Abstract
Currently, older adults comprise nearly one third of prevalent U.S. dialysis patients, and this proportion will increase as the population ages. Older dialysis patients experience greater morbidity and mortality than non-dialysis patients of the same age, and in part, it is related to progressive functional decline. Progressive functional decline, characterized by need for assistance with more than two activities of daily living, contributes to risk of hospitalization, further functional decline, and subsequent nursing home placement when a patient no longer functions independently at home. Progressive functional decline may appear to be unavoidable for older dialysis patients; however, comprehensive geriatric assessment may alleviate the prevalence and severity of functional decline. This editorial summarizes common risk factors of functional decline and introduces comprehensive geriatric assessment as a potentially transformative approach to breaking the cycle of functional decline in older dialysis patients.
Introduction
Currently, older adults comprise nearly one third of prevalent U.S. dialysis patients, and this proportion will increase as the population ages. Although a small proportion of older dialysis patients reside in nursing homes,1 those who live independently in the community have a greater risk of nursing home placement compared to older adults without kidney failure because of a substantially faster rate of functional decline over time.2,3 Functional decline is a geriatric syndrome that is characterized as the process of loss of physical and/or cognitive function and manifest as needing assistance with basic activities (e.g., walking, dressing, or remembering to take medication).4 Dialysis patients with functional limitations often experience recurrent hospitalizations, which in turn, can yield further functional decline.
Whether functional decline is acute (e.g., post-hospitalization or acute infection) or insidious, one functional loss can lead to others in a “snowball” manner resulting in dependence in activities of daily living.5 This cycle is frequently manifest as recurrent hospitalizations, subsequent inpatient rehabilitation, further complications from chronic conditions or geriatric syndromes, impaired quality of life, and a subsequent trajectory of illness that hastens death.6,7 Despite this common course, functional decline can be reversed in some instances.4,8 In this editorial, we assert that the cycle of functional decline can be broken by identifying patients at risk and incorporating geriatric assessment into routine dialysis care and then using the results of the assessment to guide management.
Framework of Risk Factors of Functional Decline
There is no risk calculator to estimate an individual dialysis patient’s risk of functional decline; however, there is sufficient evidence to inform a framework through which we can characterize risk factors. This framework defines five categories of risk factors of functional decline: 1) frailty, 2) geriatric syndromes, 3) comorbidity burden, 4) disability, and 5) low physical activity (Figure 1). Geriatric syndromes develop as a result of accumulation of multiple risk factors that initiate different pathophysiological pathways to yield a final common pathway.9 Examples of geriatric syndromes include falls, incontinence, delirium, and pressure ulcers.
Figure 1. Risk Factors for Functional Decline in Older Dialysis Patients.
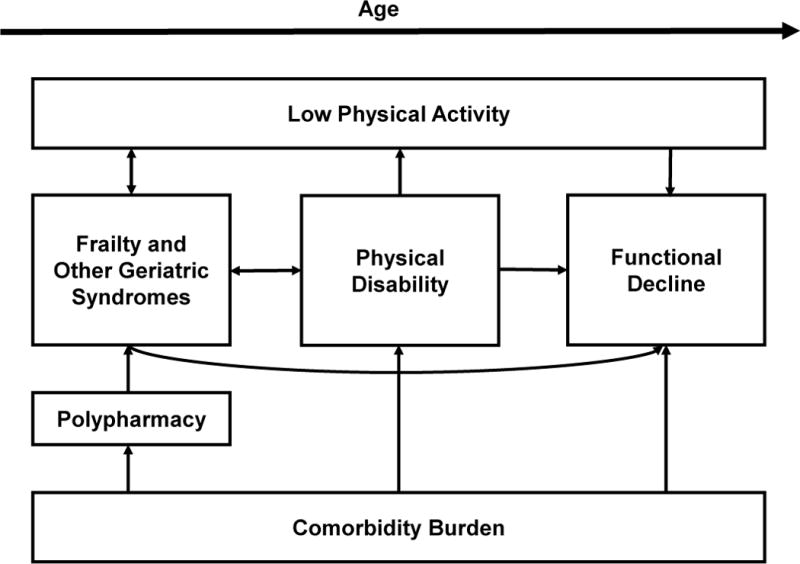
This figure depicts a conceptual framework of risk factors for functional decline (frailty, other geriatric syndromes, physical disability, comorbidity burden, and low physical activity) that interact and contribute to functional decline in a synergistic manner. Increasing age yields a patient who is more sensitive to these risk factors. Geriatric syndromes include falls, incontinence, delirium, and pressure ulcers. Physical disability refers to diminished capacity in vision, hearing, or mobility.
