Abstract
All of the canonical transient receptor potential channels (TRPC) with the exception of TRPC 2 are expressed in hypothalamic neurons and are involved in multiple homeostatic functions. Although the metabotropic glutamate receptors have been shown to be coupled to TRPC channel activation in cortical and sub-cortical brain regions, in the hypothalamus multiple amine and peptidergic G protein-coupled receptors (GPCRs) and growth factor/cytokine receptors are linked to activation of TRPC channels that are vital for reproduction, temperature regulation, arousal and energy homeostasis. In addition to the neurotransmitters, circulating hormones like insulin and leptin through their cognate receptors activate TRPC channels in POMC neurons. Many of the post-synaptic effects of the neurotransmitters and hormones are regulated in different physiological states by expression of TRPC channels in the post-synaptic neurons. Therefore, TRPC channels are key targets not only for neurotransmitters but circulating hormones in their vital role to control multiple hypothalamic functions, which is the focus of this review.
Keywords: TRPC channels, GnRH, kisspeptin, neurokinin B, POMC, orexin, 17β-estradiol, leptin, insulin
1. Introduction
The mammalian canonical transient receptor potential (TRPC) channel family consists of seven members, TRPC1–7 that appear to function as receptor-operated channels, analogous to the TRP channels involved in Drosophilia phototransduction (Clapham, 2003). With the exception of TRPC 2, these channels are widely distributed in the mammalian brain (Venkatachalam and Montell, 2007). The TRP channels are made of subunits with six membrane-spanning domains that co-assemble as tetrameric complexes similar to what has been described for K+ channels (Clapham et al., 2005; Clapham et al., 2001). TRPC channels co-assemble as heteromeric channels consisting of the TRPC 1, 4 and 5 sub-family (Plant and Schaefer, 2003; Strubing et al., 2001) as well as TRPC 3, 6 and 7 sub-family (Berg et al., 2007; Trebak et al., 2003). Interestingly, TRPC 4 and 5 share ~73% homology, and TRPC 3, 6 and 7 share ~75% homology (Clapham, 2003). The functional distinction between these channel subtypes in CNS neurons has been problematic because of a lack of selective pharmacological reagents (Clapham et al., 2005). However, a unique feature of the heteromeric complexes of TRPC 1 + 4 or TRPC 1 + 5 subunits expressed in HEK cells is current-voltage relationship with a characteristic negative slope conductance and pronounced outward rectification (Clapham, 2003; Strubing et al., 2001). This biophysical characteristic has been exploited to identify TRPC 1 + 4 channel activation by kisspeptin in GnRH neurons (Zhang et al., 2008), and TRPC 1 + 5 channel activation by leptin and insulin in arcuate POMC and kisspeptin neurons (Qiu et al., 2011; Qiu et al., 2010; Qiu et al., 2014). However, it is also important to use molecular techniques like single-cell RT-PCR to help verify expression of TRPC channel transcripts in individual hypothalamic neurons to complement the pharmacological and biophysical characterization (Table 1).
Table 1.
Summary of TRPC channel subtype expression and their cell-type dependent effects
| CELL TYPE | EXPRESSION LEVEL OF TRPC CHANNEL | EFFECT ON CELL | STIMULATOR | PHYSIOLOGICAL FUNCTION | REFERENCE |
|---|---|---|---|---|---|
| GnRH neurons (m) | 4>1>5 | Increases excitability | Kisspeptin | Reproduction | Zhang 2008, 2013; Bosch 2013 |
| POMC neurons (m, g) | 5>1>4>7>6 (m) | Increases excitability | Leptin, insulin, | Energy homeostasis; anorexigenic | Qiu 2010 (m); Qiu 2014 (g); Gao 2017 (m) |
| ARH Kiss1 neurons (m,g) | 5 (m) | Increases excitability | Senktide, insulin | Reproduction | Qiu (new data, m); Qiu 2014 |
| Hypocretin/Orexin neurons (r) | 4,5>7>1>3 | Increases excitability | Unknown, but controls excitability | Arousal | Cvetkovic-Lopes 2010 |
| Vasopressin neurons (r) | 4 | Increases excitability | Water deprivation | Body fluid homeostasis | Nedungadi 2014 |
| Glutamatergic MnPO neurons (m) | 1,5,7; PCR only | Increase excitability | Histamine | Thermoregulation | Tabarean 2012 |
| Glucose-excited neurons in MBH (m) | 3 | Increases excitability | Glucose | Energy homeostasis | Chretien 2017 |
| PMV neurons (m) | Putative, pharmacology only | Increases excitability | Leptin | Reproduction | Williams 2011 |
| GABAergic neurons in LH (m) | Putative, pharmacology only | Increases excitability results in MCH neuron inhibition | TRH | Anorexic and arousal actions | Zhang 2012 |
ARH, arcuate nucleus; GnRH, gonadotropin- releasing hormone; LH, lateral hypothalamus ; MBH, mediobasal hypothalamus ; MCH, melanin-concentrating hormone; MnPO, median preoptic nucleus; PCR, polymerase chain reaction ; PMV, ventral premammillary nucleus ; POMC, pro-opiomelanocortin; TRH, thyrotropin-releasing hormone. (m), mouse; (r), rat; (g), guinea pig.
The mammalian TRPC channels can be activated by G protein-coupled receptors and receptor tyrosine kinases (see Ambudkar and Ong, 2007; Clapham, 2003 for review). TRPC channels are one of the major targets for group I metabotropic glutamate receptor (mGluR1) signaling in CNS neurons (Bengtson et al., 2004; Berg et al., 2007; Faber et al., 2006; Tozzi et al., 2003). For example, in substantia nigra dopamine neurons TRPC 1 and 5 channels are highly expressed, and the mGluR1 agonist dihydroxyphenylglycine-induced current yields a double-rectifying (“S” shape) current-voltage plot (Tozzi et al., 2003). In addition, the peptide cholecystokinin via its receptor (CCK2) activates TRPC 1, 4 and 5 channels in amygdala neurons with the characteristic double-rectifying I/V (Meis et al., 2007). Both the mGluR1 and CCK2 receptors are Gq-coupled to PLC activation which leads to hydrolysis of phosphatidylinositol 4,5-bisphosphate (PIP2) to diacylglycerol (DAG) and inositol 1,4,5 triphosphate (IP3). A unique feature of TRPC 4 and 5 channels is that they are sensitive (potentiated) by the lanthanide lanthanum (La3+), which blocks TRPC 3, 6 and 7 channels (Clapham et al., 2005). This unique pharmacology has been used to characterize TPRC 5 signaling in POMC neurons (Qiu et al., 2010; Qiu et al., 2014). Classically, the TRPC 3, 6 and 7 sub-family is DAG sensitive (Ambudkar and Ong, 2007; Clapham, 2003; Clapham et al., 2005). Interestingly, TRPC channels are minimally Ca2+ selective, but can associate with Orai calcium channels to form calcium release-activated calcium channels (see below) (Birnbaumer, 2009).
2. Calcium homeostasis and TRPC channel activity
TRPC channels form either receptor-operated cation channels (activated by membrane delimited receptors) or store-operated calcium channels (activated by depletion of calcium stores), which is dependent on their association with the endoplasmic reticulum protein stromal-interaction molecule 1 (STIM1) and Orai calcium channels (Birnbaumer, 2009; Salido et al., 2011). STIM1 is localized to the endoplasmic reticulum (ER) membrane of cells and its N-terminal domain contains an EF-hand that protrudes into the lumen of the endoplasmic reticulum to sense changes in ER Ca2+ concentrations (Salido et al., 2011). Upon depletion of endoplasmic reticulum Ca2+, STIM1 undergoes a conformational change, oligomerizes and then interacts with plasma membrane Orai and TRPC channels to become plasma membrane calcium release-activated calcium (Icrac) channels (Huang et al., 2006; Salido et al., 2011; Yuan et al., 2007). In cerebellar Purkinje neurons, activation of mGluR1 receptors evokes IP3 receptor-mediated Ca2+ release from endoplasmic reticulum stores, activation of STIM1, recruitment of plasma membrane TRPC 1 and Orai channels, and the refilling of endoplasmic reticulum Ca2+ stores (Hartmann et al., 2014). This is critical for Purkinje neuron excitability since specific deletion of Stim1 in Purkinje neurons causes significant motor impairments in mice (Hartmann et al., 2014). The STIM1 Ca2+ sensing is also involved in the control of L-type Ca2+ channel activity in hippocampal pyramidal neurons (Dittmer et al., 2017). Depolarization by glutamate activates L-type calcium channels and release of Ca2+ from the endoplasmic reticulum stores that activates STIM1, which drives aggregation of the calcium channels to inhibit L-type channel activity (Dittmer et al., 2017).
