Abstract
Purpose:
Tendon tears are common injuries that heal with scar formation. Interestingly, MRL/MpJ mice heal without scar in several tissues, including tendon. Most hypotheses regarding scarless healing implicate the systemic environment. However, the tissue-specificity of this regenerative response and our previous findings showing regeneration of subrupture tendon injuries, which lack an overt systemic response, motivate a tissue-driven hypothesis. Our objective is to investigate the potential of the local tendon environment in driving scarless healing (1) by comparing the systemic response and the healing capacity associated with ear and tendon injuries in MRL/MpJ mice, and (2) by comparing intrinsic healing properties between MRL/MpJ and normal healer C57Bl/6 tendons.
Methods:
We examined the systemic inflammatory and local structural environments of ear and tendon punch injuries in MRL/MpJ and C57Bl/6 mice. Systemic differences were analyzed to assess effects of different injuries on the inflammatory response. Correlations were assessed between MRL/MpJ ear and tendon injuries to compare the extent of healing between regenerative tissues.
Results:
Analysis showed similarities between the systemic environment in MRL/MpJ post ear or tendon injuries. However, comparable inflammatory responses did not translate into analogous healing between tissues, suggesting that the systemic environment is not the driver of regeneration. Supporting the regenerative role of the local environment, healing MRL/MpJ tendons exhibited improved matrix and cell alignment and a distinct composition of growth factors and Hyaluronan from C57Bl/6.
Conclusion:
These findings support the tissue-driven hypothesis for MRL/MpJ tendon regeneration and motivate further investigation regarding specific roles of extracellular factors in scarless healing.
Keywords: Growth factors, Hyaluronan, matrix alignment, Murphy Roths Large Mice (MRL/MpJ), scarless healing, tendon regeneration
Introduction
Tendon tears are common musculoskeletal injuries that impact athletes and the general population (1). Canonical tendon healing is characterized by scar formation, with disorganized structure and inferior mechanical function, predisposing the healing tendon to re-injury and rupture (2). Extensive attempts to recapitulate the regenerative tendon healing environment have been unsuccessful due to the lack of a guiding model of adult mammalian scarless healing (3). While regenerative tissue healing has been identified in amphibians and fetal mammals, the large discrepancies of the mechanical environment and immune response between these models and the adult mammalian healing environment have hindered the development of effective tendon therapeutics. Consequently, identification of Murphy Roths Large (MRL/MpJ) mice as an adult model of mammalian regeneration holds much potential for advancement of therapeutics in this area.
While the driver of the regenerative properties of MRL/MpJ mice is largely unknown, the immunocompromised environment of this mouse strain has led to the prevailing hypothesis that these phenotypes are interdependent. Supporting this hypothesis, injuries in which circulating inflammatory cytokines from the blood thoroughly infiltrate the damaged region, such as ear cartilage and full thickness articular cartilage wounds, have been noted to recuperate naïve-like structure and composition (4,5). However, no improvements have been identified in injuries that lacked blood flow such as partial-thickness articular cartilage lacerations (5).
Conversely, the fact that regenerative healing of the MRL/MpJ mouse is tissue specific, and does not extend to skin, dopaminergic neurons, or certain types of myocardial and cartilage injury, supports an alternative hypothesis wherein the local environment of the injured tissue may be integral to the regenerative outcome (5–8). For instance, the growth factor-rich cell-free extracellular matrix (ECM) generated in vitro by isolated MRL/MpJ ear blastema cells stimulated scarless dorsal cutaneous wound healing in C57Bl/6 mice (9). This suggests the potential role of the MRL/MpJ tissue-specific growth factor environment in driving a regenerative response. Furthermore, very weak correlations were found between extent of healing in articular cartilage and closure of ear punch injury despite their shared systemic environment (5). Recently, Sereysky et al. examined the healing capacity of tendons from MRL/MpJ in response to both acute injury, which exhibits the typical systemic inflammatory cascade, and sub-rupture fatigue injury, which is largely characterized by a biological response that is intrinsic to the tendon (10). Interestingly, the improved healing capacity of these mice extended to both types of injuries, as exhibited by restoration of structural and mechanical integrity, providing evidence that implicates the tissue’s local environment as a driver of the improved healing response.
Moreover, structure and composition have also been identified as vital players in regeneration of tissues such as the liver, fetal skin, and oral mucosa (11–13). Namely, Hyaluronan (HA), a key structural glycoprotein of the ECM, has been shown to modulate the local inflammatory healing phase, provide structural matrix integrity, and regulate availability and activation of growth factors such as TGF-β, FGF, and PDGF during the regenerative healing response of these tissues (14–16). These key growth factors are upregulated during repair and play a major role in modulating cell proliferation, angiogenesis and fibrosis. Gaining a better understanding of the role of this glycoprotein and growth factors in the regenerative tendon healing response of the MRL/MpJ could elucidate promising targets for the development of effective tendon therapeutics.
Accordingly, we hypothesize that the structural and compositional differences in the provisional ECM of MRL/MpJ mice orchestrate a biological environment that stimulates cell activity to ultimately promote improved tendon healing. As a first step toward testing this hypothesis, the objectives of this study are to (1) compare the systemic inflammatory response associated with different injuries, (2) evaluate the correlation in the extent of healing between tendon and ear punch injuries, and (3) assess the alterations in the intrinsic tendon healing response of MRL/MpJ and C57Bl/6 mice as determined by HA content, growth factor environment, matrix alignment, and cell alignment.
Methods
In vivo patellar tendon punch and ear punch injuries
Under IACUC approval, skeletally mature 8–9-week-old male “super-healer” MRL/MpJ mice (n = 41) and “normal-healer” C57Bl/6 mice (n = 28) underwent left unilateral ear punch at the start of the 4-week study period using a standard 2-mm ear tag punch (Fisher Scientific, Waltham, MA, USA). In addition, all animals underwent left unilateral full-thickness, excisional 0.75-mm patellar tendon punch as described previously (17,18) and were allowed to heal for either 1 week or 4 weeks so that all animals were sacrificed at the completion of the 4-week study period. Ear punches were imaged using a digital SLR camera (Nikon D7100, Tokyo, Japan). Images were analyzed for degree of ear wound closure in MRL/MpJ and C57Bl/6 mice. Following bulk ear analysis, some MRL/MpJ (n = 13) and C57Bl/6 (n = 4) were allocated for other experiments not encompassed in this study.