These geriatric syndromes, including frailty, have shared risk factors which support the idea of common underlying pathophysiology, such as sarcopenia and chronic inflammation. Although not mutually exclusive, these categories of risk factors of functional decline are interrelated, yielding a synergistic impact on its rate. For example, a frail older adult with ESRD, arthritis, and diabetes (comorbidity) who uses a walker (disability) may experience an injurious fall (geriatric syndrome) requiring hospitalization and surgery (limited physical activity). This patient is likely to have problems with physical function sooner than someone who had the same comorbidity and disability profile and did not experience a fall. Below, we briefly describe the framework of risk factors of functional decline.
Frailty and Other Geriatric Syndromes
Although considered a “geriatric syndrome,” a higher than expected prevalence of frailty has been described even in younger adults with ESRD.10 Frailty is an overarching syndrome of decreased physiological reserve and resistance to stressors resulting in an increased vulnerability to hospitalization and mortality.11 A commonly used phenotype defines a patient as frail if they manifest at least three of the following five components: unintentional weight loss, exhaustion, low physical activity, weakness, and slow gait speed. This phenotype can be assessed by self-report (weight loss, exhaustion, and low physical activity) and objective (weakness and slow gait speed) measures. Frailty screening tools categorize individuals as not frail, pre-frail (defined as presence of any two of the five components), or frail.10 In one study, dialysis patients who were pre-frail had fewer comorbidities and less disability than those who were frail.10
Underlying mechanisms of frailty include impaired nutrition and sarcopenia which are also common outcomes for older adults with advancing chronic kidney disease (CKD).12 Thus, CKD is considered a risk factor for frailty. Frailty is associated with functional decline through limited muscle function which commonly affects mobility first and basic activities of daily living subsequently.11 Additionally, frailty at dialysis initiation is associated with a decline in cognitive function during the first year of dialysis;13 this loss of cognitive function may be an additional pathway linking frailty to functional decline.
Older dialysis patients who do not meet frailty criteria may nevertheless be at risk of functional decline from other geriatric syndromes. An important contributor to development of geriatric syndromes is polypharmacy. Common in older dialysis patients, polypharmacy increases the likelihood of adverse events, such as falls or delirium, from inappropriate use of medications, such as benzodiazepines or antipsychotics.14 Thus, geriatric syndromes are associated with functional decline through their contribution to a patient’s overall vulnerability to adverse events; the presence of one geriatric syndrome often leads to the development of additional geriatric syndromes. For example, dialysis patients who are frail are at an increased risk of falls.15
Comorbidity, Disability, and Low Physical Activity
The more numerous and severe comorbid conditions are, the greater is the risk of functional decline. Specific comorbid conditions associated with functional decline include obesity, diabetes, coronary artery disease, cerebrovascular disease, arthritis, depression, and cognitive impairment.16–19 Among these conditions, obesity, diabetes, vascular diseases, and arthritis are associated with functional decline through low-grade systemic inflammation and manifest as increased rates of both physical disability and low physical activity.20–22 Common forms of physical disability that are associated with functional decline are visual impairment, hearing impairment, and mobility impairment (i.e., unable to ambulate without assistance).16,23,24 Mobility impairment contributes to fall risk, but also reduces an older patient’s interest in participating in exercise or moderate physical activity. Thus, low physical activity, either resulting from thrice weekly hemodialysis or as a result of an acute hospitalization, reduces muscle strength and contributes to functional decline.25,26
Identifying Dialysis Patients at Risk of Functional Decline
