Abstract
Healthy tendons are maintained in homeostasis through controlled usage of glucose for energy and redox equilibrium. Tendon cell stress imposed by overuse injury or vascular insufficiency is accompanied by activation of wound healing pathways which facilitate an adaptive response and the restoration of homeostasis. To understand this response at the gene expression level we have studied the in vivo effects of injected TGF-β1 in a murine model of tendinopathy, as well as treatment of murine tendon explants with either TGF-β1 or hypoxia in vitro. We provide evidence (from expression patterns and immunohistochemistry) that both in vivo and in vitro, the stress response in tendon cells may be metabolically controlled in part by glycolytic reprogramming. A major feature of the response to TGF-β1 or hypoxia is activation of the Warburg pathway which generates lactate from glucose under normoxia and thereby inhibits mitochondrial energy production. We discuss the likely outcome of this major metabolic shift in terms of the potential benefits and damage to tendon and suggest how incorporation of this metabolic response into our understanding of initiation and progression of tendinopathies may offer new opportunities for diagnosis and the monitoring of therapies.
Keywords: Tendinopathy, Transforming growth factor beta 1, Hypoxia, Metabolic Reprogramming, Glucose Metabolism
Introduction
An association between HIF1A accumulation and cellular apoptosis with the severity of human rotator cuff tendinopathy (1) provided early support for the hypothesis that hypoxic stress is a common feature of all tendinopathies. More recent evidence has emerged from supraspinatus tendon samples suggesting that changes in HIF1A are accompanied concurrently by altered abundance of other injury response factors including alarmins, such as S100A9, IL-33, and HMGB1 (2). Moreover, these alarmins are localized to macrophage-like cells (CD68+) which produce a variety of growth factors, including TGF-β1, resulting in the activation of stromal fibroblasts (CD68-) and progenitor cells that facilitate tissue repair. Indeed, markers of stromal fibroblast activation, including the tumor markers podoplanin, VCAM1, and endosialin, are increased in tendinopathic samples (3) and such fibroblasts can affect immune cell behaviors (4), neovascularization (5), and fibrosis (6).
While surgical samples can provide details of cellular and matrix changes in chronic tendon disease, there is a paucity of data regarding the HIF1A–responsive genes and effector pathways which operate in acute and chronic tendon injury. For example, in a macrophage-rich environment, increased intracellular HIF1A levels could result from TGF-β1-mediated inhibition of prolyl hydroxylase expression, as shown in tumor cell lines (7), or from bona-fide hypoxia through inhibition of prolyl hydroxylase activity directly (8). In this manner, as HIF1A protein levels are highly regulated at the transcriptional, post-transcriptional, translational, and post-translational (proteolysis/stabilization) levels (9, 10), it is difficult to discern the functional pathways responsible for the production of stable HIF1A protein.
To examine the potential mechanism underlying activated HIF1A signaling in the development of tendinopathy, we have assayed for HIF1A signaling changes, and downstream effects on glycolytic metabolism (glycolysis), angiogenic, and cell fate genes, using a TGF-β1-induced murine Achilles tendinopathy model (11–13). This model,which utilizes a non-surgical injury induced by a physiologically relevant growth factor, is distinct from other in vivo models which utilize partial or complete tendon transection or direct mechanical loading (e.g., overuse treadmill running). Of note, TGF-β1 injection elicits wound healing-related inflammatory responses ((14) and unpublished data), rapid collagen disorganization, chondroid deposition (within 1–2 weeks) and reduction of tendon biomechanical properties (11, 12), all of which are typical of human tendinopathies (15–20). To further delineate typical hypoxia mediated responses, and the potential crosstalk with TGF-β1 response pathways in tendon cells, we utilized a murine Achilles tendon explant system to examine cell responses to each individual stimulus.
Our previous studies with this tendinopathy model in Adamts5-deficient mice demonstrated defective healing responses of TGF-β1-injured tendons in vivo (13). Furthermore, fibroblast cultures from KO mice showed defective TGF-β1-Smad2,3-dependent regulation of collagen synthesis and altered glucose uptake and intracellular utilization for glycosaminoglycan synthesis (21). We therefore included TS5KO mice in our analyses of in vivo and in vitro HIF1A signaling responses in tendons to further validate our hypothesis for a role of TGFβ1-HIF1A crosstalk in the pathogenesis of tendinopathy.
Data obtained from the combination in vivo and in vitro studies reported here, suggest that metabolic reprogramming of tendon cells targeting primarily the glycolytic pathway and lactate production, is an integral part of a post-injury response in tendons, and downstream regulation of these activated pathways may play an important role in achieving regenerative versus tendinopathic outcomes during the treatment of such injuries.
Methods
Murine Model of Tendinopathy in WT and TS5KO Mice
All animal use described below was approved by the IACUC of Rush University. 12 week old Wild Type (WT) and Adamts5−/− (TS5KO) male mice were bred in-house, with the pups separated at weaning and distributed randomly into the experimental groups. For in vivo experiments in this study, a total of 240 WT and 210 TS5KO mice were used (Supplemental Table 1). All mice had free access to standard chow and water and were exposed to the same light cycles (12 hour light, 12 hours dark) during growth and throughout the experimental period. Tendinopathy was induced by 2 injections of 100ng active TGF-β1 (human recombinant, Peprotech, Inc, Rocky Hill, NJ) into the body of the Achilles tendon on days 0 and 2 of the experimental protocol (11). Mice were allowed normal cage activity for 3, 14, or 28 days. Control groups received no TGF-β1 injection (UI), and sham controls received needle insertion, without TGF-β1. All injections were given between 2pm-4pm and all euthanasia and tissue collections carried out between 9am-11am. After sacrifice, mice within each experimental group were randomly allocated for outcome measurements, with n=2–4/group for histology and 3 replicates of n=12–24/group for gene expression.
Murine Achilles Tendon Explant Culture
A total of 134 WT and 131 TS5KO, 12 week old male mice were used for the in vitro experiments (See Supplemental Table 1). Achilles tendons from both limbs were dissected (with intact peritenon) and placed immediately into CO2-independent medium (Gibco®) supplemented with antibiotics, and then cultured in AMEM (ThermoFisher) supplemented with 5mM glucosamine/1% FCS (basal medium) and maintained at 5% CO2/air in either 20% or 5% O2. Additional cultures were maintained in basal medium supplemented with 10 ng/mL TGF-β1 and/or 30nM TGF-β1 Receptor Kinase RI/II dual inhibitor LY2109761 (Selleckchem) (22), under 20% or 5% O2. For all experimental groups, 6–8 tendons were cultured together in 60mm2 non-adherent petri dishes containing 1mL of media per tendon. Media was changed at 24h and 72h, and cultures terminated at 96h. Explant conditioned media was stored at –20°C until further analyses. Upon explant harvest, tendons were randomly assigned for histologic (n=3–4/group), biomechanical (n=5–6/group), gene expression (n=13–22 pooled/group) or Alamar Blue (n=3/group) assays, with glucose and lactic acid assays performed on explant conditioned media from n=13–22 tendons explants per experimental group.