Serology associated with ear and tendon punch injuries was analyzed utilizing standard techniques of blood sample collection from the facial vein 24 h following ear punch and 24 h following tendon punch. Mice were then sacrificed and their left patellar tendons were dissected at the completion of the study period. Contralateral patellar tendons from the 24-h time-point of a separate study were used as “naïve” uninjured controls. Finally, following sacrifice, tendons were randomly designated to histology, or ELISA for analysis (Figure 1).
Figure 1.
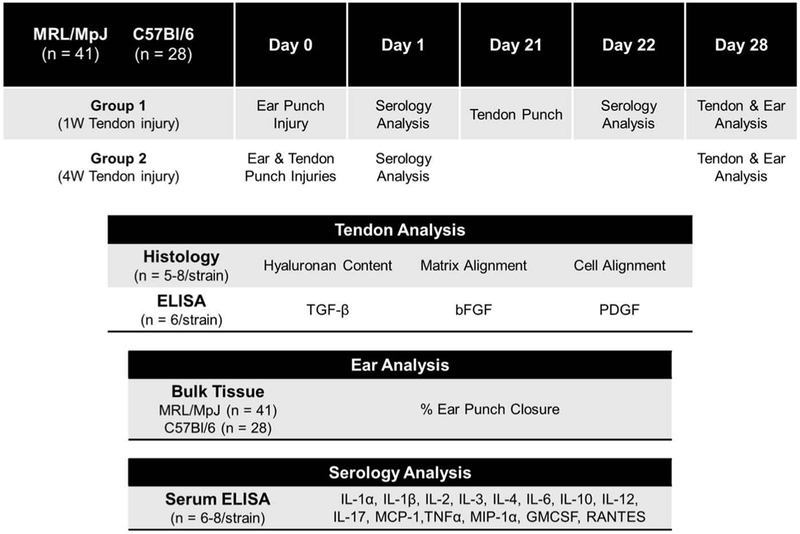
Schematic of experimental design.
Serological analysis
Previous studies have noted key differences in circulating inflammatory cytokines in the MRL/MpJ compared to C57Bl/6 after injury. These differences include decreased pro-inflammatory IL-1 (19), IL-6 and TNF-α (20), as well as increased anti-inflammatory IL-10 and IL-4 (21). Therefore, to assess the differences in the inflammatory environments between ear and tendon injuries, we utilized a comprehensive non-biased multiplex ELISA panel commercially designed to measure the concentration of the previously mentioned pro and anti-inflammatory markers as well as nine additional factors that could be implicated in tendon regeneration. This assay was utilized according to the manufacturer’s protocol (14 markers) (Quansys Biosciences, Logan, UT, USA). For each cytokine with at least half of the samples in the detectable range, levels in MRL/MpJ were compared to levels in C57Bl/6 by t-test (n = 6–8). For MRL/MpJ samples, cytokine levels following ear punch injury were compared to levels following tendon injury by t-test.
Histology and immunohistochemistry
Dissected tendons designated for histology were prepared in paraffin using standard technique and sectioned in the coronal plane at 6-μm thickness. Sections were stained for toluidine blue and hyaluronic acid (Abcam, Cambridge, United Kingdom) and imaged at 5× and 40×. Hyaluronic acid-stained sections were thresholded using MATLAB to calculate the fraction of positively stained matrix (n = 6– 9). Toluidine blue-stained sections were analyzed using a custom MATLAB code to determine fraction of aligned ECM (n = 5–8). Briefly, a digital grid was placed on each image and fast Fourier transforms were used to determine the power spectrum and entropy, so as to categorize each grid element as aligned or unaligned. Subsequently, a blinded user analyzed cell orientation in three images sampled each from regions of aligned and unaligned matrix from each tendon (n = 5–8). Binary conversion was performed utilizing ImageJ and the angle of cell alignment was calculated in MATLAB for each cell using an ellipse-fit method. The matrix alignment histomorphometry algorithm was validated by six blinded users (n = 6) tasked to grade several matrix regions as aligned or unaligned (number of regions graded per user: 73–107 Aligned, 60–90 Unaligned). The algorithm was then utilized to also perform this task on the same regions. Percent agreement between the users and the code was measured to assess the accuracy of the code (Percent Agreement for Aligned Regions = 89.6 ± 6.77%; Percent Agreement for Unaligned Regions = 90.0 ± 5.52%) (Figure S1 in Supplementary Material).
Bulk tendon analysis
Dissected tendons designated for bulk tissue analysis were flash frozen in liquid nitrogen and stored at −80°C until time of processing. Samples were lyophilized and then homogenized with mechanical disruption (2010 Geno/Grinder, SPEX Sample Prep, Metuchen, NJ, USA). Protein was extracted from homogenized tendons in 1% SDS in 1M Tris-HCl (pH 7.4) and total protein concentrations were measured using the BCA assay (Thermo Scientific Pierce, Waltham, MA, USA) at a 1:25 dilution. ELISAs for bFGF, TGF-β, and PDGF were performed on MRL/MpJ (n = 6/cytokine) and C57Bl/6 (n = 6/cytokine) according to manufacturer’s instructions (MyBioSource, San Diego, CA, USA).
Ear punch analysis
Numerous studies have identified ear punch injuries to heal regeneratively in the MRL/MpJ by assessing parameters such as the degree of wound closure (4,5,22,23). Therefore, in order to interrogate the correlations between the level of healing of tendon and ear injuries, the percent closure of ear punch areas was calculated by comparing ear punches at onset of injury and 4 weeks later (ImageJ, NIH, Bethesda, MD, USA). Percent ear closure for MRL/MpJ (n = 41) and C57Bl/6 (n = 28) were compared by t-test, and MRL/MpJ ear healing was subjected to non-subjective k-means cluster analysis using Minitab software (Minitab, State College, PA, USA). MRL/MpJ ear healing clusters were designated as “normal-healer,” “healer,” or “super-healer.” Measurements of tendon healing for MRL/MpJ animals were then segregated by corresponding ear punch healing cluster for each animal. T-tests were used to com-pare super-healer and healer clusters, as defined by ear punch closure, for each tendon parameter. Correlations were calculated between each tendon healing parameter and percent ear punch closure.
