatricide (Fisher Scientific, Item #NC0232105)
Mix 32 ml quatricide into 1968 ml of dH20 and add the mixture to the spray bottle. Contact the manufacturer (Coy Lab Products) to inquire about alternative cleaning reagents.
Spray bottle (Fisher Scientific, Item #02–993-423)
Paper towels (Uline, Item #S-7711)
Bottle brush (Fisher Scientific, Item #S13958)
Stopwatch with split lap timer feature (most smartphones have stopwatches with this feature or Fisher Scientific, Item #14–649-8)
Digital timer (Fisher Scientific, Item #14–649-17)
Animal balance with accuracy of 1 g (Ohaus Corporation Item #CS2000)
Data sheets (Figure 2)
Figure 2.
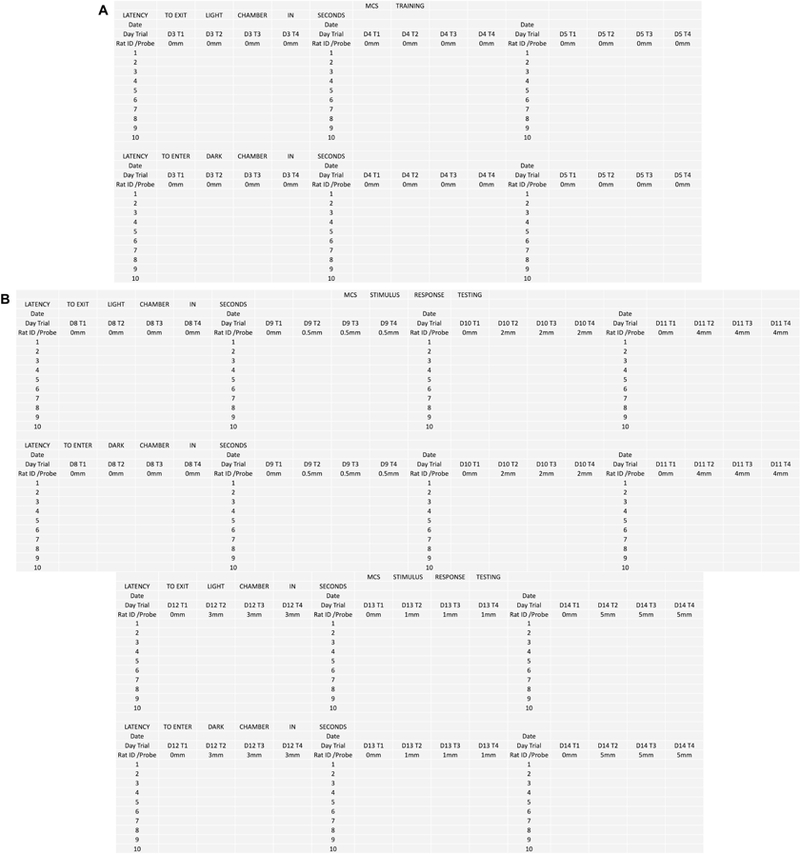
Sample data sheets. (A) Data sheet for mechanical conflict-avoidance training. (B) Data sheet for mechanical conflict-avoidance stimulus-response testing. This data sheet can be used for both baseline testing as well as dependence and withdrawal testing. The order of probe presentation can be changed as long as the following criteria are met: (1) the first probe height presented is 0mm, (2) The second probe height is not higher than 2mm, and (3) the probes are presented in non-sequential order.
Prepare animals, apparatus, and test room
Have experimental rats (~10 per group) delivered to the colony room at least 1 wk prior to the start of the experiment. Group house the rats (2 rats per cage) in the colony room in home cages with a saw wood shaving bedding (e.g. Teklad 7090 Laboratory Grade Sani Chips, Envigo, USA) and allow ad libitum access to food (e.g. Standard Purina Rat Chow, Ralston Purina, St. Louis, MO) and water. Handle them regularly (~5 min per day for 5 days) and give them 1 wk to acclimate to the colony room prior to start of the experiment. Maintain the environmental temperature at ~21ºC on a reverse *12-hr light/dark cycle (lights off at 8:00 am). All experimental procedures are performed during the dark phase of the cycle. *The experimental procedures described in this unit occur during the dark phase of a reverse 12-hr light/dark cycle, however, it is possible to test animals during the light cycle of a 12-hr light/dark cycle (see Harte et al., 2016). There were no evident differences in behavior between control animals tested under dark conditions during the dark phase (see Pahng et al., 2017) compared to control animals tested under light conditions during the light phase (see Harte et al., 2016).
Mark each rat’s tail with a unique experimental number using a dark sharpie throughout all experimental procedures. The tails should be re-marked with a sharpie on a weekly basis. Alternative methods for giving rats a unique experimental number include ear tags (Kent Scientific, Item #INSTMP-KIT), ear punches (Kent Scientific, Item #INS750078–10), or tail tattoos (Animal Identification & Marking Systems).
In the test room, place the mechanical conflict-avoidance system (Figure 1) on a raised bench with a chair provided for the experimenter. All experimental procedures are performed in the dark*, so place red lights overhead to allow the experimenter to see in the dark during all experimental procedures. Testing should be completed around the same time each day to reduce variation in diurnal patterns.
- Prepare data sheets (Figure 2) and outline the experiment in the following stages** (Figure 3):
- Handling (5 min per day for 5 days) during housing room acclimation period (1 wk)
- Habituation (days 1–2)
- Training (days 3–5) (data sheet: Figure 2a)
- Home cage rest (days 6–7)
- Baseline stimulus-response testing (7 days) (data sheet: Figure 2b)
- 0 mm probe test (day 8)
- 0.5 mm probe test (day 9)
- 2 mm probe test (day 10)
- 4 mm probe test (day 11)
- 3 mm probe test (day 12)
- 1 mm probe test t (day 13)
- 5 mm probe test t (day 14)
- Assigning dependence and control conditions (Support Protocol 1 & Figure 4) & Induction of opioid/alcohol dependence (Support Protocol 2)The time frame for this step varies depending on the drug of interest and the specific protcols used for inducing a drug-dependent state.
- Dependence and withdrawal stimulus-response testing (7 days)- repeat the order of baseline testing (data sheet: Figure 2b)
- 0 mm probe test (dependence test 1)
- 0.5 mm probe test (dependence test 2)
- 2 mm probe test (dependence test 3)
- 4 mm probe test (dependence test 4)
- 3 mm probe test (dependence test 5)
- 1 mm probe test (dependence test 6)
- 5 mm probe test (dependence test 7)
- Analyze data (Support Protocol 3)
Figure 3.
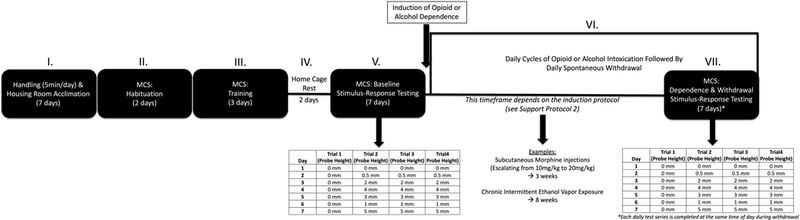
Timeline. Completion of the full protocol occurs in a series of stages: (I) Handling and housing room acclimation (7 days), (II) Habituation (2 days), (III) Training (3 days), (IV) Home cage rest (2 days), (V) Baseline stimulus-response testing (7 days), (VI) Induction of dependence and withdrawal (time frame dependent on protocol used), and (VII) Dependence and withdrawal stimulus-response testing (7 days).
Figure 4.
