Abstract
Background:
Limited data exists describing impact of body mass index (BMI) on post-LVAD outcomes. We sought to define the relationship between BMI and adverse events (AE) following LVAD implantation by examining the ISHLT Mechanically Assisted Circulatory Support (IMACS) registry.
Methods:
Patients implanted with a contemporary continuous flow (CF) LVAD were stratified into 4 groups using pre-operative BMI: underweight (UW) (BMI<=18.5 kg/m2), non-obese (NO) (BMI>18.5 kg/m2 to <30 kg/m2), obese (OB) (BMI>=30 kg/m2 to <40 kg/m2), and morbidly obese (MO) (BMI>=40 kg/m2). Freedom from AE was evaluated using the Kaplan-Meier method and risk factors for development of first AE were identified using multi-phase parametric hazard modeling. AE included infection, thromboembolic events, bleeding, device malfunction, and neurological dysfunction.
Results:
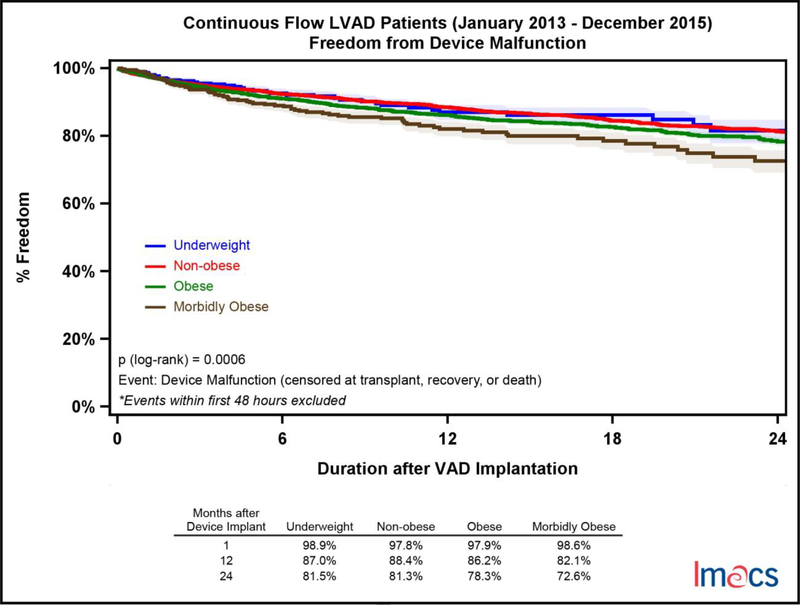
Between 2013 and 2015, 9,408 patients underwent implantation of a CF-LVAD. 368 (4%) patients were UW, 5,719 (61%) NO, 2,770 (29%) OB, and 444 (5%) MO. Survival amongst the 4 BMI cohorts was similar at 2 years (70.8%−75.8%, p=0.24). MO patients were less likely to be free from a non VAD-related infection (p<.0001) or device related infection (p=.0014) at 2 years (50.3%, 70.7%) when compared to OB (58.3%, 78.7%), NO (65.2%, 81.4%) and UW (68.9%, 77.4%). UW (81.5%) and NO (81.3%) patients were more likely to be free from device malfunction at 2 years when compared to OB (78.3%) and MO (72.6%), p=.0006. Thromboembolic events were rare and more common in the UW cohort (p=.026).
Conclusions:
While BMI was not correlated with 2-year mortality, an increased rate of infectious and device related AEs was noted in OB and MO LVAD patients. In a group with few options for transplant, the event morbidity in obese patients can be expected to impact morbidity with longer support durations.
Introduction
While heart transplantation remains the gold standard treatment for end-stage heart failure, persistence of supply-demand imbalance as well as the known additional morbidity that obesity plays on outcomes has resulted in the streaming of obese patients with end stage heart failure toward LVAD support. Cardiac transplant patients with extremes in BMI have worse long term survival when compared to normal weight recipients.[1, 2] Moreover, obese patients who are bridged to transplant with a left ventricular assist device (LVAD) have an increased risk of mortality during the post-transplantation period.[3] Patient size influences programmatic selection for recipient eligibility and in fact, international guidelines recommended that obese patients with a BMI greater than 35 kg/m2 lose weight prior to transplantation.[4] Consequently, obesity predisposes recipients to significantly longer wait times when compared to normal sized patients.[1] As the obesity epidemic persists, we hypothesize that there will be an increasing number of obese patients with end stage heart failure who require long term durable support as an alternative to cardiac transplantation.