Statistical analysis
Comparisons between MRL/MpJ and C57Bl/6 at each time point were assessed using parametric two-tailed t-tests. To further analyze changes within each strain, one-way ANOVA with post-hoc Bonferroni was used to compare naïve, 1-week injured, and 4-week injured. For cell alignment, F-tests were used to test for differences in variance in cell orientation between strains at each time point. Statistical significance was set at p < 0.05 (denoted by *) and a trend was set at p < 0.1 (denoted by #).
Results
Systemic inflammatory response to injury in different tissues
As expected, systemic responses to tendon injury and ear cartilage injury were not different in 8 of 10 detectable cytokines in MRL/MpJ, suggesting a generally similar systemic response between these injuries (Figure 2). More specifically, only higher levels of IL-6 and lower levels of RANTES were observed in response to tendon injury compared to ear injury. In addition, the systemic response in MRL/MpJ due to tendon or ear injury differed from that of C57Bl/6 injuries. More specifically, MRL/MpJ tendon injury elicited a lower response than C57Bl/6 in 7 of 12 detectable cytokines whereas MRL/MpJ ear injury elicited a lower response than C57Bl/6 in 3 of 8 detectable cytokines (Figure 3).
Figure 2.
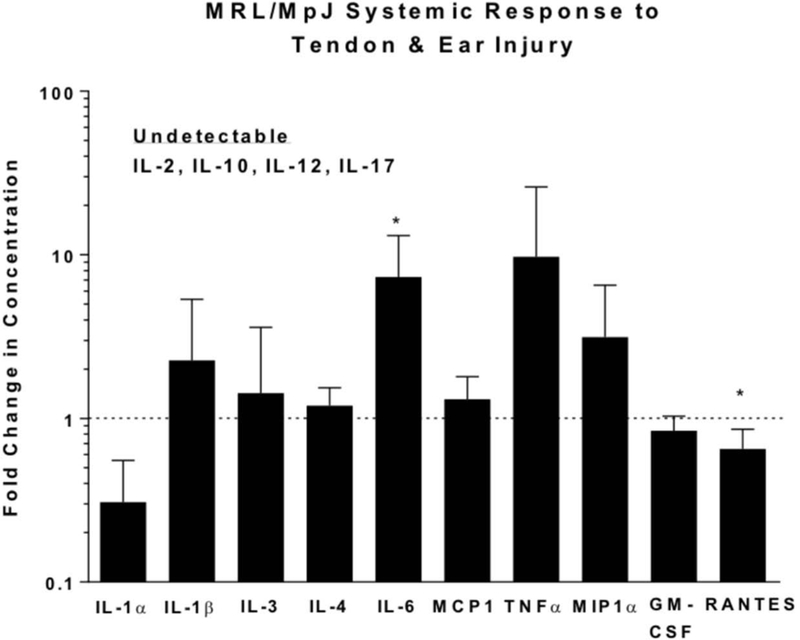
Serological measurement of cytokine and chemokine levels in MRL/MpJ 24 h following ear and tendon injuries. For an individual cytokine, each data point from the tendon injury was normalized to the average measurement of the same cytokine in the ear injury. Therefore, data are shown as fold change in serologic response of MRL/MpJ tendon injury relative to MRL/MpJ ear injury. The dotted line along the y-axis represents equal cytokine levels between ear and tendon serum post injury. Systemic responses to tendon injury and ear cartilage injury were not different in 8 of 10 detectable cytokines in MRL/MpJ. *Statistically significant difference compared to MRL/MpJ ear injury (p < 0.05).
Figure 3.
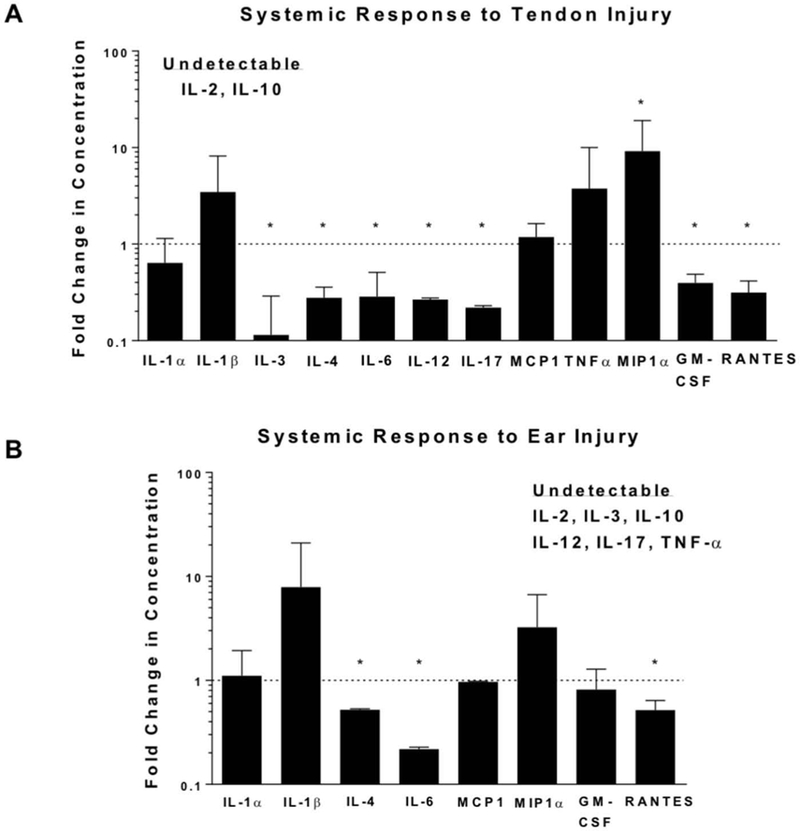
Serological measurement of cytokine and chemokine levels 24 h following tendon injury (A) and ear injury (B). Data are shown as fold change in serologic response of MRL/MpJ relative to C57Bl/6. Equal cytokine serum levels between strains after either tendon (A) or ear (B) injury are represented by the dotted line. MRL/MpJ tendon and ear injuries elicited a lower response than C57Bl/6 in 7 of 12, and 3 of 8 detectable cytokines, respectively. *Statistically significant difference compared to C57Bl/6 (p < 0.05).