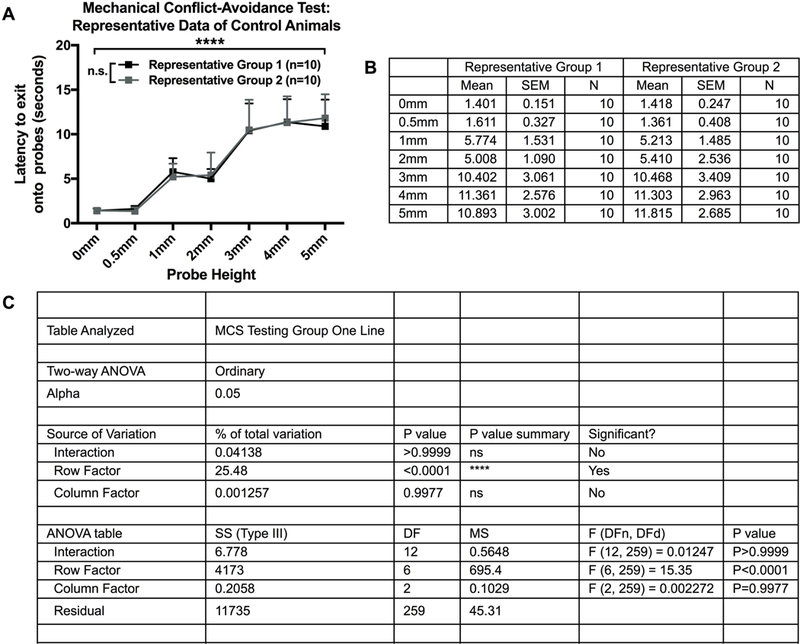
Representative stimulus-response data of control animals (A) Stimulus-response curves of control animals split into two equivalent groups based on ‘latency to exit onto the probes’ data. Represents anticipated baseline stimulus-response curves after proper group splitting, which produces similar stimulus-response curves between groups and non-significant differences at each probe height (N=20, n=10/group). B) Descriptive statistics of mean, standard error of the mean, and sample size of ‘latency to exit onto the probes’ data from control animals split into two equivalent groups (N=20, n=10/group). (C) Statistical analysis of a two-way between-subjects ANOVA for anticipated baseline stimulus-response data after splitting animals split into two equivalent groups (N=20, n=10/group). There should be a significant effect of probe (row factor; p<0.05), indicating the latency to exit onto the probes (pain avoidance) changes at different probe heights. If the groups were split correctly, there should not be a significant effect of group (column factor; p>0.05) or an interaction effect (interaction; p>0.05).
**Use an animal balance to track the weight of each rat throughout the experimental stages by weighing all animals at least once a week or more.
Habituation (2 days)
-
5.
During habituation, move the home cages from the colony room and place them in the testing room (lights off) at least 30 min prior to testing. Chose the time of day to start around the anticipated withdrawal timepoint during dependence and withdrawal stimulus-response testing. Habituation should be completed around the same time each day to reduce variation in diurnal patterns. Allow group housed animals to have free access to water and food in the home cage throughout habituation.
-
6.
Set nociceptive probe chamber of the mechanical conflict-avoidance system to 0 mm.
-
7.
Close the guillotine door next to the start chamber and open the guillotine door next to the dark goal chamber.
-
8.
Place the lids on the nociceptive probe chamber and goal chamber.
-
9.
Pick the rat up by the base of the tail and place the rat on the arm of the experimenter to walk to the mechanical conflict-avoidance system.
-
10.
Gently place the rat in the start chamber of the mechanical conflict-avoidance system by supporting the rat’s upper torso. The rats should be placed in the start chamber facing away from the door.
-
11.
Start the digital timer as soon as the rat is placed in the start chamber and the start chamber is covered with the lid that contains the LED light.
-
12.
Turn the LED light on after 10 s. By the end of habituation and training, well trained animals will generally turn towards the guillotine door once the LED light is turned on in the start chamber.
-
13.
After 20 s of being in the brightly lit start chamber, open the guillotine door.
-
14.
Allow the rat to freely explore the three chambers for 5 min. Keep the LED light turned on during this 5 min period.
-
15.
Remove the rat from the mechanical conflict-avoidance system by removing the lids and picking the rat up by the upper torso. Place the rat back into the appropriate home cage.
-
16.
Spray a paper towel with a small amount of diluted quatricide and wipe down the inside of the chambers to eliminate urine or feces left from the previous animal. Use a bottle brush to wipe away fur and bedding in the chambers.
-
17.
Repeat steps 7–16 for each animal.
-
18.
Return all animals to the colony room. Wash the mechanical conflict-avoidance system with soap and water. Leave it to dry overnight.
-
19.
On day 2 of the experiment, repeat steps 5–18. Rats naturally prefer the dark, so most rats will show a preference for the dark side of the mechanical conflict-avoidance system by the end of habituation.
Training (3 days)
-
20.
During training (days 3–5), move the home cages from the colony room and place them in the testing room (lights off) at least 30 min prior to testing. Training should be completed around the same time each day as habituation. Allow group housed animals to have free access to water and food in the home cage throughout training.
-
21.
Set the nociceptive probe chamber of the mechanical conflict-avoidance system to 0 mm.
-
22.
Close the guillotine door next to the start chamber and open the guillotine door next to the goal chamber.
-
23.
Place the lids on the nociceptive probe chamber and goal chamber.
-
24.
Pick the rat up by the base of the tail and place the rat on the arm of the experimenter to walk to the mechanical conflict-avoidance system.
-
25.
Gently place the rat in the start chamber of the mechanical conflict-avoidance system by supporting the rat’s upper torso. The rats should be placed in the start chamber facing away from the door.
-
26.
Start the digital timer as soon as the rat is placed in the start chamber and the start chamber is covered with the lid that contains the LED light.
-
27.
Turn the LED light on after 10 s.
-
28.
After 20 s of being in the brightly lit start chamber, start the split lap timer and open the guillotine door at the same time.
-
29.
Hit the lap button (lap 1) as soon as the rat places all four paws in the nociceptive probe chamber. This is your ‘latency to exit onto the probes’ measure. If the rat does not place all four paws in the nociceptive probe chamber after 30 s, then return the animal to the home cage and write down 30 s on the data sheet.
-
30.
Hit the lap button again (lap 2) as soon as the rat places all four paws in the dark goal chamber and simultaneously close the guillotine door next to the goal chamber. This is your ‘latency to enter dark goal chamber’ measure. If the rat does not place all four paws in the dark goal chamber measure after 60 s, then return the animal to the home cage and write down 60 s on the data sheet. Be very careful when closing the guillotine door next to the goal chamber. Closing the door too loudly/quickly or when the rat is not completely in the goal chamber may cause the rat to become fearful of the goal chamber.
-
31.
After 20 s of being in the dark goal chamber, remove the rat from the mechanical conflict-avoidance system by removing the goal chamber lid and picking the rat up by the upper torso. Return the rat to the home cage.
-
32.
Using the values on split lap timer, write down the ‘latency to exit onto the probes’ (e.g. lap 1= 2.61 s) and ‘latency to enter dark goal chamber’ (e.g. lap 2= 24.57 s) on the data sheet (Figure 2a).
-
33.
Spray a paper towel with a small amount of diluted quatricide and wipe down the inside of the chambers to eliminate urine or feces left from the previous animal. Use a bottle brush to wipe away excess fur and bedding in the chambers.
-
34.
For each run of training, repeat steps 22–33 for each animal.
-
35.
After each animal has completed run 1, complete run 2 for each animal by repeating steps 22–34. Repeat this procedure two more times. Animals should have a minimum of 10 min between each run of the mechanical conflict-avoidance task. At the end of day 3 of the experiment, each animal should have completed four runs of the training at 0 mm probes.
-
36.
Return all animals to the colony room. Wash the mechanical conflict-avoidance system with soap and water. Leave it to dry overnight.
-
37.
On day 4 of the experiment, repeat steps 20–36.
-
38.