Literature describing long-term outcomes and adverse event profiles in patients who receive a contemporary continuous flow (CF) LVAD stratified by BMI is scarce. The largest analysis by Brewer et al. showed that survival amongst BMI cohorts was not significantly different, however, obese patients were more susceptible to sepsis and device-related infections and less likely to have major bleeding post operatively.[5] In additional single center, small cohort series, obese patients implanted with a VAD are at increased risk for sepsis and reoperation for infection[6] as well as hemolysis and hospital readmission[7]. Interestingly, Go et al. showed that normal weight patients with a CF-LVAD were more likely to undergo reoperation for bleeding and had a higher incidence of postoperative stroke or transient ischemic attack.[8] For these reasons, we queried the ISHLT Registry for Mechanically Assisted Circulatory Support (IMACS) database to analyze survival and postoperative adverse events (AE) stratified by BMI to better elucidate the relationship between BMI and CF-LVAD support.
Methods
Data was prospectively collected and maintained in the ISHLT Mechanically Assisted Circulatory Support (IMACS) registry. All adult patients (age > 18 years) who underwent primary implantation with a contemporary CF-LVAD from January 1st, 2013 through December 31st, 2015, were included in the analysis. We stratified the patient cohort into 4 groups using preoperative BMI categories: underweight (UW, BMI<=18.5 kg/m2), non-obese (NO, BMI>18.5 kg/m2 to <30 kg/m2), obese (OB, BMI>=30 kg/m2 to <40 kg/m2), and morbidly obese (MO, BMI>=40 kg/m2). Follow-up period ended December 31, 2017.
Primary outcomes include mortality within two years and adverse events after implantation. Adverse events included infection, thromboembolic events, bleeding, device malfunction, and neurological dysfunction defined per IMACS.[9] Prevalence of adverse events by early onset (<=3 months) and late onset (>3 months) with repeated event calculations was conducted. Each adverse event category was analyzed separately and patients were censored at the first event. An analysis of adverse events between BMI categories was conducted using the Kaplan-Meier method with the log-rank test.
Statistical analyses: Continuous data are presented as means +/− standard deviation or medians [25, 75th] as appropriate. Categorical data are presented as frequencies (n, %). Baseline characteristics were compared using two-tailed t-tests to compare means and chi-square for comparisons of proportions. The hazard function for each outcome was estimated using parametric hazard modeling. A multivariable parametric hazard analyses for each morbid event (infection, bleeding, device malfunction, thromboembolic event) was performed using preoperative BMI and clinically relevant baseline variables. All the variables examined and displayed in table 1 were use in the multivariable risk models for prediction various outcomes. Risk factors with p-value <0.05 for each outcome are listed in the associated table. These analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, USA). Significance was assigned at p <0.05. Local institutional review board protocols were followed for this data analysis.
Table 1.
Baseline characteristics and preoperative variables stratified by pre-implant BMI category.