Correlations between extent of healing in tendon and ear punch injuries
After 4 weeks of healing, MRL/MpJ ears healed significantly more (64% area closed) compared to C57Bl/6 ears (8% area closed, P < 0.0001, Figure 4). Analysis with k-means clustering non-subjectively divided MRL/MpJ ear healing capacity into “normal-healers,” “healer,” and “super-healer” clusters. Note that 95.12% of MRL/MpJ mice fell into the “healers” or “super-healers” category, which both far exceed the range of C57Bl/6 healing. While distinctly superior to the healing of control C57Bl/6 mice, the variation in MRL/MpJ ear punch healing enables correlations to be made within the strain.
Figure 4.
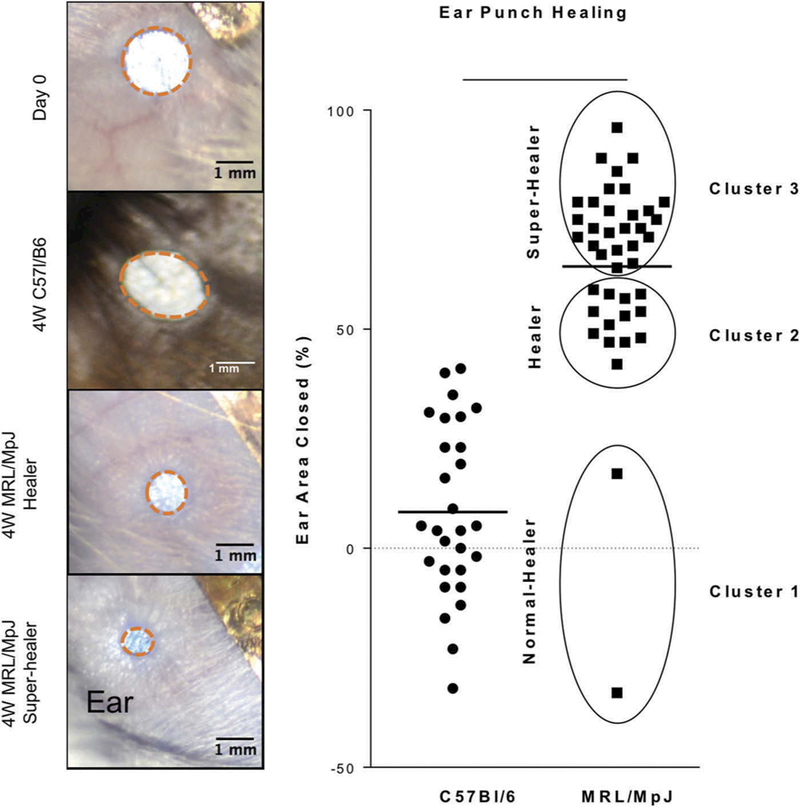
Ear punch healing. (A) Representative images of ear punch healing after 4 weeks in C57Bl/6 and MRL/MpJ. (B) Quantification of ear punch closure indicates significantly greater closure in MRL/MPJ and clustering of healing ability in MRL/MpJ. Cluster analysis separated MRL/MpJ into (1) normal-healer, (2) healer, and (3) super-healer. Bar indicates average.
Tendon alignment, HA content, and growth factor measurements did not differ between super-healer and healer groups as defined by ear punch healing clusters (Figure 5), suggesting that extent of healing in the tendon injury does not correlate with that of the ear injury. Furthermore, correlations between ear healing in MRL/MpJ and HA content, growth factor levels, and cell activity indicate that of the 10 correlations conducted, only PDGF levels in the tendon at 4 weeks correlated with ear healing (P = 0.03, r2 = 0.64; Table 1).
Figure 5.
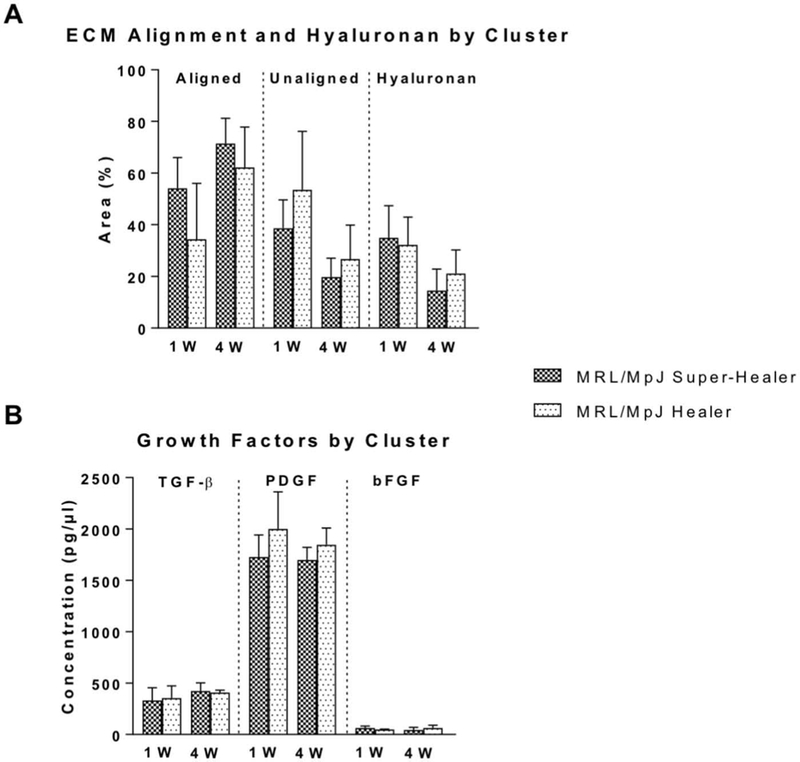
(A) Tendon alignment, hyaluronan, and (B) growth factor levels separated into two groups as determined by ear punch healing cluster analysis. No differences were detected between groups determined by ear healing cluster analysis for any of the tendon parameters evaluated at either 1 week (1W) and 4 weeks (4W) post-injury.
Table 1.