On day 5 of the experiment, repeat steps 20–36. Rats find the bright light naturally aversive, so all animals should exit start chamber and enter the nociceptive probe chamber by the end of training. A few animals may re-enter the brightly lit start chamber or only halfway enter the dark chamber then re-explore the probe chamber. However, this exploratory behavior should decrease over the course of training.
Home cage rest (2 days)
-
39.
Give animals at least 48 hrs of rest before the start of baseline stimulus-response testing.
Baseline stimulus-response testing (7 days)
-
40.
During baseline testing (days 8–14), move the home cages from the colony room and place them in the testing room (lights off) at least 30 min prior to testing. Baseline stimulus-response testing should be completed around the same time each day as habituation and training. Allow group housed animals to have free access to water and food in the home cage throughout baseline stimulus-response testing.
-
41.
Set the nociceptive probe chamber of the mechanical conflict-avoidance system to 0 mm for run 1. The nociceptive probe chamber is always set to 0 mm on run 1 of stimulus-response testing.
-
42.
Close the guillotine door next to the start chamber and open the guillotine door next to the goal chamber.
-
43.
Place the lids on the nociceptive probe chamber and the goal chamber.
-
44.
Remove the rat from the home cage and place the rat in the start chamber of the mechanical conflict-avoidance system. The rats should be placed in the start chamber facing away from the door.
-
45.
Start the digital timer as soon as the rat is in the start chamber and the start chamber is covered.
-
46.
Turn the LED light on after 10 s.
-
47.
After 20 s of being in the brightly lit start chamber, start the split lap timer and open the guillotine door at the same time.
-
48.
Hit the lap button (lap 1) as soon as the rat places all four paws in the nociceptive probe chamber. This is your ‘latency to exit onto the probes measure’. If the rat does not place all four paws in the nociceptive probe chamber after 30 s, then return the animal to the home cage and write down 30 s on the data sheet.
-
49.
Hit the lap button again (lap 2) as soon as the rat places all four paws in the dark goal chamber and simultaneously close the guillotine door next to the goal chamber. This is your ‘latency to enter dark goal chamber’ measure. If the rat does not place all four paws in the dark goal chamber measure after 60 s, then return the animal to the home cage and write down 60 s on the data sheet.
-
50.
After 20 s of being in the dark goal chamber, return the rat to the home cage.
-
51.
Using the values on split lap timer, write down the ‘latency to exit onto the probes’ (e.g. lap 1= 2.61 s) and ‘latency to enter dark goal chamber’ (e.g. lap 2= 24.57 s) on the data sheet (Figure 2b).
-
52.
Spray a paper towel with a small amount of diluted quatricide and wipe down the inside of the chambers to eliminate urine or feces left from the previous animal. Use a bottle brush to wipe away excess fur and bedding in the chambers.
-
53.
For runs 2–4, set the nociceptive probe chamber of the mechanical conflict-avoidance system to the assigned probe heights for each day of testing (day 8: 0 mm, day 9: 0.5 mm, day 10: 2 mm, day 11: 4 mm, day 12: 3 mm, day 13: 1 mm, day 14: 5 mm). This is the order of probe presentation that was used in Pahng et al., 2017. The criteria for this semi-randomized order of probe presentation is (1) the first probe height is 0 mm, (2) the second probe height must not be greater than 2mm (as recommended by the manufacturer), and (3) the presentation of probes must be completed in non-sequential order (e.g. 0 mm, 0.5 mm, 2 mm, 4 mm, 3 mm, 1 mm, 5 mm). The third criterion has been added to reduce the potential confound of amplification of pain avoidance over dependence and withdrawal testing. The order of probe presentation may be changed as long as these criteria are met. The order of probe presentation must remain consistent between baseline stimulus-response testing and dependence and withdrawal stimulus-response testing. Additionally, all animals must receive the same order of probe presentation.
-
54.
Complete each additional run at an assigned probe height by repeating steps 42–53. Animals should have a minimum of 10 min between each run of the mechanical conflict-avoidance system.
-
55.
Return all animals to the colony room. Wash the mechanical conflict-avoidance system with soap and water. Leave it to dry overnight.
-
56.
On day 9 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 0.5 mm).
-
57.
On day 10 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 2 mm).
-
58.
On day 11 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 4 mm).
-
59.
On day 12 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 3 mm).
-
60.
On day 13 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 1 mm).
-
61.
On day 14 of the experiment, repeat steps 40–55 (run 1: 0 mm, runs 2–4: 5 mm).
Induction of drug dependence
-
62.
Calculate the baseline stimulus-response curves (Support Protocol 1)
-
63.
Based on the baseline stimulus-response curves, split animals into equivalent groups to maintain non-significant differences at each probe height and similar stimulus-response curves between groups (Support Protocol 1 & Figure 4).
-
64.
Randomly assign the two groups to either dependence or control conditions (Support Protocol 1).
-
65.
Initiate procedures for the induction of opioid or alcohol dependence (Support Protocol 2).
Dependence and withdrawal stimulus-response testing (7 days)
-
66.
During dependence testing (7 days), all testing will take place when drug dependence animals are in acute withdrawal (e.g. 8 hr or 24 hr). Move the home cages from the colony room and place them in the testing room (lights off) at least 30 minutes prior to testing. Dependence and withdrawal stimulus-response testing should be completed around the same time each day as habituation, training, and baseline stimulus-response testing. Allow group housed animals to have free access to water and food throughout dependence and withdrawal testing.
-
67.Repeat steps 41–61 to complete each dependence test.
- Dependence test 1 (run 1: 0 mm, runs 2–4: 0 mm).
- Dependence test 2 (run 1: 0 mm, runs 2–4: 0.5 mm).
- Dependence test 3 (run 1: 0 mm, runs 2–4: 2 mm).
- Dependence test 4 (run 1: 0 mm, runs 2–4: 4 mm).
- Dependence test 5 (run 1: 0 mm, runs 2–4: 3 mm).
- Dependence test 6 (run 1: 0 mm, runs 2–4: 1 mm).
- Dependence test 7 (run 1: 0 mm, runs 2–4: 5 mm).
-
68.
At the end of each dependence test, put animals through a period of opioid or alcohol intoxication followed by withdrawal (Support Protocol 2).
SUPPORT PROTOCOL 1: SPLITTING ANIMALS INTO TWO GROUPS AND ASSIGNING GROUPS TO EITHER DEPENDENCE OR CONTROL CONDITIONS.
Materials
Microsoft Office Excel (or Mac OS Numbers)
Statistical analysis software (e.g. Prism 7 Graph Pad, RRID:SCR_002798)
Data Sheet (Figure 2b) with baseline stimulus-response data
-
1.For each animal’s baseline stimulus-response data, calculate the average ‘latency to exit onto the probes’ at each probe height:
- 0 mm (average of runs 1–4 on day 8 of the experiment)
- 0.5 mm (average runs 2–4 on day 9 of the experiment)
- 1 mm (average runs 2–4 on day 13 of the experiment)
- 2 mm (average runs 2–4 on day 10 of the experiment)
- 3 mm (average runs 2–4 on day 12 of the experiment)
- 4 mm (average runs 2–4 on day 11 of the experiment)
- 5 mm (average runs 2–4 on day 14 of the experiment)
-
2.
Create a stimulus-response curve based on baseline stimulus-response data. Make the y-axis the latency to exit onto the probes (measure of pain avoidance) and make the x-axis the different probe heights (0 mm – 5 mm). Use Microsoft Office Excel, Mac OS Numbers, or statistical analysis software to create stimulus-response curves.
-
3.
Split animals into equivalent groups based on similar baseline stimulus-response curves (Figure 4a & 4b). The best way to do this is to add a few animals to each group at a time and try to maintain similar averages and standard error for each probe height. It may be necessary to remove some animals from the study in order to get equivalent groups. Since the stimulus-response testing will be repeated after animals become dependent, it is essential that ‘dependent’ and ‘control’ groups have equivalent baseline measures. Group housed animals do not need to be restricted to the same condition (dependence or control conditions) unless the dependence induction protocol requires animals in the same cage to receive the same treatment (e.g. chronic intermittent ethanol vapor exposure vs. room air exposure in the home cage).
