Abstract
The multidimensional nature of pain in sickle cell disease (SCD) has rendered its therapeutic management extremely challenging. In this study, we explored the role of five single nucleotide polymorphisms (SNPs) of candidate gene GCH1 in SCD pain. Composite pain index (CPI) scores and acute care utilization rates were used as phenotype markers. Rs8007267 was associated with chronic pain (additive model: B = −3.76, p = 0.037; dominant model: B = −5.61, p = 0.021) and rs3783641 (additive model: incident rate ratio [IRR] = 1.37, p = 0.024; recessive model: IRR = 1.81, p = 0.018) with utilization rate. These associations persisted when subjects with HbSS and HbS−β genotype only were analyzed. We also identified two haploblocks (rs10483639[G>C]-rs752688 [C>T]-rs4411417[T>C] and rs3783641[T>A]-rs8007267[T>C]) with SNPs in high linkage disequilibrium. Of these, haplotype T-C of haploblock rs3783641-rs8007267 showed significant association with rate of utilization (odds ratio [OR] = 0.31, p = 0.001). Our study indicates potential contribution of GCH1 polymorphisms to the variability of pain in African Americans with SCD.
Sickle cell disease (SCD) affects millions of individuals worldwide, including about 100,000 Americans [1,2]. It is an autosomal-recessive disorder in which a single nucleotide mutation of the beta-globin gene causes the deoxygenated form of hemoglobin to polymerize. This results in rigid, sickle-shaped red blood cells that aggregate and occlude microvasculature. Therefore, blood flow to organs is obstructed, culminating in frequent episodes of severe acute pain, also referred to as acute crisis pain [3,4].
Patients experiencing crisis pain require hospitalization [5] and the frequency of these vaso-occlusive events increases with age [6]. Not only does this affect quality of life, but it is also associated with higher mortality [7,8]. In addition to acute pain, a large number of SCD patients experience persistent chronic pain [9–11]. Acute and chronic pain are alike, however, in their multifaceted nature, exhibiting a striking inconsistency in severity and frequency among individuals. Unfortunately, our understanding of the precipitating factors and mechanisms that contribute to it is limited.
Because of the vast variability in these pain phenotypes and the constraints of prescribing opioids, therapeutic management of SCD pain has been challenging and largely suboptimal [9,12]. Research in the field of pain genetics has therefore strived to account for the individual differences in pain sensitivity and susceptibility [13]. To better understand the genetic factors contributing to pain heterogeneity in SCD, we investigated the role of genetic polymorphisms of GCH1 in SCD pain.
Table 5.
Negative binomial regression model evaluating the effect of GCH1 SNPs on acute crisis pain
| Additive |
Dominant |
Recessive |
||||
|---|---|---|---|---|---|---|
| IRR (95% CI)a | p-value | IRR (95% CI)a | p-value | IRR (95% CI)a | p-value | |
| rs752688 | 1.21 (0.88, 1.71) | 0.273 | 1.15 (0.75, 1.78) | 0.516 | 1.99 (0.88, 5.33) | 0.127 |
| rs3783641 | 1.37 (1.05, 1.81) | 0.024 | 1.36 (0.90, 2.06) | 0.144 | 1.81 (1.11, 3.05) | 0.018 |
| rs4411417 | 1.08 (0.79, 1.51) | 0.641 | 1.02 (0.67, 1.55) | 0.938 | 1.54 (0.70, 3.90) | 0.317 |
| rs8007267 | 0.78 (0.60, 1.03) | 0.078 | 0.76 (0.53, 1.10) | 0.149 | 0.66 (0.37, 1.19) | 0.151 |
| rs10483639 | 1.02 (0.79, 1.33) | 0.859 | 0.97 (0.67, 1.40) | 0.855 | 1.17 (0.71, 2.01) | 0.550 |
IRR and 95% CI.
Regression models are adjusted for age, sex, and sickle cell type.
The gene of interest, GCH1, encodes the enzyme GTP cyclohydrolase (GTPCH). GTPCH is as a crucial component in the tetrahydrobiopterin (BH4)-mediated production of pain-modulating molecules such as nitric oxide and monoamines, wherein it serves as a rate-limiting catalyst in the biosynthesis of the critical cofactor BH4 [14,15]. Upon neuronal damage, GCH1 is upregu-lated and there is an increased production of BH4 in the injured sensory neurons [16]. Intracellular levels of GCH1 and BH4 thereby correlate to pain sensitivity. For example, overexpression of BH4 in sensory neurons increases thermal pain sensitivity [16] and an intrathecal administration of BH4 aggravates neuropathic pain [17]. Conversely, pharmacological inhibition of BH4 synthesis [16,17] or downregulation of GTPCH [18] can alleviate pain.
Association studies have reported that several genetic polymorphisms in GCH1 affect pain sensitivity. Among other single nucleotide polymorphisms (SNPs) of GCH1 studies have identified and established pain-protective alleles that are associated with reduced pain sensitivity [17,19–1], although a few have failed to find a positive correlation between the two [22–26].