Among the risk factors for functional decline, geriatric syndromes are least familiar to the dialysis provider and more likely to be under recognized. Developed as a tool for identification of older patients at risk for nursing home placement, comprehensive geriatric assessment (CGA) is the gold standard for identifying functional decline and other geriatric syndromes that can contribute to functional decline.27 The standard CGA is characterized by a multi-disciplinary team (e.g., a geriatrician, nurse, and social worker) that conducts a comprehensive multi-dimensional evaluation.8,28 The essential dimensions include functional status, cognition, comorbidities, mood, falls, polypharmacy, nutrition, social support, and financial well-being.29 The evaluation also can extend to caregiver burden, spirituality, sensory impairments, goals of care, advance care planning, and identification of additional geriatric syndromes. This comprehensive approach uncovers geriatric syndromes that otherwise may remain undiagnosed. When implemented in a study of four Dutch dialysis units, the CGA identified polypharmacy, poor nutrition, disability (vision, hearing, or mobility), and depression each had a prevalence of at least 25%.30 These findings show that CGA can identify geriatric syndromes and other risk factors for functional decline in older dialysis patients.
Because CGA implementation requires additional time and personnel, there are alternatives for identifying patients at risk of functional decline. The most common alternatives are instruments that screen for frailty. While there are numerous approaches to identify if a person is frail, none have shown both high sensitivity and specificity in older dialysis patients when compared to CGA.31 In an evaluation of six different screening approaches, the Identification of Seniors at Risk (ISAR) instrument had both moderate sensitivity (72%) and specificity (79%); however, more than half (52%) of dialysis patients who are not frail by ISAR were still found to have two or more geriatric problems (e.g., cognitive impairment, depression, mobility impairment, poor nutrition, or limitations in activities of daily living).31 Although these alternatives may not perform as well as CGA for identifying geriatric problems, some providers apply them to screen for patients who would benefit from CGA.32
Model Comprehensive Geriatric Assessment Program for Dialysis Patients
The value of CGA goes beyond identification of patients at risk of functional decline. In other clinical settings, such as oncology, CGA can be used for prognostication and individualization of treatment regimens.28 Most importantly, CGA is valuable for addressing functional decline and other geriatric syndromes through an individualized care plan. Practically, the multidisciplinary team meets to develop an individualized care plan from the geriatric problems and contextual factors uncovered by the multidimensional evaluation (essential dimensions listed above). The evaluation typically involves screening tests to identify specific impairments warranting intervention in one of two ways: 1) by helping a patient regain a loss (e.g., corrective lenses for visual deficits), or 2) by reducing the demand of specific tasks (e.g., bedside commode or wheelchair ramp).29 Additional aspects of a care plan might include referral to occupational therapy for home safety evaluation to minimize risk of falls in the home or engagement of a family caregiver for assistance in medication administration for a patient with cognitive impairment and polypharmacy. Table 1 lists examples of CGA evaluation and management options that address common geriatric syndromes found in dialysis patients.33–35
Table 1.
Examples of Comprehensive Geriatric Assessment Evaluation and Management
| Dimension | Evaluation Tool(s)a | Management Option(s) |
|---|---|---|
| Physical Function | Vulnerable Elders Scale-13 Survey48 Gait speed49 Two Minute Walk test50 |
Physical therapy; Referrals to home health and/or community services |
| Cognition | Modified Mini-Mental State Test (memory, language, attention)51 Clock Drawing Test (executive function)52 |
Referral to dementia specialist Home safety evaluation; Referrals to home health and/or community services |
| Mood Disorders | Geriatric Depression scale53 | Antidepressant; Psychotherapy |
| Risk of Falling | Timed Up and Go test54 History of falls |
Physical therapy Assistive devices Home safety evaluation Vision and Hearing screening |
| Nutrition | Mini nutritional assessment short form55 | Nutritional supplements Referral to dietician |
| Polypharmacy | Screening tool of older persons’ potentially inappropriate prescriptions (STOPP) criteria56 | Taper potentially inappropriate medications and provide alternatives |
Table modified with permission from American Family Physician.34
References for evaluation tool are provided to facilitate application in clinical practice.