-
4.
Use statistical analysis software to run a two-way between-subjects ANOVA with ‘probe height’ and ‘group’ as factors for ‘latency to exit onto the probes’ (Figure 4c). There should be a significant effect of probe (row factor), indicating the latency to exit onto the probes (pain avoidance) changes at different probe heights. If the groups were split properly, there should not be a significant effect of group (column factor). It is expected that each group will have a normal sampling distribution. To test for violations of normality, we recommend using the D’Agostino-Pearson normality test or using the Shapiro-Wilk normality test as an alternative test. In the event of a non-normal sampling distribution, we recommend completing a data transformation (e.g. log transformation, square-root transformation).
SUPPORT PROTOCOL 2: ALCOHOL- AND MORPHINE-DEPENDENCE INDUCTION PROTOCOLS
Materials
Ethanol vapor inhalation chambers (La Jolla Alcohol Research)
Morphine sulfate (Sigma-Aldrich, Item #1448005–500MG)
Sterile Saline (0.9%) Solution (Sigma-Aldrich, Item #S8776–100ML)
BD 1ml syringes (Fisher Scientific, Item #14–823-434)
BD PrecisionGlide 26-gauge needles (Fisher Scientific, Item #14–826-10)
Specific procedures for the induction of dependence varies depending on the drug of interest. The general procedure is to put animals through a period of intoxication (drug on board) followed by a period of spontaneous withdrawal in a single day, and to repeat this cycle until animals are dependent. Dependence is defined by the manifestation of withdrawal symptoms at the termination of drug exposure. Opioid and alcohol dependence is associated with the emergence of negative affective states, that parallels an escalation of opioid and alcohol use over time (Koob and Le Moal, 1997; Edwards and Koob, 2013). Accordingly, in most induction procedures, it is necessary for animals to either freely escalate drug intake over time or to be given an escalating dose regime to achieve a state of dependence. Some common procedures for the induction of alcohol dependence in rats include chronic intermittent ethanol vapor exposure (CIEV) (Unit 9.29) (Gilpin et al., 2008) and 2-bottle choice intermittent access to 20% ethanol (Simms et al., 2008), which produce a robust escalation of drinking (Simms et al., 2008; Edwards et al., 2012a) and hyperalgesia (Edwards et al., 2012b; Fu et al., 2015). Some measures for induction of alcohol intoxication in rats include intragastric intubation (Unit 9.28) (Faingold, 2008), operant ethanol self-administration with 0.1 ml fluid delivery per lever press of 10% (w/v) ethanol (Edwards et al., 2012a), and intraperitoneal administration of 1g/kg of 15% (w/v) ethanol alcohol (McGinn et al., 2016). Common procedures for the induction of opioid dependence include intravenous self-administration of opioids (Unite 9.20) (Thomsen & Caine, 2005) and subcutaneous administration (Park et al., 2015; Pahng et al., 2017). Intravenous self-administration (12-hr access) of fentanyl (2.5μg/kg/infusion; 5mg/kg/infusion), oxycodone (150μg/kg/infusion; 300μg/kg/infusion), and heroin (60μg/kg/infusion), but not buprenorphine produces a robust escalation of opioid intake (Wade et al., 2015). Intravenous self-administration (12-hr access) of heroin (0.06mg/kg/infusion) produces hyperalgesia (Edwards et al., 2012b). Subcutaneous administration of an escalating dose of morphine (10mg/kg to 20mg/kg) can be used to produce hyperalgesia (Craft et al., 1999) and pain avoidance (Pahng et al., 2017). Similarly, subcutaneous administration of heroin (1.25mg/kg) and fentanyl (20–100μg/kg) produces hyperalgesia (Celerier et al., 2000; Park et al., 2015).
For the current protocol, we utilize the rat alcohol dependence-induction protocol from (Unit 9.29) (Gilpin et al., 2008), being sure to maintain peak blood alcohol levels between 150–200 mg/dL. This protocol typically produces alcohol dependence in ~4 weeks. ~8 weeks is recommended for mechanical conflict-avoidance testing, based on evidence of decreased paw withdrawal thresholds (mechanical hypersensitivity) following von Frey testing in alcohol-dependent animals at 8 weeks (Edwards et al 2012b). Behavioral testing occurs as soon as blood alcohol levels approach zero (6–8 hours following termination of alcohol vapors). Repeated exposure to room air serves as the control condition. For morphine dependence-induction, we use an escalating dose regimen protocol from (Pahng et al., 2017). Here, 10 mg/kg (1 ml/kg) of morphine sulfate dissolved in sterile saline is injected subcutaneously for one week daily. Following this first week, on each subsequent day, 20 mg/kg (2 ml/kg) of morphine sulfate dissolved in sterile saline is injected subcutaneously for the remainder of the experiment in the same rats each day to mimic an escalating dose regimen. Behavioral testing occurs approximately 24 hours following the last morphine injection (just prior to that day’s injection). Repeated injections of sterile saline serve as the control condition.
SUPPORT PROTOCOL 3: ANALYZING DATA
Materials
Microsoft Office Excel (or Mac OS Numbers)
Statistical analysis software (e.g. Prism 7 Graph Pad, RRID:SCR_002798)
Data Sheet (Figure 2b) with dependence and withdrawal stimulus-response data
-
1.For each animal’s dependence and withdrawal stimulus-response data, calculate the ‘average latency to exit onto the probes’ and ‘latency to enter dark goal chamber’ at each probe height:
- 0 mm (average of runs 1–4 during dependence test 1)
- 0.5 mm (average runs 2–4 during dependence test 2)
- 1 mm (average runs 2–4 during dependence test 6)
- 2 mm (average runs 2–4 during dependence test 3)
- 3 mm (average runs 2–4 during dependence test 5)
- 4 mm (average runs 2–4 during dependence test 4)
- 5 mm (average runs 2–4 during dependence test 7)
-
2.
Use statistical analysis software (Prism 7 Graph Pad) to run a two-way between-subjects ANOVA with probe height (row factor) and group (column factor) as factors for ‘latency to exit onto the probes’. A significant effect of probe height demonstrates that the latency to exit onto the probe (pain avoidance) changes at different probe heights. This indicates that there is a stimulus-response measure of pain avoidance based on probe height. A significant effect of group, indicates the latency to exit onto the probes (pain avoidance) is altered following induction of dependence. Group differences in performance in the absence of the probes (0 mm, no noxious stimulus) may indicate differences in motivation, anxiety, and/or locomotor activity.
-
3.
Use statistical analysis software (Prism 7 Graph Pad) to run a two-way between-subjects ANOVA with probe height (row factor) and group (column factor) as factors for ‘latency to enter the dark chamber’. This is an additional measure that can be examined to look at differences in locomotor activity.
-
4.
Read the Understanding Results section of the Commentary for a summary of test measures, potential outcomes, and interpretations of the pain avoidance results.
REAGENTS AND SOLUTIONS
Ethanol (95% v/v): Store securely indefinitely at room temperature. Note: Do not expose research animals to 100% (v/v) ethanol
Quatricide (diluted): Mix 32 ml quatricide into 1968 ml of dH20 and add the mixture to the spray bottle. Store up to 12 months at room temperature. Other disinfectants may be used.
Morphine Sulfate: Powder can be stored at room temperature indefinitely. For purposes of the current protocol, fresh solutions for injection should be made daily and not stored. Morphine is readily soluble in physiological saline.
