Abstract
Aims
Adolescents with conduct and substance use problems are at increased risk for premature mortality, but the extent to which these risk factors reflect family- or individual-level differences and account for shared or unique variance is unknown. The authors examined common and independent contributions to mortality hazard in adolescents ascertained for conduct and substance use problems, their siblings, and community controls, hypothesizing that individual differences in CD and SUD severity would explain unique variation in mortality risk beyond that due to clinical/control status and demographic factors.
Design
Mortality analysis in a prospective study (Genetics of Antisocial Drug Dependence Study) that began in 1993.
Setting
Multi-site sample recruited in San Diego, California and Denver, Colorado.
Participants
1,463 clinical probands were recruited through the juvenile correctional system, court-mandated substance abuse treatment programs, and correctional schools, along with 1,399 of their siblings, and 904 controls.
Measurements
Mortality and cause-of-death were assessed via National Death Index search (released October, 2017).
Findings
There were 104 deaths documented among 3,766 (1,168 female) adolescents and young adults (average age 16.79 years at assessment, 32.69 years at death/censoring). Mortality hazard for clinical probands and their siblings was 4.99 times greater than that of controls (95% CI: 2.40 to 10.40; p < .001). After accounting for demographic characteristics, site, clinical status, familial dependence, and shared contributions of conduct disorder and substance use disorder, conduct disorder independently predicted mortality hazard, whereas substance use disorder severity did not.
Conclusions
Youth ascertained for conduct and substance use problems and their siblings face far greater risk of premature death than demographically similar community controls. In contrast to substance use disorder severity, conduct disorder is a robust predictor of unique variance in all-cause mortality hazard beyond other risk factors. Comprehensive psychiatric and social services are necessary to address these potentially preventable deaths among young people.
Introduction
Adolescence and early adulthood are periods of elevated risk for unnatural death; homicide, suicide, and unintentional injury accounted for 71% of the 936,000 deaths of young persons in the United States (age 15–29 years) between 1999 and 20161. However, the risk of early death is not distributed evenly. American youth who engage in risk behaviors, such as substance abuse and criminal activities, are 2–9 times more likely to die prematurely than their general population counterparts2–5, and their deaths are disproportionately caused by homicide, legal intervention, and motor vehicle accidents2–4,6–9,5,10. Youth with conduct- or substance-related problems (hereafter referred to as adolescents with externalizing problems) can often be identified through involvement in juvenile correction systems and/or placement in treatment programs; therefore, they comprise a prime target for intervention and prevention efforts. Nevertheless, our current understanding of the independent contributions of potentially modifiable risk factors for mortality hazard—information crucial to the success of such efforts—is incomplete.
Previous research has identified numerous demographic variables and individual differences that predict premature mortality among adolescents and young adults with externalizing problems, including male sex3,4,7,11, minority ethnic status (i.e., Black/African American or Latino)2–4,7,9,11, substance abuse2,3,5,6,11–13, and previous criminal history2,7,9. These findings mirror studies of mortality in previously incarcerated individuals14–20. However, with few exceptions6,11, recent studies have compared the mortality risk for youth with externalizing problems to those from retrospectively obtained population-level data rather than prospectively ascertained comparison cohorts2–4,7,12,21–24. Additionally, no prior study has analyzed the independent contributions of conduct disorder [CD] and substance use disorders [SUD] among legally-ascertained youth to determine hazard of premature death despite evidence of substantial psychiatric morbidity in delinquent youth populations25 and elevated mortality risk in clinically-ascertained youth5,11. Further, although both genetic and environmental familial factors contribute to conduct problems and substance abuse26–28, no study has examined whether these identified risk factors for premature death account for independent or redundant variance beyond that explained by demographics, or whether familial effects remain salient after accounting for individual differences. In particular, it remains unclear whether severity of substance abuse increases mortality hazard after accounting for CD, a point of clinical relevance given that several researchers have suggested that further dissemination and implementation of SUD treatments will reduce mortality in youth with externalizing problems5,13. Finally, it is unknown whether risk for premature death is heightened among siblings of adolescents with externalizing problems, and whether any differences between clinically-ascertained youth and their siblings are evident after accounting for individual differences in CD and SUD severity.
In light of these current limitations, the present study sought to examine the independent mortality hazard conferred by previously identified risk factors. We addressed the following aims in a multi-site, longitudinal, prospective cohort study of youth ascertained for CD and SUD (clinical probands), their siblings, and matched community controls:
Comparison of mortality risk and cause of death between clinical probands, their siblings, and controls. We hypothesized that mortality risk will be highest in the clinical probands and that shared familial factors will place their siblings at elevated risk with respect to controls (though not at the level of the clinical probands). Additionally, we expected that CD- and SUD-related causes of death (e.g., homicide, legal intervention, and overdose) would account for group differences.
Examination of the independent and simultaneous risk for premature death conferred by CD and SUD severity, beyond demographic variables and controlling for within-family dependence, among youth with externalizing problems and community controls.
We hypothesized that individual differences in CD and SUD severity would explain unique variation in mortality risk beyond that due to clinical/control status and demographic factors.