Correlations between MRL/MpJ tendon and ear healing environment.
| 1 week | 4 weeks | |
|---|---|---|
| ECM alignment | P = 0.47 | P = 0.74 |
| r2 = 0.09 | r2 = 0.02 | |
| Hyaluronan | P = 0.27 | P = 0.18 |
| r2 = 0.06 | r2 = 0.14 | |
| TGF-β | P = 0.35 | P = 0.35 |
| r2 = 0.04 | r2 = 0.04 | |
| PDGF | P = 0.12 | P = 0.03 * |
| r2 = 0.32 | r2 = 0.64 | |
| bFGF | P = 0.13 | P = 0.11 |
| r2 = 0.31 | r2 = 0.35 |
Statistically significant difference between the tendon environment of MRL/MpJ super-healer compared to normal-healer mice (p < 0.05).
Deviations in the innate tendon healing response between mrl/mpj and c57bl/6
Consistent with findings from tendon laceration studies (10), MRL/MpJ tendons exhibited a significantly larger area of aligned ECM (1 week, P = 0.0164; 4 weeks, P < 0.0001; Figure 6A), and a significantly smaller area of unaligned ECM (1 week, P = 0.0281; 4 weeks, P < 0.0001; Figure 6B) compared to C57Bl/6 tendons at both 1 and 4 weeks. Cells in MRL/MpJ matrix demonstrated significantly lower variation in alignment at 1 week and 4 weeks in both highly aligned and unaligned matrix compared to cells in C57Bl/6 matrix (1 week aligned, P < 0.0001; 4 weeks aligned, P < 0.0001; 1 week unaligned, P < 0.0001; 4 weeks unaligned, P < 0.0001; Figure 6C–D). As expected, HA levels were not different between naïve C57Bl/6 and naïve MRL/MpJ (Figure 6E, P = 0.1010). HA levels in C57Bl/6 tendons were elevated compared to naïve levels only after 4 weeks (P = 0.0003). In MRL/MpJ, HA levels were elevated after 1 week compared to naïve, but returned to levels not different compared to naïve by 4 weeks (P = 0.0144). Representative images are shown for each histological stain and group (Figure S2 in Supplementary Material).
Figure 6.
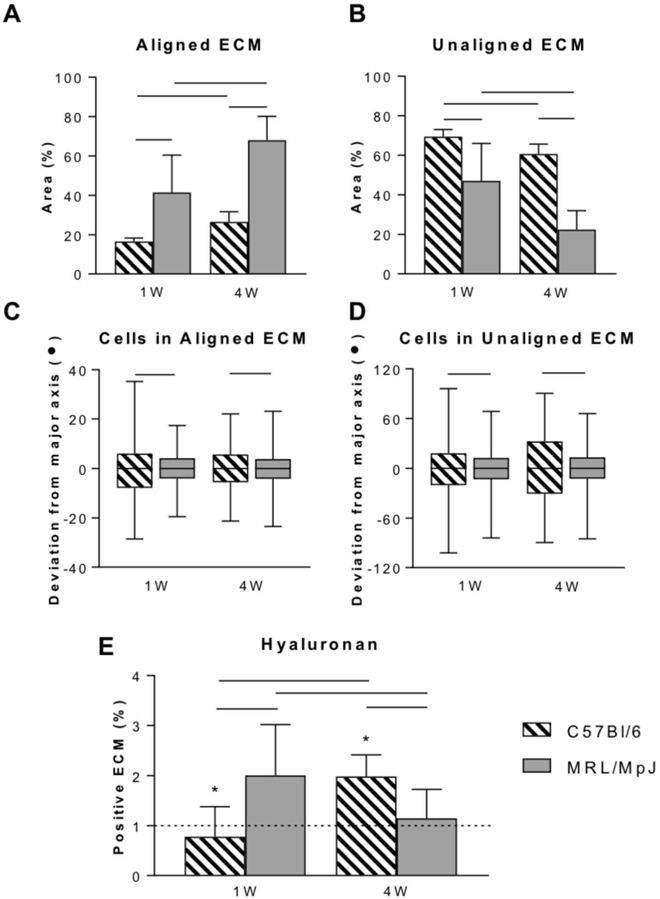
ECM alignment, cell alignment, and hyaluronan content of provisional ECM in MRL/MpJ and C57Bl/6 tendons 1 and 4 weeks after injury. MRL/MpJ tendons exhibited higher area of aligned matrix (A) and lower area of unaligned matrix (B) compared to C57Bl/6. Lower variation in MRL/MpJ cell alignment was observed in both aligned (C) and unaligned (D) matrix compared to C57Bl/6. Hyaluronan levels (E) were elevated at 1 week in MRL/MpJ tendons and at 4 weeks in C57Bl/6 tendons. *Statistically significant difference compared to naïve (P < 0.05). Lines indicate statistically significant difference between groups (P < 0.05).
Assessment of key growth factors using ELISA showed that MRL/MpJ tendons exhibited a decrease in TGF-β at 1 week that returned to naïve levels by 4 weeks (P = 0.0342) (Figure 7). In contrast, C57Bl/6 tendons exhibited elevated TGF-β only at 4 weeks (P < 0.0001). PDGF levels in MRL/MpJ tendons were similar to naïve levels (P = 0.1943) after 1 and 4 weeks but higher than C57Bl/6 at 4 weeks (P = 0.0087). On the other hand, PDGF levels in C57Bl/6 tendons were significantly lower than naïve only at 4 weeks (P = 0.0058). bFGF levels in C57Bl/6 tendons were not changed at 1 or 4 weeks. However, MRL/MpJ tendons exhibited marked elevations in bFGF at both 1 and 4 weeks compared to both naïve (P = 0.0041) and C57Bl/6 tendons (P = 0.0022).
Figure 7.