COMMENTARY
Background Information
Limited and controlled use of opioids or alcohol can have analgesic properties. However, excessive and uncontrolled use of opioids or alcohol can lead to paradoxical increases in pain sensitivity, termed hyperalgesia (Angst and Clark, 2006; Shurman et al., 2010; Egli et al., 2012). The development of hyperalgesia is the result of pronociceptive central nervous system sensitization, which manifests as pain hypersensitivity (Woolf, 2011). Furthermore, the development of hyperalgesia often coincides with escalation of drug intake, compulsive drug seeking, and the emergence of negative affective states (e.g. depression, dysphoria), which are key diagnostic criteria for opioid and alcohol use disorders (Koob and Le Moal, 1997; Edwards et al., 2010; Edwards and Koob, 2013). It is thought that the escalation of drug use over time is driven by negative reinforcement mechanisms where individuals will seek drugs in increasing amounts to attenuate the symptoms associated with drug dependence (disease state) and withdrawal (Edwards et al., 2016). The symptoms of drug dependence including escalation of intake, compulsive drug seeking, negative affective states, and hyperalgesia can be reliably modeled in rats. Traditional behavioral measures of hyperalgesia in rats include von Frey (Chaplan et al., 1994) and Hargreaves (Hargreaves et al., 1988), which measure mechanical hypersensitivity and thermal hypersensitivity, respectively. In these tasks, the rat’s hind paw is stimulated with ether a von Frey filament or a thermal light until the animal retracts the hind paw from the nociceptive source. These methods represent reflex-based methods commonly used in measuring differences in sensory pain during drug withdrawal-induced somatic hypersensitivity (e.g., Edwards et al., 2012b; Roltsch Hellard et al., 2016).
Pain is a multidimensional experience beyond sensory nociception (Figure 5). Negative affective states (e.g. dysphoria, depression) can coincide with chronic pain states, which contribute to increased distress from experiencing pain as well as increased perception of pain (Shurman et al., 2010; Egli et al., 2012). A coping strategy often used to deal with heightened pain states is avoidance from everyday activities. Activity avoidance is cognitive strategy where individuals will avoid engaging in activities for fear of experiencing pain, which can contribute to worsening health outcomes (Philips et al., 1987). Accordingly, chronic pain can engender a reorganization of cognitive strategies to avoid pain, while relief from pain can be rewarding (Becerra and Borsook, 2008; King et al., 2009; Navratilova et al., 2015; Porreca and Navratilova, 2017). These relationships between pain and negative affective states may drive the addictive potential of drugs of abuse. Specifically, drugs of abuse may be sought after and taken in excessive amounts to alleviate pain and associated negative affective states (Edwards & Koob, 2010; Massaly et al., 2016). These complex cognitive processes involve organized central control of behavior. Accordingly, there have been recent efforts to quantify the affective and motivational aspects of pain beyond sensory nociception in rodents.
Figure 5.
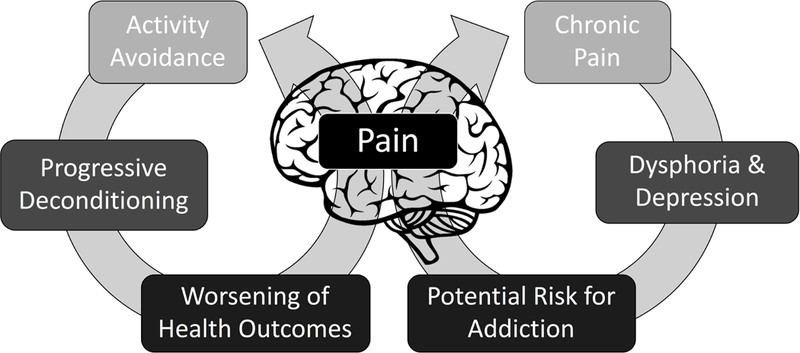
Pain is a multidimensional experience beyond sensory nociception. Negative affective states (e.g. dysphoria, depression) can coincide with chronic pain, which contribute to increased perception of pain and distress from experiencing pain. These relationships between pain and negative affective states may drive the addictive potential of drugs of abuse. Consequently, drugs of abuse may be sought after and taken in excessive amounts to alleviate pain and negative affective states. Chronic pain can also engender a reorganization of cognitive strategies to avoid pain, which drives avoidance from everyday activities and contributes to worsening health outcomes.
The mechanical conflict-avoidance system was first used for assessing differences in nociceptive behavior between male Sprague-Dawley rats with a chronic constriction nerve injury (CCI) and naïve controls (Harte et al., 2016). They found that the latency to exit onto the probes increased in both naïve and CCI rats, as the probe height was increased. However, the latency to exit onto the probes was increased across all probe heights in CCI rats compared to naïve rats, indicating increased pain avoidance-like behavior in CCI rats. In this same study, Harte and colleagues found that acute administration analgesic drugs (e.g. morphine, pregabalin) prior to the 3mm probe height test reduced pain avoidance in CCI rats.
More recently, the mechanical conflict-avoidance system was used to measure the motivation to avoid noxious stimuli during withdrawal from chronic morphine administration (Pahng et al., 2017). Adult male Wistar rats were administered an escalating dose regimen (10mg/kg to 20mg/kg) of morphine (opioid-dependent group) or repeated saline (control group) over three-week period and were tested for mechanical hypersensitivity (von Frey) and pain avoidance-like behavior (mechanical conflict-avoidance task) during acute morphine withdrawal (24 hrs after injections). Opioid-dependent rats demonstrated significant decreases in paw withdrawal thresholds (von Frey) during opioid escalation, while saline controls did not, indicating increased mechanical hypersensitivity in opioid-dependent animals during withdrawal. During mechanical conflict-avoidance testing, opioid-dependent rats exhibited significant increases in pain avoidance during withdrawal compared to saline controls. To test the concurrent validity of the mechanical conflict-avoidance model compared to von Frey testing, Pahng and colleagues correlated pain avoidance-like behavior with changes in mechanical sensitivity (Von Frey test). Changes in mechanical sensitivity (Von Frey test) over the week prior to mechanical conflict-avoidance testing negatively correlated with individual levels of subsequent pain avoidance-like behavior (r=−0.4413, p=0.0452), demonstrating that indicators of increased pain hypersensitivity as measured by von Frey or mechanical conflict-avoidance testing were significantly correlated. This indicates that the two models may measure overlapping, but not entirely identical behavioral measures of pain.
Dependence has been previously described as a chronic pain disorder (Egli et al., 2012). There is a clear neurobiological connection between chronic pain and drug dependence, namely the shared neuronal circuitry mediating nociception and negative reinforcement mechanisms associated with addiction (Egli et al., 2012). To test the neurobiological correlates of pain avoidance-like behavior, Pahng and colleagues investigated individual relationships between pain avoidance and alterations in protein phosphorylation in central motivation-related brain areas (Pahng et al., 2017). They found that pain avoidance was significantly correlated with alterations in phosphorylation status of protein kinases (ERK, CaMKII), transcription factors (CREB), presynaptic markers of neurotransmitter release (Synapsin), and the rate-limiting enzyme for dopamine synthesis (TH) across specific brain regions including the dorsomedial prefrontal cortex, ventromedial prefrontal cortex, dorsal striatum, ventral striatum, and dorsal hippocampus. These alterations in phosphorylation status were specific to behavioral output from the mechanical conflict-avoidance task, as von Frey thresholds did not significantly correlate with the phosphoprotein levels examined. This suggests different neuronal mechanisms may be mediating these pain behaviors as measured by von Frey and mechanical conflict-avoidance tests.
Continued use of the mechanical conflict-avoidance model may allow us to better understand the affective and motivational dimensions of pain associated with dependence and withdrawal. Compared to traditional paw withdrawal measures of pain, the mechanical conflict-avoidance model may provide additional translational validity with regard to the interaction of pain and motivational (or goal-directed) behavior as well as provide more suitable treatment options for affective pain beyond sensory nociception. It is important to consider the testing timeframe for mechanical conflict-avoidance task when using dependence models with shorter transition periods to dependence. Testing procedures for the mechanical conflict-avoidance task occurs over seven days and it is possible that acute withdrawal symptoms may get worse over the course of testing. To avoid the confound of a potential amplification of pain avoidance over testing it is important to run the daily tests of probe heights in non-sequential order (e.g. 0 mm, 0.5 mm, 2 mm, 4 mm, 3 mm, 1 mm, 5 mm).