Methods
Participants
This prospective cohort study examined a sample of youth ascertained for CD and SUD (clinical probands; n = 1463 [254 female]), their siblings (n = 1399 [651 female]), community controls matched to clinical proband demographics by age and sex (n = 401 [35 female]), and their siblings (n = 503 [228 female]; distinguishing between matched controls and their siblings failed to impact any of our results substantially, so we collapsed these individuals into a single designation of controls for clarity; results presented in Table S1 illustrate that this choice did not affect primary conclusions) participating in a study of familial transmission and genetic linkage of SUD and CD between 1993 and 2016 (Genetics of Antisocial Drug Dependence Study)29–32. Clinical probands were recruited from individuals currently involved in or referred to residential and outpatient treatment facilities for substance abuse and delinquency in the Denver, Colorado area (n = 941), from adjudicated adolescents involved in the Colorado juvenile correctional system (n = 288), and from two schools for troubled youth in the San Diego, California area (n = 234). Within the Denver residential and outpatient treatment site, three separate rounds of ascertainment occurred following original funding and funding renewals; we treat these as separate sites in our analyses. Within the San Diego site, participants’ schools were not recorded, preventing modeling efforts to account for additional dependence due to nesting of observations within institutions, though analyses excluding the San Diego site were consistent with primary results (Table S2). To be admitted to the study, clinical probands had to be judged by staff as not currently psychotic, severely developmentally delayed, suicidal, or homicidal and to have no physical illness or current intoxication which would prevent participation in treatment or evaluation.
Research staff contacted those who met eligibility requirements for the study and invited them to participate. Written consent from a parent or guardian and assent from the patient were obtained for all subjects after complete description of the study. Subjects were paid between $20 and $100 for participation, with payment increasing over time. The respective institutional review boards approved all procedures. The mortality data-collection period lasted through the end of 2016, with average an age at assessment of 16.79 years (SD = 2.75) and an average age at study conclusion or death of 32.69 years (SD = 5.04). Additional demographic information is presented in Table 1.
Table 1.
Participant characteristics
| Clinical Probands | Siblings** | Controls | |
|---|---|---|---|
| Sample size | 1463 | 1399 | 904 |
| Sex [percent female] | 254 [17.36%] | 651 [46.53%] | 263 [20.09%] |
| Race/Ethnicity* | |||
| African-American | 119 [8.13%] | 81 [5.79%] | 47 [5.12%] |
| Non-Latino Caucasian | 605 [41.35%] | 476 [33.81%] | 386 [42.70%] |
| Multi-ethnic | 233 [15.93%] | 181 [12.94%] | 257 [28.43%] |
| Other/Unreported | 506 [34.59%] | 661 [47.25%] | 214 [23.67%] |
| Age at ascertainment† | 16.33 [1.29] | 17.50 [3.45] | 16.50 [3.17] |
| Age at death or at end of observation period† | 31.49 [4.22] | 32.17 [5.31] | 35.44 [4.82] |
| Lifetime conduct disorder symptoms at ascertainment†‡ | 5.36 [2.85] | 2.51 [2.40] | 1.31 [1.77] |
| Lifetime conduct disorder diagnosis at ascertainment*‡ | 1198 [64.34%] | 508 [27.28%] | 156 [8.38%] |
| Substance Dependence diagnosis ascertainment* | 1127 [70.44%] | 409 [25.56%] | 64 [4.00%] |
| Total substance abuse/dependence symptoms across substance at ascertainment† | 17.51 [12.32] | 6.71 [9.75] | 1.32 [3.33] |
| Number of substances used > 5 times at ascertainment† | 3.92 [1.94] | 2.24 [2.00] | 1.06 [1.48] |
| Substance abuse/dependence vulnerability index at ascertainment† | 4.23 [2.09] | 1.70 [1.80] | 0.53 [1.08] |
Note: Total substance abuse/dependence symptoms reflects the sum of symptom counts for multiple substances. Substance abuse/dependence vulnerability index reflects the total symptom count divided by the number of substances used greater than five times.
Number [percent of sample]
Siblings indicates siblings of clinical probands
Mean [standard deviation]
Participants aged 18 or above at assessment were assessed for conduct disorder symptoms retrospectively.
Measures
Demographic measures
Information regarding age, sex, race, and ethnicity was collected by self-report at enrollment.
Substance abuse/dependence
Substance use was assessed via the Composite International Diagnostic Interview-Substance Abuse Module (CIDI-SAM)33. The CIDI-SAM provided diagnostic data regarding the lifetime occurrence of four abuse symptoms and seven dependence symptoms on ten different drug classes (tobacco, alcohol, cannabis, cocaine, amphetamines, sedatives, opiates, PCP, hallucinogens, and inhalants), according to DSM-IV criteria34,35. The number of substances used on at least five separate occasions and the abuse/dependence symptom count per such substance were obtained. From this, we obtained a substance abuse/dependence vulnerability (SADV) index by calculating participants’ average number of abuse/dependence symptoms per substance, an approach that has been shown to maximize trait heritability32. The decision to use SADV was made a priori. Relevant results were tested for sensitivity to this approach by repeating analyses with multiple alternative variables: the total number of abuse/dependence symptoms across substances, the maximum number of abuse/dependence symptoms for a given substance across substances, and substance-dependence diagnosis (e.g., see Table S3 for model based on dependence diagnosis); these substitutions did not influence our results and we present analyses that used SADV.