CGA can help community-dwelling older adults with moderate functional limitations regain autonomy after functional decline, reduce hospitalizations, and limit nursing home placement.36–39 However, other groups of older adults may not see these benefits. Specifically, community-dwelling older adults who are independent and have no comorbidities are less likely to benefit from CGA. Older adults who are completely dependent on others for activities of daily living, have advanced dementia, or terminal illness are also less likely to benefit from CGA. Although there is no validated approach to identify patients who should undergo CGA, CGA should be conducted in vulnerable older adults when a provider suspects a potential problem, subsequent to a hospitalization or new medical problem, or annually.40,41 Ideally, early implementation of CGA in a patient with small decrements in function would slow progression of functional impairment. However, when a patient has significant difficulty with activities of daily living, CGA becomes most critical for helping the patient find community and social service resources that can fill their unmet needs. Older adults with total dependence in activities of daily living often require nursing home placement for long-term care. Such patients may no longer benefit from a CGA to regain function; however, a CGA focus on goals of care and advance care planning then becomes the highest priority.
Irrespective of findings from a CGA, the older dialysis patient who is at risk of functional decline may recover or maintain functional status through physical activity. Dialysis patients have had improved function through engaging in physical therapy, exercise, resistance training, and gymnastics.42,43 The benefits from physical activity include increased mobility, preserved cognitive function, handgrip strength, motivation to exercise, and independence in household activities.44
Because CGA in non-dialysis populations has proven to minimize functional decline, there is promise that CGA can be effective in the dialysis setting; however, we must first address important challenges to implementation of CGA in this population. First, we must identify which patients are most likely to benefit from CGA. Second, there is a need for a pragmatic clinical trial that demonstrates the feasibility and effectiveness of CGA for older dialysis patients and the key aspects (e.g., multidisciplinary team, patient engagement, or staff training) that yield positive outcomes. Finally, implementation of CGA for dialysis patients requires careful consideration of mode of delivery, resources required, and estimation of cost-benefit ratio.
Resources needed for implementation of CGA are an important barrier to adoption in the dialysis setting. The resources include time, space, and personnel to conduct CGA, available geriatric expertise, and infrastructure for care coordination and implementation of care plans. CGA does increase costs, but cost-effectiveness is unclear because of heterogeneity of approach across studies.45 However, there is one representative cost analysis of a CGA program in the outpatient setting that has demonstrated a reduction in healthcare utilization.46 This CGA program involves a geriatrics nurse practitioner and social worker team who conduct in-home CGA, and it shows the program costs were offset by reduction in acute care visits in older adults who are high risk for hospital readmissions.39,47 Because older dialysis patients are often high risk for hospitalizations, CGA may be cost-neutral or even cost-savings.
Key components of CGA are a multidisciplinary team that includes geriatrics expertise, multidimensional evaluation, and an individualized care plan8; therefore, the approach to delivering these key components is flexible for diverse care settings and resources available. Additional research is needed to understand the approaches that are acceptable in the dialysis setting. One potentially acceptable mode of delivery that could be adapted to the dialysis setting is a multidisciplinary team that conducts an outpatient consultation and relays findings and treatment plan to the primary provider (i.e., nephrologist).37 Because of the limited geriatric workforce to meet the demand of the aging population, a multidisciplinary team meeting may not be feasible in all settings. Thus, an abbreviated approach to a CGA program could involve a single trained nurse performing data collection during dialysis sessions and serving as a liaison between the patient, nephrologist, and geriatric provider. Alternatively, nurse home visits on non-dialysis days may be an acceptable approach.30 Irrespective of approach, a CGA program that provides additional oversight for vulnerable older dialysis patients and initiates care processes to manage geriatric problems has potential to improve and prevent functional decline.