Critical Parameters and Troubleshooting
Experimental conditions
The mechanical conflict-avoidance system has been validated for testing mechanical pain avoidance in rats (Harte et al., 2016, Pahng et al., 2018), but not mice. In the mechanical conflict-avoidance apparatus, the probes are positioned evenly within 1 cm in any direction so that rats have to traverse over the probes to reach the dark goal chamber. Accordingly, the current positioning of the probes is too spaced out to account for the smaller size of mice. There are changes that can be made to the current chamber with a mouse adapter kit (Coy Laboratory Products Inc.). The kit includes a small tunnel that fits in the probe chamber and instructions on other changes to make to the mechanical conflict-avoidance apparatus. To function as the aversive stimuli, the kit comes with sand paper instead of probes. Modified cold and thermal nociception tests in mice using the conflict-avoidance system have been described (Zhou & Carlton, 2012; Carlton & Zhou, 2013). In these modified versions of the task, the light intensity was increased, the space under the chamber doors was reduced, and the middle chamber was changed to test either cold or heat sensitivity. The mechanical conflict-avoidance system has only been validated in male Sprague Dawley rats (Harte et al., 2016) and male Wistar rats (Pahng et al., 2017), but it can easily be tested in other male rat strains and in female rats.
All animals should be group housed with a maximum of 2 rats per cage (standard housing size). Completion of the first part of Basic Protocol 1 (from ordering animals to completing of baseline stimulus-response testing) occurs in 21 days. Completion of the second part of Basic Protocol 1 (from induction of dependence and withdrawal to completing dependence and withdrawal stimulus-response testing) occurs in 7 days plus the time needed to produce dependence (see Support Protocol 2). Accordingly, rats will continue to gain weight throughout the course of experimental procedures. It is optimal to house 2 rats per cage to avoid having to re-house the rats part way through the experiment due to age-related weight gain. To decrease animal stress during training and testing, it is essential that rats are familiar with the experimenter. Rats should be handled regularly (~5min per day for 5 days) prior to the start of experimental procedures. All testing procedures should be completed in a quiet behavior room. During training and testing, it is important to be very careful when closing the guillotine door next to the goal chamber. Closing the door too loudly/quickly or when the rats is not completely in the goal chamber may cause the rat to become fearful of the goal chamber.
Baseline measures of pain avoidance-like behavior
Animals will demonstrate individual variability in behavioral performance during stimulus-response testing of the mechanical conflict-avoidance task. This is why it is critical to measure baseline pain avoidance-like behavior before splitting animals into dependent and non-dependent groups (Support Protocol 1). It is also important to have sufficient animal numbers at the beginning of the experiment, because it may be necessary to remove animals from the study that are poor performers (see Table 1 for more details on identifying poor performers). It may also be necessary to remove animals from the study in order to have two equivalent groups, which will eventually be the dependent and non-dependent groups. After properly splitting animals into two groups, there will be no significant group differences in baseline pain avoidance-like behavior and both groups will have similar stimulus-response curves (i.e. latency to exit onto the probes increases as probe height increases) (Support Protocol 1).
Table 1.
Troubleshooting
| Potential Problems | Commentary |
|---|---|
| Animals remain in the brightly lit start chamber and do not exit onto the probes after 30 s |
This may occur a few times during testing, particularly at the highest probe heights. In this case, an animal should be placed back into the home cage and the latency to exit onto the probes will be 30 s (max time). However, animals that do not exit the start chamber in the absence of the probes (0 mm) during baseline testing can be removed from study. If the majority of the animals do not exit the start chamber in the absence of the probes (0 mm), it may be necessary to adjust the brightness of the bulb (contact the manufacturer). This was not a problem in Pahng et al., 2017, but some adjustments to test parameters would need to be made if this problem occurs (consult with Coy Laboratory Products Inc. before making adjustments that may damage the equipment). |
| Animals do not place all four paws in the nociceptive probe chamber |
In some cases, animals may not initially place all four paws in the nociceptive probe chamber. This behavior of testing the probes with the front two paws and sometimes all but one paw before proceeding is common. It is essential that the ‘latency to exit onto the probes’ is only measured when animals place all four paws and thus full body weight on the probes. |
| Animals do not enter the dark chamber after 60 s or only partially enter the dark chamber |
In this case, an animal should be placed back into the home cage and the latency to enter the dark chamber will be 60 s (max time). If animals only partially enter the dark chamber, then leave the animal alone until the 60 s has elapsed or the animal has placed all four paws in the dark goal chamber, which is the ‘latency to enter the dark chamber’ measure. |
| Animals enter the dark chamber after 60 s, but are in the way of closing the guillotine door. |
Sometimes animals may have all four paws in the dark chamber, but the animal’s backside is in the guillotine doorway. In this case, the door should be closed slowly to avoid startling the animal. Fortunately, there is space under the chamber doors so there is room to close the door without the animal’s tail being caught. |
| Animals return to the light chamber after entering the dark chamber |
This is less common, but still possible. This behavior is most likely to occur during training and is indicative of exploratory behavior, which should decrease over the course of training. |
Statistical Analyses
By the end of training (step 38), all animals should be consistently exiting the start chamber in the absence of probes (0mm probe height). Any animal that is exceeding the maximum time to exit the start chamber (30 s) in the absence of probes, should be excluded from the study and not proceed to baseline stimulus-response testing. After the production of baseline stimulus-response curves, animals are split into equivalent groups to produce similar stimulus-response curves for each group (Support Protocol 1). A two-way between-subjects ANOVA with probe height (0mm, 0.5mm, 1mm, 2mm, 3mm, 4mm, 5mm) and group (group 1, group 2) as factors for ‘latency to exit onto the probes’ is used to verify that there are no significant differences between groups (Support Protocol 1). Accordingly, both the inclusion and exclusion criteria for animals at this stage is based on producing two statistically equivalent groups, which can then be randomly assigned to either dependence or control conditions.
As described in Support Protocol 3, a two-way between-subjects ANOVA with probe height (0mm, 0.5mm, 1mm, 2mm, 3mm, 4mm, 5mm) and group (dependent rats, non-dependent rats) as factors is used to quantify differences in ‘latency to exit onto the probes’ and ‘latency to enter the dark goal chamber’. Tukey’s multiple comparisons test is recommended for post-hoc analyses of significant probe height, group, or interaction effects. The completion of these tests assumes a normal sampling distribution. To test for violations of normality, we recommend using the D’Agostino-Pearson normality test or using the Shapiro-Wilk normality test as an alternative test. In the event of a non-normal sampling distribution, we recommend completing a data transformation (e.g. log transformation, square-root transformation).
Presentation of probes
As described in Basic Protocol 1, the order of probe presentation during stimulus-response testing must meet the following criteria: (1) the first probe height is 0 mm, (2) the second probe height must not be greater than 2mm, and (3) the presentation of probes must be in non-sequential order (Table 2). The order of probe presentation may be changed as long as these criteria are met. Each session occurs on separate days (e.g. session 1 = day 1, session 2 = day 2, session 3 = day 3 etc…) (Table 2). This order of probe presentation has been shown to produce a stimulus-response curve during both baseline testing and post-dependence testing (i.e. latency to exit onto the probes increases as probe height increases) (Pahng et al., 2017). It is critical that animals complete the first run of each session in the absence of probes (0 mm). This re-establishes the behavior to move through the probe chamber to reach the dark goal chamber. Animals will demonstrate similar latencies to exit onto the probes at 0 mm during sessions 1–7.