Conduct disorder
Lifetime CD symptom count was assessed using the DISC according to DSM-III-R or DSM-IV criteria depending on the time of study enrollment36,37. Participants were asked to answer questions about individual CD symptoms. The DSM-III-R criteria included 13 symptoms of CD, and the DSM-IV included two additional symptoms. Earlier samples using the DSM-III-R criteria (n = 916) were scored using the DSM-IV criteria for compatibility with newer samples (with the earlier 916 participants missing scores for two items). Among clinical probands who were assessed for all DSM-IV criteria, DSM-III-R- and DSM-IV-derived symptom counts were strongly correlated (r = 0.98, p < .001). We present only results based on DSM-IV criteria.
Mortality
Mortality and cause of death data were obtained through a search of the National Death Index for all participants from the year of study enrollment through the end of 2016 (released October 2017)38 for determination of mortality status as suggested by the National Center for Health Statistics39. International Classification of Disease 10 (ICD-10) classifications were hand coded as falling into eight non-mutually-exclusive categories: (non-traffic) physical accidents, medical conditions, traffic accidents, assault, suicide, substance-related incidents, legal intervention-related incidents, and firearm-related incidents40. For example, death due to "intentional self-harm by handgun discharge” was coded as both suicide- and firearm-related. Specific coding used was verified by a licensed physician and patterns of overlap are presented in Figure S1.
Analyses
Primary analyses were conducted using the coxme package41 in the R computing environment42. Frailty models (also known as Cox proportional hazards models with Gaussian random effects) were used to examine the association between mortality hazard and predictor variables and covariates while controlling for dependence between siblings via a random intercept (frailty) term, among both clinical and control subjects. First, univariate frailty models were employed to (Table 2) examine the contributions of sex, sample, ethnicity, clinical/control designation, proband/sibling designation, substance/abuse dependence vulnerability, and CD symptom count to hazard of mortality. Next, multivariate frailty models (Table 3) were used to examine evidence of independent contributions of these predictors. In all models, site was included via the fixed effects of a set of orthogonal contrast codes (see Table 2 for further details). To elucidate patterns of redundancy among predictors, further analyses were conducted with several informative subsets of predictors (Table S5). Proportional hazards assumptions were validated using the survival package43. Listwise deletion was employed in primary analyses (202 participants were missing measures of SADV or CD symptoms), but possible consequences of non-random missing data were examined in supplementary sensitivity analyses (Table S4). For all models, an alpha-level of .05 was used to determine significance.
Table 2.
Univariate frailty models
| HR | 95% CI | SEβ | z/χ2 | df | p | |
|---|---|---|---|---|---|---|
| Site* | – | – | – | .470 | 4 | .976 |
| Sex | 2.81 | 1.59 – 4.95 | 0.29 | 3.56 | – | < .001 |
| Ethnicity | – | – | – | 3.59 | 3 | .309 |
| Clinical Probands vs. Clinical Siblings† | 1.95 | 1.27 – 2.98 | 0.22 | 3.07 | .002 | |
| Clinical Probands vs. Controls† | 6.97 | 3.30 – 14.74 | 0.38 | 5.08 | – | < .001 |
| Clinical Siblings vs. Controls† | 3.58 | 1.64 – 7.79 | 0.40 | 3.27 | – | .001 |
| Clinical Probands and Siblings vs. Controls† | 4.99 | 2.40 – 10.40 | 0.37 | 4.30 | – | < .001 |
| Substance Abuse/Dependence Vulnerability‡ | 1.16 | 1.08 – 1.26 | 0.04 | 3.92 | – | < .001 |
| Conduct Disorder Symptoms‡ | 1.18 | 1.12 – 1.25 | 0.03 | 5.68 | – | < .001 |
Note: Each row represents a separate frailty model estimating the contribution of the predictor to mortality hazard on the multiplicative scale while accounting for dependence due to family.
Probands recruited from residential and outpatient treatment facilities for substance abuse and delinquency in the Denver, Colorado area were ascertained in the context of three separate waves of funding, each of which was treated as a separate site in case of cohort-specific differences, resulting in five total sites and four orthogonal contrasts.
Group variables were estimated using orthogonal codes together in a single analysis of deviance (χ2(2) = 39.53, p < .001). We present four contrasts of interest above, though only two contrasts are identifiable in the context of a single model.
Slopes are unstandardized and are to be interpreted relative to unit increases in average abuse/dependence symptoms per substance and in total symptom counts for substance abuse/dependence vulnerability and conduct disorder symptoms, respectively.
Table 3.