HIF1A Immunohistochemistry
As previously described (12), in vivo specimens were fixed in formaldehyde, decalcified using EDTA, processed, embedded in paraffin, and sectioned through the entire ankle joint using 5 #03BC;m thin sagittal sections (6 sections analyzed per in vivo specimen with n=2–4 specimens in each experimental group). Explanted tendons were embedded in HistoGel (Thermo) before fixation, processed, paraffin embedded, and then sectioned longitudinally (6 sections analyzed per in vitro specimen with n=3–4 specimens in each experimental group). For immunohistochemistry, sections were deparaffinized and incubated overnight at 4°C with anti-HIF1A (ab114977, rabbit polyclonal against the human C-terminal (50-residues), Abcam, San Francisco, CA) followed by biotinylated anti-rabbit IgG as a secondary antibody. All sections were counterstained with methyl green.
QPCR Gene Expression Assays
Immediately following sacrifice or explant, Achilles tendons (with peritenon intact) were placed in RNALater and stored at −20o C. RNA was isolated from tissue pools containing 10–24 tendons for each experimental group (Supplemental Table 1), as previously described (11, 12). Briefly, pooled tissue was fragmented under liquid nitrogen in a Bessman Tissue Pulverizer and extracted in 1 mL of Trizol by vortexing for 60 seconds. RNA purification was done with the RNeasy MiniKit (Qiagen, Cat #:74104, Valencia, CA), with yields of approximately 305ng /tendon for uninjured, 805ng/tendon 3 days post-injury, 1800ng/tendon 14 days post-injury, and 800ng/tendon 28 days post-injury. For explant tissues, yields were approximately 148 ng/tendon for freshly excised, 219ng/tendon for basal, 537ng/tendon for basal + TGF-β1, 468ng/tendon for 2.5% O2, 599ng/tendon for basal + TGF-β1 + LY2109761, and 440ng/tendon for 2.5% O2 + LY2109761.
RNA quality (A260:A280) was greater than 1.90 for all preparations, and 500ng of each RNA preparation was converted to cDNA using the RT2 First Strand Kit (Qiagen). Hypoxia signaling pathway and downstream target gene transcript abundances were determined using SYBR qt-PCR array plates (PAMM-032ZA, Qiagen). The list of genes (n=86), provided in Supplemental Table 2, included 25 genes involved in HIF1A Signaling, 26 genes for glycolytic metabolism, 16 genes for angiogenesis and coagulation, and 19 genes for cell fate. The reproducibility of the QPCR assay was confirmed by triplicate assays of a typical sample (i.e., WT 3 day Pool #3) which showed a coefficient of variation of less than 5% for all genes. For explant tissues, single tube assays using the Taqman expression assays (In Vitrogen) were also performed for Col1a1, Col1a2, Col3a1, and Has2 as previous described (12). Due to a low coefficient of variation between triplicate pools for each in vitro experimental group, only one pool per group was utilized in the present study for in vitro experiments. It should be noted that the data for individual pools represents the average expression from 13–22 individual tendons (11).
Changes in transcript abundance (ΔCt=Ct for transcript of interest minus Ct for the housekeeping gene, B2m) were used to calculate the fold change (2^-ΔΔCt) relative to un-injured or basal levels for in vivo and in vitro experimental groups, respectively. Of note, three additional housekeeping genes (Gapdh, Actb, and Gusb) were included on the custom array plate, with only B2m demonstrating minimal variation in Ct values across experimental samples assayed. A 1-way ANOVA with Tukey’s post-hoc test was conducted using GraphPad Prism 5 (La Jolla, CA) on the ΔCt values to determine the significance (p<0.05) in expression of genes in the post-injury (3d, 14d and 28d) compared to uninjured (UI) groups for each genotype. An unpaired Student’s t-test was used to compare TS5KO and WT values for each experimental group.
Alamar Blue Cell Viability Assay of Explanted Tendons
The Alamar Blue assay was used to determine cell viability of explants by measuring the reducing activity (as NADH/NADPH) via conversion of resazurin to resorufin. Individual tendons removed at 72 hours of explant culture were incubated at 37°C in 20% O2 for 24h in 12 well plates containing 1 mL of fresh medium with 10% (v/v) Alamar Blue Reagent (ThermoFisher). The media was removed and the fluorescence was measured (excitation: 530nm, emission: 590nm). Freshly harvested tendons, undergoing the same protocol were used as controls. Within each genotype, groups were compared with a 1-way ANOVA followed by Tukey’s post-hoc tests (*p<0.05) using GraphPad Prism 5 (La Jolla, CA). Differences between TS5KO and WT mice for each experimental group were determined using an unpaired Student’s t-test.
Biomechanical Testing
The cross-sectional area of each tendon was measured using a precision caliper (width) and laser displacement sensor (thickness), assuming a rectangular geometry (23). Tendons were clamped in custom grips using an initial grip-to-grip length of 3.75mm and placed in an isotonic saline bath within an electromechanical testing system (MTS, Eden Prairie, MN) equipped with a 10 lb load cell. Following a ten minute equilibration in the saline bath and a preload of 0.05N for 2 minutes, tendons were loaded to failure at 0.05mm/sec. Following testing, maximum stress and elastic modulus was determined. Within each genotype, groups were compared using a 1-way ANOVA and Tukey’s post hoc tests (p<0.05). For genotypic comparisons an unpaired Student’s t-test was used to compare TS5KO and WT values for each experimental group.
Lactate and Glucose Assays of Explant Culture Medium
Lactate concentration in explant conditioned media collected after 0–24 hours, 24–72 hours, and 72–96 hours of culture was measured with a colorimetric kit obtained from Abcam (ab65331). This assay gives a linear A410 absorbance range between 0–10 nmole of lactate standard. Accuracy of the reaction was determined by assaying n=4 of each standard (0.1, 0.5, 1.0, 2.0, 4.0, 8.0 and 10 nmol lactate) or 10 μL of unconditioned blank medium (coefficient of variation less than 1%). 10 μL portions of blank or conditioned media from 2 separate explant cultures for each experimental condition (Supplemental Figure 1) were assayed in duplicate, and a less than 4.2% coefficient of variation between duplicate assays was obtained for each experimental condition. The amount of lactate secreted at each time-point was calculated as the average A410 Conditioned Media minus the A410 Media Blank and normalized to the number of tendons in that explant culture. The total lactate accumulated by each culture over the 96h culture period was calculated by adding the lactate produced at each time-frame (0–24h, 24–72h, and 72–96).
Glucose concentration in blank and explant conditioned media was measured using a colorimetric kit obtained from In Vitrogen. No significant differences in concentration of medium glucose were detected in each of the conditioned media relative to the medium blank. This is likely due to the large excess of glucose provided to each culture, relative to the amount used metabolically in vitro by explanted tissues of low cellularity, such as tendon.