Studies and clinical trials exploring the influence of GCH1 SNPs and haplotypes on SCD pain are scarce and focused only on acute crisis pain [27,28]. In a recent study, Belfer et al. noted that, contrary to prior findings, the previously identified pain-protective allele T of rs8007267 was associated with more frequent episodes of crisis pain. Biochemical assays and pulmonary function tests revealed that this risk allele associated with higher BH4 levels in vitro and altered endothelial function in females. This study also brought into light the stark contrast in allele frequencies of this SNP in populations of different ethnicities and identified a distinct pain-associated haplotype present in only in African subjects [27]. In this study, we analyzed the possible association of five SNPs of GCH1 (rs752688, rs4411417, rs3783641, rs8007267, and rs10483639) with both acute and chronic SCD pain.
Methods
Subject recruitment
This study was approved by University of Illinois at Chicago’s institutional review board. Patients were recruited upon obtaining written informed consent. Either blood or buccal swab samples were collected from recruited patients during their scheduled visits at the University of Illinois Hospital and Health Sciences System of Chicago.
Baseline chronic pain assessment
Subjects recorded the number of pain sites; pain pattern and quality; and least, worst, and current pain intensity on PAIN-ReportIt, an adaptation of the McGill Pain Questionnaire for the assessment of baseline pain [29–31]. Responses were used to compute the CPI score as a measure of chronic pain [32–34].
Acute crisis pain assessment
Acute care utilization, that is, the number of visits to the emergency department (ED) or acute care center due to crisis pain, was used as an indicator of acute pain in this study. The number of utilizations for each patient over a period of 12 months from their baseline pain assessment was obtained from medical records maintained at University of Illinois or at the ED that the patient visited or via periodic telephone calls to the recruited subjects.
Genotyping
A modified phenol/chloroform method was used to extract DNA from buccal swabs [35], whereas DNA extraction from blood samples was performed by either a modified salting out procedure [36] or by the QuickGene DNA whole-blood extraction method (AutoGen, Holliston, MA). The Quick-Gene-mini80 isolation device was employed for the latter. Further, the MassARRAY iPLEX Platform (Sequenom, San Diego, CA) was used for genotyping.
Statistical analysis
All statistical analysis was performed on R version 3.4.0 (R Foundation for Statistical Computing, Vienna, Austria) [37]. We included data from 131 subjects with complete genotype and pain phenotype information. We used three different genetic models, additive, dominant, and recessive, to assess the effect of the risk allele on the outcome. CPI scores for chronic pain were modelled with multiple linear regression, whereas acute pain utilization data was modeled with negative binomial regression. Each association study was adjusted for covariates such as age, sex, and sickle cell type. A subgroup of subjects with SCD SS and S/Sβ° (SS type, n =109) were also analyzed for association. Both chi-squared test and exact test were used to determine whether the SNPs were in Hardy–Weinberg equilibrium. Linkage disequilibrium analysis was plotted using Haploview version 4.2 (Broad Institute, Cambridge, MA, USA) [38]. Haplotype analysis was performed using R package “hapassoc” [39].
Results
This study included 131 African-American subjects ages 15–70. As seen from the patient demographics in Table 1, approximately 65% of the participants were female and 78% of the study population had homozygous hemoglobin SS disease, which is the most prevalent type of SCD.
Table 1.
Patient demographics
| Age (years) | Range | 15 –70 | |
|---|---|---|---|
| Mean ± SD | 34.3 ± 11.8 | ||
| Sex, n (%) | Female | 86 (65.6) | |
| Male | 45 (34.4) | ||
| Sickle Cell Type, n (%)a | SS type | SCD-SS | 102 (77.9) |
| SCD-Sβ° | 7 (5.3) | ||
| Others | SCD-SC | 15 (11.5) | |
| SCD-Sβ+ | 7 (5.3) | ||
| CPI (0–100) | Range | 14.8–86.5 | |
| Mean ± SD | 40.6 ± 13.4 | ||
| Utilization | Range | 0–38 | |
| Mean ± SD | 4.5 ± 5.3 | ||
| Utilization Groups, n (%) | Zero (0) | 19 (14.5) | |
| Low (1–3) | 56 (42.7) | ||
| High (4–38) | 56 (42.7) | ||
Sickle cell types: SCD–SS (SCD–homozygous hemoglobin S, sickle cell anemia) and SCD–Sβ° (SCD–sickle β° thalassemia); others include SCD–SC (SCD–sickle hemoglobin C) and SCD–Sβ+ (SCD–sickle β+ thalassemia).
The major and minor allele frequencies for each of the five SNPs: rs752688 [C>T], rs3783641 [T>A], rs4411417 [T>C], rs8007267 [T>C], and rs10483639 [G>C] are listed in Table 2. Major alleles were used as reference with the minor alleles being the risk allele in our analyses.
Table 2.
GCH1 SNP genotype and allele frequencies
| dbSNP ID | Major Allele, n (%) |
Minor Allele, n (%) |
Major Homozygote, n (%) |
Heterozygote, n (%) | Minor Homozygote, n (%) |
|---|---|---|---|---|---|
| rs752688 | C, 174 (80) | T, 44 (20) | CC, 70 (64) | CT, 34 (31) | TT, 5 (5) |
| rs3783641 | T, 143 (63) | A, 85 (37) | TT, 48 (42) | TA, 47 (41) | AA, 19 (17) |
| rs4411417 | T, 196 (80) | C, 48 (20) | TT, 80 (66) | TC, 36 (30) | CC, 6 (5) |
| rs8007267 | T, 168 (65) | C, 90 (35) | TT, 54 (42) | TC, 60 (47) | CC, 15 (12) |
| rs10483639 | G, 165 (63) | C, 95 (37) | GG, 53 (41) | GC 60 (45) | CC, 18 (14) |
The five SNPs studied were found to be in Hardy–Weinberg equilibrium because the chi-squared and exact test showed no significant deviation (p > 0.05) as seen in Table 3.