Full multivariate frailty model
| HR | 95% CI | SEβ | z/χ2 | df | GVIF1/2df | p | |
|---|---|---|---|---|---|---|---|
| Site* | – | – | – | 4.77 | 4 | 1.06 | .323 |
| Sex | 2.09 | 1.11 – 3.92 | 0.32 | 2.29 | – | 1.07 | .020 |
| Ethnicity | – | – | – | 1.87 | 3 | 1.07 | .599 |
| Clinical Probands vs. Clinical Siblings | 1.14 | 0.65 – 2.01 | 0.29 | 0.47 | – | 1.29 | .640 |
| Clinical Probands and Siblings vs. Controls | 4.20 | 1.80 – 9.79 | 0.43 | 3.32 | – | 1.16 | .001 |
| Substance Abuse/Dependence Vulnerability† | 0.98 | 0.89 – 1.09 | 0.05 | −0.30 | – | 1.25 | .770 |
| Conduct Disorder Symptoms† | 1.09 | 1.01 – 1.18 | 0.04 | 2.20 | – | 1.23 | .028 |
Note: Fully saturated frailty model estimating the simultaneous independent contributions of the predictors to mortality hazard on the multiplicative scale while accounting for dependence due to family. GVIF1/2df (generalized variance inflation factor) is a linear indicator of multicollinearity of predictors; greater values indicate greater collinearity with other predictors53.
Probands recruited from residential and outpatient treatment facilities for substance abuse and delinquency in the Denver, Colorado area were ascertained in the context of three separate funding efforts, each of which was treated as a separate site in case of cohort-specific differences.
Slopes are unstandardized and are to be interpreted relative to unit increases in average abuse/dependence symptoms per substance and in total symptom counts for substance abuse/dependence vulnerability and conduct disorder symptoms, respectively.
Results
Cause of death
Substance-related deaths comprised the plurality of observed deaths overall and across male and female participants, accounting for 20 of 62 deaths among clinical probands (32%), 7 of 34 deaths among their siblings (21%), and 1 of 8 deaths among controls (13%; Table 4). Traffic accidents were the second most prominent cause, accounting for 12 deaths among clinical probands (19%), 7 among their siblings (21%), and 1 among controls (13%). Violent deaths (related to suicide, assault, or legal intervention) together accounted for 26 deaths among clinical probands (42%), 10 among their siblings (31%), and 2 among controls (26%). Note that percentages for controls are poor estimates as mortality was relatively uncommon. Additionally, three deaths classified as suicides were also classified as substance-related, as overdose was the mechanism of suicide (see Figure S1 for patterns of overlap between causes).
Table 4.
Cause of death by group and by sex
| Cause of Death | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
Substance related |
Traffic related |
Suicide | Assault |
Medical Condition |
Firearm |
Non-traffic Physical Accident |
Legal Intervention |
Unspecified |
Totals (non- overlapping) |
|
| Group | ||||||||||
| Clinical Probands | 20 | 12 | 13 | 12 | 3 | 6 | 3 | 1 | 1 | 62 |
| Clinical Probands’ Siblings | 7 | 7 | 4 | 3 | 10 | 4 | 0 | 3 | 0 | 34 |
| Controls | 1 | 3 | 2 | 0 | 1 | 1 | 1 | 0 | 0 | 8 |
| Sex | ||||||||||
| Female | 5 | 3 | 0 | 2 | 4 | 0 | 0 | 0 | 0 | 14 |
| Male | 23 | 19 | 19 | 13 | 10 | 11 | 4 | 4 | 1 | 90 |
| Totals per cause | 28 | 22 | 19 | 15 | 14 | 11 | 4 | 4 | 1 | 104 |
Note: Observed cause-of-death information as reported by the National Death Index split by group and by sex. Causes were established by categorizing International Disease Classification 10 codes and were verified by a licensed physician. Not all categories were mutually exclusive; for example, death due to "intentional self-harm by handgun discharge” was coded as both suicide- and firearm-related. Patterns of overlap are presented in Figure S1.
Univariate frailty models
After accounting for lack of independence due to family, all predictors other than site (χ2(4) = 0.47, p = .976) and ethnicity (χ2(3) = 3.59, p = .309) evidenced contributions to hazard of mortality (Table 2). Males had 2.81 times the expected hazard as females (z = 3.56, p < .001) and clinical probands had 1.95 times the expected hazard as their siblings (z = 3.07, p = .002), who in turn had 3.58 times the expected hazard as did controls (z = 3.27, p = .001). Single average symptom per substance in SADV and single symptom increases in CD were respectively associated with 1.16- and 1.18-fold increases in expected hazard (z = 3.92, p < .001; z = 5.68, p < .001).