Results
TGF-β1 injection induces Hif1a gene expression and protein accumulation in vivo in WT and TS5KO tendon cells
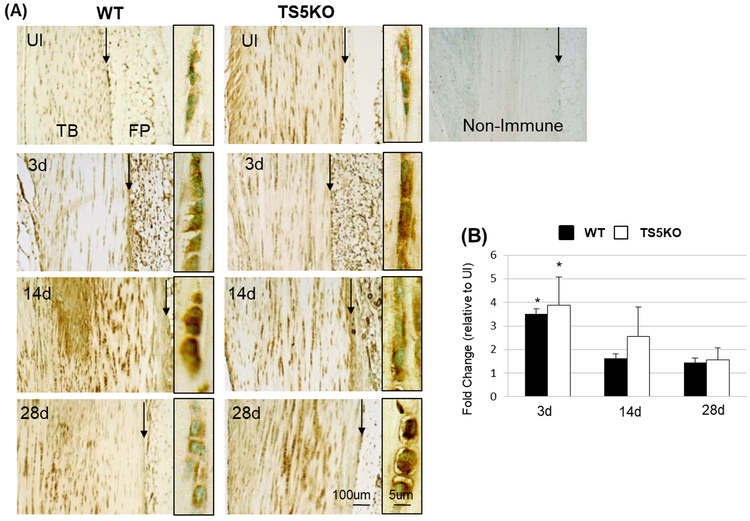
HIF1A cellular immunostaining was increased at 3 and 14 days after TGF-β1 injection, in both WT and TS5KO mice relative to un-injured levels (Figure 1A). Notably, whereas the staining for HIF1A had decreased by 28 days in WT mice, it remained high in TS5KO, most prominently in the rounded cells of the tendon body. Consistent with the protein staining, in both genotypes, Hif1a expression was increased at 3 days for both WT and TS5KO mice (3.5-fold and 3.9-fold, respectively) and returned to un-injured levels at 14 and 28 days (Figure 1B). No significant differences were detected between WT and TS5KO tendons at each time-point (Un-injured, 3 days, 14 days, or 28 days). Since TS5KO mice exhibit a more severe TGF-β1-induced tendinopathic response (13), the presence of long-lived HIF1A protein in TS5KO tendons is consistent with a central role for HIF1A-inducible genes in the pathology.
Figure 1:
(A) Representative images (from 12–24 sections per experimental group) of HIF1A histochemical localization in Achilles tendons from WT and TS5KO mice, before (UI) or after injection of TGF-β1 (3d, 14d, and 28d). Framed panels (high magnification) show tendon cell-associated staining from areas in the tendon body. (*) Negative control with Non-Immune Rabbit IgG. Abbreviations: Tendon body (TB), Peritenon (black arrow), and fat pad (FP). (B) Average (STD) fold change in expression of Hif1a (taken from the hypoxia signaling pathway QPCR array) in WT and TS5KO Achilles tendons after injection of TGF-β1 (3d, 14d, and 28d) relative to before injection. *p<0.05 relative to UI.
TGF-β1 injection into tendons in vivo induces expression of hypoxia signaling pathway, angiogenesis and glycolytic metabolism genes
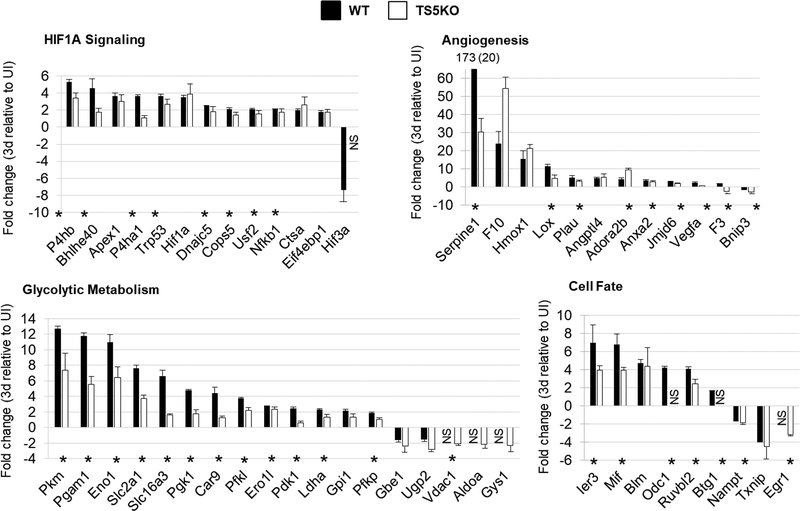
The expression (ΔCt) pattern of 86 hypoxia signaling genes in un-injured and post-injury (3 days, 14 days, and 28 days) tendons for WT and TS5KO mice can be seen in Supplemental Figure 1. The majority of genes were either activated greater than 2-fold or unaffected by the injury, with activation most evident at 3 days and normalization occurring over the 14 and 28 day post-injury period. Furthermore, a greater number of genes were activated in WT compared to TS5KO mice, and particularly in the angiogenesis and glycolytic metabolism groups. The genes which exhibited a statistically significant fold-activation (over un-injured) are summarized in Figure 2, and the fold-change values at 3 days in each group for WT and TS5KO mice are shown in Figure 3. Taken together, the gene expression data strongly suggest that TGF-β1 injection in tendon in vivo induces a metabolic response that mimics hypoxic conditions, classically associated with a switch from mitochondrial oxidative phosphorylation to cytosolic lactate production and secretion (24). Notably, the degree of this metabolic switch was repressed in TS5KO relative to WT tendons.
Figure 2:
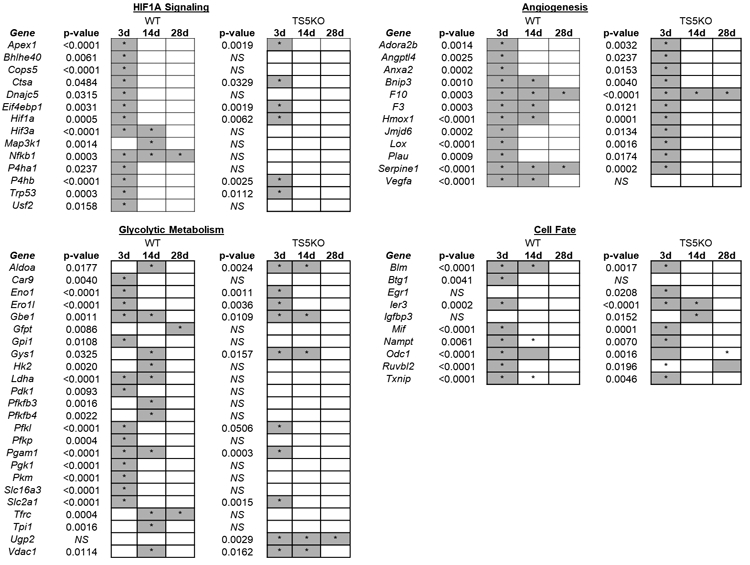
Summary of statistically significant changes (*p<0.05) in hypoxia responsive genes of WT and TS5KO tendons after injection of TGF-β1 (3d, 14d, and 28d) relative to un-injured. Genes are separated based on function: Hif1a signaling, angiogenesis, glycolytic metabolism, and cell fate.
Figure 3:
Average (STD) fold-change in expression of significantly altered hypoxia signaling genes in Achilles tendons 3 days post- TGF-β1 injection relative to un-injured. Genes are separated based on function: HIF1A Signaling, Angiogenesis, Glycolytic Metabolism, and Cell Fate. NS = no significance relative to un-injured. Statistically significant differences (p<0.05) between WT and TS5KO tendon responses are shown by an asterisk (*).
TGF-β1-induced changes in expression of hypoxia-related genes in vivo can be reproduced by TGF-β1 or hypoxia in tendon explants.