STIM1 is also dynamically regulated by other endogenous activity. Phosphorylation of STIM1 is required for oligomerization, and 17β-estradiol (E2) inhibits the phosphorylation of STIM1 and consequently its interaction with plasma membrane Orai and TRPC channels and hence store-operated Ca2+ entry (Salido et al., 2011; Yuan et al., 2007). Pathologically, Stim1 mRNA is up-regulated in human glomerular mesangial cells in a diabetic (hyperglycemia) model (Chaudhari et al., 2014) and in smooth muscle cells of hypertensive mice that exhibit vascular dysfunction and high (systolic) blood pressure (Kassan et al., 2016). Therefore, STIM1 regulates plasma membrane calcium (and TRPC) channels in both physiological and pathological conditions.
Plasma membrane voltage-gated calcium channels can also be part of TRPC channel signaling complexes in neurons under normal physiological conditions (Hartmann et al., 2014; Sun et al., 2017). In substantia nigra dopamine neurons the activities of TRPC 1 and L-type calcium (Cav1.3) channels are coupled (Sun et al., 2017), and TRPC 3 and P/Q-type calcium (Cav2.1) channels are linked in cerebellar Purkinje neurons (Hartmann et al., 2014). The T-type calcium channel Cav3.1 underlies burst firing in hypothalamic kisspeptin neurons (Zhang et al., 2013b) and facilitates TRPC 4 channel activation in GnRH neurons (Zhang et al., 2013a; Zhang et al., 2008). In POMC neurons it appears that the T-type calcium channel is coupled to TRPC 5 channel activation by insulin (Qiu et al., 2018a). Therefore, voltage-gated calcium channels can be part of the TRPC channel signaling complex.
3. TRPC channels in reproductive circuits
3.1 Gonadotropin Releasing Hormone (GnRH) neurons
The somas of GnRH neurons are localized to the medial septum, rostral preoptic area, anterior hypothalamus and medial basal hypothalamus with the exception that in mice and rats there are a few scattered GnRH neurons in the medial basal hypothalamus (Silverman et al., 1987; Silverman et al., 1979). The majority of GnRH neurons project to the median eminence where they release GnRH peptide into the portal blood in a pulsatile manner to control gonadotropin secretion from the pituitary gland and ultimately fertility (Clarke and Cummins, 1985; Levine et al., 1985; Levine and Ramirez, 1980). Because GnRH cell bodies are widely scattered throughout the hypothalamic-septum continuum, the control of the synchronous firing of these neurons, the so-called “pulse generator,” had been debated over two decades (see (Moenter et al., 2003) for review). Then in 2003 the puberty peptide kisspeptin was discovered and its ability to stimulate GnRH release via signaling through GPR54, a.k.a. Kiss1R (De Roux et al., 2003; Seminara et al., 2003). Indeed, kisspeptin is the most potent and efficacious neuropeptide/neurotransmitter to excite GnRH neurons (Han et al., 2005; Pielecka-Fortuna et al., 2008; Zhang et al., 2008), and Kiss1 neurons may be the presynaptic pacemaker neurons that drive GnRH neurons (Figure 1) (Liu et al., 2011; Qiu et al., 2016; Wang et al., 2016; Zhang et al., 2015). Although single action potential-generated calcium influx is sufficient to spark the release of classical neurotransmitters, burst firing or tetanic stimulation is required for the release of neuropeptides. High frequency electrical stimulation is required to evoke peptide release as originally demonstrated in the frog ganglion by Jan et al. (Jan et al., 1979) (see Arrigoni and Saper, 2014 for review). In this respect, high frequency electrical stimulation of anteroventral periventricular/periventricular preoptic nucleus Kiss1 (Kiss1AVPV/PeN) neurons increases GnRH cell firing, which is absent in Kiss1R knockout mice and blocked by Kiss1R partial agonist peptide 234 (Liu et al., 2011). High frequency photostimulation (20 Hz) of Kiss1AVPV/PeN neurons releases kisspeptin, which excites GnRH neurons directly (Qiu et al., 2016). This high-frequency photostimulation of Kiss1AVPV/PeN neurons evokes a slow EPSP in GnRH neurons that is characterized by the tell-tale double rectifying I/V plot and antagonism by the Kiss1R partial agonist peptide 234 (Qiu et al., 2016; Roseweir et al., 2009; Zhang et al., 2008).
Figure 1. A model by which activation of Kiss1 neurons governs GnRH neuronal excitability through TRPC channels.
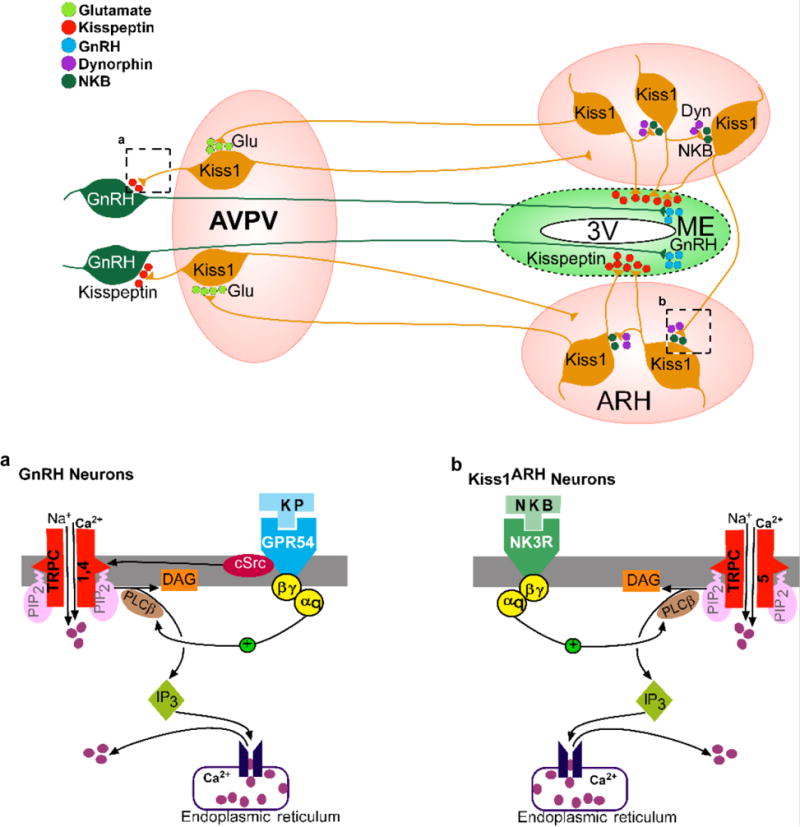
Activation of Kiss1 neurons in the ARH releases neurokinin B (NKB) that depolarizes and recruits other Kiss1ARH neurons. Dynorphin is co-released and acts presynaptically to modulate (inhibit) the release of NKB. Together the two peptides govern the synchronous activity of Kiss1ARH neurons and promote kisspeptin release that stimulates GnRH release in the median eminence (ME). Kiss1ARH neurons also communicate with the Kiss1AVPV/PeN neurons via the fast neurotransmitter glutamate, which stimulates burst-firing of Kiss1AVPV/PeN neurons. Activation of these rostral Kiss1 neurons releases kisspeptin to robustly excite GnRH neurons via activation of the GPR54 (Kiss1R) signaling cascade, thereby stimulating the release of GnRH at the time of the preovulatory surge. Kisspeptin, GPR54, NKB, TacR3 and GnRH are all required for normal fertility. [Modified from Fig. 12, Qiu et al., 2016. Elife doi: 10.7554/eLife.16246.] (a) Kisspeptin excites GnRH neurons through TRPC 4 channels. Kisspeptin binds to the Gq-coupled GPR54 receptor to activate phospholipase Cβ (PLCβ), which catabolizes PIP2, potentiates TRPC channel activity and inhibits the inwardly rectifying K+ channel activity. cSRC kinase, which is activated by kisspeptin/GRP54 signaling, potentiates the activity of TRPC 4 channels; and (b) Neurokinin B excites Kiss1ARH neurons through TRPC 5 channel activation.
Pharmacologically, Kisspeptin-54 and the smaller peptide fragments (e.g., kisspeptin 14, 13 and 10) bind with low nanomolar affinities to rat and human Kiss1R (GPR54) expressed in Chinese hamster ovary cells and are Gq-coupled to stimulate PIP2 hydrolysis, Ca2+ mobilization, arachidonic acid release, and phosphorylation of mitogen-activated protein kinase (Kotani et al., 2001). In native GnRH neurons, kisspeptin causes excitation primarily through activation of TRPC channels and to a lesser extent inhibition of inwardly rectifying K+ channels (Pielecka-Fortuna et al., 2008; Zhang et al., 2008). The activation of TRPC channels in GnRH neurons by kisspeptin is not affected by buffering intracellular calcium levels by the calcium chelators EGTA (ethylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid) or BAPTA (1,2-bis(o-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid) or by depleting intracellular calcium stores (Zhang et al., 2013a). Therefore, store release of calcium does not appear to play a critical role in the kisspeptin-mediated activation of TRPC channels. However, the kisspeptin-activated TRPC current is attenuated by the general calcium channel blocker Cd2+ and by the low voltage-activated calcium channel blocker Ni2+, but not by the high voltage-activated calcium channel blocker amlodipine. This would indicate that low voltage-activated (T-type) calcium channels are a trigger for activating TRPC channels in GnRH neurons and are part of the TRPC signaling complex as described above. However, reducing extracellular calcium to nominally calcium free has no effect on the kisspeptin-activated TRPC current, an indication that very little calcium is needed to spark the opening of TRPC channels in GnRH neurons. This is consistent with the small, but persistent, T-type calcium channel current around the resting membrane potential of GnRH neurons (Zhang et al., 2009). Therefore, kisspeptin excites GnRH neurons primarily through the opening of a TRPC channel that is independent of intracellular calcium store release, but appears to be dependent on calcium influx through T-type calcium channels. This ensures fast and sustained depolarization of GnRH neurons.