Conclusion
The prevalence of older adults receiving dialysis is increasing. Co-existence of functional decline in these patients has wide consequences including morbidity, limited quality of life, and increased healthcare costs. These consequences may be minimized through modification of risk factors for functional decline. Among the risk factors described, we posit that geriatric syndromes may be modified through introduction of CGA. Through identification and management of geriatric syndromes, a CGA program has potential to prevent or slow progression of functional decline in older dialysis patients. We are conducting preliminary studies to access the feasibility of physical and cognitive function evaluations in older dialysis patients, the acceptability of home visits, and the needs and preferences of patients and staff in development of a CGA program. These studies should inform design of a pilot CGA program to identify the key elements and personnel that can be easily adopted across dialysis unit settings. Long-term, evidence that this model of care demonstrates recovery or maintenance of functional status could transform the clinical course of vulnerable older dialysis patients.
Acknowledgments
Funding Sources: Dr Hall receives support from Duke University CTSA KL2 Scholar program (NIH grant KL2TR001115), the Duke University Claude D. Pepper Older Americans Independence Center (NIH grant P30AG028716), and Grant 2015207 from the Doris Duke Charitable Foundation. Dr. McAdams-DeMarco is supported by NIH grants R01AG055781 and R01DK114074. Dr. McAdams-Demarco was also supported by the Johns Hopkins University Claude D. Pepper Older Americans Independence Center (P30AG021334) and the National Institute on Aging (K01AG043501).
References
- 1.Hall RK, O’Hare AM, Anderson RA, Colon-Emeric CS. End-stage renal disease in nursing homes: a systematic review. J Am Med Dir Assoc. 2013;14(4):242–247. doi: 10.1016/j.jamda.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook WL, Jassal SV. Functional dependencies among the elderly on hemodialysis. Kidney Int. 2008;73(11):1289–1295. doi: 10.1038/ki.2008.62. [DOI] [PubMed] [Google Scholar]
- 3.Jassal SV, Chiu E, Hladunewich M. Loss of independence in patients starting dialysis at 80 years of age or older. N Engl J Med. 2009;361(16):1612–1613. doi: 10.1056/NEJMc0905289. [DOI] [PubMed] [Google Scholar]
- 4.Hébert R. Functional decline in old age. Cmaj. 1997;157(8):1037–1045. [PMC free article] [PubMed] [Google Scholar]
- 5.McAdams-Demarco MA, Law A, Garonzik-Wang JM, et al. Activity of daily living disability and dialysis mortality: better prediction using metrics of aging. J Am Geriatr Soc. 2012;60(10):1981–1982. doi: 10.1111/j.1532-5415.2012.04161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hall RK, Toles M, Massing M, et al. Utilization of acute care among patients with ESRD discharged home from skilled nursing facilities. Clin J Am Soc Nephrol. 2015;10(3):428–434. doi: 10.2215/CJN.03510414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schell JO, O’Hare AM. Illness trajectories and their relevance to the care of adults with kidney disease. Current Opinion in Nephrology and Hypertension. 2013;22(3):316–324. doi: 10.1097/MNH.0b013e32835ffaaf. [DOI] [PubMed] [Google Scholar]