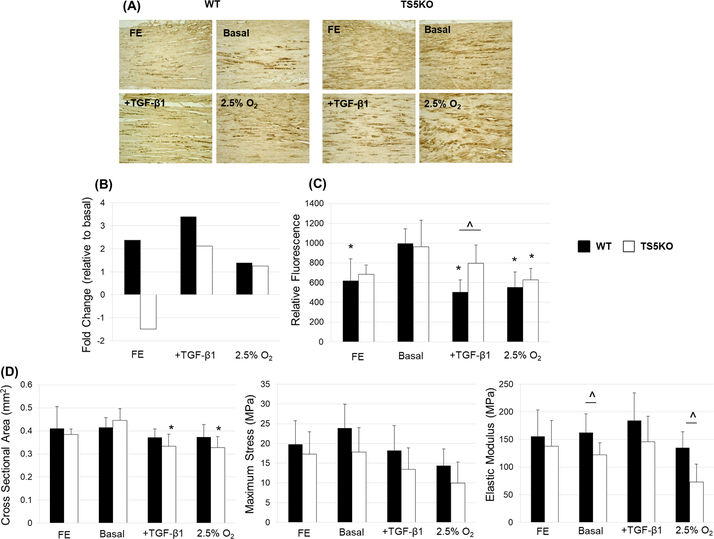
We next examined whether these HIF1A-related metabolic responses of TGF-β1 in vivo are the result of a transient hypoxic condition in the injured tendon, or whether TGF-β1 itself can cause a metabolic reprogramming via HIF1A signaling. For this purpose, a short term explant culture under low serum conditions was developed for murine Achilles tendons. HIF1A localization (Figure 4A) of freshly excised and explant-maintained WT and TS5KO tendons showed increased distribution of HIF1A protein in basal conditions relative to freshly excised tendons, but gave no indication that the abundance or cell/matrix distribution of HIF1A protein was markedly affected by TGF-β1 or low O2 culture conditions. Further, the fold-change in expression of Hif1a (relative to basal culture conditions) in explants under TGF-β1 or low O2 culture conditions (Figure 4B) showed no major differences. As assessed by Alamar Blue, an indicator of cell viability, maintenance under normoxic conditions (basal) showed significantly (p<0.05) high metabolic activity over 72 hours (Figure 4C) compared to freshly excised (WT only), +TGF-β1 (WT only), and low O2 (WT and TS5KO) explants. Metabolic activity was not significantly altered by the addition of LY2109761, a TGF-β Receptor kinase I/II dual inhibitor (data not shown). Notably, in the presence of TGF-β1, TS5KO explants displayed significantly (p<0.05) higher oxidative metabolic activity relative to WT, which could be related to dysregulated glucose uptake mechanisms in TS5KO cells (21). Furthermore, there was no detectable tissue swelling or loss of biomechanical properties under any culture condition (Figure 4D). However, TS5KO tendons exhibited significantly (p<0.05) decreased cross-sectional area with the addition of TGF-β1 and/or low O2 relative to basal media conditions. Additionally, while biomechanical properties were not broadly affected by culture conditions, TS5KO tendons exhibited a significantly (p<0.05) decreased elastic modulus relative to WT tendons, but only in the absence of TGF-β1 (basal and low O2 groups).
Figure 4:
(A) Representative images (from 18–24 sections per experimental group) of HIF1A localization of WT and TS5KO freshly excised Achilles tendons and tissue maintained for 4 days in explant culture under basal (20% O2), basal + TGF-β1, or 2.5% O2 conditions. (B) Fold-change (relative to basal culture conditions) expression of Hif1a in freshly excised and 4 day explanted WT and TS5KO Achilles tendons. (C) Alamar Blue assay for viability of freshly excised and explanted WT (p=0.0002) and TS5KO (p=0.0027) Achilles tendons. * = significant (p<0.05) differences relative to basal conditions. ^ = significant (p<0.05) differences between WT and TS5KO. (D) Geometric and material properties of freshly excised and explanted WT (CSA: p=0.5057, Max Stress: p=0.0840, Elastic Modulus: p=0.2958) and TS5KO (CSA: p=0.0009, Max Stress: p=0.1132, Elastic Modulus: p=0.0266) Achilles tendons. * = significant (p<0.05) differences relative to basal conditions. ^ = significant (p<0.05) differences between WT and TS5KO.
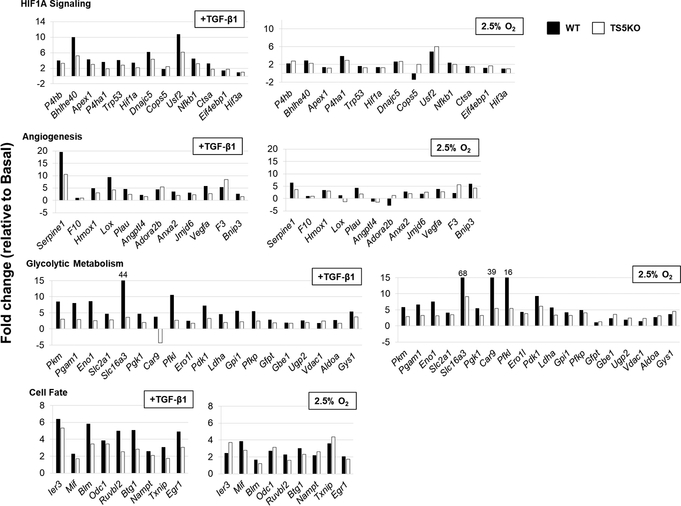
Additionally, explanted WT and TS5KO tendons were assayed after the 96h culture period using the hypoxia signaling gene array. ΔCt data from all culture conditions are summarized in heatmap format (Supplemental Figure 2), with the data obtained from un-injured and 3 days post TGF-β1 injection in vivo tendons (Supplemental Figure 1) also shown for comparison. For all gene groups, TGF-β1 or low O2 resulted in activated expression relative to basal culture conditions.
The fold-change in expression produced by TGF-β1 or low O2 conditions, relative to basal conditions in vitro, are summarized in Figure 5. To facilitate comparison with the in vivo data (Figure 3), the affected genes are arranged in the same order on both Figures. Although the basal level of expression (ΔCt values) had decreased upon explant, likely due to generalized depression of protein synthesis in the serum-free conditions, the degree of activation by TGF-β1 in vitro was generally similar (with some exceptions) to that observed in vivo. Furthermore, the greater activation seen in vivo in the WT relative to TS5KO tendons was also broadly reproduced in explants under TGF-β1 (or low O2), particularly for the glycolytic metabolism gene group (Figure 5). This genotypic difference further supports the suitability of the explants for further mechanistic studies. In this respect, a range of HIF1A target genes modified by TGF-β1 in the explant were also affected by low O2 (Figure 5, right hand panels). For WT tissues, these included P4ha1, Usf2, Serpine1, Plau, Vegfa, Bnip3, Mif, Txnip, and the majority of genes in the Glycolytic Metabolism group such as Pkm, Pgam1, Eno1, Slc2a1, Slc16a3, Pgk1, Car9, Pfkl, Ero1l, Pdk1, Ldha, Gpi1 Pfkp, and Gys1. Most notably, whereas the degree of activation for the metabolism genes in WT explants was similar for TGF-β1 and low O2 culture conditions, in TS5KO explants the degree of activation by low O2 was consistently greater than with TGF-β1. This observation is consistent with our earlier studies (25) showing a decrease in TGFβ1-Smad2 signaling in the skin fibroblasts of TS5KO mice.
Figure 5:
Fold-change in expression of hypoxia responsive genes altered by TGF-β1 or 2.5% O2 in explanted WT and TS5KO Achilles tendons relative to basal culture conditions (20% O2). Genes are separated based on function: HIF1A Signaling, Angiogenesis, Glycolytic Metabolism, and Cell Fate.