Single-cell RT-PCR analysis shows that GnRH neurons express primarily TRPC 1,4,5 channels (Table 1) (Zhang et al., 2008). TRPC 1 channels form heteromeric complexes with TRPC 4 and/or TRPC 5 channels (Plant and Schaefer, 2003; Strubing et al., 2001). Quantitative PCR analysis shows that Trpc4 mRNA is expressed at levels 4-fold higher than Trpc1 and Trpc5 in GnRH neurons, and Trpc4 mRNA expression is significantly increased in E2-treated, ovariectomized mice (Bosch et al., 2013). Phosphatidylinositol 4,5-bisphosphate is an important regulator of TRPC channels, and hydrolysis of PIP2 is required for kisspeptin-induced TRPC channel activation in GnRH neurons (Zhang et al., 2013a). In addition to PIP2 depletion, kisspeptin activation of TRPC channels is also dependent on the non-receptor tyrosine (cSrc) kinase activation (Fig. 1), since both global tyrosine kinase inhibitors such as genistein and the specific cSrc kinase inhibitor PP2 (4-amino-5-(4-chlorophenyl)-7-(dimethylethyl)pyrazolo[3,4-d]pyrimidine) attenuate (inhibit) kisspeptin currents in GnRH neurons (Zhang et al., 2013a). cSrc kinase directly regulates TPRC 4 channel activity through tyrosine phosphorylation, which causes rapid insertion of TRPC 4 channels into the plasma membrane (Odell et al., 2012). Therefore, cSrc kinase appears to be a key signaling molecule in the kisspeptin-mediated activation of TRPC 4 channels in GnRH neurons, and Kiss1R activation of TRPC 4 channels appears to be a major cellular mechanism by which kisspeptin causes a robust, sustained depolarization of GnRH neurons to generate a surge for maintaining reproductive function (d’Anglemont de Tassigny and Colledge, 2010). TRPC 5 channels are also expressed in the pituitary gonadotropes and mediate, at least in part, the depolarizing effects of GnRH (Götz et al., 2017).
3.2 Arcuate Kiss1 (Kiss1ARH) neurons
As stated earlier since the discovery of the pulsatile release of GnRH, the source of the hypothalamic “pulse generator” activity had been an open question. More recently, Kiss1ARH neurons (a.k.a KNDy neurons since they co-express neurokinin B and dynorphin) have been proposed to be the “pulse-generator” neurons that stimulate pulsatile secretion of GnRH (Figure 1) (Lehman et al., 2010; Navarro et al., 2009), and unilateral optogenetic stimulation of Kiss1ARH neurons is efficacious to generate pulsatile release of LH (driven by pulsatile GnRH) in the mouse (Han et al., 2015). Therefore, to study the synaptic connections between Kiss1 neurons, we have utilized a Kiss1Cre mouse expressing channel rhodopsin unilaterally in Kiss1 neurons (Kiss1-Cre mice were injected with an AAV-DIO-ChR2:mCherry virus) to study the circuitry (Qiu et al., 2016). These injections labeled ChR2-mCherry-positive (Kiss1) cells and fibers ipsilaterally, but only ChR2-mCherry-positive fibers in the contralateral ARH. In addition to the ChR2:mCherry labeling ipsilaterally, control AAV-DIO-YFP was injected into the contralateral ARH in order to identify Kiss1 neurons for whole cell recordings. Low frequency (1 Hz) photostimulation of the ChR2 fibers evoked a glutamatergic fast EPSC in the contralateral ARH, but high frequency stimulation (20 Hz) generated a peptidergic slow EPSP, which is mediated by neurokinin B based on the blockade by tachykinin receptor 3 (TacR3) antagonists. The slow EPSP was blocked by sodium channel blocker tetrodotoxin (TTX) but rescued with the addition of the K+ channels blockers 4-aminopyridine (4-AP) and tetraethylammonium (TEA), demonstrating direct communication between Kiss1ARH neurons on both sides (Cousin and Robinson, 2000; Petreanu et al., 2009). The TacR3 is coupled to the activation of TRPC channels (Figure 2). Moreover, dual patch recording revealed that Kiss1ARH neurons are excited simultaneously with photostimulation of the contralateral Kiss1ARH neuronal input even with the median eminence transected (Qiu et al., 2016). Thus, high frequency autoexcitation of Kiss1ARH neurons ipsilaterally is able to recruit Kiss1ARH neurons bilaterally, which is dependent on TRPC channel activation (Figure 2), to induce synchronization of this critical neural network that underlies the pulse generator activity in mammalian females (Clarkson et al., 2017; Qiu et al., 2016).
Figure 2. TacR3 agonist excites Kiss1ARH neurons through TRPC5 channels.
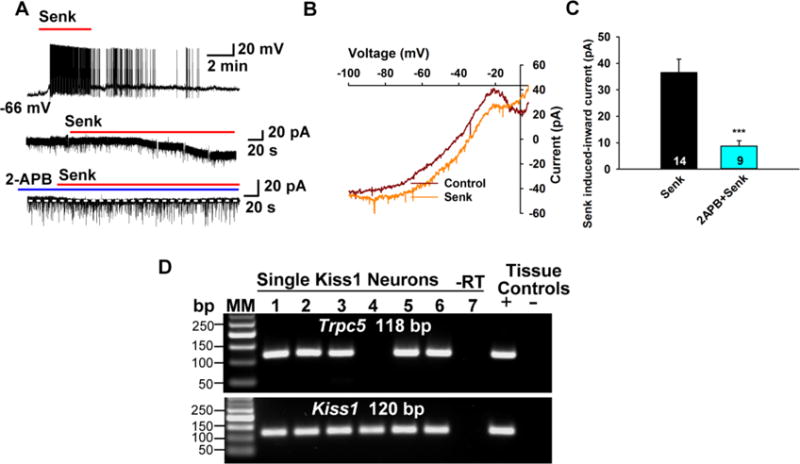
(A) TacR3 agonist senktide (500 nM) depolarizes Kiss1ARH neurons in female mice and induces firing (upper trace). In voltage clamp senktide induces an inward current (middle trace). Vhold = −60 mV. The inward current is blocked by TRPC channel blocker 2-aminoethyl diphenylborinate (2-APB; 100 μM) (lower trace). (B) The I-V relationship for the senktide (Senk)-induced inward current reverses at −10 mV and exhibits double rectification. (C) Summary of the effects of TRPC channel blocker 2-APB on the senktide-induced inward currents. The TRPC channel blocker is applied 15 min before the application of senktide (500 nM). Vhold = − 60 mV. ***p < 0.005, different from the senktide control group. Data points represent the mean ± SEM. Unpaired two-tailed t test, t(21) = 4.234, p = 0.0004. Cell numbers tested are indicated. (D) Representative gel images illustrating the mRNA expression of Trpc5 channel subunit in Kiss1ARH neurons harvested from female mice. The expected size of PCR products for Kiss1 and Trpc5 are indicated. MM is the molecular marker; −RT indicates a harvested Kiss1 neuron without RT; + indicates positive tissue control (with RT); – indicates negative tissue control (without RT) using cDNA from mouse medial basal hypothalamic tissue. RT, reverse transcriptase.
The tachykinins comprise a series of structurally related peptides that are derived from alternative processing of three Tac genes and are expressed throughout the nervous and immune systems (Steinhoff et al., 2014). Tachykinins interact with three neurokinin G protein-coupled receptors, Tacr1, Tacr2 and Tacr3. Tacr1 and Tacr3, but not Tacr2, mRNA are expressed in Kiss1ARH neurons based on single cell RT-PCR analysis (Navarro et al., 2015), and the TacR3 agonist senktide depolarizes Kiss1ARH neurons, which is blocked by TacR3 antagonists (Navarro et al., 2011; Qiu et al., 2016). As proof of principle, we recently investigated the effects of senktide to activate TRPC 5 channels directly. These channels are expressed in Kiss1ARH neurons, and bath application of the TacR3 agonist generated an inward current, which was blocked by the TRPC channel blocker 2-aminoethoxydiphenyl borate (2-APB) (Figure 2). The I/V plot for the senktide-induced cation current showed the typical characteristics of TRPC 5 channels with a reversal of -10 mV (Figure 2). Kiss1ARH neurons co-express NKB and dynorphin, and high-frequency firing of Kiss1ARH neurons co-releases NKB and dynorphin (Qiu et al., 2016). NKB binds to TacR3 in neighboring Kiss1ARH neurons to open TRPC 5 channels to cause a robust depolarization (slow EPSP); co-released dynorphin feedbacks to bind to presynaptic κ-opioid receptors to limit the release of NKB to discrete bursts of activity and presumably postsynaptic TacR3 desensitization (Qiu et al., 2016). The combination of the two peptide neurotransmitters coordinates the synchronous firing of Kiss1ARH neurons that drives the pulsatile release of GnRH into the median eminence (Figure 1) (Campos and Herbison, 2014; Clarkson et al., 2017; Qiu et al., 2016).