Table 3.
Hardy-Weinberg equilibrium test for GCH1 SNPs
|
p-value |
||
|---|---|---|
| Chi-squared test | Exact test | |
| rs752688 | 0.898 | 0.765 |
| rs3783641 | 0.244 | 0.228 |
| rs4411417 | 0.604 | 0.401 |
| rs8007267 | 0.831 | 0.848 |
| rs10483639 | 0.962 | 0.852 |
CPI scores for chronic pain, computed on a scale of 0–100, had a mean of 40.7 and were distributed over a wide range from 14.8 to 86.5 (Table 1). Linear regression used to model the chronic pain phenotype identified that the minor allele, C, of rs8007267 genotype was associated with a decrease in CPI scores for the additive (B = −3.76,95% confidence interval (CI) = [−7.28, −0.24], p = 0.037) and dominant (B = −5.64, 95% CI=[−10.31, −0.85], p = 0.021) genetic models (Table 4).
Table 4.
Multiple linear regression model evaluating the effects of GCH1 SNPs on chronic pain in SCD
| Additive |
Dominant |
Recessive |
||||
|---|---|---|---|---|---|---|
| B (95% CI)a | p-value | B (95% CI)a | p-value | B (95% CI)a | p-value | |
| rs752688 | 1.51 (−3.13, 6.15) | 0.521 | 1.81 (−3.80, 7.41) | 0.524 | 1.95 (−10.58, 14.48) | 0.758 |
| rs3783641 | 1.65 (−2.20, 5.50) | 0.397 | 2.57 (−3.11, 8.24) | 0.372 | 1.59 (−5.49, 8.67) | 0.657 |
| rs4411417 | 0.47 (−3.84, 4.78) | 0.829 | 0.96 (−4.32, 6.24) | 0.719 | −1.16 (−12.51, 10.18) | 0.839 |
| rs8007267 | −3.76 (−7.28, −0.24) | 0.037 | −5.61 (−10.37, −0.85) | 0.021 | −3.03 (−10.42, 4.36) | 0.419 |
| rs10483639 | 3.23 (−0.27, 6.72) | 0.070 | 3.56 (−1.33, 8.45) | 0.152 | 5.46 (−1.48, 12.40) | 0.122 |
Unstandardized regression coefficient and 95% CI. Regression models are adjusted for age, sex, and sickle cell type.
Conversely, the marker for acute crisis pain was a count score reported as the number of utilizations per year and ranged from 0 to 38 utilizations. We performed a negative binomial regression on the utilization counts and computed the IRR. It was found that the minor allele, A, of rs3783641 was associated with an increase in the number of utilizations per year in the additive model (IRR= 1.37, 95% CI= [1.05, 1.81], p = 0.024) as well as in the recessive model (IRR =1.81, 95% CI = [1.11, 3.05], p = 0.018), as seen in Table 5.
When association tests for utilization and CPI (Table 6) were performed in the SS type (SCD-SS and SCD-Sβ°) cohort (n = 109), we observed that, similar to the findings in the overall study population, risk allele C of rs8007267 associated with decreased CPI scores in the additive (B = −4.26, 95% CI= [−8.27, −0.25], p = 0.037) and dominant (B = −6.43, 95% CI = [−11.67, − 1.18], p = 0.017) models, whereas variant A of rs3783641 associated with increased utilization rate in the additive (IRR =1.44, 95% CI = [1.11, 1.89], p = 0.012) and recessive (IRR= 1.97, 95% CI=[1.19, 3.36], p = 0.013) model. The other SNPs failed to show significant associations.
Table 6.
Association tests evaluating the effect of GCH1 SNPs rs8007267 and rs3783641 on CPI and utilization in SS type (SCD-SS and SCD-Sβ°) cohort
| Additive |
Dominant |
Recessive |
||||
|---|---|---|---|---|---|---|
| B (95% CI)a | p-value | B (95% CI)a | p-value | B (95% CI)a | p-value | |
| rs3783641 | 1.65 (−2.48, 5.77) | 0.430 | 3.37 (−2.62, 9.35) | 0.267 | 0.20 (−7.63, 8.02) | 0.960 |
| rs8007267 | −4.26 (−8.27, −0.25) | 0.037 | −6.43 (−11.67, −1.18) | 0.017 | −2.50 (−11.14, 6.14) | 0.567 |
| IRR (95% CI)b | p–value | IRR (95% CI)a | p–value | IRR (95% CI)a | p–value | |
| rs3783641 | 1.44 (1.11, 1.89) | 0.012 | 1.53 (1.01, 2.32) | 0.054 | 1.97 (1.19, 3.36) | 0.013 |
| rs8007267 | 0.76 (0.57, 1.02) | 0.076 | 0.69 (0.47, 1.02) | 0.065 | 0.75 (0.40, 1.49) | 0.385 |
Unstandardized regression coefficient and 95% confidence interval.