- 8.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342(8878):1032–1036. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 9.Inouye SK, Studenski S, Tinetti ME, Kuchel GA. Geriatric syndromes: clinical, research, and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55(5):780–791. doi: 10.1111/j.1532-5415.2007.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McAdams-DeMarco MA, Law A, Salter ML, et al. Frailty as a novel predictor of mortality and hospitalization in individuals of all ages undergoing hemodialysis. J Am Geriatr Soc. 2013;61(6):896–901. doi: 10.1111/jgs.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 12.Kim JC, Kalantar-Zadeh K, Kopple JD. Frailty and protein-energy wasting in elderly patients with end stage kidney disease. J Am Soc Nephrol. 2013;24(3):337–351. doi: 10.1681/ASN.2012010047. [DOI] [PubMed] [Google Scholar]
- 13.McAdams-DeMarco MA, Tan J, Salter ML, et al. Frailty and Cognitive Function in Incident Hemodialysis Patients. Clin J Am Soc Nephrol. 2015;10(12):2181–2189. doi: 10.2215/CJN.01960215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corsonello A, Onder G, Maggio M, Corica F, Lattanzio F. Medications affecting functional status in older persons. Curr Pharm Des. 2014;20(19):3256–3263. doi: 10.2174/13816128113196660695. [DOI] [PubMed] [Google Scholar]
- 15.McAdams-DeMarco MA, Suresh S, Law A, et al. Frailty and falls among adult patients undergoing chronic hemodialysis: a prospective cohort study. BMC Nephrol. 2013;14:224. doi: 10.1186/1471-2369-14-224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunlop DD, Manheim LM, Sohn MW, Liu X, Chang RW. Incidence of functional limitation in older adults: the impact of gender, race, and chronic conditions. Arch Phys Med Rehabil. 2002;83(7):964–971. doi: 10.1053/apmr.2002.32817. [DOI] [PubMed] [Google Scholar]
- 17.Ishizaki T, Yoshida H, Suzuki T, et al. Effects of cognitive function on functional decline among community-dwelling non-disabled older Japanese. Arch Gerontol Geriatr. 2006;42(1):47–58. doi: 10.1016/j.archger.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 18.Kamper AM, Stott DJ, Hyland M, Murray HM, Ford I. Predictors of functional decline in elderly people with vascular risk factors or disease. Age Ageing. 2005;34(5):450–455. doi: 10.1093/ageing/afi137. [DOI] [PubMed] [Google Scholar]
- 19.Volpato S, Maraldi C, Fellin R. Type 2 diabetes and risk for functional decline and disability in older persons. Curr Diabetes Rev. 2010;6(3):134–143. doi: 10.2174/157339910791162961. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi L, Zuliani G, Volpato S. Physical disability in the elderly with diabetes: epidemiology and mechanisms. Curr Diab Rep. 2013;13(6):824–830. doi: 10.1007/s11892-013-0424-6. [DOI] [PubMed] [Google Scholar]
- 21.Corsonello A, Garasto S, Abbatecola AM, et al. Targeting inflammation to slow or delay functional decline: where are we? Biogerontology. 2010;11(5):603–614. doi: 10.1007/s10522-010-9289-0. [DOI] [PubMed] [Google Scholar]
- 22.Guarner V, Rubio-Ruiz ME. Low-grade systemic inflammation connects aging, metabolic syndrome and cardiovascular disease. Interdiscip Top Gerontol. 2015;40:99–106. doi: 10.1159/000364934. [DOI] [PubMed] [Google Scholar]
- 23.Hoogerduijn JG, Buurman BM, Korevaar JC, Grobbee DE, de Rooij SE, Schuurmans MJ. The prediction of functional decline in older hospitalised patients. Age Ageing. 2012;41(3):381–387. doi: 10.1093/ageing/afs015. [DOI] [PubMed] [Google Scholar]
- 24.Spiers NA, Matthews RJ, Jagger C, et al. Diseases and impairments as risk factors for onset of disability in the older population in England and Wales: findings from the Medical Research Council Cognitive Function and Ageing Study. J Gerontol A Biol Sci Med Sci. 2005;60(2):248–254. doi: 10.1093/gerona/60.2.248. [DOI] [PubMed] [Google Scholar]