4. TRPC channels in other homeostatic circuits
4.1 Orexin neurons and arousal
Orexin neurons are maintained in a depolarized state, independent of synaptic input, by a cation current generated by the expression of TRPC channels (Table 1) (Cvetkovic-Lopes et al., 2010). These spontaneously firing orexin neurons are thought to maintain arousal states (Adamantidis et al., 2007; Saper et al., 2001) and are inhibited during sleep states by GABAergic synaptic input (Eggermann et al., 2003). The orexin neurons project to the tuberomammillary histamine neurons, and high frequency photostimulation of ChR2-expressing orexin neurons evokes a slow EPSC in these histamine neurons that is abrogated by a selective orexin receptor 2 antagonist (Schone et al., 2014). Although the underlying cellular (channels) mechanism mediating the slow EPSC has not been elucidated, it is possibly mediated by TRPC channels. The orexin neurons also excite local lateral hypothalamic GABA neurons that inhibit the REM sleep-promoting melanin-concentrating hormone (MCH) neurons. High frequency optogenetic stimulation of orexin neurons inhibits MCH cell firing, which is presumed to be via these inhibitory GABA interneurons (Apergis-Schoute et al., 2015). In addition, Thyrotropin-releasing Hormone (TRH), which increases arousal states and locomotor activity, also excites the local GABA neurons, and based on the pharmacological profiling with a number of channels blockers is thought to be mediated by TRPC channels (Zhang and Van den Pol, 2012).
Therefore, TRPC channels play a prominent role in the orexin-MCH neuronal circuitry in controlling arousal/sleep states (Table 1).
4.2 Preoptic neurons and temperature regulation
The preoptic area of the hypothalamus is involved in many physiological and behavioral homeostatic processes (McKinley et al., 2015). One main function of the preoptic area is to govern core body temperature in response to changes in ambient temperature, which is achieved in part by controlling brown adipose tissue thermogenesis. Thermoregulatory neurons of median preoptic nucleus (MnPO) are a target at which histamine modulates body temperature, and MnPO GABAergic neurons tonically inhibit warm-sensitive neurons in the medial preoptic area (Morrison et al., 2014). Histamine fibers from the tuberomammillary nucleus densely innervate the MnPO, and activation of H3 histamine receptors reduces the firing frequency of MnPO GABAergic neurons by augmenting an A-type current conducted by Kv4.2 channels, which decreases inhibitory input to the warm-sensitive neurons and ultimately sympathetic drive for brown adipose tissue (BAT) thermogenesis (Lundius et al., 2010; Sethi et al., 2011). In addition, histamine activates H1 and H2 receptors in MnPO glutamatergic neurons, which increases their firing frequency and excitatory input to the warm-sensitive neurons and BAT thermogenesis (Lundius et al., 2010; Tabarean et al., 2012). The activation of H1 histamine receptors on MnPO glutamatergic neurons excites these neurons through TRPC 1,5 channels (Tabarean, 2012). Interestingly, Kiss1ARH neurons project to the medial preoptic area (Krajewski et al., 2010; Yeo and Herbison, 2011), and local infusion of a selective neurokinin B agonist activates preoptic neurons and reduces core body temperature (Dacks et al., 2011). The TacR3 is highly expressed in the medial preoptic area (Dacks et al., 2011) and presumably is coupled to TRPC channel activation (Figure 2). Neurokinin B expression is up-regulated in post-menopausal states and is implicated in the etiology of menopausal hot flashes (Rance et al., 2013) such that TacR3 antagonists are in phase 2 clinical trials for treatment of postmenopausal hot flushes (Prague et al., 2017). Therefore, TRPC channels play a critical role in the control of temperature homeostasis.
4.3 POMC neurons and energy homeostasis
In the early part of this century it was discovered that leptin excites/depolarizes POMC neurons by opening non-selective cation channels, which is a vital action of the metabolic hormone to suppress food intake (Cowley et al., 2001). Ten years later it was discovered that TRPC 5 channels constitute the non-selective cation channels mediating the response, which became the focus of numerous metabolic studies (Gao et al., 2017; Qiu et al., 2011; Qiu et al., 2010; Qiu et al., 2014; Sohn et al., 2011). POMC and the orexigenic NPY/AgRP neurons are major CNS targets of insulin and leptin actions (Belgardt and Bruning, 2010; Morton et al., 2006; Qiu et al., 2014; Schwartz et al., 2000). Insulin depolarizes mouse, guinea pig and monkey POMC neurons in both males and females via activation of TRPC 5 channels (Figure 3), and hyperpolarizes NPY/AgRP neurons via activation of KATP channels (Qiu et al., 2018b; Qiu et al., 2014), activities that are complementary for mediating the anorexigenic effects of insulin. In addition, the serotonin 5HT2C receptor is Gq-coupled to activate TRPC 5 channels in POMC neurons (Sohn et al., 2011), and deletion of TRPC 5 channels specifically in POMC neurons results in a decrease in energy expenditure and increase in food intake and weight gain in male mice (Gao et al., 2017). The increase in POMC cell excitability induced by insulin translates into heightened transcriptional activity—i.e., an increase in c-Fos expression in the arcuate nucleus and specifically in POMC neurons following icv insulin (Qiu et al., 2014). Insulin delivered directly into the third ventricle uniformly decreases food intake in guinea pigs (Qiu et al., 2014), mice (Benoit et al., 2002; Brown et al., 2006) and rats (Clegg et al., 2011). The insulin-induced decrease in food intake is correlated with alterations in energy expenditure as manifested by increases in O2 consumption, CO2 production and metabolic heat production (Qiu et al., 2014). The catabolic effects of insulin are blocked by melanocortin receptor 3, 4 antagonists, which is evidence that actions of insulin are mediated by POMC neurons (Benoit et al., 2002). Although optogenetic and pharmacogenetic stimulation of NPY/AgRP neurons rapidly increases food consumption (Aponte et al., 2011; Krashes et al., 2011), prolonged stimulation of POMC neurons attenuates food intake (Aponte et al., 2011). The effects of leptin and insulin in POMC neurons are vital for both the short term (excitability) and long term (transcriptional) modulation of POMC neuronal activity and the control of food intake and ultimately energy homeostasis.
Figure 3. A cellular model of insulin (and leptin) signaling via TRPC 5 channel activation in POMC neurons.
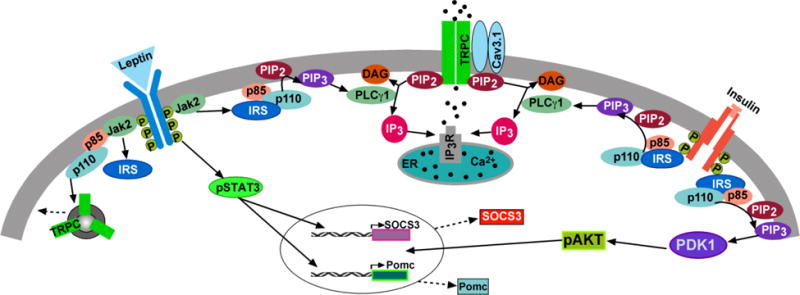
Insulin signals via IRS-PI3K to activate TRPC 5 channels in POMC neurons, which generates a robust inward cationic current to depolarize POMC neurons and increase their excitability. Similarly, leptin binding to its receptor (LRb) triggers the recruitment of the tyrosine kinase Janus kinase (JAK) 2, leading to the activation of the JAK/signal transducer and activator of transcription (STAT) signaling pathway and simultaneously activation of PI3K, which also activates TRPC 5 channels. PI3K (p85/p110) will also accelerate the rapid insertion of TPRC 5 channels into the plasma membrane. PDK1, 3-phosphoinositide–dependent protein kinase-1. [Modified from Fig. 8, Qiu et al., 2018a. Endocrinology 159, 647-664.]
In POMC neurons the insulin receptor (InsR) couples to phosphoinositide 3-kinase (PI3K) p110β activation (Al-Qassab et al., 2009; Xu et al., 2005), and the InsR-mediated excitation of POMC neurons is abrogated by inhibition of PI3K activity (Al-Qassab et al., 2009; Hill et al., 2008; Qiu et al., 2010; Qiu et al., 2014). Activation of PI3K generates PIP3, which stimulates phospholipase C (PLC) and protein kinase B (Akt) (Bae et al., 1998; Falasca et al., 1998; Qiu et al., 2014; Rameh et al., 1998). PLC also hydrolyzes PIP2, which modulates TRPC 4, 5 channel activity (Qiu et al., 2014; Rodríguez-Menchaca et al., 2012; Zhang et al., 2013a). In addition, it is thought that PI3K rapidly increases the insertion of TRPC 5 channels into the plasma membrane from intracellular vesicular pools to further boost depolarization and Ca2+ entry into neurons (Figure 3) (Bezzerides et al., 2004). Collectively, all of these PI3K-mediated effects are critically involved in the activation of TRPC 5 channels by insulin actions in POMC neurons (Qiu et al., 2018b).