The effect of TGF-β1 RI/II kinase inhibitor on TGF-β1 or hypoxia-mediated activation of glycolytic metabolism and matrix genes in explanted tendons
To identify TGF-β1 (and potentially low O2) mediated gene expression changes which are transduced via the TGF-β1 receptor pathway, the TGFβ1RI/II kinase inhibitor LY2109761 was utilized in explant cultures. The fold-effects of the inhibitor on gene expression levels in the TGF-β1 or low O2 culture conditions are shown in Figure 6. In WT explants maintained in TGF-β1, the expected inhibitory effect of LY2109761 was seen for only two metabolism genes, Ugp2 (UDP glucose synthesis) and Gfpt (hexosamine synthesis) with two additional genes, Slc2a1 (glucose transporter GLUT1) and Eno1 (glycolytic enzyme), also inhibited in TS5KO explants (Figure 6, left hand panel). Unexpectedly, the inhibitor had no effect on TGF-β1-activated expression of 5 and 15 genes in WT and TS5KO tendons, respectively. Moreover TGF-β1-activated transcript levels were further activated by LY2109761 for 13 genes in WT and 1 gene (Carb9, carbonic anhydrase for pH regulation) in TS5KO explants.
Figure 6:
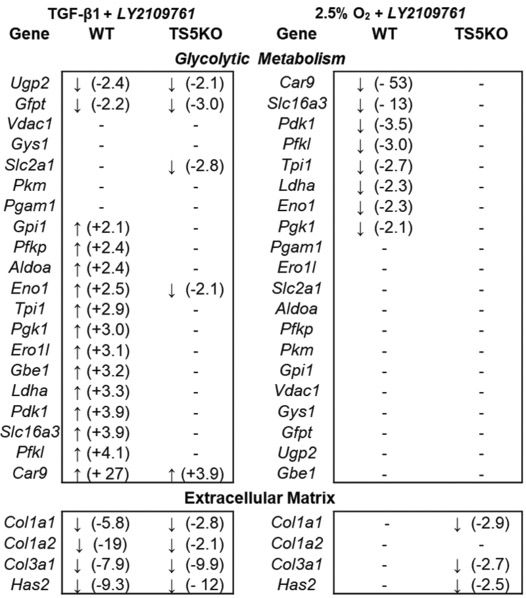
Effect of TGF-β1 receptor I/II dual inhibitor LY2109761 on expression of glycolytic metabolism and ECM genes WT and TS5KO Achilles tendons maintained in TGF-β1 or 2.5% O2. Data are expressed as fold-change relative to no inhibitor conditions.
Since low O2 activated the same set of genes, we also tested the effect of LY2109761 on explants maintained in low O2 culture conditions. (Figure 6A, right hand panels). A distinctly different pattern from the mixed effects on TGF-β activation was evident. Firstly, in WT explants, the inhibitor lowered transcript abundance (>2 fold) for 8 hypoxia activated metabolism genes, suggesting crosstalk between hypoxia and TGF-βI/II receptor signaling in their regulation. The remaining hypoxia-activated metabolism genes were, however, unaffected by LY2109761, and unlike for TGF-β1 activated metabolic genes, no genes were further stimulated in its presence. Of particular interest was the complete lack of an effect of the inhibitor on TS5KO tendon explants maintained in low O2.
The diverse effects of LY2109761 on TGF-β1 signaling have been previously described for both its in vitro and in vivo usage (26–30), in which LY2109761 binding to the TGF-β receptors was reported to initiate cross-talk between TGF-β1 and other signaling pathways. Indeed, such mechanisms might be operating during regulation of the metabolic state of cells under stress from physical or ischemic injuries. The unexpected effects of LY2109761 on TGF-β1 activations, does not appear to be attributable to a lack of experimental control since the expected inhibitory specificity on TGF-β1-mediated activation of three collagen genes (Col1a1, Col1a2, Col3a1) in WT and TS5KO explants was observed (Figure 6). In support of its specificity for the TGFbRI/II complex, the inhibitor had essentially no effect on the low O2-mediated stimulation of the collagen genes assayed here.
Evidence for activation of aerobic glycolysis and lactate secretion in tendon cells after TGF-β1 treatment
The glycolytic metabolism genes activated in tendon cells by TGF-β1 injection in vivo and in vitro (Figures 4 and 7, respectively), were also modified under low O2 conditions in explanted tendons (Figure 5). These genes included Pdk1 (which inactivates PDH, thereby blocking pyruvate entry to the TCA cycle), Ldha, (the protein product of which converts pyruvate to lactate), and Slc16a3 (encoding a transporter that catalyzes lactate export from the cell). These modulations suggest that the primary end product of glucose metabolism in tendon cells under TGF-β1 or low O2 is lactate. While this pathway is expected to operate under hypoxia (due to insufficient oxygen for mitochondrial activity) it can also be activated under normoxic conditions and this situation is referred to as glycolytic reprogramming or the Warburg effect (31). Notably, Warburg metabolism is a common pathway in fibrotic tissues (32, 33) and also operates in tumor cell survival (34).
Figure 7:
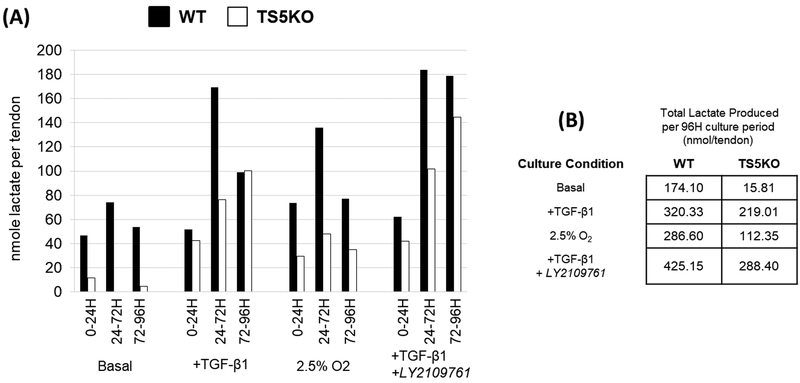
Lactate secretion (nmol/tendon) into the medium of explanted WT and TS5KO Achilles tendons maintained in (basal (20% O2), basal + TGF-β1, 2.5% O2, or basal + TGF-β1 + LY2109761) conditions. (A) Lactate secretion was measured following each media change (0–24h, 24–72h, and 72–92h), (B) with the cumulative total produced over the 92h culture period provided.
The extent to which this pathway may be operating in tendon cells during exposure to TGF-β1 or low O2 in explant culture was determined by assaying lactate production from a non-limiting supply of extracellular glucose. Indeed, TGF-β1 or low O2 culture conditions stimulated the secretion of lactate into explant medium (Figure 7). Notably, the production of lactate was lower in TS5KO tendon explants, which is consistent with the broadly lower expression of glycolytic and lactate-related enzymes (Ldha, Slc16a3) in the TS5KO tissues (Figure 5). Furthermore, addition of LY2109761 to TGF-β1 supplemented cultures enhanced the production of lactate. This, together with a lack of an inhibitory effect on transcript levels for the glycolytic enzymes (Figure 6), suggests that the TGF-β1-stimulated Warburg effect may not be due to canonical TGF-β1 signaling, but rather due to crosstalk with other pathways, possibly those involving AKT and ID2-mediated control of gene expression (35). Overall, the independent and combined effects of TGF-β1, low O2, and LY2109761 on the amount of lactate produced in explant culture strongly supports a pivotal role for glycolytic reprogramming in tendon cell responses to injurious and hypoxic stress.