- 25.Gill TM, Allore H, Guo Z. Restricted activity and functional decline among community-living older persons. Arch Intern Med. 2003;163(11):1317–1322. doi: 10.1001/archinte.163.11.1317. [DOI] [PubMed] [Google Scholar]
- 26.Wu HY, Sahadevan S, Ding YY. Factors associated with functional decline of hospitalised older persons following discharge from an acute geriatric unit. Ann Acad Med Singapore. 2006;35(1):17–23. [PubMed] [Google Scholar]
- 27.Parker SG, McCue P, Phelps K, et al. What is Comprehensive Geriatric Assessment (CGA)? An umbrella review. Age Ageing. 2018;47(1):149–155. doi: 10.1093/ageing/afx166. [DOI] [PubMed] [Google Scholar]
- 28.Pilotto A, Cella A, Pilotto A, et al. Three Decades of Comprehensive Geriatric Assessment: Evidence Coming From Different Healthcare Settings and Specific Clinical Conditions. J Am Med Dir Assoc. 2017;18(2):192.e191–192.e111. doi: 10.1016/j.jamda.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 29.Colon-Emeric CS, Whitson HE, Pavon J, Hoenig H. Functional decline in older adults. Am Fam Physician. 2013;88(6):388–394. [PMC free article] [PubMed] [Google Scholar]
- 30.Parlevliet JL, Buurman BM, Pannekeet MM, et al. Systematic comprehensive geriatric assessment in elderly patients on chronic dialysis: a cross-sectional comparative and feasibility study. BMC Nephrol. 2012;13:30. doi: 10.1186/1471-2369-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Loon IN, Goto NA, Boereboom FTJ, Bots ML, Verhaar MC, Hamaker ME. Frailty Screening Tools for Elderly Patients Incident to Dialysis. Clin J Am Soc Nephrol. 2017;12(9):1480–1488. doi: 10.2215/CJN.11801116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Loon IN, Wouters TR, Boereboom FT, Bots ML, Verhaar MC, Hamaker ME. The Relevance of Geriatric Impairments in Patients Starting Dialysis: A Systematic Review. Clin J Am Soc Nephrol. 2016;11(7):1245–1259. doi: 10.2215/CJN.06660615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurella Tamura M, Larive B, Unruh ML, et al. Prevalence and correlates of cognitive impairment in hemodialysis patients: the Frequent Hemodialysis Network trials. Clin J Am Soc Nephrol. 2010;5(8):1429–1438. doi: 10.2215/CJN.01090210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li M, Tomlinson G, Naglie G, Cook WL, Jassal SV. Geriatric comorbidities, such as falls, confer an independent mortality risk to elderly dialysis patients. Nephrol Dial Transplant. 2008;23(4):1396–1400. doi: 10.1093/ndt/gfm778. [DOI] [PubMed] [Google Scholar]
- 35.Parker K, Aasebo W, Stavem K. Potentially Inappropriate Medications in Elderly Haemodialysis Patients Using the STOPP Criteria. Drugs Real World Outcomes. 2016;3(3):359–363. doi: 10.1007/s40801-016-0088-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mattke S, Han D, Wilks A, Sloss E. Medicare Home Visit Program Associated With Fewer Hospital And Nursing Home Admissions, Increased Office Visits. Health Aff (Millwood) 2015;34(12):2138–2146. doi: 10.1377/hlthaff.2015.0583. [DOI] [PubMed] [Google Scholar]
- 37.Reuben DB, Frank JC, Hirsch SH, McGuigan KA, Maly RC. A randomized clinical trial of outpatient comprehensive geriatric assessment coupled with an intervention to increase adherence to recommendations. J Am Geriatr Soc. 1999;47(3):269–276. doi: 10.1111/j.1532-5415.1999.tb02988.x. [DOI] [PubMed] [Google Scholar]
- 38.Stuck AE, Egger M, Hammer A, Minder CE, Beck JC. Home visits to prevent nursing home admission and functional decline in elderly people: Systematic review and meta-regression analysis. Journal of the American Medical Association. 2002;287(8):1022–1028. doi: 10.1001/jama.287.8.1022. [DOI] [PubMed] [Google Scholar]