| Variable | Morbidly Obese n=444 | Obese n=2770 | Non-obese n=5719 | Underweight n=368 | P value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age at Implant | 48 (20, 77) | 55 (19, 82) | 58 (19, 88) | 53 (19, 87) | <0.0001 |
| Male | 311 (70%) | 2185 (79%) | 4590 (80%) | 256 (70%) | <0.0001 |
| Dilated cardiomyopathy | 418 (95%) | 2509 (91%) | 5043 (89%) | 315 (88%) | <0.0001 |
| Device Strategy | |||||
| Bridge to Transplant | 170 (39%) | 1546 (56%) | 3339 (59%) | 254 (70%) | <0.0001 |
| Destination Therapy | 266 (61%) | 1197 (44%) | 2311 (41%) | 110 (30%) | <0.0001 |
| Type of device - centrifugala | 75 (17%) | 764 (28%) | 1926 (34%) | 139 (38%) | <0.0001 |
| Concomitant surgery | 153 (35%) | 1013 (37%) | 2386 (42%) | 153 (42%) | <0.0001 |
| Comorbid Conditions | |||||
| Chronic renal disease | 95 (22%) | 632 (23%) | 1078 (20%) | 47 (16%) | 0.001 |
| Pulmonary disease | 45 (10%) | 268 (10%) | 427 (8%) | 26 (7%) | 0.002 |
| Pulmonary hypertension | 101(23%) | 639 (24%) | 1201 (22%) | 81 (22%) | 0.154 |
| Severe diabetes | 64 (15%) | 339 (13%) | 447 (8%) | 31 (9%) | <0.0001 |
| Liver dysfunction | 6 (1%) | 102 (4%) | 204 (4%) | 8 (3%) | 0.026 |
| Major stroke | 15 (3%) | 99 (4%) | 253 (5%) | 8 (3%) | 0.080 |
| Coronary artery disease | 16 (4%) | 146 (5%) | 360 (6%) | 20 (6%) | 0.044 |
| Limited social support | 24 (6%) | 139 (5%) | 192 (4%) | 10 (3%) | 0.003 |
| Non-compliance | 22 (5%) | 78 (3%) | 136 (3%) | 12 (4%) | 0.009 |
| History of smoking | 112 (26%) | 809 (31%) | 1840 (34%) | 105 (30%) | <0.01 |
| Current smoking | 15 (4%) | 133 (5%) | 308 (6%) | 21 (6%) | 0.176 |
| Severe depression | 19 (4%) | 89 (3%) | 123 (2%) | 7 (2%) | 0.007 |
| Patient Acuity | |||||
| Pre-implant IABP | 107 (24%) | 648 (24%) | 1491 (27%) | 79 (27%) | 0.004 |
| Pre-implant dialysis | 8 (2%) | 73 (3%) | 134 (2%) | 11 (4%) | 0.444 |
| Pre-implant ventilator/intubation | 41 (9%) | 293 (11%) | 560 (10%) | 36 (12%) | 0.652 |
| Pre-implant ECMO | 16 (4%) | 127 (5%) | 250 ( 5%) | 19 (6%) | 0.383 |
| Pre-implant cardiac arrest | 17 (4%) | 124 (5%) | 235 (4%) | 19 (6%) | 0.402 |
| Pre-implant major MI | 10 (2%) | 98 (4%) | 222 (4%) | 17 (5%) | 0.104 |
| Pre-implant major infection | 29 (7%) | 138 (5%) | 303 (5%) | 13 (4%) | 0.409 |
| IV inotropes within 48 hours of implant | 342 (78%) | 2092 (77%) | 4489 (80%) | 301 (82%) | 0.001 |
| Intermacs Patient Profile 1 | 57 (13%) | 367 (13%) | 825 (14%) | 65 (18%) | 0.086 |
| Intermacs Patient Profile 2 | 140 (32%) | 873 (32%) | 2029 (36%) | 133 (36%) | 0.002 |