Multivariate frailty models
Not all predictors evidenced independent contributions to hazard in models with greater saturation (Table S5). Specifically, independent contributions of SADV were not evident in any models including group or CD symptoms simultaneously (min p = .190). The fully saturated model (Table 3) provided evidence for independent contributions of sex (HR = 2.09, z = 2.29, p = .020), clinical/control contrast (HR = 4.20, z = 3.32, p = .001), and CD symptoms (HR = 1.09, z = 2.20, p = .028), but not for SADV (HR = 0.98, z = −0.30, p = .770) or for clinical proband/sibling contrast (HR = 1.14, z = 0.47, p = .640). However, siblings of clinical probands continued to evidence greater adjusted hazards than controls (HR = 3.58, z = 3.21, p = .001). Using total number of abuse/dependence symptoms or maximal number of abuse/dependence symptoms for any given substance not change this pattern of results (max p = .037 for terms associated CD symptom count, min p = 0.54 for terms associated with alternative substance abuse variables). Effects of site or ethnicity were not evident in any of the models (Tables 2, S5). Additionally, we conducted a series of sensitivity analyses examining possible consequences of multiple schemes of non-random missingness of baseline predictors. Our results suggested such artifacts were unlikely to have driven our primary conclusions (Table S4, Figure S2).
As we found little evidence for an independent contribution of SADV after accounting CD symptoms and additional covariates, we re-estimated the full multivariate frailty model twice, once substituting binary diagnoses of substance dependence and CD for SADV and CD symptoms, respectively (Table S3), and again including a SADV-by-CD symptoms product term in addition to their simple effects (Table S6). Results derived from the diagnosis-based model were directionally consistent with those of the primary frailty model, but with greater uncertainty regarding slope estimates. Specifically, CD diagnosis was no longer significant (HR = 1.43, z = 1.29, p = .200), presumably due to the reduction in power associated with dichotomizing continuous predictors and greater collinearity with the clinical versus control contrast (Table S3). There was no strong evidence of an interaction between SADV and CD symptoms (HR = 1.01, z = 0.49, p = .330) and simple effects were directionally consistent with the primary model excluding the interaction term (Table S6).
Discussion
The present prospective, multi-site study examined demographic and psychiatric predictors of early mortality in a sample of youth ascertained for CD and SUD, their siblings, and community controls. This study is the first to distinguish between the relative risk for premature death conferred by individual-differences versus familial factors and to examine the independent risk conferred by SADV after accounting for CD symptoms, demographic factors, and dependence due to family. In line with hypotheses, clinical probands evidenced significantly higher mortality hazard than their siblings, who in turn evidenced greater hazard than controls. Substance-related and violent causes accounted for the majority of deaths among clinical probands and their siblings, with the substance-related causes accounting for 32% of deaths among clinical probands (Table 4). Results from univariate models confirmed previous findings that male sex3,4,7,11, conduct problems2,7,9, and SAD2–7,11–13 each are associated with increased mortality risk (Table 2).
Contrary to expectation, multivariate model results suggested that the risk conferred by individual differences in SADV was largely redundant with that due to CD (Tables 3, S5). That is, there was no discernible independent effect of SADV in any models accounting for CD symptoms or clinical ascertainment status, though the latter accounted for independent variance regardless of which covariates were presented in the model. However, consistent with previous findings5,13,19,44–46, substance-related causes comprised the largest proportion of observed deaths an4d occurred disproportionately among clinical probands and their siblings vis-a-vis controls. In light of these findings, we echo previous researchers’ recommendations5,13,16 that intervention and prevention efforts include SUD treatment within a constellation treatment foci including additional psychiatric resources and social services.
Further complicating this discussion, the determination of cause of death as substance-related versus suicide is often ambiguous with respect to available evidence and misclassification errors are ubiquitous47–49. Some researchers have suggested that substance-related suicides are particularly likely to be erroneous classified as accidental or undetermined48,49. The degree to which deaths classified as physical or traffic accidents might have indirect consequences of substance use is unknown. Additional limitations to the present study include the lack of a comprehensive measure of socioeconomic status (SES), inadequate measures of race/ethnicity among multiethnic individuals (Table 1), and potential regional specificity to site locations (urban Colorado and southern California), though the first was partially mitigated by the inclusion of familial random intercepts and there was no evidence of site differences among our samples. Furthermore, the contrasts between clinical probands and their siblings may have reflected ascertainment procedures rather than individual differences. That is, it is possible that the elder or younger siblings of probands might have themselves been ascertained as probands had ascertainment occurred at a different date. Additionally, as the present study focused on individuals ascertained specifically for severe externalizing behaviors, their siblings, and community controls, the extent to which our results might generalize to individuals with moderate externalizing problems is unclear. That is, our results concerning the independent risk attributable to substance versus conduct problems might not generalize to youth with subclinical externalizing problems or who avoid legal or clinical attention. Further, our results do not reflect the diagnostic criteria currently employed in the DSM-5, though we are skeptical that employing DSM-V criteria would have dramatically altered our results; symptoms for CD remained unchanged and substance abuse/dependence symptoms saw only the replacement of the legal troubles criterion with a craving criterion50,51. The utility of the callous-unemotional traits CD subtype specifier in predicting premature mortality remains unknown. Lastly, the National Death Index search likely failed to identify some deceased participants; a family member reported the death of one participant that our search did not identify as deceased. In the present study, the full name and date-of-birth were available for all participants, and social security numbers were available for a subset of participants. Previous research suggests that the sensitivity and specificity of NDI searches are above 90% even for those missing social security numbers52. However, whether these estimates generalize to samples with relatively high incidences of unnatural death early in life is unknown.