IRR and 95% CI.
Regression models are adjusted for age and sex.
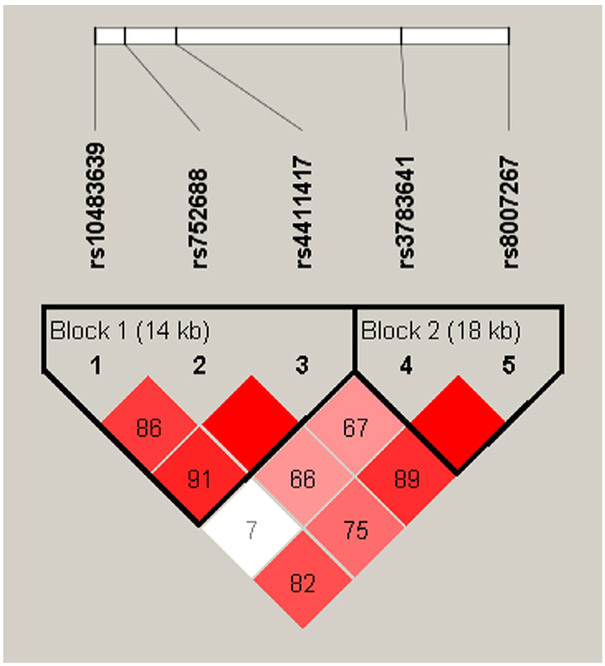
Furthermore, linkage disequilibrium and haplotype analysis were performed (Figure 1). We identified two haploblocks in our sample. Block 1 spanned 14 kb consisted of three SNPs, rs10483639, rs752688, and rs4411417. Block 2 spanned 18 kb consisted of two SNPs, rs3783641 and rs8007267.
Figure 1.
Linkage disequilibrium plot for GCH1 SNPs: Linkage disequilibrium plot was generated from Haploview 4.2 using the standard D’/LOD color scheme. D’ values show the linkage disequilibrium coefficient. LOD= logarithm of odds of two loci having linkage disequilibrium. Red= high D’ and high LOD, white= low D’ and low LOD; shades of pink/red= low D’ and high LOD.
We also analyzed the African-American cohort in publicly available database 1000Genomes for haplo-types and validated the prevalence of haploblock 1. The utilization scores were categorized in two ways for regression analyses: zero utilization versus one or more utilizations and zero to three utilizations versus four or more utilizations. Logistic regression was performed for both scenarios, as seen in Table 7 and 8.
Table 7.
GCH1 block 1 (14kb) haplotype analysis with CPI and utilization groups
| Haplotype | Frequency (%) | 0 vs 1 or More Utilizations |
0–3 vs 4 or More Utilizations |
CPI |
|||
|---|---|---|---|---|---|---|---|
| OR | p-value | OR | p-value | Estimate | p-value | ||
| CCT | 18 | 1.01 | 0.989 | 1.09 | 0.819 | 4.02 | 0.066 |
| CTC | 19 | 1.75 | 0.321 | 2.04 | 0.050 | 2.33 | 0.282 |
SNP order in haplotype (rs10483639[G>C]– rs752688[C>T]–rs4411417[T>C]). Reference haplotype was GCT (frequency = 61%). Regression models are adjusted for covariates (age, sex, and sickle cell type).
Table 8.
GCH1 block 2 (18kb) haplotype analysis with CPI and utilization groups
| Haplotype | Frequency (%) | 0 vs 1 or More Utilizations |
0– 3 vs 4 or More Utilizations |
CPI |
|||
|---|---|---|---|---|---|---|---|
| OR | p-value | OR | p-value | Estimate | p-value | ||
| TC | 35 | 0.80 | 0.637 | 0.31 | 0.001 | −3.61 | 0.060 |
| TT | 28 | 0.64 | 0.367 | 0.54 | 0.089 | 0.30 | 0.886 |
SNP order in haplotype (rs3783641[T>A]– rs8007267[T>C]). Reference haplotype was AT (frequency = 37%). Regression models are adjusted for covariates (age, sex, and sickle cell type).
For haploblock 1 (rs10483639[G>C]- rs752688 [C>T]-rs4411417[T>C]), we found that the haplotype CTC (OR = 2.04, p = 0.050) showed a trend of association with high (four or more) utilization compared with reference haplotype GCT. For haploblock 2 (rs3783641 [T>A]- rs8007267[T>C]), haplotype TC (OR = 0.31, p = 0.001) was significantly less likely to have high (four or more) utilizations as compared with reference haplotype AT. Interestingly, we observed only a trend associated with CPI scores, but the findings were not statistically significant.
Discussion
Studies have shown that GCH1 affects BH4 production and, as a result, modulates pain through a nitric oxide (NO)-mediated pathway [14,17,27]. Therefore, it has been hypothesized that genetic variants of GCH1 impair its function, thereby decreasing BH4 production and ultimately decreasing pain sensitivity. This was in agreement with the findings that the common alleles were associated with more pain, whereas the minor alleles, in other words, the polymorphic variants, conferred pain protective properties [17,20,21].