| Intermacs Patient Profile 3 | 153 (35%) | 935 (34%) | 1879 (33%) | 124 (34%) | 0.806 |
| Intermacs Patient Profile 4 | 70 (16%) | 449 (16%) | 739 (13%) | 32 (9%) | <0.0001 |
| Intermacs Patient Profile 5 | 15 (3%) | 65 (2%) | 124 (2%) | 7 (2%) | 0.390 |
| Intermacs Patient Profile 6 | 0 | 21 (0.8%) | 34 (0.6%) | 1 (0.3%) | 0.213 |
| Intermacs Patient Profile 7 | 2 (0.5%) | 13 (0.5%) | 26 (0.5%) | 2 (0.5%) | 0.996 |
| Pre-implant creatinine mg/dL | 438 (1.4+/−0.6) | 2736 (1.4+/−0.7) | 5595 (1.4+/−0.7) | 367 (1.2+/−0.6) | <0.0001 |
| Total Bilirubin mg/dL | 405 (1.3+/−1.2) | 2565 (1.3+/−1.4) | 5282 (1.4+/−1.4) | 346 (1.4+/−1.4) | 0.077 |
| Albumin mg/dL | 397 (3.4+/−0.7) | 2514 (3.5+/−0.7) | 5078 (3.4+/−0.7) | 338 (3.4+/−0.6) | 0.013 |
compared to axial flow devices
Results
Study Cohort
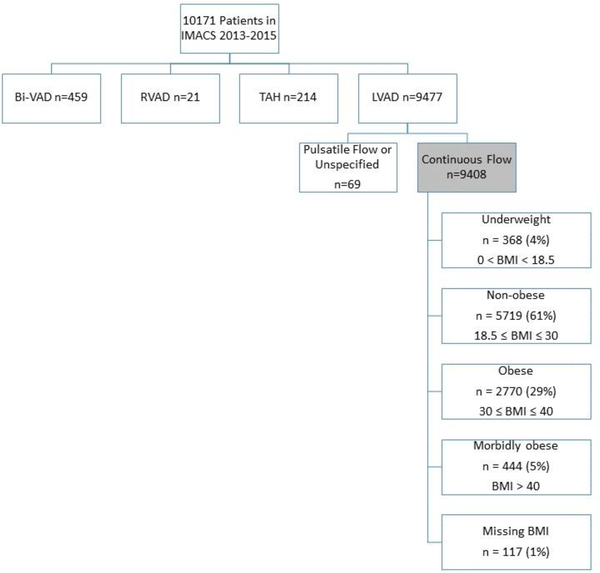
Between 2013 and 2015, 10,171 patients were registered in the IMACS database. The final study cohort included 9,408 patients who were implanted with a contemporary CF-LVAD. Patients were excluded if they required biventricular support, isolated right ventricular support or a total artificial heart (Figure 1). A majority of patients were non-obese (NO) 5,719 (61%) while 2,770 (29%) were obese (OB). Patients with extremes of BMI were less common, underweight (UW) 368 (4%) and morbidly obese (MO) 444 (5%).
Figure 1.
Distribution of patients by ventricular support and body mass index, IMACS, 2013–2015, n=10171.
Baseline Characteristics
Preoperative variables and baseline characteristics are shown in Table 1 and we compared these variables’ means or proportions across different obesity groups and p-values indicate the significance level of the comparisons. NO patients were most likely to be male (80%) and were on average the oldest of the BMI cohorts, 58 years. MO patients were the youngest with a mean age of 48 years old. Women were more common in the MO or UW group comprising 30% of the patients in each group. Destination therapy (DT) as an indication for device implantation was significantly more common in larger patients, occurring 61% of the time in the MO cohort versus 30% of the time in the UW cohort, p<0.0001. The acuity of patient illness was similar between the cohorts with similar incidence of pre-implant ventilatory support and ECMO support. There was a slightly higher incidence of pre-implant IABP use in the UW and NO cohort when compared to the OB and MO patients, 27% vs 24% respectively (p=0.004). Centrifugal flow devices were most commonly implanted in UW patients (38%) when compared to NO (34%), OB (28%), and MO (17%), p<0.0001.