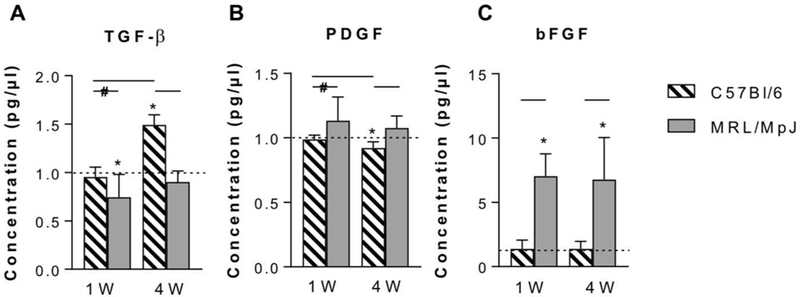
Assessment of key growth factors in healing MRL/MpJ and C57Bl/6 tendons. (A) TGF-β was decreased in MRL/MpJ tendons at 1W but elevated in C57Bl/6 tendons at 4W. (B) PDGF was elevated in MRL/MpJ tendons. (C) bFGF was elevated in MRL/MpJ tendons at 1W and 4W. Dotted line indicates levels of naïve for the appropriate strain. *Statistically significant difference compared to naïve (P < 0.05). Lines indicate statistically significant difference between groups (P < 0.05). Lines with # indicate trend (P < 0.1).
Discussion
The discovery of the adult regenerative healing response observed in the MRL/MpJ mouse provides a promising template to elucidate the biological and structural cues necessary to achieve tissue regeneration. Recent studies on the MRL/MpJ have led to the development of two common hypotheses regarding the driver of its regenerative healing response. The first hypothesis attributes these regenerative properties to the systemic inflammatory environment, while the second credits the role of the local tissue environment as the driver for scarless healing.
Consequently, the objective of this study was to analyze the systemic and local tendon healing properties to provide support for the prominent role of the extracellular environment in driving the regenerative healing response of the MRL/MpJ strain. Consistent with previous studies, initial evaluation of the systemic environment showed that MRL/MpJ exhibited a decreased inflammatory response compared to C57Bl/6 mice 24-h post-tendon and ear injury (21). This deviation from canonical healing has become a major contributor to the idea that the systemic environment acts as a driver for tissue regeneration in the MRL/MpJ. However, contrary to this hypothesis further analysis showed that although the systemic environment between MRL/MpJ ear and tendon injuries was similar, no correlations were found between the level of healing of ear punch injuries and levels of tendon alignment, HA content, growth factor concentration, or cell behavior. These findings suggest that the extent of the regenerative healing trait seen in this mouse strain differs between tissues despite a similar systemic environment, alluding that while it may aid in the mechanisms of regeneration; the systemic response does not drive this process. A major limitation of this study is that the majority of cytokines and chemokines in naïve MRL/MpJ and C57Bl/6 mice were found to be undetectable (21,24) and were therefore not analyzed herein. Additionally, the systemic response to ear injury in MRL/MpJ elicited minimal responses in several of the cytokines that were below the detectable threshold, which made assessment of correlations impossible for some of the cytokines in this study, but is consistent with previously reported literature describing the muted inflammatory response to injury in MRL/MpJ. Future studies investigating the specific role of the inflammatory environment in MRL/MpJ regeneration could benefit from wide-ranging assays such as RNAseq to identify potential mechanistic pathways that may contribute to scarless healing.
Surprisingly, IL-10 was undetectable in all samples. While low naïve levels of IL-10 are expected, other studies have shown its upregulation in MRL/MpJ after injury, noting its important anti-inflammatory role (21,24). Interestingly, we also found a decrease in the presence of circulating anti-inflammatory cytokine IL-4 after both ear and tendon injury. While this result is not consistent with other studies in ear and articular cartilage, we expect that this discrepancy could be due to the difference in timing of data collection after injury between studies.
Supporting the role of the local environment in tissue regeneration, we have demonstrated that MRL/MpJ tendons exhibited superior healing than normal-healer C57Bl/6 tendons with earlier and sustained improved cell and ECM alignment. This improved alignment is consistent with the enhanced tendon healing noted in other studies and insinuates that the provisional ECM of MRL/MpJ tendons harnesses unique structural cues that drive scarless tendon healing (10,25). In-vitro studies have also noted the benefit of proper matrix alignment on cell behavior, with increased alignment of cells in culture leading to improved cell morphology, matrix deposition and tenogenic marker expression over time (26,27).
Furthermore, the tendon field has shown a growing interest in the use of small extracellular molecules as potential therapeutics (3). For instance, TGF-β has been shown to play an important role in the healing process, and much study has been devoted to the role of each of its three isoforms. Whereas TGF-β1 and TGF-β2 are generally considered to promote fibrosis, TGF-β3 has been shown to reduce scarring in both fetal and adult wounds (28–30). bFGF and PDGF on the other hand have been reported to affect matrix properties by modulating cell proliferation, cell migration and angiogenesis during early phases of healing (31). However, while the expression pattern of these individual growth factors is important; their synergistic relationship, timing, and function are responsible for their overall effect on cell behavior throughout healing. Together, the early decreased levels of TGF-β and sustained elevation of PDGF and bFGF that we describe in MRL/MpJ could suggest local growth factor mechanisms that underlie tenocyte mitogenesis without initiating fibrotic pathways. Characterization of the of time course of these growth factors throughout the healing of MRL/MpJ tendons in this study provides further insight into the properties of the local environment necessary to guide regeneration and allows for the ability to identify connections between adult and fetal scarless healing models.
Compositionally, we also found changes in HA content between MRL/MpJ and C57Bl/6 mice throughout injury. This glycoprotein has been identified to modulate the structural environment of regeneratively healing fetal skin and adult liver wounds (16,32). Similar to the results found in MRL/MpJ, early increases in HA in the peri-cellular and extra-cellular space during early fetal healing modulates the cell–matrix relationship, and can increase availability and efficiency of receptor-ligand interactions necessary to modulate cell behavior and induce regeneration (15,16,32). In adulthood, however, prolonged increases in HA, similar to what is seen in scar-mediated healing C57Bl/6 tendons at 4-week post-injury, have been implicated in lung, liver, and kidney fibrosis labeling this glycoprotein as a marker for disease progression (33–35). These conflicting results suggest that changes in HA concentration at different stages of maturation could play an integral role of modulating the tissue environment throughout healing and highlight the necessity to further explore the role of HA in tissue regeneration.