In this study, two of the five GCH1 SNPs that we analyzed showed significant association with pain in SCD patients. We explored these associations in all 131 subjects irrespective of sickle type as well as in subjects with SS type (SCD-SS and SCD-Sβ°) only. HbSS and Hb Sβ° genotypes are known to present severe clinical symptoms, including pain [40–42]. Also, in their work researching GCH1 polymorphisms in SCD, Belfer et al. evaluated subjects with sickle type SS only [27]. We observed comparable significance of associations and directionality for both the overall study population and the SS type cohort, indicating the contribution of sickle type in SCD pain.
The minor allele of rs8007267 was associated with a pain-protective property in the additive and dominant models. In the additive model, each C allele contributed to a threefold to fivefold decrease in CPI scores. Whereas in the dominant model, the presence of either one or both C alleles decreased the CPI score by fivefold to sevenfold. This finding, although in conflict with initial studies [17,19–21], is in agreement with the recent findings by Belfer et al. in African Americans with SCD. The T allele of rs8007267 was found to be the minor allele in previous studies in Caucasian populations and was associated with lower biopterin levels and decreased pain [17]. Contrary to this, the variant T was found to be the common allele among African Americans [27,43]. Moreover, in their study on crisis pain in SCD, Belfer et al. found that the previously identified pain protective T allele was instead associated with higher in vitro BH4 levels and increased episodes of acute crisis pain in a dominant genetic model [27]. Consistent with the latter, our study found that the variant C of rs8007267 is the minor allele and variant T the common allele in the African Americans. Like-wise, the risk allele C was associated with decreased chronic pain in subjects carrying this allele. We did not, however, detect an association of rs8007267 with utilization. The latter may be attributed to the relatively small sample size of our study; therefore, it did not refute the findings reported by Belfer et al. [27]. A large prospective study with sufficient power may be needed to validate the findings.
Conversely, the minor allele of rs3783641 was found to be associated with increased utilization score. In the additive model, each A allele resulted in a 37–44% increase in utilization rate and, in the recessive model, there was an 81–97% increase in the utilization rate when homozygous with A. Once again, we observed that, unlike prior work by Tegeder et al. and Lotsch et al. in populations of predominantly Caucasian ethnicity in which the variant A was the common allele [17,19,21], in our cohort of African Americans, the variant A was the minor allele. However, the direction of association remained the same. In other words, the variant T had been previously associated with lower pain scores [17,19], consistent with our finding that the variant A was associated with increased painful crisis.
These findings are not surprising because allele frequencies in racially different populations have been reported to often vary to a great extent [25,27,44] and studies on populations of African ancestry have been unable to replicate past findings [24,26,45]. In addition to the obvious differences in ethnicity, disease pathophysiology possibly plays a significant role in the observed outcomes. As Kim et al. have previously suggested, the direction and strength of association of genetic polymorphisms with pain might depend on the pain-inducing techniques and/or the underlying physiology of the condition being studied [24].
The relationship of BH4 and NO with sickle cell pain is a two-edged sword. Previous findings have indicated that BH4 modulates pain via a NO-dependent pathway and these studies were focused on the nociceptive and proinflammatory effects of neuronal [17] and inducible nitric oxide synthase (iNOS) [19]. However, BH4 is also critical in endothelial nitric oxide synthase (eNOS)-dependent vasodilation and inhibition of platelet aggregation [46]. Unlike its Neuronal nitric oxide synthase (nNOS)- or iNOS-mediated role in nociception, BH4-coupled eNOS maintains proper endothelial functioning [47]. In fact, SNPs in the eNOS gene have been found to be associated with acute chest syndrome and vaso-occlusive crisis in SCD patients [48,49]. Moreover, the depletion of glutamine and L-arginine result in decreased availability of NO, contributing to vascular dysfunction and crisis events in SCD [50–52]. The recently U.S. Food and Drug Administration-approved L-glutamine therapy for SCD significantly reduced painful crises events compared with the placebo group [53]. Therefore, one might speculate that the variant allele, upon impairing GCH1 activity, would decrease BH4 production in the endothelium. This BH4 deficiency would consequently lead to uncoupling of eNOS, thereby generating oxidative stress and precipitating painful crises. The variant/minor allele A of rs3783641 associates with increased utilization rate, a surrogate marker of acute crisis pain. This role of pathophysiology of SCD combined with the specific ethnicity of the study populations could potentially explain the difference in outcome that we observed in our study compared with earlier studies [17,54].
Even though we did not detect a significant correlation between rs8007267 and utilization rate, the minor allele C of rs8007267 exhibited an association with decreased chronic pain. Chronic pain is not a prolonged form of crisis pain, so the role of BH4 in endothelial functioning may not be the contributing factor in this case and the proinflammatory effects of NO or monoamines might be driving this outcome. Belfer et al. found that the T allele of rs8007267, which was earlier found to decrease BH4 and confer pain protection in Caucasians, in fact showed higher BH4 levels in African Americans with SCD [27]. It would be interesting to investigate whether the same holds true in our study population and if the association with decreased persistent pain in subjects carrying the minor allele C could be attributed to that. Although chronic pain and crisis pain are distinct pain phenotypes [11] that are likely driven by different mechanisms, we have previously reported that chronic pain CPI scores can serve as a predictor of acute care utilization wherein subjects with lesser chronic pain intensity exhibited lower utilization rates [29]. This is an important consideration to make when interpreting the findings of this study from a clinical aspect.