Discussion
The current study was motivated by the need to uncover cellular response mechanisms which are common to clinical tendinopathies, animal models of tendinopathy, and in vitro tendon analyses. While stress response mediators (HIF1A (36), S100A9 (2), and HMGB1/TLR4 (37)), inflammatory markers (IL-17A (38) and IL-33 (39)), and stromal fibroblast markers (PDPN, CD248, and CD106 (3)) have been detected in human tendinopathic samples, it is unclear whether inflammatory markers provide useful insight into disease mechanism. In this regard, a recent short-term Ibuprofen study in chronic human Achilles tendinopathy (40) found no evidence that treatment provided any benefit in tendon pain or function.
Therefore, an alternative to searching for additional markers is to develop metabolic pathway expression profiles which can be used in conjunction with the assay of metabolic end products. With a view to developing this approach for tendon diseases, we have used a murine model to examine the effect of tendinopathic injury on cellular energy production and the expression of multiple enzymes controlling the fate of cellular glucose. The murine model of Achilles tendinopathy studied here (12) is initiated by TGF-β1 injection. Our use of TGF-β1 as the initiating agent appears to be counter-intuitive since this growth factor is generally regarded as the archetypal factor for ECM assembly and repair, whereas in human tendinopathy the collagen matrix becomes disorganized and the tissue is characterized by pathological remodeling, including chondroid, osteoid, and lipid deposition (41). In fact, the data reported here strongly support the idea that the metabolic changes which follow TGF-β1 injection in the tendon (including activation of HIF1A-responsive genes and matrix genes) are characteristic of a cellular stress response.
Overall, the data reported here led us to the novel observation that glycolytic reprogramming and increased lactate secretion by tendon cells may represent a robust metabolic marker of disease activity. Figure 10 summarizes the observed expression changes of enzymes involved in glycolytic and related pathways which were found to be modified by both TGF-β1 and low O2. In addition, our studies with explanted tendon tissue using a TGF-β1RI/II receptor LY2109761 supported other published in vivo and in vitro studies (24, 26–28). Thus, binding of the inhibitor to the TGF-β receptors can initiate cross-talk between TGF-β1 and other signaling pathways such as growth factor mediated activation of PI3K/AKT. Indeed, such mechanisms might be operating during regulation of the metabolic state of tendon cells undergoing proliferation or responding to stresses induced by physical and ischemic injuries.
The Warburg response to stress under normoxic conditions, which results in a redirecting of glucose usage away from mitochondrial oxidative phosphorylation toward lactate production, actually represents a major loss in ATP yield (30mol/mol glucose (OxPhos) and about 4mol/mol (Warburg)) (31). Though energy-inefficient, Warburg metabolism has been considered as an adaptive response to cell stress which confers benefit to respiring cells, including the capacity for rapid ATP production and ready maintenance of redox homeostasis under stress. However, Warburg metabolism is also a major feature of cells involved in tissue fibrosis (32, 33), immune function (42), and tumorigenesis (43), and in these cases, reversal of the Warburg effect via enhancement of mitochondrial oxidative phosphorylation has arisen as a potential therapeutic intervention (44).
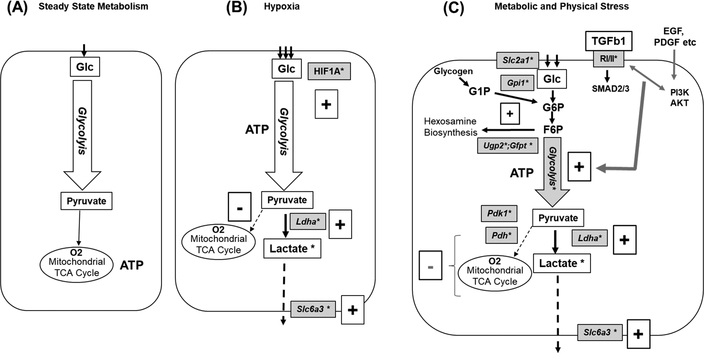
In the current context, it is important to note that the cellular switch to Warburg metabolism in tendons can be activated by either hypoxia (low O2) or TGF-β1 (Figure 8), which is consistent with the literature showing that it is not simply a response to hypoxia but also to growth factor stimulation under normoxic conditions. For example, glycolytic reprogramming accompanies TGF-β1-mediated fibroblast to myofibrobast transition (32, 33), PDGF-enhanced proliferation of smooth muscle cells (31), and EGF-stimulated tumorigenesis (45, 46). The relevance of this response to diseased tendons is underscored by the reported accumulation of lactate in painful and chronic Achilles tendinopathy (47). Hence, the development of a murine tendon explant model which under hypoxia or TGF-β1 treatment reproduces the glycolytic reprogramming seen in vivo provides a framework for future studies directed toward understanding tendon-specific features of this widely observed stress response mechanism. Additionally, the use of the mouse model for in vivo monitoring of post-injury lactate production (anaerobic glycolysis) in relation to glucose uptake during reparative (e.g. cell proliferation, ECM production) responses in WT tendons vs pathogenic responses (collagen disorganization, chondroid metaplasia, reduced mechanical function) in TS5KO tendons would further inform on using metabolic programming as an indicator for prevention vs development of chronic tendinopathies.
Figure 8:
Schematic Illustrating Potential Pathways for Glucose Utilization and Energy Production by Tendon Cells. (A) Steady State Metabolism (Pyruvate Supply for Mitochondrial Oxidative Phosphorylation); (B) Hypoxia/HIF1A Induced switch to anaerobic glycolysis, with cytosolic production of lactate from pyruvate; (C) Stress-Induced Aerobic Glycolysis with cytosolic production of lactate from pyruvate. Injury related growth factors such as TGF-βs, EGF and PDGF signal via receptors and cross-talk involving PI3K/AKT and Smads. This results in activation of genes for glucose uptake (Slc2a1), glycolytic production of pyruvate (Pfkp, Aldoa, Pgk1, Pgam1, Eno1, Pkm), conversion of pyruvate to lactate (Ldha) and lactate removal from the cell (Slc16a3). At the same time, Pdk1 expression is activated, which blocks conversion of pyruvate to acetylCoA by Pdh, which thereby prevents usage of glucose for mitochondrial oxidative phosphorylation. (*) Indicates genes and metabolites assayed in the current study. Plus (+) and minus (–) signs denote activation or deactivation of pathway components, respectively. Abbreviations:Glc:glucose;G1P:glucose-1-phosphate; G6P:glucose-6-phosphate ; F6P:fructose-6-phosphate; Gpi1:glucose-phosphate isomerase; Ugp2:UDP-glucose pyrophosphorylase 2; Gfpt: glutamine fructose-6-phosphate transaminase; Ldha ;lactate dehydrogenase A; Pdh: pyruvate dehydrogenase Slc2a1;solute carrier family 2 (facilitated glucose transporter), member 1 ; Slc6a3;solute carrier family 16 (monocarboxylic acid transporters), member 3; RI/II : TGFb1 receptor I & 2 kinases, (blocked by LY2109761).