Motivated by this current study suggesting a role for the ECM in driving regenerative healing, future studies will elucidate the key ECM structural, compositional and functional parameters that correspond to the extent of tendon regeneration in MRL/MpJ. For instance, in addition to alignment, fiber quality as determined by fiber diameter and density play a significant part in modulating cell behavior throughout injury and should therefore be interrogated. Moreover, while we evaluated HA due to its role in the early deposited provisional ECM of regeneratively healing tissues such as the liver, the role of HA in moderating inflammatory cytokines has also been described. In this context high molecular weight HA has been noted to promote induction of anti-inflammatory cells, while cleaved fragments of HA lead to the production of pro-inflammatory cytokines (36). Therefore, assessing the concentrations of different HA isoforms throughout MRL/MpJ injury could help elucidate the role of this glycoprotein in influencing the local inflammatory environment during regenerative healing. The presence of other major structural proteins should be interrogated. For example, proteoglycans such as Decorin and Heparan sulfate, which sequester TGF-β and FGF/PDGF, respectively, could play a role in moderating the growth factor environment leading to the changes in concentration between MRL/MpJ and C57Bl/6 observed in this study (37,38). Furthermore, while PDGF, FGF, and TGF-β play major roles in modulating tendon homeostasis, other growth factors and glycoproteins such as VEGF or Tenomodulin should be investigated to further elucidate the role of vascularity and collagen fibrillogenesis in tendon regeneration (31,39,40) In addition, a multi-scale evaluation of tendon mechanics should be conducted, particularly at the early time-points, to identify early differences in mechanical stability, functionality and tissue properties between scar-mediated C57Bl/6 and scarless healing MRL/MpJ mice. Finally, cell behavior such as proliferation, apoptosis and cell morphology should be studied to make connections between these parameters and the previously mentioned structural components. Appropriate cellular stimulation is necessary to enhance subsequent matrix deposition, therefore assessing the synergistic role between cells and matrix could further elucidate the mechanisms of tendon regeneration.
Overall, the findings of this study further support the role of the intrinsic tendon properties as the drivers of regenerative tendon healing in MRL/MpJ mice. The lack of correlation between healing of different tissues in MRL/MpJ or between the systemic responses to these injuries highlights the importance of each tissue’s individual environment in the resulting healing response. Further work is warranted to determine the specific functional, structural, and temporal role of the local ECM environment in driving the scarless tendon healing response in MRL/MpJ.
Supplementary Material
Acknowledgments
The authors thank Dr Rebecca Bell, Dr Alice Huang, Meagan Robles-Harris, and Corey Suraci for their contributions to this work.
Funding
This work was supported by NIH and NIAMS [R01-AR068301 and R01-AR052743].
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- 1.Khan KM, Maffulli N. Tendinopathy: an Achilles’ heel for athletes and clinicians. Clin J Sport Med 1998;8 (3):151–154. [PubMed] [Google Scholar]
- 2.Wang JH-C, Guo Q, Li B. Tendon biomechanics and mechanobiology – a minireview of basic concepts and recent advancements. J Hand Ther 2012;25(2):133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paredes JJ, Andarawis-Puri N. Therapeutics for tendon regeneration: a multidisciplinary review of tendon research for improved healing. Ann N Y Acad Sci 2016;1383(1):125–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davis TA, Longcor JD, Hicok KC, Lennon GG. Prior injury accelerates subsequent wound closure in a mouse model of regeneration. Cell Tissue Res 2005;320(3):417–426. [DOI] [PubMed] [Google Scholar]
- 5.Fitzgerald J, Rich C, Burkhardt D, Allen J, Herzka AS, Little CB. Evidence for articular cartilage regeneration in MRL/MpJ mice. Osteoarthr Cartil 2008;16(11):1319–1326. [DOI] [PubMed] [Google Scholar]
- 6.Colwell AS, Krummel TM, Kong W, Longaker MT, Lorenz HP. Skin wounds in the MRL/MPJ mouse heal with scar. Wound Repair Regen 2006;14(1):81–90. [DOI] [PubMed] [Google Scholar]
- 7.Hampton DW, Seitz A, Chen P, Heber-Katz E, Fawcett JW. Altered CNS response to injury in the MRL/MpJ mouse. Neuroscience 2004;127(4):821–832. [DOI] [PubMed] [Google Scholar]
- 8.Oh Y-S, Thomson LEJ, Fishbein MC, Berman DS, Sharifi B, Chen P-S. Scar formation after ischemic myocardial injury in MRL mice. Cardiovasc Pathol 2004;13(4):203–206. [DOI] [PubMed] [Google Scholar]
- 9.Vorotnikova E, McIntosh D, Dewilde A, Zhang J, Reing JE, Zhang L, Cordero K, Bedelbaeva K, Gourevitch D, Heber-Katz E, Badylak S, Braunhut SJ. Extracellular matrix-derived products modulate endothelial and progenitor cell migration and proliferation in vitro and stimulate regenerative healing in vivo. Matrix Biol 2010;29(8):690–700. [DOI] [PubMed] [Google Scholar]
- 10.Sereysky JB, Flatow EL, Andarawis-Puri N. Musculoskeletal regeneration and its implications for the treatment of tendinopathy. Int J Exp Pathol 2013;94 (4):293–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vrochides D, Papanikolaou V, Pertoft H, Antoniades AA, Heldin P. Biosynthesis and degradation of hyaluronan by nonparenchymal liver cells during liver regeneration. Hepatology 1996;23(6):1650–1655. [DOI] [PubMed] [Google Scholar]
- 12.West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol 1997;29(1):201–210. [DOI] [PubMed] [Google Scholar]
- 13.Yamada Y, Itano N, Hata K, Ueda M, Kimata K. Differential regulation by IL-1β and EGF of expression of three different hyaluronan synthases in oral mucosal epithelial cells and fibroblasts and dermal fibroblasts: quantitative analysis using real-time RT-PCR. J Invest Dermatol 2004;122(3):631–639. [DOI] [PubMed] [Google Scholar]
- 14.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol 2014. March;11(5):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rolfe K, Grobbelaar AO. A Review of Fetal Scarless Healing. Vol. 2012 In: ISRN dermatology 2012. p. 698034 PMC. Web. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pritchard MT, McCracken JM. Identifying novel targets for treatment of liver fibrosis: what can we learn from injured tissues which heal without a scar? Curr Drug Targets 2015;16(12):1332–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beason DP, Kuntz AF, Hsu JE, Miller KS, Soslowsky LJ. Development and evaluation of multiple tendon injury models in the mouse. J Biomech 2012;45(8):1550–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George N Distinctly different molecular profile associated with regerative than scar-mediated tendon healing. ORS 2014;1–5.