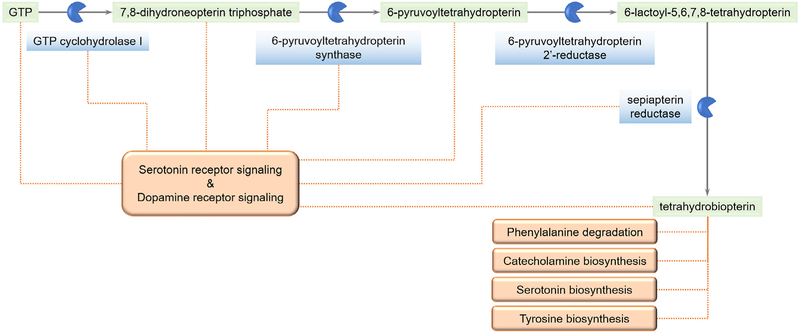
The effect of BH4 modulation on pain exerted via NO-mediated pathways is, however, just one of the many potential ways in which GCH1 variants can play a functional role. There are other less explored mechanisms that might throw light on the causal aspect of this association, as can be visualized from a look at the BH4 biosynthesis pathway and the associated pathways (Figure 2). GCH1 variants can potentially affect a number of neurotransmitter biosynthesis and signaling pathways involved in pain.
Figure 2.
Tetrahydrobiopterin biosynthesis and associated pathways: Schematic representation of some of the different pathways associated with components involved in the synthesis of tetrahydrobiopterin (BH4).
We also observed haplotype patterns that were different from the previously identified pain-protective haplotype rs10483639-rs3783641-rs8007267. As noted by Lazarev et al. in their study on the severity of pain in chronic pancreatitis, the pattern of GCH1 haplotype is considerably different between Caucasian and non-Caucasian populations [25]. In fact, Belfer et al. observed population-based differences in basal GCH1 expression in the brain [27]. We validated our findings by analyzing the African-American cohort in the 1000Genomes database. Both the public database and our sample had three SNPs in high linkage disequilibrium (rs10483639[G>C]- rs752688[C>T]-rs4411417[T>C]). This haploblock, when regressed against utilization counts, showed a trend of association with higher utilization. More interestingly, we identified yet another haplotype in our cohort (rs3783641[T>A]- rs8007267[T>C]). Haplotype TC, the T allele of rs3783641 and the C allele of rs8007267, was less likely to be associated with high utilization compared with the reference AT, the A allele of rs3783641 and the T allele of rs8007267.
The relatively small size of our study is a limitation. Future studies should also take into account factors such as hematocrit, hemoglobin, and ferritin levels, which have been shown to associate strongly with episodes of painful crisis [27,40,55,56] and are potential confounders. Furthermore, admixture proportions within a population can explain phenotypic associations and haplotype patterns. As has been previously noted by Belfer et al., subjects of African ancestry carry a pain-specific haplotype that is not detected in subjects of European ancestry [27].
Currently, the majority of literature studying genetic variants of GCH1 is centered around Caucasian populations, with very little information on its role in SCD and all of which are focused on crisis pain. Our study explores the association of GCH1 polymorphisms with chronic SCD pain along with that of crisis pain. Our findings indicate that specific SNPs and haplotypes of GCH1 associate with acute and chronic pain in SCD and might explain the heterogenous nature of this pain. Rs8007267 is associated with chronic pain and rs3783641 with acute crisis pain. These associations persisted when subjects with HbSS and HbSβ° genotype only were analyzed. We also identified two haploblocks (rs10483639[G>C]-rs752688[C>T]-rs4411417[T>C], and rs3783641[T>A]-rs8007267[T>C]) with SNPs in high linkage disequilibrium. Of these, haplo-type T-C of haploblock rs3783641-rs8007267 showed a significant association with rate of utilization. These findings on GCH1 polymorphisms will help in our understanding of and in the design of personalized medicine for acute crisis pain and chronic pain in SCD.
Acknowledgments
This work was supported in part by grants from the Illinois Department of Public Health (IDPH) and the National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health (NIH) (R01HL124945, R01HL098141, and R35HL140031). EJ was supported by a predoctoral fellowship from the National Institute of Dental and Craniofacial Research (NIDCR) (T32DE018381). YH is supported by a Pathway to Independence Award (K99HL133590). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the IDPH, NIH, NHLBI, NIDCR, or the Veteran’s Administration.