Table 2.
Presentation of Probes
| Run 1 | Run 2 | Run 3 | Run 4 | |
|---|---|---|---|---|
| Session 1 | 0 mm | 0 mm | 0 mm | 0 mm |
| Session 2 | 0 mm | 0.5 mm | 0.5 mm | 0.5 mm |
| Session 3 | 0 mm | 2 mm | 2 mm | 2 mm |
| Session 4 | 0 mm | 4 mm | 4 mm | 4 mm |
| Session 5 | 0 mm | 3 mm | 3 mm | 3 mm |
| Session 6 | 0 mm | 1 mm | 1 mm | 1 mm |
| Session 7 | 0 mm | 5 mm | 5 mm | 5 mm |
Time considerations with dependence and withdrawal stimulus-response testing
After induction of dependence, the time point chosen to complete dependence and withdrawal stimulus-response testing is highly dependent on drug of interest and the induction protocol conditions (Support Protocol 2). Tests of hyperalgesia (e.g. von Frey, Hargreaves) can be used to determine when testing should occur. Prior to stimulus-response testing, Pahng and colleagues measured changes in mechanical hypersensitivity (von Frey test) over the week prior to mechanical conflict-avoidance testing to determine when morphine-dependent animals demonstrated decreased paw withdrawal thresholds consistent with opioid withdrawal-induced hyperalgesia (Pahng et al., 2017). An alternative test of hyperalgesia that could be used is the Hargreaves test of thermal hypersensitivity (Hargreaves et al., 1988; Roltsch Hellard et al., 2016). A literature search of the opioid- and alcohol-hyperalgesia in rodent models of dependence could also help narrow the testing timepoint. Fortunately, the mechanical conflict-avoidance task is easily repeatable as is evident by similar stimulus-response curves during baseline and post-dependence testing in non-dependent animals.
Stimulus-response testing should be completed during the same time each day and when animals are in acute withdrawal. The general procedure is to put animals through a period of intoxication followed by a period of spontaneous withdrawal (natural occurring withdrawal) in a single day. In contrast to spontaneous withdrawal, precipitated withdrawal is when a withdrawal state is induced by a drug that blocks the effects of intoxication. For example, naloxone (opioid antagonist) can be given to rapidly reverse the effects of opioid drugs and quickly induce symptoms of withdrawal. Pain avoidance-like behavior has only been testing during spontaneous withdrawal in opioid-dependent animals (Pahng et al., 2017), however, precipitated withdrawal is another option that could be used with the mechanical conflict-avoidance task. A potential drawback of using precipitated withdrawal is that it may create a strong association between the testing procedure and the rapid induction of withdrawal symptoms. Finally, testing pain avoidance with other drugs of abuse not discussed in this unit (e.g. cocaine, nicotine, THC etc.) is an alternative and viable option for future investigations
Understanding Results
Below is a summary of test measures, potential outcomes, and interpretations of the pain avoidance results when measuring the ‘latency to exit onto the probes’ in the mechanical conflict-avoidance task (Table 3). The ‘latency to enter the dark goal chamber’ measure can be examined to look at differences in locomotor activity. It has previously been reported that during baseline testing and after induction opioid dependence testing, there was not a significant effect of ‘probe height’ or ‘group’ when measuring the ‘latency to enter the dark goal chamber’, indicating that the ‘latency to exit onto the probes’ was a better stimulus-response measure of pain avoidance than ‘latency to enter the dark chamber’ (Pahng et al., 2017). Additionally, these findings suggest that there were no differences in the locomotor capacity to cross over the probes to reach the dark goal chamber between groups. However, this measure would be of interest to examine in studies examining different dependence induction protocols or drugs of abuse.
Table 3:
Understanding Results
| Latency to exit onto the probes | ||
|---|---|---|
| Test Measures |
Potential Outcomes |
Interpretations and Commentary of Findings |
| Two-way ANOVA: | ||
| Effect of probe height |
Significant | There is stimulus-response measure of pain avoidance based on probe height. This indicates that latency changes as the probe height changes. It is expected that the latency to exit onto the probes will increase as the probe height increases (as seen in Pahng et al., 2017, p<0.0001) |
| Not Significant | There is no stimulus-response measure of pain avoidance based on probe height. This is an unexpected effect. It is important that animals demonstrate a significant effect of probe height during baseline testing before proceeding to dependence induction protocols. |
|
| Effect of group |
Significant | There is a difference in pain avoidance between groups after induction of dependence. It is expected that the latency to exit onto the probes will be greater in dependent animals during acute withdrawal (as seen in Pahng et al., 2017, p<0.0001) |
| Not Significant | There is a no difference in pain avoidance between groups after induction of dependence. It may be possible that animals are not hyperalgesic at the time of testing. If animals are not hyperalgesic, then the test can be repeated at a later time point, the withdrawal timepoint could be extended, or the dependence induction parameters may need to be adjusted. If animals are hyperalgesic, then alterations in pain sensitivity may only increase sensory pain instead of affective and motivational pain. |
|
| Interaction effect |
Significant | There is a significant interaction of group and probe height. This suggests that groups may only appear different at certain probe higher (e.g. groups differ at higher probe heights, but not lower probe heights). |
| Not Significant | There is no interaction of group and probe height (as seen in Pahng et al., 2017, p=0.2490). |
|
| t-test, Tukey multiple comparisons: | ||
| Effect of group at 0 mm probe height |
Significant | There is a difference in the motivation to exit the start chamber in the absence of the probes. Changes in the motivation to exit the light chamber in the absence of the probes may suggest group differences in anxiety, motivation, or locomotor capacity. |
| Not Significant | There is no difference in the motivation to exit the brightly lit start chamber in the absence of the probes (as seen in Pahng et al., 2017, p=0.1862). This suggests that both groups exhibit similar locomotor capacity and were motivated to exit the light chamber and reach the goal box in the absence of the probes. |
|
Time Considerations
Completion of the full protocol occurs in a series of stages (Figure 3): (I) Handling and housing room acclimation (5–7 days), (II) Habituation (2 days), (III) Training (3 days), (IV) Home cage rest (2 days), (V) Baseline stimulus-response testing (7 days), (VI) Induction of dependence and withdrawal (time frame dependent on protocol used), and (VII) Dependence and withdrawal stimulus-response testing (7 days). It is recommended that animals have 1 wk to acclimate to a novel housing environment prior to the start of experimental procedures. During this acclimation period, rats should be handled by the experimenter for 5 min a day for 5 days. As mentioned in Basic Protocol 1, the weights of each animal should be tracked throughout the experiment by weighing all animals at least once a week or more (~25 min per week). Habituation occurs over 2 days. On each day, animals are placed in the start chamber for 10 s, the light is turned for 20 s, and the door is opened allowing the rat to explore for 5 min. This procedure is completed once per day for each rat (anticipated time per day: ~2 hrs for 20 rats). Training occurs over 3 days. Training consists of placing rats in the dark start chamber for 10 s, turning on the light for 20 s, and opening the start chamber until the ra\ reaches the goal chamber or exceeds max time (latency to exit onto the probes = 30 s, latency to enter onto the dark goal chamber = 60 s). This procedure is completed four times per day for each rat (anticipated time per day: ~2.5 hrs for 20 rats). Baseline stimulus-response testing occurs over 7 days and involves the same procedure as training, but the probe heights are adjusted during specific trials and days (Table 2) (anticipated time per day: ~2.5 hrs for 20 rats). In total, this first part of the protocol can be completed in 21 days.