Survival
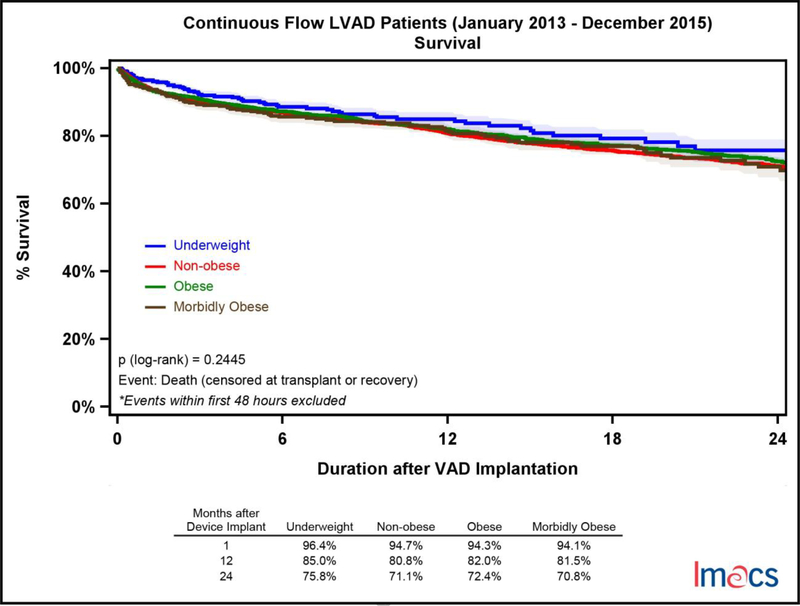
Survival amongst BMI cohorts was similar with no statistically significant difference, p = 0.2445 (Figure 2). Short-term survival at 30 days was in the range of 94.1% to 96.4% for all cohorts. Long-term survival at 2 years ranged from 70.8% (MO) to 75.8% (UW).
Figure 2.
Survival analysis by pre-implant BMI category in continuous flow LVADs in IMACS, 2013–2015. Underweight (n=368, death=77), non-obese (n=5,719, death=1,152), obese (n=2,769, death=543), and morbidly obese (n=445, death=97).
Adverse Events
The prevalence of adverse events and event rate per 100 patient months of support, stratified by early (<3 months after device implantation) and late (>3 months after device implantation), are shown in Table 2.
Table 2.
Adverse events rates by early onset (<=3 months) and late onset (>3 months).
| Infection | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 22.80% | 12.32 | 17.90% | 3.27 |
| Non-Obese | 22.10% | 11.5 | 18.60% | 3.2 |
| Obese | 24.90% | 13.42 | 22.60% | 4.08 |
| Morbidly Obese | 30.00% | 16.8 | 28.40% | 4.97 |
| Bleeding | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 27.40% | 15.69 | 13.30% | 2.59 |
| Non-Obese | 24.40% | 13.42 | 15.20% | 2.97 |
| Obese | 21.90% | 12.4 | 16.50% | 3.08 |
| Morbidly Obese | 24.10% | 13.34 | 17.10% | 3.2 |
| Neurological Dysfunction | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 11.10% | 4.77 | 7.60% | 1.08 |
| Non-Obese | 9.70% | 4.23 | 9.00% | 1.21 |
| Obese | 9.30% | 3.82 | 8.30% | 1.12 |
| Morbidly Obese | 10.80% | 4.9 | 10.80% | 1.43 |
| Respiratory Failure | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 13.90% | 6.36 | 3.00% | 0.4 |
| Non-Obese | 12.10% | 5.53 | 3.10% | 0.4 |
| Obese | 14.10% | 6.39 | 4.10% | 0.51 |
| Morbidly Obese | 18.90% | 8.19 | 5.00% | 0.65 |
| Device Malfunction | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 4.30% | 1.79 | 6.30% | 0.9 |
| Non-Obese | 5.10% | 2.21 | 6.80% | 0.96 |
| Obese | 5.80% | 2.44 | 8.80% | 1.16 |
| Morbidly Obese |
6.30% | 2.79 | 11.70% | 1.54 |
| Arterial Non-CNS Thromboembolism | Early Onset | Late Onset | ||
|---|---|---|---|---|
| Patient Percent (%) | Event Rate (per 100 patient months) | Patient Percent (%) | Event Rate (per 100 patient months) | |
| Underweight | 1.40% | 0.6 | 1.40% | 0.15 |
| Non-Obese | 0.80% | 0.34 | 0.20% | 0.02 |
| Obese | 0.70% | 0.25 | 0.40% | 0.04 |
| Morbidly Obese | 0.50% | 0.17 | 0.50% | 0.04 |
Within the first three months of support, patients most commonly experienced a bleeding event or an infectious complication. Infectious events occurred with an event rate of 11.5 to 16.8 per 100 patient months, most commonly in the morbidly obese cohort. Bleeding events were just as common, 12.4 to 15.69 per 100 patient months of support, most often in the underweight cohort. Device malfunction, neurological dysfunction, and respiratory failure were less common. Arterial thromboembolic events that were not strokes were exceedingly rare in 0.5–1.4% of the cohorts. Recurrent events were most common in patients suffering bleeding complications with 3,326 events in 2,233 patients over the first three months of support. Overall, event rate for all complications was higher within the first three months of support. For patients who were supported with a device for more than three months, infection was the most common adverse event with 3.54 events per 100 patient months of support.