Insulin resistance (IR) is at the core of the metabolic syndrome and causes abnormal insulin signaling in cells throughout the body. Neurons, similar to fat and muscle cells, can develop hyperinsulinemia-induced IR, which results in severe injury to the nervous system as seen in diabetic neuropathies (Kim and Feldman, 2012). Moreover, males exhibit a higher incidence of metabolic syndrome than women in early adult life, but this sex difference diminishes sharply in hypo-estrogenic states (Gustafsson et al., 2011; Janssen et al., 2008). Exposing rodents to a high fat diet over 6-10 weeks invariably causes diet-induced obesity (DIO) and dramatic alterations in multiple physiological systems. The DIO model has been exploited for electrophysiological studies of NPY/AgRP and POMC neurons (Baver et al., 2014; Konner and Bruning, 2012; Parton et al., 2007; Plum et al., 2006; Tsaousidou et al., 2014). Diet-induced obese PomcEGFP mice have been used for comprehensive cellular and molecular studies in order to dissect deficits in the insulin signaling pathway (Qiu et al., 2018a). Although there are no differences in the resting membrane potential or input resistance between the control and DIO male groups, there is a significant attenuation in the insulin response (TRPC channel-mediated current) in POMC neurons from DIO males, which is an indication that these anorexigenic neurons have become insulin resistant (Qiu et al., 2018a).
Interestingly, TRPC 5 channels are fully expressed and operational in DIO males since the thiazolidinedione rosiglitazone (Bon and Beech, 2013) is able to fully activate the channels in POMC neurons (Qiu et al., 2018a). The inward current generated by rosiglitazone mimics insulin’s effects in control animals with the same reversal potential, indicative of activation of a non-selective cationic (i.e., TRPC) channel. Therefore, it is clear that TRPC 5 channels are not downregulated in POMC neurons from DIO males, but rather there is an uncoupling of insulin receptors from channel activation. Indeed, there is no difference in Trpc5 mRNA expression in POMC neurons from DIO versus control males based on qPCR of POMC neurons (Qiu et al., 2018a).
However, there is a pronounced sex difference in the response to insulin with diet-induced obesity. Similar to the males, there are no differences in the resting membrane potential or input resistance of POMC neurons between control and DIO female mice; but in contrast to males, the steady-state response (inward current) to insulin is not attenuated with DIO (Qiu et al., 2018a). The reversal potential for the insulin-induced inward current is the same for both control fed and DIO females and as expected is antagonized by the TRPC channel blocker 2-APB (Clapham et al., 2005). Moreover, insulin robustly depolarizes and increases the firing activity of POMC neurons from DIO females similar to the controls. Hence, there is no attenuation of the insulin response (i.e., TRPC 5 channel activation) in POMC neurons from DIO, proestrous females, which indicates that there is a clear sex difference in the development of insulin resistance by POMC neurons in obesity.
In order to explore the role of the gonadal steroids in preserving the insulin response in DIO females, we ovariectomized female PomcEGFP mice after they had been on a high fat diet for 10 weeks, and one week following ovariectomy, we gave an estradiol benzoate treatment (s.c. injection regimen that yields proestrous serum levels of E2) (Bosch et al., 2013) or oil vehicle. In contrast to ovariectomized females fed a control diet, POMC neurons from ovariectomized, DIO females are completely resistant to insulin such that there is no inward current (Qiu et al., 2018a). However, the TRPC 5 channel opener rosiglitazone is able to generate a robust inward current indicating that TRPC 5 channels are expressed and functional in POMC neurons from DIO, ovariectomized females. In contrast, POMC neurons from E2-treated, ovariectomized, DIO females maintain their sensitivity to insulin—i.e., insulin induces a robust inward current and depolarizes POMC neurons. Therefore, in the absence of E2 there appears to be an uncoupling of InsR from activating TRPC 5 channels in obese females, but the insulin response is rescued with E2 replacement. Importantly, E2 treatment significantly down-regulated the expression of Stim1, which as discussed above (Section 2), shifts TRPC 5 channels from being store-operated to receptor (InsR)-operated channels (Birnbaumer, 2009; Salido et al., 2011).
Deletion of both insulin and leptin receptors in POMC neurons causes overt systemic insulin resistance in both male and female mice (Hill et al., 2010). If left untreated, insulin-deficiency leads to hyperglycemia, polyuria, ketoacidosis and death as seen in type 1 diabetes. However, insulin-deficient rodents are viable with leptin monotherapy, and this life-saving therapy is effective in mice expressing leptin receptors only in hypothalamic POMC and GABAergic neurons (Fujikawa et al., 2013). Coppari and colleagues (Fujikawa et al., 2013) postulated that leptin and insulin must engage the same hypothalamic circuitry to maintain glucose homeostasis and hepatic function, which at the cellular level means that insulin and leptin engage a common effector such as TRPC 5 channels to excite POMC neurons to maintain homeostatic function (Qiu et al., 2014). In addition, TRPC 3 channels are involved in mediating the glucose-mediated excitation of ventromedial hypothalamic neurons (Table 1) (Chretien et al., 2017), which provide an additional excitatory drive to POMC neurons (Conde et al., 2017; Tong et al., 2007). Therefore, TRPC channels play a pivotal role in maintaining energy homeostasis.
5. Conclusions
Although TRPC channels are probably one of the major targets for group I metabotropic glutamate receptor (mGluR1) signaling in CNS neurons (Bengtson et al., 2004; Berg et al., 2007; Faber et al., 2006; Tozzi et al., 2003), they are key players in multiple homeostatic functions in the hypothalamus. As such they are activated by G protein-coupled receptors similar to other CNS structures (see Ambudkar and Ong, 2007; Clapham, 2003 for review) and in addition by tyrosine kinase receptors, which can be expressed in the same neuron. In Kiss1ARH neurons, for example, leptin and insulin through their cognate receptors signal via PI3K to activate TRPC 5 channels in different metabolic states (Qiu et al., 2011; Qiu et al., 2014), whereas the Gq-coupled TacR3 activates TRPC 5 channels in reproductive states (Figure 2) (Qiu et al., 2016). Likewise in POMC neurons, the serotonin 5HT2C receptor is Gαq-coupled to activate TRPC 5 channels in POMC neurons (Sohn et al., 2011), and deletion of TRPC 5 channels specifically in POMC neurons results in a decrease in energy expenditure and increase in food intake and weight gain in male mice, a clear metabolic phenotype (Gao et al., 2017). Furthermore, the importance of preserving this critical metabolic signaling pathway is exemplified by the fact that E2 is able to protect females from the development of insulin resistance through preserving TRPC 5 channel coupling to the insulin receptor (Figure 3). Therefore, there are multiple systems that impinge on TRPC channel signaling in hypothalamic peptidergic neurons. A future challenge will be to develop specific tools (e.g., selective pharmacological agents, genetic engineering) for probing TRPC channel function in different physiologic and pathophysiologic states.
Highlights.
TRPC channels are highly expressed in hypothalamic neurons
TRPC channels maintain the excitability of hypothalamic neurons
Multiple G protein-coupled receptors are coupled to TRPC channels
Receptor tyrosine kinases activate TRPC channels
Receptor coupling to TRPC channels depends on physiological state
Acknowledgments
This research was funded by US National Institute of Health (NIH) grants: NS038809 (to MJK), NS043330 (to OKR) and DK068098 (to MJK and OKR). The authors would like to recognize Ms. Martha Bosch for her help in preparing the figures.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The authors have nothing to disclose.