The degree to which forensic artifacts surrounding cause-of-death classification account for the incidence of substance-related deaths among clinically- and legally-ascertained youth comprises a prime target for future research efforts. Additionally, future work should examine the independent contributions of SUD and CD to premature mortality in the context of thorough measures of SES. Finally, we wish to caution that though it remains unclear which domains comprise the most pressing target for intervention and prevention efforts (e.g., targeting SUD versus general psychiatric care), it is clear that youth identified with conduct problems are at extreme risk for premature mortality and in critical need of greater resources.
Supplementary Material
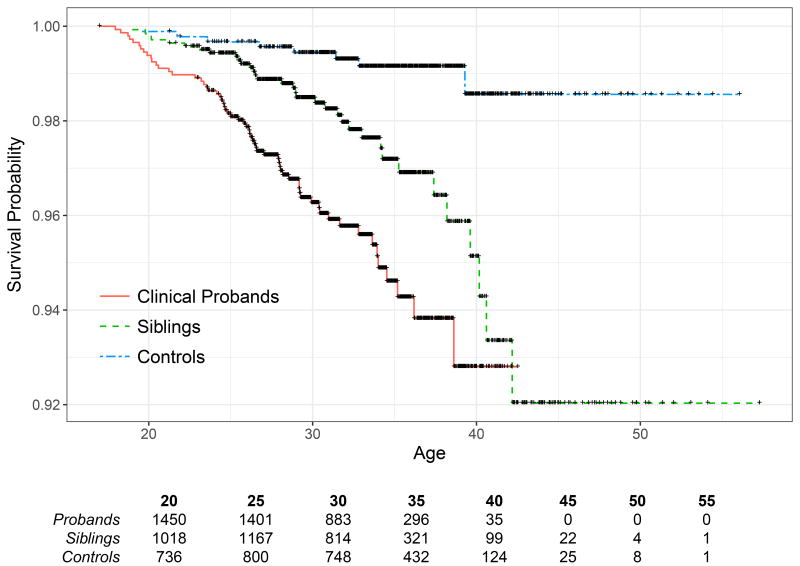
Figure 1.
Observed mortality among clinical probands, their siblings, and controls
Note. Black cross marks indicate censoring and the bottom table indicates the number of participants per group at each age. Mortality hazard differed by group after accounting for dependence due to family (χ2(2) = 39.53, p < .001). For additional contrasts see Table 2.
Acknowledgments
We thank Dr. Ryan Masters for his thoughtful commentary and the Colorado Department of Corrections for their assistance (Project 11-323).
Footnotes
Disclosures: The authors report no conflicts of interest.
References
- 1.Centers for Disease Control and Prevention, National Center for Health Statistics. Underlying Cause of Death: 1999–2016 on CDC WONDER Online Database. [Accessed January 14, 2018];CDC WONDER Online Database. http://wonder.cdc.gov/ucd-icd10.html. Published 2017.
- 2.Lattimore PK, Linster RL, MacDonald JM. Risk of Death among Serious Young Offenders. J Res Crime Delinquency. 1997;34(2):187–209. doi: 10.1177/0022427897034002002. [DOI] [Google Scholar]
- 3.Teplin LA, Jakubowski JA, Abram KM, Olson ND, Stokes ML, Welty LJ. Firearm homicide and other causes of death in delinquents: A 16-year prospective study. Pediatrics. 2014;134(1):63–73. doi: 10.1542/peds.2013-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teplin LA, McClelland GM, Abram KM, Mileusnic D. Early violent death among delinquent youth: a prospective longitudinal study. Pediatrics. 2005;115(6):1586–1593. doi: 10.1542/peds.2004-1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manninen M, Pankakoski M, Gissler M, Suvisaari J. Adolescents in a residential school for behavior disorders have an elevated mortality risk in young adulthood. Child Adolesc Psychiatry Ment Health. 2015;9:46. doi: 10.1186/s13034-015-0078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laub JH, Vaillant GE. Delinquency and mortality: a 50-year follow-up study of 1,000 delinquent and nondelinquent boys. [Accessed February 23, 2016];Am J Psychiatry. 2000 doi: 10.1176/ajp.157.1.96. http://ajp.psychiatryonline.org/doi/pdf/10.1176/ajp.157.1.96. [DOI] [PubMed]
- 7.Teplin LA, McClelland GM, Abram KM, Mileusnic-Polchan D, Olson ND, Harrison AJ. Violent Death in Delinquent Youth After Detention. US Department of Justice, Office of Justice Programs, Office of Juvenile Justice and Delinquency Prevention; 2015. [Accessed February 26, 2016]. http://shadowproof.com/wp-content/uploads/2015/09/cook-county-youth-violent-death-after-detention.pdf. [Google Scholar]
- 8.Jennings WG, Piquero AR, Reingle JM. On the overlap between victimization and offending: A review of the literature. Aggress Violent Behav. 2012;17(1):16–26. doi: 10.1016/j.avb.2011.09.003. [DOI] [Google Scholar]
- 9.Aalsma MC, Lau KSL, Perkins AJ, et al. Mortality of Youth Offenders Along a Continuum of Justice System Involvement. Am J Prev Med. 2016;50(3):303–310. doi: 10.1016/j.amepre.2015.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Elonheimo H, Sillanmäki L, Sourander A. Crime and mortality in a population-based nationwide 1981 birth cohort: Results from the FinnCrime study. Crim Behav Ment Health. 2017;27(1):15–26. doi: 10.1002/cbm.1973. [DOI] [PubMed] [Google Scholar]