A potential technical limitation of the current study is that due to the small size of the murine Achilles tendon, residual cell populations from surrounding sources (peritenon, fat pad, and/or synovium) may have been included in excised tendons from both the in vivo and in vitro specimens analyzed. Since we previously showed that cells in the peritenon are also affected by TGF-β1 injection (11), the extent to which the metabolic responses seen in this model are generated by a single or multiple cell populations remains to be determined. Future work will focus on identifying the contributions of individual cell populations from associated tissues, such as the Achilles enthesis and the retrocalcaneal bursa, to further understand the overall response of the Achilles to tendon body injury. In addition, a more detailed analysis of cell-specific responses would confirm the expression of metabolic reprogramming during post-injury activation of tendon progenitor cells (48, 49).
With respect to the continued clinical challenge of treatments for tendinopathies, in vivo imaging of glucose utilization (50) and pyruvate to lactate production (51) to detect the onset and extent of metabolic reprogramming in affected tendons, might provide a new approach to evaluating disease initiation, the extent of progression versus healing, and the effectiveness of targeted interventions.
Supplementary Material
Acknowledgements:
Funding:
This study was funded by NIH AR63144 (VMW), Rush Arthritis Institute (AP), and Katz Rubschlager Endowment for OA Research (AP).
Glossary:
- WT
Wild-Type
- TS5KO
Adamts5−/−
- UI
Un-injured
- 3d
3 days post-injury
- 14dCA
14 days post-injury
- 28dCA
28d post-injury
- TGF-β1
Transforming Growth Factor beta 1
- ECM
Extracellular Matrix
- HIF1A
hypoxia-inducible factor-1a
- LY2109761
TGF-β1 Receptor Kinase RI/II dual inhibitor
- Col1a1
Collagen Type I alpha 1 gene
- Col2a1
Collagen Type II alpha 1 gene
- Col3a1
Collagen Type III alpha 1 gene
- Has2
Hyaluronan Synthase 2 gene
Footnotes
Declaration of Interest:
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper
References:
- 1.Benson RT, McDonnell SM, Knowles HJ, Rees JL, Carr AJ, Hulley PA. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. The Journal of bone and joint surgery British volume. 2010;92(3):448–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosca MJ, Carr AJ, Snelling SJB, Wheway K, Watkins B, Dakin SG. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1alpha in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc Med. 2017;3(1):e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dakin SG, Buckley CD, Al-Mossawi MH, Hedley R, Martinez FO, Wheway K, et al. Persistent stromal fibroblast activation is present in chronic tendinopathy. Arthritis research & therapy. 2017;19(1):16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley CD, Filer A, Haworth O, Parsonage G, Salmon M. Defining a role for fibroblasts in the persistence of chronic inflammatory joint disease. Ann Rheum Dis. 2004;63 Suppl 2: ii92–ii5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bagley RG, Honma N, Weber W, Boutin P, Rouleau C, Shankara S, et al. Endosialin/TEM 1/CD248 is a pericyte marker of embryonic and tumor neovascularization. Microvasc Res. 2008;76(3):180–8. [DOI] [PubMed] [Google Scholar]
- 6.Smith SW, Eardley KS, Croft AP, Nwosu J, Howie AJ, Cockwell P, et al. CD248+ stromal cells are associated with progressive chronic kidney disease. Kidney international. 2011;80(2):199–207. [DOI] [PubMed] [Google Scholar]
- 7.McMahon S, Charbonneau M, Grandmont S, Richard DE, Dubois CM. Transforming growth factor beta1 induces hypoxia-inducible factor-1 stabilization through selective inhibition of PHD2 expression. The Journal of biological chemistry. 2006;281(34):24171–81. [DOI] [PubMed] [Google Scholar]
- 8.Berra E, Benizri E, Ginouves A, Volmat V, Roux D, Pouyssegur J. HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 2003;22(16):4082–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuschel A, Simon P, Tug S. Functional regulation of HIF-1alpha under normoxia--is there more than post-translational regulation? Journal of cellular physiology. 2012;227(2):514–24. [DOI] [PubMed] [Google Scholar]
- 10.Bardos JI, Ashcroft M. Negative and positive regulation of HIF-1: a complex network. Biochimica et biophysica acta. 2005;1755(2):107–20. [DOI] [PubMed] [Google Scholar]
- 11.Trella KJ, Li J, Stylianou E, Wang VM, Frank JM, Galante J, et al. Genome-wide analysis identifies differential promoter methylation of Leprel2, Foxf1, Mmp25, Igfbp6 and Peg12 in murine tendinopathy. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2017;35(5):947–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell R, Li J, Gorski DJ, Bartels AK, Shewman EF, Wysocki RW, et al. Controlled treadmill exercise eliminates chondroid deposits and restores tensile properties in a new murine tendinopathy model. Journal of biomechanics. 2013;46(3):498–505. [DOI] [PubMed] [Google Scholar]
- 13.Bell R, Li J, Shewman EF, Galante JO, Cole BJ, Bach BR Jr., et al. ADAMTS5 is required for biomechanically-stimulated healing of murine tendinopathy. Journal of Orthopaedic Research. 2013;31(10):1540–8. [DOI] [PubMed] [Google Scholar]
- 14.Bitterman A, Gao S, Trella KJ, Li J, Galante J, Lee S, et al. Time related effects of non-steroidal anti-inflammatory drugs on Achilles tendinopathy in a murine model. Foot & Ankle Orthopaedics 2017;2(2). [Google Scholar]
- 15.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73:1507–25. [PubMed] [Google Scholar]
- 16.Corps AN, Robinson AH, Movin T, Costa ML, Hazleman BL, Riley GP. Increased expression of aggrecan and biglycan mRNA in Achilles tendinopathy. Rheumatology. 2006;45(3):291–4. [DOI] [PubMed] [Google Scholar]
- 17.Khan KM, Cook JL, Bonar F, Harcourt P, Åstrom M. Histopathology of common tendinopathies. Sports Medicine. 1999;27(6):393–408. [DOI] [PubMed] [Google Scholar]
- 18.Khan M, Bonar F, Desmond PM, Cook JL, Young DA, Visentini PJ, et al. Patellar tendinosis (jumper’s knee): Findings at bistopathologic examination, US, and MR lmaging. Musculoskelet Rad. 1996;200:821–7. [DOI] [PubMed] [Google Scholar]
- 19.Attia M, Scott A, Carpentier G, Lian O, Van Kuppevelt T, Gossard C, et al. Greater glycosaminoglycan content in human patellar tendon biopsies is associated with more pain and a lower VISA score. British journal of sports medicine. 2014;48(6):469–75. [DOI] [PubMed] [Google Scholar]
- 20.Jarvinen M, Jozsa L, Kannus P, Jarvinen TL, Kvist M, Leadbetter W. Histopathological findings in chronic tendon disorders. Scandinavian journal of medicine & science in sports. 1997;7(2):86–95. [DOI] [PubMed] [Google Scholar]
- 21.Gorski DJ, Xiao W, Li J, Luo W, Lauer M, Kisiday J, et al. Deletion of ADAMTS5 does not affect aggrecan or versican degradation but promotes glucose uptake and proteoglycan synthesis in murine adipose derived stromal cells. Matrix biology : journal of the International Society for Matrix Biology. 2015;47:66–84. [DOI] [PubMed] [Google Scholar]