References
- Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Qassab H, Smith MA, Irvine EE, Guillermet-Guibert J, Claret M, Choudhury AI, Selman C, Piipari K, Clements M, Lingard S, Chandarana K, Bell JD, Barsh GS, Smith AJH, Batterham RL, Ashford MLJ, Vanhaesebroeck B, Withers DJ. Dominant role of the p110β isoform of PI3K over p110α in energy homeostasis regulation by POMC and AgRP neurons. Cell Metab. 2009;10:343–354. doi: 10.1016/j.cmet.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambudkar IS, Ong HL. Organization and function of TRPC channelsomes. Pflügers Arch. 2007;455:187–200. doi: 10.1007/s00424-007-0252-0. [DOI] [PubMed] [Google Scholar]
- Apergis-Schoute J, Iordanidou P, Faure C, Jego S, Schone C, Aitta-Aho T, Adamantidis A, Burdakov D. Optogenetic evidence for inhibitory signaling from orexin to MCH neurons via local microcircuits. J Neurosci. 2015;35:5435–5441. doi: 10.1523/JNEUROSCI.5269-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigoni E, Saper CB. What optogenetic stimulation is telling us (and failing to tell us) about fast neurotransmitters and neuromodulators in brain circuits for wake-sleep regulation. Curr Opin Neurobiol. 2014;29C:165–171. doi: 10.1016/j.conb.2014.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae YS, Cantley LG, Chen CS, Kim SR, Kwon KS, Rhee SG. Activation of phospholipase C-γ by phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:4465–4469. doi: 10.1074/jbc.273.8.4465. [DOI] [PubMed] [Google Scholar]
- Baver SB, Hope K, Guyot S, Bjorbaek C, Kaczorowski C, O’Connell KM. Leptin modulates the intrinsic excitability of AgRP/NPY neurons in the arcuate nucleus of the hypothalamus. J Neurosci. 2014;34:5486–5496. doi: 10.1523/JNEUROSCI.4861-12.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belgardt BF, Bruning JC. CNS leptin and insulin action in the control of energy homeostasis. Ann N Y Acad Sci. 2010;1212:97–113. doi: 10.1111/j.1749-6632.2010.05799.x. [DOI] [PubMed] [Google Scholar]
- Bengtson CP, Tozzi A, Bernardi G, Mercuri NB. Transient receptor potential-like channels mediate metabotropic glutamate receptor EPSCs in rat dopamine neurones. J Physiol. 2004;555:323–330. doi: 10.1113/jphysiol.2003.060061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoit SC, Air EL, Coolen LM, Strauss R, Jackman A, Clegg DJ, Seeley RJ, Woods SC. The catabolic action of insulin in the brain is mediated by melanocortins. J Neurosci. 2002;22:9048–9052. doi: 10.1523/JNEUROSCI.22-20-09048.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AP, Sen N, Bayliss DA. TrpC3/C7 and Slo2.1 are molecular targets for metabotropic glutamate receptor signaling in rat striatal cholinergic interneurons. J Neurosci. 2007;27:8845–8856. doi: 10.1523/JNEUROSCI.0551-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezzerides VJ, Ramsey IS, Kotecha S, Greka A, Clapham DE. Rapid vesicular translocation and insertion of TRP channels. Nat Cell Biol. 2004;6:709–720. doi: 10.1038/ncb1150. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. The TRPC class of ion channels: a critical review of their roles in slow, sustained increases in intracellular Ca2+ concentrations. Annu Rev Pharmacol Toxicol. 2009;49:395–426. doi: 10.1146/annurev.pharmtox.48.113006.094928. [DOI] [PubMed] [Google Scholar]
- Bon RS, Beech DJ. In pursuit of small molecule chemistry for calcium-permeable non-selective TRPC channels – mirage or pot of gold? Br J Pharmacol. 2013;170:459–474. doi: 10.1111/bph.12274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch MA, Tonsfeldt KJ, Rønnekleiv OK. mRNA expression of ion channels in GnRH neurons: subtype-specific regulation by 17β-Estradiol. Mol Cell Endocrinol. 2013;367:85–97. doi: 10.1016/j.mce.2012.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, Clegg DJ, Benoit SC, Woods SC. Intraventricular insulin and leptin reduce food intake and body weight in C57BL/6J mice. Physiol Behav. 2006;89:687–691. doi: 10.1016/j.physbeh.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Campos P, Herbison AE. Optogenetic activation of GnRH neurons reveals minimal requirements for pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2014;111:18387–18392. doi: 10.1073/pnas.1415226112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari S, Wu P, Wang Y, Ding Y, Yuan J, Begg M, Ma R. High glucose and diabetes enhanced store-operated Ca2+ entry and increased expression of its signaling proteins in mesangial cells. Am J Physiol Renal Physiol. 2014;306:F1069–F1080. doi: 10.1152/ajprenal.00463.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chretien C, Fenech C, Lienard F, Grall S, Chevalier C, Chaudy S, Brenachot X, Berges R, Louche K, Stark R, Nedelec E, Laderriere A, Andrews ZB, Benani A, Flockerzi V, Gascuel J, Hartmann J, Moro C, Birnbaumer L, Leloup C, Penicaud L, Fioramonti X. Transient Receptor Potential Canonical 3 (TRPC3) Channels Are Required for Hypothalamic Glucose Detection and Energy Homeostasis. Diabetes. 2017;66:314–324. doi: 10.2337/db16-1114. [DOI] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Julius D, Montell C, Schultz G. International Union of Pharmacology. XLIX Nomenclature and structure-function relationships of transient receptor potential channels. Pharmacol Rev. 2005;57:427–450. doi: 10.1124/pr.57.4.6. [DOI] [PubMed] [Google Scholar]
- Clapham DE, Runnels LW, Strubing C. The TRP ion channel family. Nat Rev Neurosci. 2001;2:387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- Clarke IJ, Cummins JT. Increased gonadotropin-releasing hormone pulse frequency associated with estrogen-induced luteinizing hormone surges in ovariectomized ewes. Endocrinology. 1985;116:2376–2383. doi: 10.1210/endo-116-6-2376. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, Porteous RW, Kim JS, Colledge WH, Iremonger KJ, Herbison AE. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci USA. 2017;114:E10216–E10223. doi: 10.1073/pnas.1713897114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg DJ, Gotoh K, Kemp C, Wortman MD, Benoit SC, Brown LM, D’Alessio D, Tso P, Seeley RJ, Woods SC. Consumption of a high-fat diet induces central insulin resistance independent of adiposity. Physiol Behav. 2011;103:10–16. doi: 10.1016/j.physbeh.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde K, Fabelo C, Krause WC, Propst R, Goethel J, Fischer D, Hur J, Meza C, Ingraham HA, Wagner EJ. Testosterone Rapidly Augments Retrograde Endocannabinoid Signaling in Proopiomelanocortin Neurons to Suppress Glutamatergic Input from Steroidogenic Factor 1 Neurons via Upregulation of Diacylglycerol Lipase-α. Neuroendocinology. 2017;105:341–356. doi: 10.1159/000453370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousin MA, Robinson PJ. Ca2+ influx inhibits dynamin and arrests synaptic vesicle endocytosis at the active zone. J Neurosci. 2000;20:949–957. doi: 10.1523/JNEUROSCI.20-03-00949.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- Cvetkovic-Lopes V, Eggermann E, Uschakov A, Grivel J, Bayer L, Jones BE, Serafin M, Muhlethaler M. Rat hypocretin/orexin neurons are maintained in a depolarized state by TRPC channels. PLOS One. 2010;5:e15673. doi: 10.1371/journal.pone.0015673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Anglemont de Tassigny X, Colledge WH. The role of kisspeptin signaling in reproduction. Physiology. 2010;25:207–217. doi: 10.1152/physiol.00009.2010. [DOI] [PubMed] [Google Scholar]
- Dacks PA, Krajewski SJ, Rance NE. Activation of neurokinin 3 receptors in the median preoptic nucleus decreases core temperature in the rat. Endocrinology. 2011;152:4894–4905. doi: 10.1210/en.2011-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS 1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100:10972–10976. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittmer PJ, Wild AR, Dell’Acqua ML, Sather WA. STIM1 Ca2+ Sensor Control of L-type Ca2+-Channel-Dependent Dendritic Spine Structural Plasticity and Nuclear Signaling. Cell Rep. 2017;19:321–334. doi: 10.1016/j.celrep.2017.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bayer L, Serafin M, Saint-Mleux B, Bernheim L, Machard D, Jones BE, Muhlethaler M. The wake-promoting hypocretin-orexin neurons are in an intrinsic state of membrane depolarization. J Neurosci. 2003;23:1557–1562. doi: 10.1523/JNEUROSCI.23-05-01557.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber ES, Sedlak P, Vidovic M, Sah P. Synaptic activation of transient receptor potential channels by metabotropic glutamate receptors in the lateral amygdala. Neuroscience. 2006;137:781–794. doi: 10.1016/j.neuroscience.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Falasca M, Logan SK, Lehto VP, Baccante G, Lemmon MA, Schlessinger J. Activation of phospholipase Cγ by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 1998;17:414–422. doi: 10.1093/emboj/17.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujikawa T, Berglund ED, Patel VR, Ramadori G, Vianna CR, Vong L, Thorel F, Chera S, Herrera PL, Lowell BB, Elmquist JK, Baldi P, Coppari R. Leptin engages a hypothalamic neurocircuitry to permit survival in the absence of insulin. Cell Metab. 2013;18:431–444. doi: 10.1016/j.cmet.