- 19.Donnelly RP, Levine J, Hartwell DQ, Frendl G, Fenton MJ, Beller DI. Aberrant regulation of IL-1 expression in macrophages from young autoimmune-prone mice. J Immunol 1990;145(10):3231–3239. Available from http://www.jimmunol.org/content/145/10/3231 [PubMed] [Google Scholar]
- 20.Alleva DG, Kaser SB, Beller DI. Aberrant cytokine expression and autocrine regulation characterize macrophages from young MRL+/+ and NZB/W F1 lupus-prone mice. J Immunol 1997;159(11):5610–5619. Available from www.jimmunol.org/content/159/11/5610 [PubMed] [Google Scholar]
- 21.Ward BD, Furman BD, Huebner JL, Kraus VB, Guilak F, Olson SA. Absence of posttraumatic arthritis follow-ing intraarticular fracture in the MRL/MpJ mouse. Arthritis Rheum 2008;58(3):744–753. [DOI] [PubMed] [Google Scholar]
- 22.Clark LD, Clark RK, Heber-Katz E. A New Murine Model for mammalian wound repair and regeneration. Clin Immunol Immunopathol 1998;88(1):35–45. Available from http://www.sciencedirect.com/science/article/pii/S0090122998945196 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Mohan S, Gu W, Baylink DJ. Analysis of gene expression in the wound repair/regeneration process. Mamm Genome 2001. January;12(1):52–59. doi: 10.1007/s003350010230 [DOI] [PubMed] [Google Scholar]
- 24.Zins SR, Amare MF, Anam K, Elster EA, Davis TA. Wound trauma mediated inflammatory signaling attenuates a tissue regenerative response in MRL/MpJ mice. J Inflamm 2010;7:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalley AL, Dyment NA, Kazemi N, Kenter K, Gooch C, Rowe DW, Butler DL, Shearn JT. Improved biomechanical and biological outcomes in the MRL/MpJ murine strain following a full-length patellar tendon injury. J Orthop Res 2015;33(11):1693–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Foolen J, Wunderli SL, Loerakker S, Snedeker JG. Tissue alignment enhances remodeling potential of tendon-derived cells – lessons from a novel microtissue model of tendon scarring. Matrix Biol 2017;65:14–29. [DOI] [PubMed] [Google Scholar]
- 27.Zhu J, Li J, Wang B, Zhang WJ, Zhou G, Cao Y, Liu W. The regulation of phenotype of cultured tenocytes by microgrooved surface structure. Biomaterials 2010;31 (27):6952–6958. [DOI] [PubMed] [Google Scholar]
- 28.Li W-Y, Huang E, Chong S, Dudas M, Warburton D, Anderson K, Kaartinen V, Tuan TL. Fetal scarless healing: the role of TGF-beta 3 and pro-fibrotic molecule PAI-1. J Am Coll Surg 2004;199(3):56. [Google Scholar]
- 29.Cowin AJ, Holmes TM, Brosnan P, Ferguson MW. Expression of TGF-beta and its receptors in murine fetal and adult dermal wounds. Eur J Dermatology 2001;11(5):424. [PubMed] [Google Scholar]
- 30.Penn JW, Grobbelaar AO, Rolfe KJ. The role of the TGF-β family in wound healing, burns and scarring: a review. Int J Burns Trauma 2012. February 5;2(1):18–28. [PMC free article] [PubMed] [Google Scholar]
- 31.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sport Med 2003;33(5):381–394. [DOI] [PubMed] [Google Scholar]
- 32.Longaker MT, Chiu ES, Adzick NS, Stern M, Harrison MR, Stern R. Studies in fetal wound healing. V. A prolonged presence of hyaluronic acid characterizes fetal wound fluid. Ann Surg 1991;213(4):292–296. (8): 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McHutchison JG, Blatt LM, De Medina M, Craig JR, Conrad A, Schiff ER, Tong MJ, Group TCIS. Measurement of serum hyaluronic acid in patients with chronic hepatitis C and its relationship to liver histology. J Gastroenterol Hepatol 2000;15 (8):945–951. [DOI] [PubMed] [Google Scholar]
- 34.Akin D, Ozmen S, Yilmaz ME. Hyaluronic acid as a new biomarker to differentiate acute kidney injury from chronic kidney disease. Iran J Kidney Dis 2017;11(6):409–413. [PubMed] [Google Scholar]
- 35.Dentener M, Vernooy J, Hendriks S, Wouters E. Enhanced levels of hyaluronan in lungs of patients with COPD: relationship with lung function and local inflammation. Thorax 2005. February;60(2):114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Albano G, Bonanno A, Cavalieri L, Ingrassia E, Di Sano C, Siena L, Riccobono L, Gagliardo R, Profita M. Effect of high, medium, and low molecular weight hyaluronan on inflammation and oxidative stress in an In Vitro model of human nasal epithelial cells. Mediators Inflamm 2016;2016:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-β by the proteoglycan decorin. Nature 1990. July;19(346):281. [DOI] [PubMed] [Google Scholar]
- 38.Walker A, Turnbull JE, Gallagher JT. Specific heparan sulfate saccharides mediate the activity of basic fibroblast growth factor. J Biol Chem 1994;269(2):931–935. [PubMed] [Google Scholar]
- 39.Kaux J-F, Janssen L, Drion P, Nusgens B, Libertiaux V, Pascon F, Heyeres A, Hoffmann A, Lambert C, Le Goff C, Denoël V, Defraigne JO, Rickert M, Crielaard JM, Colige A. Vascular Endothelial Growth Factor-111 (VEGF-111) and tendon healing: preliminary results in a rat model of tendon injury. Muscles Ligaments Tendons J 2014. May 8;4(1):24–28. Available from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4049645/ [PMC free article] [PubMed] [Google Scholar]
- 40.Docheva D, Hunziker EB, Fässler R, Brandau O. Tenomodulin is necessary for tenocyte proliferation and tendon maturation. Mol Cell Biol 2005. January 15;25(2):699–705. Available from http://www.ncbi. nlm.nih.gov/pmc/articles/PMc543433/ [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