- 11.Clark DB, Martin CS, Cornelius JR. Adolescent-Onset Substance Use Disorders Predict Young Adult Mortality. J Adolesc Health. 2008;42(6):637–639. doi: 10.1016/j.jadohealth.2007.11.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chassin L, Piquero AR, Losoya SH, Mansion AD, Schubert CA. Joint Consideration of Distal and Proximal Predictors of Premature Mortality Among Serious Juvenile Offenders. J Adolesc Health. 2013;52(6):689–696. doi: 10.1016/j.jadohealth.2012.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Auerswald CL, Lin JS, Parriott A. Six-year mortality in a street-recruited cohort of homeless youth in San Francisco, California. PeerJ. 2016;4:e1909. doi: 10.7717/peerj.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patterson EJ. The dose–response of time served in prison on mortality: New York State, 1989–2003. Am J Public Health. 2013;103(3):523–528. doi: 10.2105/AJPH.2012.301148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krinsky CS, Lathrop SL, Brown P, Nolte KB. Drugs, detention, and death: a study of the mortality of recently released prisoners. Am J Forensic Med Pathol. 2009;30(1):6–9. doi: 10.1097/PAF.0b013e3181873784. [DOI] [PubMed] [Google Scholar]
- 16.Lim S, Seligson AL, Parvez FM, et al. Risks of Drug-Related Death, Suicide, and Homicide During the Immediate Post-Release Period Among People Released From New York City Jails, 2001–2005. Am J Epidemiol. 2012;175(6):519–526. doi: 10.1093/aje/kwr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson EJ. Incarcerating death: Mortality in US state correctional facilities, 1985–1998. Demography. 2010;47(3):587–607. doi: 10.1353/dem.0.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spaulding AC, Seals RM, McCallum VA, Perez SD, Brzozowski AK, Steenland NK. Prisoner Survival Inside and Outside of the Institution: Implications for Health-Care Planning. Am J Epidemiol. 2011;173(5):479–487. doi: 10.1093/aje/kwq422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zlodre J, Fazel S. All-cause and external mortality in released prisoners: systematic review and meta-analysis. Am J Public Health. 2012;102(12):e67–e75. doi: 10.2105/AJPH.2012.300764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Binswanger IA, Stern MF, Deyo RA, et al. Release from Prison — A High Risk of Death for Former Inmates. N Engl J Med. 2007;356(2):157–165. doi: 10.1056/NEJMsa064115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramchand R, Morral AR, Becker K. Seven-year life outcomes of adolescent offenders in Los Angeles. Am J Public Health. 2009;99(5):863–870. doi: 10.2105/AJPH.2008.142281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piquero AR, MacDonald J, Dobrin A, Daigle LE, Cullen FT. Self-Control, Violent Offending, and Homicide Victimization: Assessing the General Theory of Crime. J Quant Criminol. 2005;21(1):55–71. doi: 10.1007/s10940-004-1787-2. [DOI] [Google Scholar]
- 23.Loeber R, DeLamatre M, Tita G, Cohen J, Stouthamer-Loeber M, Farrington DP. Gun injury and mortality: The delinquent backgrounds of juvenile victims. Violence Vict. 1999;14(4):339–352. [PubMed] [Google Scholar]
- 24.Stoddard-Dare P, Tedor MF, Quinn L, Mallett C. An assessment of risk factors for early death among a sample of previously incarcerated youth. Crim Justice Stud. 2014;27(2):191–209. doi: 10.1080/1478601X.2014.885756. [DOI] [Google Scholar]
- 25.Teplin LA, Abram KM, McClelland GM, Dulcan MK, Mericle AA. Psychiatric disorders in youth in juvenile detention. Arch Gen Psychiatry. 2002;59(12):1133–1143. doi: 10.1001/archpsyc.59.12.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hopfer CJ, Crowley TJ, Hewitt JK. Review of Twin and Adoption Studies of Adolescent Substance Use. J Am Acad Child Adolesc Psychiatry. 2003;42(6):710–719. doi: 10.1097/01.CHI.0000046848.56865.54. [DOI] [PubMed] [Google Scholar]
- 27.Advances in Genetics. Vol. 55. Elsevier; 2005. [Accessed March 6, 2016]. E. Moffitt T. Genetic and Environmental Influences on Antisocial Behaviors: Evidence from Behavioral–Genetic Research; pp. 41–104. http://linkinghub.elsevier.com/retrieve/pii/S006526600555003X. [DOI] [PubMed] [Google Scholar]
- 28.Rhee SH, Waldman ID. Genetic and environmental influences on antisocial behavior: A meta-analysis of twin and adoption studies. Psychol Bull. 2002;128(3):490–529. doi: 10.1037//0033-2909.128.3.490. [DOI] [PubMed] [Google Scholar]
- 29.Hopfer CJ, Stallings MC, Hewitt JK, Crowley TJ. Family transmission of marijuana use, abuse, and dependence. J Am Acad Child Adolesc Psychiatry. 2003;42(7):834–841. doi: 10.1097/01.CHI.0000046874.56865.85. [DOI] [PubMed] [Google Scholar]
- 30.Young SE, Friedman NP, Miyake A, et al. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118(1):117. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hartman CA, Lessem JM, Hopfer CJ, Crowley TJ, Stallings MC. The family transmission of adolescent alcohol abuse and dependence. J Stud Alcohol. 2006;67(5):657–664. doi: 10.15288/jsa.2006.67.657. [DOI] [PubMed] [Google Scholar]