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Yao T, Deng Z, Sohn JW, Sun J, Huang Y, Kong X, Yu KJ, Wang RT, Chen H, Guo H, Yan J, Cunningham KA, Chang Y, Liu T, Williams KW. TrpC5 mediates acute leptin and serotonin effects via pomc neurons. Cell Rep. 2017;18:583–592. doi: 10.1016/j.celrep.2016.12.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götz V, Qiao S, Beck A, Boehm U. Transient receptor potential (TRP) channel function in the reproductive axis. Cell Calcium. 2017;67:138–147. doi: 10.1016/j.ceca.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Persson M, Hammarstrom A. Life course origins of the metabolic syndrome in middle-aged women and men: the role of socioeconomic status and metabolic risk factors in adolescence and early adulthood. Ann Epidemiol. 2011;21:103–110. doi: 10.1016/j.annepidem.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Han SK, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, Clifton DK, Steiner RA, Herbison AE. Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. J Neurosci. 2005;25:11349–11356. doi: 10.1523/JNEUROSCI.3328-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SY, McLennan T, Czieselsky K, Herbison AE. Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proc Natl Acad Sci USA. 2015;112:13109–13114. doi: 10.1073/pnas.1512243112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann J, Karl RM, Alexander RP, Adelsberger H, Brill MS, Rühlmann C, Ansel A, Sakimura K, Baba Y, Kurosaki T, Misgeld T, Konnerth A. STIM1 controls neuronal Ca2+ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron. 2014;82:635–644. doi: 10.1016/j.neuron.2014.03.027. [DOI] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Bruning JC, Elmquist JK. Direct insulin and leptin action on pro-poiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab. 2010;11:286–297. doi: 10.1016/j.cmet.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Williams KW, Ye C, Luo J, Balthasar N, Coppari R, Cowley MA, Cantley LC, Lowell BB, Elmquist JK. Acute effects of leptin require PI3K signalng in hypothalamic proopiomelanocortin neurons in mice. J Clin Invest. 2008;118:1796–1805. doi: 10.1172/JCI32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GN, Zeng W, Kim JY, Yuan JP, Han L, Muallem S, Worley PF. STIM1 carboxyl-terminus activates native SOC, Icrac and TRPC1 channels. Nat Cell Biol. 2006;8:1003–1010. doi: 10.1038/ncb1454. [DOI] [PubMed] [Google Scholar]
- Jan YN, Jan LY, Kuffler SW. A peptide as a possible transmitter in sympathetic ganglia of the frog. Proc Natl Acad Sci USA. 1979;76:1501–1505. doi: 10.1073/pnas.76.3.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen I, Powell LH, Crawford S, Lasley B, Sutton-Tyrrell K. Menopause and the metabolic syndrome: the Study of Women’s Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. doi: 10.1001/archinte.168.14.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassan M, Ait-Aissa K, Radwan E, Mali V, Haddox S, Gabani M, Zhang W, Belmadani S, Irani K, Trebak M, Matrougui K. Essential Role of Smooth Muscle STIM1 in Hypertension and Cardiovascular Dysfunction. Arter Thromb Vasc Biol. 2016;36:1900–1909. doi: 10.1161/ATVBAHA.116.307869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Feldman EL. Insulin resistance in the nervous system. Trends Endocrinol Metab. 2012;23:133–141. doi: 10.1016/j.tem.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16:144–152. doi: 10.1016/j.cmet.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Kotani M, Detheux M, Vandenbogaerde A, Communi D, Vanderwinden JM, Le Poul E, Brezillon S, Tyldesley R, Suarez-Huerta N, Vandeput F, Blanpain C, Schiffmann SN, Vassart G, Parmentier M. The metastasis suppressor gene KiSS-1 encodes kisspeptins, the natural ligands of the orphan G protein-coupled receptor GPR54. J Biol Chem. 2001;276:34631–34636. doi: 10.1074/jbc.M104847200. [DOI] [PubMed] [Google Scholar]
- Krajewski SJ, Burke MC, Anderson MJ, McMullen NT, Rance NE. Forebrain projections of arcuate neurokinin B neurons demonstrated by anterograde tract-tracking and monosodium glutamate lesions in the rat. Neuroscience. 2010;166:680–697. doi: 10.1016/j.neuroscience.2009.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151:3479–3489. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JE, Norman RL, Gliessman PM, Oyama TT, Bangsberg DR, Spies HG. In vivo gonadotropin-releasing hormone release and serum luteinizing hormone measurements in ovariectomized, estrogen-treated rhesus macaques. Endocrinology. 1985;117:711–721. doi: 10.1210/endo-117-2-711. [DOI] [PubMed] [Google Scholar]
- Levine JE, Ramirez VD. In vivo release of luteinizing hormone-releasing hormone estimated with push-pull cannulae from the mediobasal hypothalami of ovariectomized, steroid-primed rats. Endocrinology. 1980;107:1782–1790. doi: 10.1210/endo-107-6-1782. [DOI] [PubMed] [Google Scholar]
- Liu X, Porteous R, d’Anglemont de Tassigny X, Colledge WH, Millar R, Petersen SL, Herbison AE. Frequency-dependent recruitment of fast amino acid and slow neuropeptide neurotransmitter release controls gonadotropin-releasing hormone neuron excitability. J Neurosci. 2011;31:2421–2430. doi: 10.1523/JNEUROSCI.5759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundius EG, Sanchez-Alavez M, Ghochani Y, Tabarean IV. Histamine influences body temperature by acting at H1 and H3 receptors on distinct populations of preoptic neurons. J Neurosci. 2010;30:4369–4381. doi: 10.1523/JNEUROSCI.0378-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinley MJ, Yao ST, Uschakov A, McAllen RM, Rundgren M, Martelli D. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiol. 2015;214:8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- Meis S, Munsch T, Sosulina L, Pape HC. Postsynaptic mechanisms underlying responsiveness of amygdloid neurons to cholecystrokinin are mediated by a transient receptor potential-like current. Mol Cell Neurosci. 2007;35:356–367. doi: 10.1016/j.mcn.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Moenter SM, DeFazio AR, Pitts GR, Nunemaker CS. Mechanisms underlying episodic gonadotropin-releasing hormone secretion. Front Neuroendocrinol. 2003;24:79–93. doi: 10.1016/s0091-3022(03)00013-x. [DOI] [PubMed] [Google Scholar]
- Morrison SF, Madden CJ, Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metab. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- Navarro VM, Bosch MA, León S, Simavli S, True C, Pinilla L, Carroll RS, Seminara SB, Tena-Sempere M, Rønnekleiv OK, Kaiser UB. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156:627–637. doi: 10.1210/en.2014-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA. Regulation of gonadotropin-releasing hormone secretion by kisspeptin/dynorphin/neurokinin B neurons in the arcuate nucleus of the mouse. J Neurosci. 2009;29:11859–11866. doi: 10.1523/JNEUROSCI.1569-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro VM, Gottsch ML, Wu M, García-Galiano D, Hobbs SJ, Bosch MA, Pinilla L, Clifton DK, Dearth A, Rønnekleiv OK, Braun RE, Plamiter RD, Tena-Sempere M, Alreja M, Steiner RA. Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology. 2011;152:4265–4275. doi: 10.1210/en.2011-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedungadi TP, Cunningham JT. Differential regulation of TRPC 4 in the vasopressin magnocellular system by water deprivation and hepatic cirrhosis in the rat. Am J Physiol Regul Integr Comp Physiol. 2014;306:R304–R314. doi: 10.1152/ajpregu.00388.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell AF, Scott JL, Van Helden DF. Epidermal growth factor induces tyrosine phosphorylation, membrane insertion, and activation of transient receptor potential channel 4. J Biol Chem. 2012;280:37974–37987. doi: 10.1074/jbc.M503646200. [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Lee CE, Elmquist JK, Cowley MA, Lowell BB. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature. 2007;449:228–232. doi: 10.1038/nature06098. [DOI] [PubMed] [Google Scholar]
- Petreanu L, Mao T, Sternson SM, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielecka-Fortuna J, Chu Z, Moenter SM. Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology. 2008;149:1979–1986. doi: 10.1210/en.2007-1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plant TD, Schaefer M. TRPC4 and TRPC5: receptor-operated Ca2+-permeable nonselective cation channels. Cell Calcium. 2003;33:441–450. doi: 10.1016/s0143-4160(03)00055-1. [DOI] [PubMed] [Google Scholar]