Induction of dependence and withdrawal involves putting animals through intermittent cycles of intoxication over an extended time frame, which produces the manifestation of withdrawal symptoms. The duration required to produce dependence in animals is highly variable and largely determined by the drug of interest, the protocol used to induce dependence, and parameters chosen by the experimenter (Support Protocol 2). For example, subcutaneous injections of an escalating dose regimen of morphine (10mg/kg to 20mg/kg) will produce symptoms of opioid withdrawal after 2 weeks. Similar to baseline stimulus-response testing, dependence and withdrawal stimulus-response testing occurs over 7 days and involves a specific presentation of probe heights (Table 2) (anticipated time per day: ~2.5 hrs for 20 rats). The main change during this stage is that animals are tested during withdrawal and undergo a period of intoxication (e.g. drug injections, vapor exposure etc.) following completion of testing each day.
SIGNIFCANCE STATEMENT:
Excessive use of either opioids or alcohol produces the emergence of heightened pain sensitivity to noxious stimuli, termed hyperalgesia. This unit describes the use of the mechanical conflict-avoidance task for measuring pain avoidance-like behavior in opioid- and alcohol-dependent rats during withdrawal. In this conflict task, rats run across probes of varying heights to avoid a bright aversive light and to reach a dark goal chamber. A longer latency to exit onto the probes reflects increased pain avoidance-like behavior. Compared to traditional reflex-based assays where an animal elicits a withdrawal response when a stimulus reaches a noxious threshold, the mechanical conflict-avoidance system provides a better method for modeling the motivational and affective dimensions of pain that are beyond the sensory component of pain.
Acknowledgments
Acknowledgements: This article is based on research generously supported by research and training grants from the National Institute on Alcohol Abuse and Alcoholism (T32AA007577, ARP; R00AA020839, SE) and by LSUHSC School of Medicine start-up funds.
Footnotes
Author Disclosure Statement: The authors declare no competing financial interests or potential conflicts of interest.
LITERATURE CITED
- Angst MS, & Clark JD (2006). Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology, 104(3), 570–587. [DOI] [PubMed] [Google Scholar]
- Becerra L, & Borsook D (2008). Signal valence in the nucleus accumbens to pain onset and offset. Eur J Pain, 12(7), 866–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, & Simonnet G (2000). Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology, 92(2), 465–472. [DOI] [PubMed] [Google Scholar]
- Carlton S & Zhou S (2013). A novel operant method to test acute heat hypersensitivity in mice using a modification of the Coy Operant Mechanical Conflict Avoidance System. Journal of Pain, 14(4), s43. [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, & Yaksh TL (1994). Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods, 53(1), 55–63. [DOI] [PubMed] [Google Scholar]
- Craft RM, Stratmann JA, Bartok RE, Walpole TI, & King SJ (1999). Sex differences in development of morphine tolerance and dependence in the rat. Psychopharmacology (Berl), 143(1), 1–7. [DOI] [PubMed] [Google Scholar]
- Edwards S, & Koob GF (2010). Neurobiology of dysregulated motivational systems in drug addiction. Future Neurol, 5(3), 393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, & Koob GF (2012a). Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol, 17(1), 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, Vendruscolo LF, Schlosburg JE, Misra KK, Wee S, Park PE, Schulteis G, & Koob GF (2012b). Development of mechanical hypersensitivity in rats during heroin and ethanol dependence: alleviation by CRF(1) receptor antagonism. Neuropharmacology, 62(2), 1142–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S, & Koob GF (2013). Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol, 24(5–6), 356–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards S (2016). Reinforcement principles for addiction medicine; from recreational drug use to psychiatric disorder. Prog Brain Res, 223, 63–76. [DOI] [PubMed] [Google Scholar]
- Egli M, Koob GF, & Edwards S (2012). Alcohol dependence as a chronic pain disorder. Neurosci Biobehav Rev, 36(10), 2179–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold CL (2008). The Majchrowicz binge alcohol protocol: an intubation technique to study alcohol dependence in rats. Curr Protoc Neurosci, Chapter 9, Unit 9.28. [DOI] [PubMed]
- Fu R, Gregor D, Peng Z, Li J, Bekker A, & Ye J (2015). Chronic intermittent voluntary alcohol drinking induces hyperalgesia in Sprague-Dawley rats. Int J Physiol Pathophysiol Pharmacol, 7(3), 136–144. [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Richardson HN, Cole M, & Koob GF (2008). Vapor inhalation of alcohol in rats. Curr Protoc Neurosci, Chapter 9, Unit 9.29. [DOI] [PMC free article] [PubMed]
- Hargreaves K, Dubner R, Brown F, Flores C, & Joris J (1988). A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain, 32(1), 77–88. [DOI] [PubMed] [Google Scholar]
- Harte SE, Meyers JB, Donahue RR, Taylor BK, & Morrow TJ (2016). Mechanical Conflict System: A Novel Operant Method for the Assessment of Nociceptive Behavior. PLoS One, 11(2), e0150164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King T, Vera-Portocarrero L, Gutierrez T, Vanderah TW, Dussor G, Lai J, Fields H, & Porreca F (2009). Unmasking the tonic-aversive state in neuropathic pain. Nat Neurosci, 12(11), 1364–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (1997). Drug abuse: hedonic homeostatic dysregulation. Science, 278(5335), 52–58. [DOI] [PubMed] [Google Scholar]
- Massaly N, Moron JA, & Al-Hasani R (2016). A Trigger for Opioid Misuse: Chronic Pain and Stress Dysregulate the Mesolimbic Pathway and Kappa Opioid System. Front Neurosci, 10, 480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinn MA, Paulsen RI, Itoga CA, Farooq MA, Reppel JE, Edwards KN, Whitaker AM, Gilpin NW, & Edwards S (2016). Withdrawal from Chronic Nicotine Exposure Produces Region-Specific Tolerance to Alcohol-Stimulated GluA1 Phosphorylation. Alcohol Clin Exp Res, 40(12), 2537–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Atcherley CW, & Porreca F (2015). Brain Circuits Encoding Reward from Pain Relief. Trends Neurosci, 38(11), 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahng AR, Paulsen RI, McGinn MA, Edwards KN, & Edwards S (2017). Neurobiological correlates of pain avoidance-like behavior in morphine-dependent and non-dependent rats. Neuroscience, 366, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park PE, Schlosburg JE, Vendruscolo LF, Schulteis G, Edwards S, & Koob GF (2015). Chronic CRF1 receptor blockade reduces heroin intake escalation and dependence-induced hyperalgesia. Addict Biol, 20(2), 275–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips HC (1987). Avoidance behaviour and its role in sustaining chronic pain. Behav Res Ther, 25(4), 273–279. [DOI] [PubMed] [Google Scholar]
- Porreca F, & Navratilova E (2017). Reward, motivation, and emotion of pain and its relief. Pain, 158 Suppl 1, S43–s49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roltsch Hellard EA, Impastato RA, & Gilpin NW (2016). Intra-cerebral and intra-nasal melanocortin-4 receptor antagonist blocks withdrawal hyperalgesia in alcohol-dependent rats. Addict Biol. [DOI] [PubMed]
- Shurman J, Koob GF, & Gutstein HB (2010). Opioids, pain, the brain, and hyperkatifeia: a framework for the rational use of opioids for pain. Pain Med, 11(7), 1092–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, & Bartlett SE (2008). Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res, 32(10), 1816–1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, & Caine SB (2005). Chronic intravenous drug self-administration in rats and mice. Curr Protoc Neurosci, Chapter 9, Unit 9.20. [DOI] [PubMed]
- Wade CL, Vendruscolo LF, Schlosburg JE, Hernandez DO, & Koob GF (2015). Compulsive-like responding for opioid analgesics in rats with extended access. Neuropsychopharmacology, 40(2), 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ (2011). Central sensitization: implications for the diagnosis and treatment of pain. Pain, 152(3 Suppl), S2–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S & Carlton S (2012). A novel operant method to test acute cold hypersensitivity in mice using a modification of the Coy Operant Mechanical Conflict Avoidance System. Journal of Pain, 13(4), s46. [Google Scholar]