- 32.Stallings MC, Corley RP, Hewitt JK, et al. A genome-wide search for quantitative trait loci influencing substance dependence vulnerability in adolescence. Drug Alcohol Depend. 2003;70(3):295–307. doi: 10.1016/s0376-8716(03)00031-0. [DOI] [PubMed] [Google Scholar]
- 33.Crowley TJ, Mikulich SK, Ehlers KM, Whitmore EA, Macdonald MJ. Validity of structured clinical evaluations in adolescents with conduct and substance problems. J Am Acad Child Adolesc Psychiatry. 2001;40(3):265–273. doi: 10.1097/00004583-200103000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Association AP DSM-III-R: Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association; 1980. [Google Scholar]
- 35.First MB. Diagnostic and statistical manual of mental disorders. DSM IV-4th Ed APA. 1994 1994. [Google Scholar]
- 36.Shaffer D, Schwab-Stone M, Fisher P, et al. The Diagnostic Interview Schedule for Children-Revised Version (DISC-R): I. Preparation, Field Testing, Interrater Reliability, and Acceptability. J Am Acad Child Adolesc Psychiatry. 1993;32(3):643–650. doi: 10.1097/00004583-199305000-00023. [DOI] [PubMed] [Google Scholar]
- 37.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, Differences from Previous Versions, and Reliability of Some Common Diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 38.National Center for Health Statistics. National Death Index. Hyattsville, MD: Centers for Disease Control and Prevention; [Google Scholar]
- 39.National Center for Health Statistics. National Death Index User’s Guide. Centers for Disease Control and Prevention; 2013. [Google Scholar]
- 40.World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva, Switzerland: World Health Organization; 1992. [Google Scholar]
- 41.Therneau TM. [Accessed January 7, 2018];Coxme: Mixed Effects Cox Models. 2018 https://cran.r-project.org/web/packages/coxme/index.html.
- 42.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2016. https://www.R-project.org/ [Google Scholar]
- 43.Therneau TM. [Accessed January 7, 2018];Survival: Survival Analysis. 2017 https://cran.r-project.org/web/packages/survival/index.html.
- 44.Teplin LA, Jakubowski JA, Abram KM, Olson ND, Stokes ML, Welty LJ. Firearm Homicide and Other Causes of Death in Delinquents: A 16-Year Prospective Study. PEDIATRICS. 2014;134(1):63–73. doi: 10.1542/peds.2013-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merrall ELC, Kariminia A, Binswanger IA, et al. Meta-analysis of drug-related deaths soon after release from prison: Drug-related deaths after release from prison. Addiction. 2010;105(9):1545–1554. doi: 10.1111/j.1360-0443.2010.02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rosen DL, Schoenbach VJ, Wohl DA. All-cause and cause-specific mortality among men released from state prison, 1980–2005. Am J Public Health. 2008;98(12):2278–2284. doi: 10.2105/AJPH.2007.121855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Breiding MJ, Wiersema B. Variability of undetermined manner of death classification in the US. Inj Prev. 2006;12(Suppl 2):ii49–ii54. doi: 10.1136/ip.2006.012591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rockett IRH, Hobbs GR, Wu D, et al. Variable Classification of Drug-Intoxication Suicides across US States: A Partial Artifact of Forensics? PLoS ONE. 2015;10(8) doi: 10.1371/journal.pone.0135296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohberg A, Lonnqvist J. Suicides hidden among undetermined deaths. Acta Psychiatr Scand. 1998;98(3):214–218. doi: 10.1111/j.1600-0447.1998.tb10069.x. [DOI] [PubMed] [Google Scholar]
- 50.Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, et al. DSM-5 Criteria for Substance Use Disorders: Recommendations and Rationale. Am J Psychiatry. 2013 Aug 1;170(8):834–51. doi: 10.1176/appi.ajp.2013.12060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®) American Psychiatric Pub; 2013. May 22, [Google Scholar]
- 52.Williams BC, Demitrack LB, Fries BE. The accuracy of the National Death Index when personal identifiers other than Social Security number are used. Am J Public Health. 1992 Aug 1;82(8):1145–7. doi: 10.2105/ajph.82.8.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox J, Monette G. Generalized Collinearity Diagnostics. Journal of the American Statistical Association. 1992 Mar 1;87(417):178–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.