References
- 1.Tsao JC, Jacob E, Seidman LC, Lewis MA, Zeltzer LK. Psychological aspects and hospitalization for pain crises in youth with sickle-cell disease. J Health Psychol. 2014;19:407–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zempsky WT. Evaluation and treatment of sickle cell pain in the emergency department: paths to a better future. Clin Pediatr Emerg Med. 2010;11:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bunn HF. Pathogenesis and treatment of sickle cell disease. N Engl J Med. 1997;337:762–769. [DOI] [PubMed] [Google Scholar]
- 4.Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120:3647–3656. [DOI] [PubMed] [Google Scholar]
- 5.Brousseau DC, Owens PL, Mosso AL, Panepinto JA, Steiner CA. Acute care utilization and rehospitalizations for sickle cell disease. JAMA. 2010;303:1288–1294. [DOI] [PubMed] [Google Scholar]
- 6.Brandow AM, Weisman SJ, Panepinto JA. The impact of a multidisciplinary pain management model on sickle cell disease pain hospitalizations. Pediatr Blood Cancer. 2011;56:789–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darbari DS, Wang Z, Kwak M, et al. Severe painful vaso-occlusive crises and mortality in a contemporary adult sickle cell anemia cohort study. PLoS One. 2013;8:e79923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dale JC, Cochran CJ, Roy L, Jernigan E, Buchanan GR. Health-related quality of life in children and adolescents with sickle cell disease. J Pediatr Health Care. 2011;25:208–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkie DJ, Molokie R, Boyd-Seal D, et al. Patient-reported outcomes: descriptors of nociceptive and neuropathic pain and barriers to effective pain management in adult outpatients with sickle cell disease. J Natl Med Assoc. 2010;102:18–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang ZJ, Wilkie DJ, Molokie R. Neurobiological mechanisms of pain in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2010;2010:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith WR, Penberthy LT, Bovbjerg VE, et al. Daily assessment of pain in adults with sickle cell disease. Ann Intern Med. 2008;148:94–101. [DOI] [PubMed] [Google Scholar]
- 12.Okpala I, Tawil A. Management of pain in sickle-cell disease. J R Soc Med. 2002;95:456–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Adegbola M. Genomics and pain research in sickle cell disease: An explanation of heterogeneity? ISRN Nurs. 2011;2011:672579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latremoliere A, Costigan M. GCH1, BH4 and pain. Curr Pharm Biotechnol. 2011;12:1728–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Costigan M, Latremoliere A, Woolf CJ. Analgesia by inhibiting tetrahydrobiopterin synthesis. Curr Opin Pharmacol. 2012;12:92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latremoliere A, Latini A, Andrews N, et al. Reduction of neuropathic and inflammatory pain through inhibition of the tetrahydrobiopterin pathway. Neuron. 2015;86:1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tegeder I, Costigan M, Griffin RS, et al. GTP cyclohydrolase and tetrahydrobiopterin regulate pain sensitivity and persistence. Nat Med. 2006;12:1269–1277. [DOI] [PubMed] [Google Scholar]
- 18.Kim SJ, Lee WI, Lee YS, et al. Effective relief of neuropathic pain by adeno-associated virus–mediated expression of a small hairpin RNA against GTP cyclohydrolase 1. Mol Pain. 2009;5:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tegeder I, Adolph J, Schmidt H, Woolf CJ, Geisslinger G, Lotsch J. Reduced hyperalgesia in homozygous carriers of a GTP cyclohydrolase 1 haplotype. Eur J Pain. 2008;12:1069–1077. [DOI] [PubMed] [Google Scholar]
- 20.Campbell CM, Edwards RR, Carmona C, et al. Polymorphisms in the GTP cyclohydrolase gene (GCH1) are associated with ratings of capsaicin pain. Pain. 2009;141:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lotsch J, Klepstad P, Doehring A, Dale O. A GTP cyclohydrolase 1 genetic variant delays cancer pain. Pain. 2009;148:103–106. [DOI] [PubMed] [Google Scholar]
- 22.Holliday KL, Nicholl BL, Macfarlane GJ, Thomson W, Davies KA, McBeth J. Do genetic predictors of pain sensitivity associate with persistent widespread pain? Mol Pain. 2009;5:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wadley AL, Lombard Z, Cherry CL, Price P, Kamerman PR. Analysis of a previously identified “pain–protective” haplotype and individual polymorphisms in the GCH1 gene in Africans with HIV–associated sensory neuropathy: a genetic association study. J Acquir Immune Defic Syndr. 2012;60:20–23. [DOI] [PubMed] [Google Scholar]
- 24.Kim H, Dionne RA. Lack of influence of GTP cyclohydrolase gene (GCH1) variations on pain sensitivity in humans. Mol Pain. 2007;3:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lazarev M, Lamb J, Barmada MM, et al. Does the pain-protective GTP cyclohydrolase haplotype significantly alter the pattern or severity of pain in humans with chronic pancreatitis? Mol Pain. 2008;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hendry L, Lombard Z, Wadley A, Kamerman P. KCNS1, but not GCH1, is associated with pain intensity in a black southern African population with HIV-associated sensory neuropathy: A genetic association study. J Acquir Immune Defic Syndr. 2013;63:27–30. [DOI] [PubMed] [Google Scholar]
- 27.Belfer I, Youngblood V, Darbari DS, et al. A GCH1 haplotype confers sex-specific susceptibility to pain crises and altered endothelial function in adults with sickle cell anemia. Am J Hematol. 2014;89:187–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Heart, Lung, and Blood Institute. Genetics and pain severity in sickle cell disease. http://ClinicalTrials.gov Identifier: NCT01441141. https://clinicaltrials.gov/ct2/show/NCT01441141