- 22.Luo W, Sandy J, Trella K, Gorski D, Gao S, Li J, et al. Degenerative Suspensory Ligament Desmitis (DSLD) in Peruvian Paso Horses Is Characterized by Altered Expression of TGFbeta Signaling Components in Adipose-Derived Stromal Fibroblasts. PloS one. 2016;11(11):e0167069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang VM, Bell RM, Thakore R, Eyre DR, Galante JO, Li J, et al. Murine tendon function is adversely affected by aggrecan accumulation due to the knockout of ADAMTS5. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2012;30(4):620–6. [DOI] [PubMed] [Google Scholar]
- 24.Spriet LL, Howlett RA, Heigenhauser GJ. An enzymatic approach to lactate production in human skeletal muscle during exercise. Med Sci Sports Exerc. 2000;32(4):756–63. [DOI] [PubMed] [Google Scholar]
- 25.Velasco J, Li J, DiPietro L, Stepp MA, Sandy JD, Plaas A. Adamts5 deletion blocks murine dermal repair through CD44-mediated aggrecan accumulation and modulation of transforming growth factor beta1 (TGFbeta1) signaling. The Journal of biological chemistry. 2011;286(29):26016–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Esaki S, Nigim F, Moon E, Luk S, Kiyokawa J, Curry W, Jr., et al. Blockade of transforming growth factor-beta signaling enhances oncolytic herpes simplex virus efficacy in patient-derived recurrent glioblastoma models. Int J Cancer. 2017;141(11):2348–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang M, Kleber S, Rohrich M, Timke C, Han N, Tuettenberg J, et al. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71(23):7155–67. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Ma C, Zhang Q, Yu L, Ma J, Zhang L, et al. The key role of transforming growth factor-beta receptor I and 15-lipoxygenase in hypoxia-induced proliferation of pulmonary artery smooth muscle cells. Int J Biochem Cell Biol. 2012;44(7):1184–202. [DOI] [PubMed] [Google Scholar]
- 29.Bedi A, Chang X, Noonan K, Pham V, Bedi R, Fertig EJ, et al. Inhibition of TGF-beta enhances the in vivo antitumor efficacy of EGF receptor-targeted therapy. Molecular cancer therapeutics. 2012;11(11):2429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu X, Hubchak SC, Browne JA, Schnaper HW. Epidermal growth factor inhibits transforming growth factor-beta-induced fibrogenic differentiation marker expression through ERK activation. Cellular signalling. 2014;26(10):2276–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiao Y, Peng H, Hong C, Chen Z, Deng X, Wang A, et al. PDGF Promotes the Warburg Effect in Pulmonary Arterial Smooth Muscle Cells via Activation of the PI3K/AKT/mTOR/HIF-1alpha Signaling Pathway. Cell Physiol Biochem. 2017;42(4):1603–13. [DOI] [PubMed] [Google Scholar]
- 32.Xie N, Tan Z, Banerjee S, Cui H, Ge J, Liu RM, et al. Glycolytic Reprogramming in Myofibroblast Differentiation and Lung Fibrosis. American journal of respiratory and critical care medicine. 2015;192(12):1462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ding H, Jiang L, Xu J, Bai F, Zhou Y, Yuan Q, et al. Inhibiting aerobic glycolysis suppresses renal interstitial fibroblast activation and renal fibrosis. American journal of physiology Renal physiology. 2017;313(3):F561–F75. [DOI] [PubMed] [Google Scholar]
- 34.Bolanos JP. Adapting glycolysis to cancer cell proliferation: the MAPK pathway focuses on PFKFB3. The Biochemical journal. 2013;452(3):e7–9. [DOI] [PubMed] [Google Scholar]
- 35.Masoud GN, Li W. HIF-1alpha pathway: role, regulation and intervention for cancer therapy. Acta Pharm Sin B. 2015;5(5):378–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Millar NL, Reilly JH, Kerr SC, Campbell AL, Little KJ, Leach WJ, et al. Hypoxia: a critical regulator of early human tendinopathy. Ann Rheum Dis. 2012;71(2):302–10. [DOI] [PubMed] [Google Scholar]
- 37.Akbar M, Gilchrist DS, Kitson SM, Nelis B, Crowe LAN, Garcia-Melchor E, et al. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open. 2017;3(2):e000456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar NL, Akbar M, Campbell AL, Reilly JH, Kerr SC, McLean M, et al. IL-17A mediates inflammatory and tissue remodelling events in early human tendinopathy. Scientific reports. 2016;6:27149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Millar NL, Gilchrist DS, Akbar M, Reilly JH, Kerr SC, Campbell AL, et al. MicroRNA29a regulates IL-33-mediated tissue remodelling in tendon disease. Nat Commun. 2015;6:6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Heinemeier KM, Ohlenschlaeger TF, Mikkelsen UR, Sonder F, Schjerling P, Svensson RB, et al. Effects of anti-inflammatory (NSAID) treatment on human tendinopathic tissue. Journal of applied physiology. 2017:jap 00281 2017. [DOI] [PubMed] [Google Scholar]
- 41.Kannus P, Jozsa L. Histopathological changes preceding spontaneous rupture of a tendon. A controlled study of 891 patients. J Bone Joint Surg Am. 1991;73(10):1507–25. [PubMed] [Google Scholar]
- 42.Tang CY, Mauro C. Similarities in the Metabolic Reprogramming of Immune System and Endothelium. Front Immunol. 2017;8:837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allison KE, Coomber BL, Bridle BW. Metabolic reprogramming in the tumour microenvironment: a hallmark shared by cancer cells and T lymphocytes. Immunology. 2017;152(2):175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang SH, Li W, Sumien N, Forster M, Simpkins JW, Liu R. Alternative mitochondrial electron transfer for the treatment of neurodegenerative diseases and cancers: Methylene blue connects the dots. Prog Neurobiol. 2017;157:273–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xu Q, Zhang Q, Ishida Y, Hajjar S, Tang X, Shi H, et al. EGF induces epithelial-mesenchymal transition and cancer stem-like cell properties in human oral cancer cells via promoting Warburg effect. Oncotarget. 2017;8(6):9557–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang H, Du X, Sun TT, Wang CL, Li Y, Wu SZ. Lectin PCL inhibits the Warburg effect of PC3 cells by combining with EGFR and inhibiting HK2. Oncol Rep. 2017;37(3):1765–71. [DOI] [PubMed] [Google Scholar]
- 47.Alfredson H, Bjur D, Thorsen K, Lorentzon R, Sandstrom P. High intratendinous lactate levels in painful chronic Achilles tendinosis. An investigation using microdialysis technique. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002;20(5):934–8. [DOI] [PubMed] [Google Scholar]
- 48.Young M Stem cell applications in tendon disorders: a clinical perspective. Stem Cells Int. 2012;2012:637836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mienaltowski MJ, Adams SM, Birk DE. Tendon proper- and peritenon-derived progenitor cells have unique tenogenic properties. Stem Cell Res Ther. 2014;5(4):86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang TA, Xian SL, Guo XY, Zhang XD, Lu YF. Combined 18F-FDG PET/CT imaging and a gastric orthotopic xenograft model in nude mice are used to evaluate the efficacy of glycolysis-targeted therapy. Oncol Rep. 2018;39(1):271–9. [DOI] [PubMed] [Google Scholar]
- 51.Niles DJ, Gordon JW, Huang G, Reese S, Adamson EB, Djamali A, et al. Evaluation of renal metabolic response to partial ureteral obstruction with hyperpolarized (13) C MRI. NMR Biomed. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.