Amongst all BMI cohorts, infection and bleeding remain the most common adverse event in late device support. For patients supported for more than 3 months, the larger the pre-implant BMI, the more common it was to have an infection or bleeding event. In other words, the infection and bleeding event rate per 100 patient months of support was highest in the MO cohort, 4.97 and 3.20 respectively.
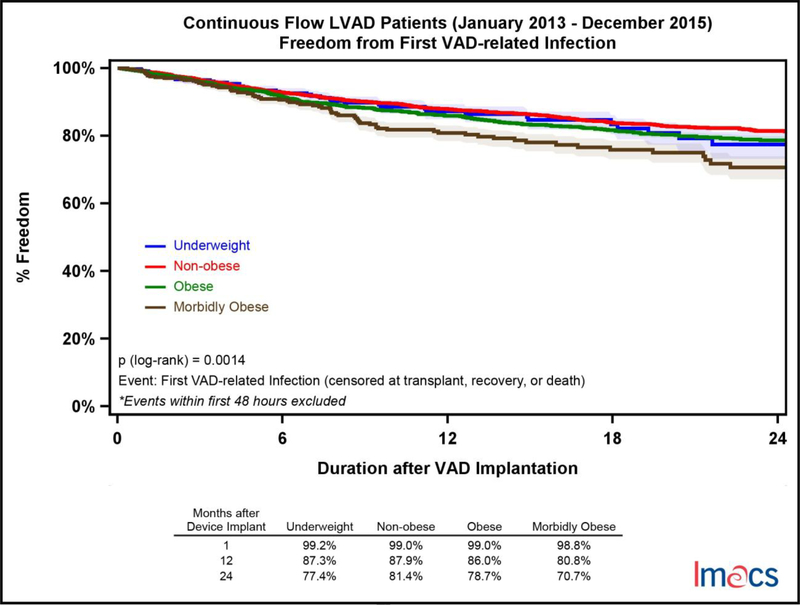
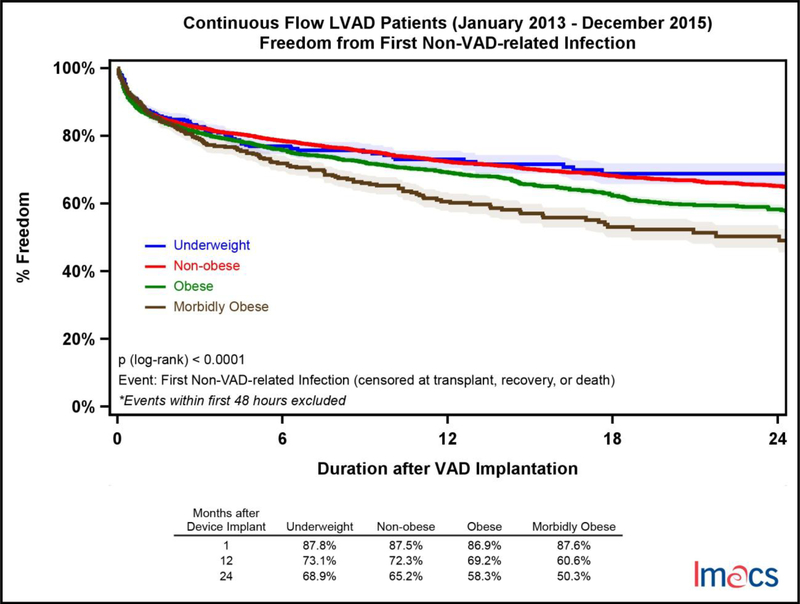
Infectious complications were stratified into non-VAD and VAD-related adverse events. VAD-related infectious adverse events were significantly more common in the MO cohort with only 70.7% of these patients free from an event at two years compared to UW (77.4%), NO (81.4%), and OB (78.7%); p-value =.0014 (Figure 3). Non-VAD-related infections were more common than VAD-related events and more likely to occur with increasing BMI category. At two years, UW patients were most likely to be free from a non-VAD-related adverse event 68.9% of the time followed by NO (65.2%), OB (58.3%) and MO (50.3%); p-value = <.0001 (Figure 4). Of note, the incidence of diabetes was significantly higher with increasing BMI, though it was not an independent predictor in the multivariable analysis. Device malfunction occurred more commonly in the MO and OB cohorts, p-value = .0006 (Figure 5). Arterial noncentral nervous system thromboembolic events were more likely to occur in the UW cohort, 95.3% free from an event at two years, compared to NO (98.7%), OB (98.4%) and MO (98.8%), p-value = .026. There was no statistically significant difference in freedom from bleeding events amongst the four cohorts at two years, UW (56.9%), NO (57.7%), OB (57.7%), and MO (55.8%), p=0.2110. Similarly, freedom from neurologic dysfunction was similar for each cohort at two years; UW (77.3%), NO (74.4%), OB (75.4%), and MO (71.5%), with no statistically significant difference, p=0.2510. Respiratory failure occurred most commonly in the MO cohort and was statistically significant, p<.0001. Freedom from respiratory failure at two years was 75.2% for MO, 80.0% in OB, 82.8% in NO, and 81.5% in the UW patients.
Figure 3.