- Plum L, Ma X, Hampel B, Balthasar N, Coppari R, Munzberg H, Shanabrough M, Burdakov D, Rother E, Janoschek R, Alber J, Belgardt BF, Koch L, Seibler J, Schwenk F, Fekete C, Suzuki A, Mak TW, Krone W, Horvath TL, Ashcroft FM, Bruning JC. Enhanced PIP3 signaling in POMC neurons causes KATP channel activation and leads to diet-sensitive obesity. J Clin Invest. 2006;116:1886–1901. doi: 10.1172/JCI27123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prague JK, Roberts RE, Comninos AN, Clarke S, Jayasena CN, Nash Z, Doyle C, Papadopoulou DA, Bloom SR, Mohideen P, Panay N, Hunter MS, Veldhuis JD, Webber LC, Huson L, Dhillo WS. Neurokinin 3 receptor antagonism as a novel treatment for menopausal hot flushes: a phase 2, randomised, double-blind, placebo-controlled trial. The Lancet. 2017;389:1809–1820. doi: 10.1016/S0140-6736(17)30823-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Meza C, Navarro UV, Nestor CC, Wagner EJ, Rønnekleiv OK, Kelly MJ. Estradiol protects proopiomelanocortin neurons against insulin resistance. Endocrinology. 2018a;159:647–664. doi: 10.1210/en.2017-00793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Bosch MA, Rønnekleiv OK, Kelly MJ. Guinea pig kisspeptin neurons are depolarized by leptin via activation of TRPC channels. Endocrinology. 2011;152:1503–1514. doi: 10.1210/en.2010-1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Fang Y, Rønnekleiv OK, Kelly MJ. Leptin excites proopiomelanocortin neurons via activation of TRPC channels. J Neurosci. 2010;30:1560–1565. doi: 10.1523/JNEUROSCI.4816-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, Rønnekleiv OK. High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excited GnRH neurons. eLife. 2016;5:e16246. doi: 10.7554/eLife.16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin and leptin excite anorexigenic pro‐opiomelanocortin neurones via activation of TRPC5 channels. J Neuroendocrinol. 2018b;30:e12501. doi: 10.1111/jne.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Zhang C, Borgquist A, Nestor CC, Smith AW, Bosch MA, Ku S, Wagner EJ, Rønnekleiv OK, Kelly MJ. Insulin excites anorexigenic proopiomelanocortin neurons via activation of canonical transient receptor potential channels. Cell Metab. 2014;19:682–693. doi: 10.1016/j.cmet.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameh LE, Rhee SG, Spokes K, Kazlauskas A, Cantley LC, Cantley LG. Phosphoinositide 3-Kinase Regulates Phospholipase Cγ-mediated Calcium Signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Rance NE, Dacks PA, Mittelman-Smith MA, Romanovsky AA, Krajewski-Hall SJ. Modulation of body temperature and LH secretion by hypothalamic KNDy (kisspeptin, neurokinin B and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. doi: 10.1016/j.yfrne.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Menchaca AA, Adney SK, Zhou L, Logothetis DE. Dual regulation of voltage-sensitive ion channels by PIP2. Front Pharmacol. 2012;3:170–170. doi: 10.3389/fphar.2012.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, Pineda R, Gottsch ML, Tena-Sempere M, Moenter SM, Terasawa E, Clarke IJ, Steiner RA, Millar RP. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29:3920–3929. doi: 10.1523/JNEUROSCI.5740-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salido GM, Jardin I, Rosado JA. The TRPC ion channels: association with Orai1 and STIM1 proteins and participation in capacitative and non-capacitative calcium entry. Adv Exp Med Biol. 2011;704:413–433. doi: 10.1007/978-94-007-0265-3_23. [DOI] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Scammell TE. The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci. 2001;24:726–731. doi: 10.1016/s0166-2236(00)02002-6. [DOI] [PubMed] [Google Scholar]
- Schone C, Apergis-Schoute J, Sakurai T, Adamantidis A, Burdakov D. Coreleased orexin and glutamate evoke nonredundant spike outputs and computations in histamine neurons. Cell Rep. 2014;7:697–704. doi: 10.1016/j.celrep.2014.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–1627. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Sethi J, Sanchez-Alavez M, Tabarean IV. Kv4.2 Mediates Histamine Modulation of Preoptic Neuron Activity and Body Temperature. PLOS One. 2011;6:e29134. doi: 10.1371/journal.pone.0029134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Jhamandas J, Renaud LP. Localization of luteinizing hormone-releasing hormone (LHRH) neurons that project to the median eminence. J Neurosci. 1987;7(8):2312–2319. [PMC free article] [PubMed] [Google Scholar]
- Silverman AJ, Krey LC, Zimmerman EA. A comparative study of the luteinizing hormone releasing hormone (LHRH) neuronal networks in mammals. Biol Reprod. 1979;20:98–110. doi: 10.1093/biolreprod/20.1.98. [DOI] [PubMed] [Google Scholar]
- Sohn JW, Xu Y, Jones JE, Wickman K, Williams KW, Elmquist JK. Serotonin 2C receptor activates a distinct population of arcuate pro-opiomelanocortin neurons via TRPC channels. Neuron. 2011;71:488–497. doi: 10.1016/j.neuron.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff MS, von Mentzer B, Geppetti P, Pothoulakis C, Bunnett NW. Tachykinins and their receptors: contributions to physiological control and the mechanisms of disease. Physiol Rev. 2014;94:265–301. doi: 10.1152/physrev.00031.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strubing C, Krapivinsky G, Krapivinsky L, Clapham DE. TRPC1 and TRPC5 form a novel cation channel in mammalian brain. Neuron. 2001;29:645–655. doi: 10.1016/s0896-6273(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sun Y, Zhang H, Selvaraj S, Sukumaran P, Lei S, Birnbaumer L, Singh BB. Inhibition of L-Type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J Neurosci. 2017;37:3364–3377. doi: 10.1523/JNEUROSCI.3010-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV. Persistent Histamine Excitation of Glutamatergic Preoptic Neurons. PLOS One. 2012;7:e47700. doi: 10.1371/journal.pone.0047700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabarean IV, Sanchez-Alavez M, Sethi J. Mechanism of H2 histamine receptor dependent modulation of body temperature and neuronal activity in the medial preoptic nucleus. Neuropharmacology. 2012;63:171–180. doi: 10.1016/j.neuropharm.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Q, Ye CP, McCrimmon RJ, Dhillon H, Choi B, Kramer MD, Yu J, Yang Z, Christiansen LM, Lee CE, Choi CS, Zigman JM, Shulman GI, Sherwin RS, Elmquist JK, Lowell BB. Synaptic glutamate release by ventromedial hypothalamic neurons is part of the neurocircuitry that prevents hypoglycemia. Cell Metab. 2007;5:383–393. doi: 10.1016/j.cmet.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tozzi A, Bengtson CP, Longone P, Carignani C, Fusco FR, Bernardi G, Mercuri NB. Involvement of transient receptor potential-like channels in responses to mGluR-I activation in midbrain dopamine neurons. Eur J Neurosci. 2003;18:2133–2145. doi: 10.1046/j.1460-9568.2003.02936.x. [DOI] [PubMed] [Google Scholar]
- Trebak M, Vazquez G, Bird GS, Putney JW., Jr The TRPC3/6/7 subfamily of cation channels. Cell Calcium. 2003;33:451–461. doi: 10.1016/s0143-4160(03)00056-3. [DOI] [PubMed] [Google Scholar]
- Tsaousidou E, Paeger L, Belgardt BF, Pal M, Wunderlich CM, Brönneke H, Collienne U, Hampel B, Wunderlich FT, Schmidt-Supprian M, Kloppenburg P, Brüning JC. Distinct roles for JNK and IKK activation in agouti-related peptide neurons in the development of obesity and insulin resistance. Cell Rep. 2014;9:1495–1506. doi: 10.1016/j.celrep.2014.10.045. [DOI] [PubMed] [Google Scholar]
- Venkatachalam K, Montell C. TRP channels. Annu Rev Biochem. 2007;76:387–417. doi: 10.1146/annurev.biochem.75.103004.142819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, DeFazio RA, Moenter SM. Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro. 2016;3 doi: 10.1523/ENEURO.0094-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams KW, Sohn J-W, Donato J, Lee CE, Zhao JJ, Elmquist JK. The acute effects of leptin require PI3K signaling in the hypothalamic ventral premammillary nucleus. J Neurosci. 2011;31:13147–13156. doi: 10.1523/JNEUROSCI.2602-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu AW, Kaelin CB, Takeda K, Akira S, Schwartz MW, Barsh GS. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J Clin Invest. 2005;115:951–958. doi: 10.1172/JCI24301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo SH, Herbison AE. Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology. 2011;152:2387–2399. doi: 10.1210/en.2011-0164. [DOI] [PubMed] [Google Scholar]
- Yuan JP, Zeng W, Huang GN, Worley PF, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK. 17β-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. J Neurosci. 2009;29:10552–10562. doi: 10.1523/JNEUROSCI.2962-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. Kisspeptin activation of TRPC4 channels in female GnRH neurons requires PIP2 depletion and cSrc kinase activation. Endocrinology. 2013a;154:2772–2783. doi: 10.1210/en.2013-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Bosch MA, Rønnekleiv OK, Kelly MJ. 17β-estradiol increases persistent Na+ current and excitability of AVPV/PeN Kiss1 neurons in female mice. Mol Endocrinol. 2015;29:518–527. doi: 10.1210/me.2014-1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Roepke TA, Kelly MJ, Rønnekleiv OK. Kisspeptin depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-like cationic channels. J Neurosci. 2008;28:4423–4434. doi: 10.1523/JNEUROSCI.5352-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Tonsfeldt KJ, Qiu J, Bosch MA, Kobayashi K, Steiner RA, Kelly MJ, Rønnekleiv OK. Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. Am J Physiol Endo Metab. 2013b;305:E1384–E1397. doi: 10.1152/ajpendo.00406.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Van den Pol AN. Thyrotropin-releasing hormone (TRH) inhibits melanin-concentrating hormone neurons: implication for TRH-mediated anorexic and arousal actions. J Neurosci. 2012;32:3032–3043. doi: 10.1523/JNEUROSCI.5966-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]