- 39.Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc. 2006;54(7):1136–1141. doi: 10.1111/j.1532-5415.2006.00791.x. [DOI] [PubMed] [Google Scholar]
- 40.Elsawy B, Higgins KE. The geriatric assessment. Am Fam Physician. 2011;83(1):48–56. [PubMed] [Google Scholar]
- 41.Stuck AE, Aronow HU, Steiner A, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med. 1995;333(18):1184–1189. doi: 10.1056/NEJM199511023331805. [DOI] [PubMed] [Google Scholar]
- 42.Kutner NG, Jassal SV. Quality of life and rehabilitation of elderly dialysis patients. Semin Dial. 2002;15(2):107–112. doi: 10.1046/j.1525-139x.2002.00034.x. [DOI] [PubMed] [Google Scholar]
- 43.Ota S, Takahashi K, Suzuki H, et al. Exercise rehabilitation for elderly patients on chronic hemodialysis. Geriatric Nephrology and Urology. 1995;5(3):157–165. [Google Scholar]
- 44.McAdams-DeMarco MA, Konel J, Warsame F, et al. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int Rep. 2018;3(1):81–88. doi: 10.1016/j.ekir.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ellis G, Whitehead MA, O’Neill D, Langhorne P, Robinson D. Comprehensive geriatric assessment for older adults admitted to hospital. Cochrane Database Syst Rev. 2011;(7):CD006211. doi: 10.1002/14651858.CD006211.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–2633. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 47.Counsell SR, Callahan CM, Tu W, Stump TE, Arling GW. Cost analysis of the Geriatric Resources for Assessment and Care of Elders care management intervention. J Am Geriatr Soc. 2009;57(8):1420–1426. doi: 10.1111/j.1532-5415.2009.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Min L, Yoon W, Mariano J, et al. The vulnerable elders-13 survey predicts 5-year functional decline and mortality outcomes in older ambulatory care patients. J Am Geriatr Soc. 2009;57(11):2070–2076. doi: 10.1111/j.1532-5415.2009.02497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49(2):M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 50.Brooks D, Parsons J, Tran D, et al. The two-minute walk test as a measure of functional capacity in cardiac surgery patients. Arch Phys Med Rehabil. 2004;85(9):1525–1530. doi: 10.1016/j.apmr.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 51.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–318. [PubMed] [Google Scholar]
- 52.Royall DR, Mulroy AR, Chiodo LK, Polk MJ. Clock drawing is sensitive to executive control: a comparison of six methods. J Gerontol B Psychol Sci Soc Sci. 1999;54(5):328–333. doi: 10.1093/geronb/54b.5.p328. [DOI] [PubMed] [Google Scholar]
- 53.Lyness JM, Noel TK, Cox C, King DA, Conwell Y, Caine ED. Screening for depression in elderly primary care patients. A comparison of the Center for Epidemiologic Studies-Depression Scale and the Geriatric Depression Scale. Arch Intern Med. 1997;157(4):449–454. [PubMed] [Google Scholar]
- 54.Bohannon RW. Reference values for the timed up and go test: a descriptive meta-analysis. J Geriatr Phys Ther. 2006;29(2):64–68. doi: 10.1519/00139143-200608000-00004. [DOI] [PubMed] [Google Scholar]
- 55.Persson MD, Brismar KE, Katzarski KS, Nordenstrom J, Cederholm TE. Nutritional status using mini nutritional assessment and subjective global assessment predict mortality in geriatric patients. J Am Geriatr Soc. 2002;50(12):1996–2002. doi: 10.1046/j.1532-5415.2002.50611.x. [DOI] [PubMed] [Google Scholar]
- 56.Hamilton H, Gallagher P, Ryan C, Byrne S, O’Mahony D. Potentially inappropriate medications defined by STOPP criteria and the risk of adverse drug events in older hospitalized patients. Arch Intern Med. 2011;171(11):1013–1019. doi: 10.1001/archinternmed.2011.215. [DOI] [PubMed] [Google Scholar]