- 29.Ezenwa MO, Molokie RE, Wang ZJ, et al. Outpatient pain predicts subsequent one-year acute health care utilization among adults with sickle cell disease. J Pain Symptom Manage. 2014;48:65–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilkie DJ, Judge MK, Berry DL, Dell J, Zong S, Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J Pain Symptom Manage. 2003;25:213–224. [DOI] [PubMed] [Google Scholar]
- 31.Jha A, Suarez ML, Ferrans CE, Molokie R, Kim YO, Wilkie DJ. Cognitive testing of PAINReportIt in adult African Americans with sickle cell disease. Comput Inform Nurs. 2010;28:141–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1:277–299. [DOI] [PubMed] [Google Scholar]
- 33.Jhun E, He Y, Yao Y, Molokie RE, Wilkie DJ, Wang ZJ. Dopamine D3 receptor Ser9Gly and catechol-o-methyltransferase Val158Met polymorphisms and acute pain in sickle cell disease. Anesth Analg. 2014;119:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkie DJ, Molokie RE, Suarez ML, Ezenwa MO, Wang ZJ. Composite pain index: reliability, validity, and sensitivity of a patient-reported outcome for research. Pain Med. 2015;16:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vandenbergh DJ, Anthony K, Whitfield KE. Optimizing DNA yield from buccal swabs in the elderly: Attempts to promote buccal cell growth in culture. Am J Hum Biol. 2003;15:637–642. [DOI] [PubMed] [Google Scholar]
- 36.Miller SA, Dykes DD, Polesky HF. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988;16:1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2017. [Google Scholar]
- 38.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. [DOI] [PubMed] [Google Scholar]
- 39.Burkett K, McNeney B, Graham J. A note on inference of trait associations with SNP haplotypes and other attributes in generalized linear models. Hum Hered. 2004;57:200–206. [DOI] [PubMed] [Google Scholar]
- 40.Platt OS, Thorington BD, Brambilla DJ, et al. Pain in sickle cell disease: Rates and risk factors. N Engl J Med. 1991;325:11–16. [DOI] [PubMed] [Google Scholar]
- 41.Rees DC, Williams TN, Gladwin MT. Sickle-cell disease. Lancet. 2010;376:2018–2031. [DOI] [PubMed] [Google Scholar]
- 42.Minireview QuinnCT. Clinical severity in sickle cell disease: The challenges of definition and prognostication. Exp Biol Med (Maywood). 2016;241:679–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Auton A, Brooks LD, Durbin RM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jhun EH, Yao Y, He Y, et al. Prevalence of pain-related single nucleotide polymorphisms in patients of African origin with sickle cell disease. Pharmacogenomics. 2015;16:1795–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wadley AL, Lombard Z, Cherry CL, Price P, Kamerman PR. Analysis of a previously identified “pain-protective” haplotype and individual polymorphisms in the GCH1 gene in Africans with HIV-associated sensory neuropathy: A genetic association study. J Acquir Immune Defic Syndr. 2012;60:20–23. [DOI] [PubMed] [Google Scholar]
- 46.Förstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. 837a–837d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akinsheye I, Klings ES. Sickle cell anemia and vascular dysfunction: The nitric oxide connection. J Cell Physiol. 2010;224:620–625. [DOI] [PubMed] [Google Scholar]
- 48.Sharan K, Surrey S, Ballas S, et al. Association of T-786C eNOS gene polymorphism with increased susceptibility to acute chest syndrome in females with sickle cell disease. Br J Haematol. 2004;124:240–243. [DOI] [PubMed] [Google Scholar]
- 49.Chaar V, Tarer V, Etienne-Julan M, Diara JP, Elion J, Romana M. ET-1 and ecNOS gene polymorphisms and susceptibility to acute chest syndrome and painful vaso-occlusive crises in children with sickle cell anemia. Haematologica. 2006;91:1277–1278. [PubMed] [Google Scholar]
- 50.Morris CR, Suh JH, Hagar W, et al. Erythrocyte glutamine depletion, altered redox environment, and pulmonary hypertension in sickle cell disease. Blood. 2008;111:402–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morris CR, Kuypers FA, Larkin S, Vichinsky EP, Styles LA. Patterns of arginine and nitric oxide in patients with sickle cell disease with vaso-occlusive crisis and acute chest syndrome. J Pediatr Hematol Oncol. 2000;22:515–520. [DOI] [PubMed] [Google Scholar]
- 52.Lopez BL, Kreshak AA, Morris CR, Davis-Moon L, Ballas SK, Ma XL. L-arginine levels are diminished in adult acute vaso-occlusive sickle cell crisis in the emergency department. Br J Haematol. 2003;120:532–534. [DOI] [PubMed] [Google Scholar]
- 53.Niihara Y, Koh HA, Tran L, et al. A phase 3 study of L-glutamine therapy for sickle cell anemia and sickle beta0-thalassemia. Blood. 2014;124:86. [Google Scholar]
- 54.Antoniades C, Shirodaria C, Van Assche T, et al. GCH1 haplotype determines vascular and plasma biopterin availability in coronary artery disease effects on vascular superoxide production and endothelial function. J Am Coll Cardiol. 2008;52:158–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Darbari DS, Onyekwere O, Nouraie M, et al. Markers of severe vaso-occlusive painful episode frequency in children and adolescents with sickle cell anemia. J Pediatr. 2012;160:286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Miller ST, Sleeper LA, Pegelow CH, et al. Prediction of adverse outcomes in children with sickle cell disease. N Engl J Med. 2000;342:83–89. [DOI] [PubMed] [Google Scholar]