Freedom from first VAD-related infection by pre-implant BMI category in continuous flow LVADs in IMACS, 2013–2015. Underweight (n=368, events=41), non-obese (n=5,719, events=611), obese (n=2,769, events=353), and morbidly obese (n=445, events=73).
Figure 4.
Freedom from first non-VAD related infection by pre-implant BMI category in continuous flow LVADs in IMACS, 2013–2015. Underweight (n=368, events=90), non-obese (n=5,719, events=1,418), obese (n=2,769, events=801), and morbidly obese (n=445, events=158).
Figure 5.
Freedom from first device malfunction by pre-implant BMI category in continuous flow LVADs in IMACS, 2013–2015. Underweight (n=368, events=38), non-obese (n=5,719, events=614), obese (n=2,769, events=358), and morbidly obese (n=445, events=75).
Multivariable Risk Analysis
Multi-phase parametric multivariable risk analysis identifying potential predictors as shown in table 1 for the development of VAD-related infection, non-VAD related infection, and device malfunction were separately analyzed and are shown in Tables 3–5. All variables in table 1 were included in the multivariable analysis and tables 3–5 display that statistically significant predictors with p-value <0.05. BMI is a significant risk factor associated with these adverse events. For every increase in BMI by 10 kg/m2, the hazard ratio for the development of a VAD-related infection was 1.11. Younger age at device implantation, chronic pulmonary disease, and pulmonary hypertension were additional risk factors. Similarly, higher BMI (an increase of 10 kg/m2) was a risk factor for non-VAD-related infections with a hazard ratio of 1.47. Additional factors that increased risk for non-VAD-related infection early after device implantation included female gender, chronic pulmonary disease, pulmonary hypertension, and concomitant surgery in addition to higher acuity patients on intra-aortic balloon pump support, intubated, or INTERMACS level 1 and 2. In addition to elevated BMI, higher pre-implant creatinine increased risk of non-VAD-related infections, hazard ratio of 1.12. With regards to device malfunction, for every10 kg/m2 increase in BMI, patients were 1.21 times more likely to have an adverse event. Younger age and history of smoking were additional risk factors for a device malfunction. An interaction term between age and BMI was examined for tables 3–5 and found to not be significant.
Table 3.
Multivariable risk factors for time to first VAD–related Infection
| Risk Factors | Hazard | |
|---|---|---|
| HR | P-value | |
| Demographics | ||
| Younger Age at Implant (10 years) | 1.22 | <0.0001 |
| Higher Pre-implant BMI (10 kg/m2) | 1.11 | 0.018 |
| Clinical Status | ||
| Chronic Pulmonary Disease | 1.26 | 0.032 |
| Pulmonary Hypertension | 1.19 | 0.016 |
Variables used in the analysis are listed in Table 1 and only predictors with p-value < 0.05 are listed in this table
Table 5.
Multi-phase parametric multivariable risk factors for time to first device malfunction. Early hazard indicates an initially high and rapidly declining hazard phase, and a constant hazard stays the same across time.
| Risk Factors | Early Hazard | Constant Hazard | ||
|---|---|---|---|---|
| HR | P-value | HR | P-value | |
| Demographics | ||||
| Younger Age at Implant (10 years) | 1.15 | <0.0001 | ||
| Higher Pre-implant BMI (10 kg/m2) | 1.21 | 0.001 | ||
| Clinical Status | ||||
| Chronic Pulmonary Disease | 1.69 | 0.007 | ||
| History of Smoking | 1.31 | 0.006 | ||
Variables used in the analysis are listed in Table 1 and only predictors with p-value < 0.05 are listed in this table
Competing Outcomes Analysis
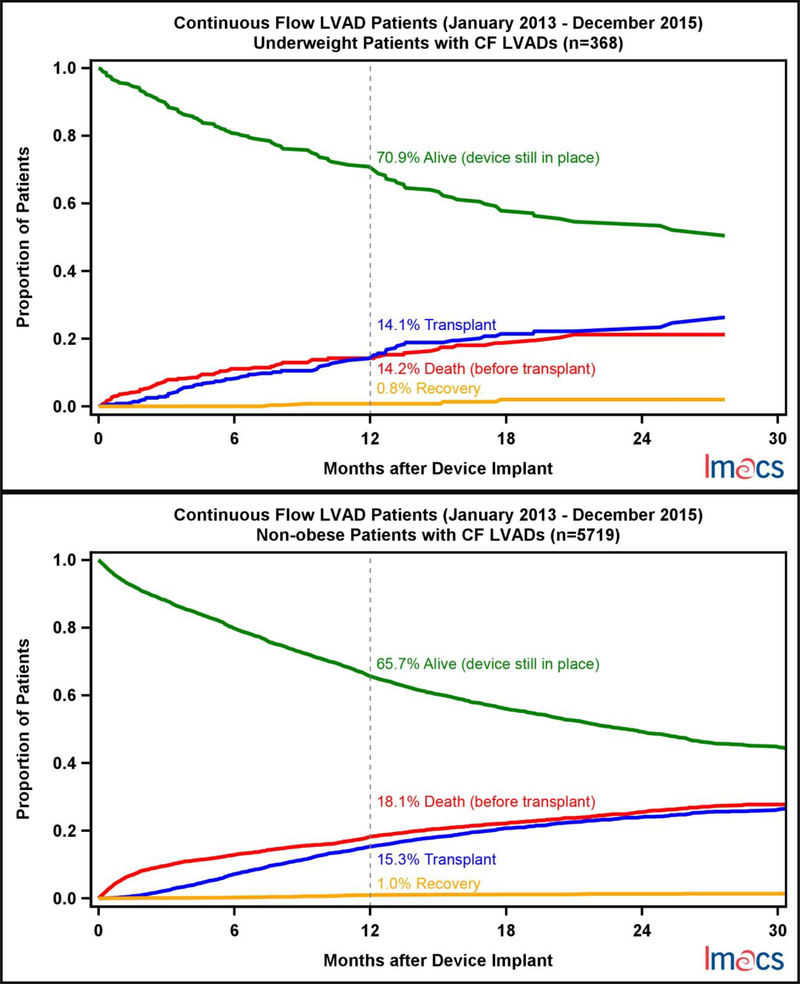
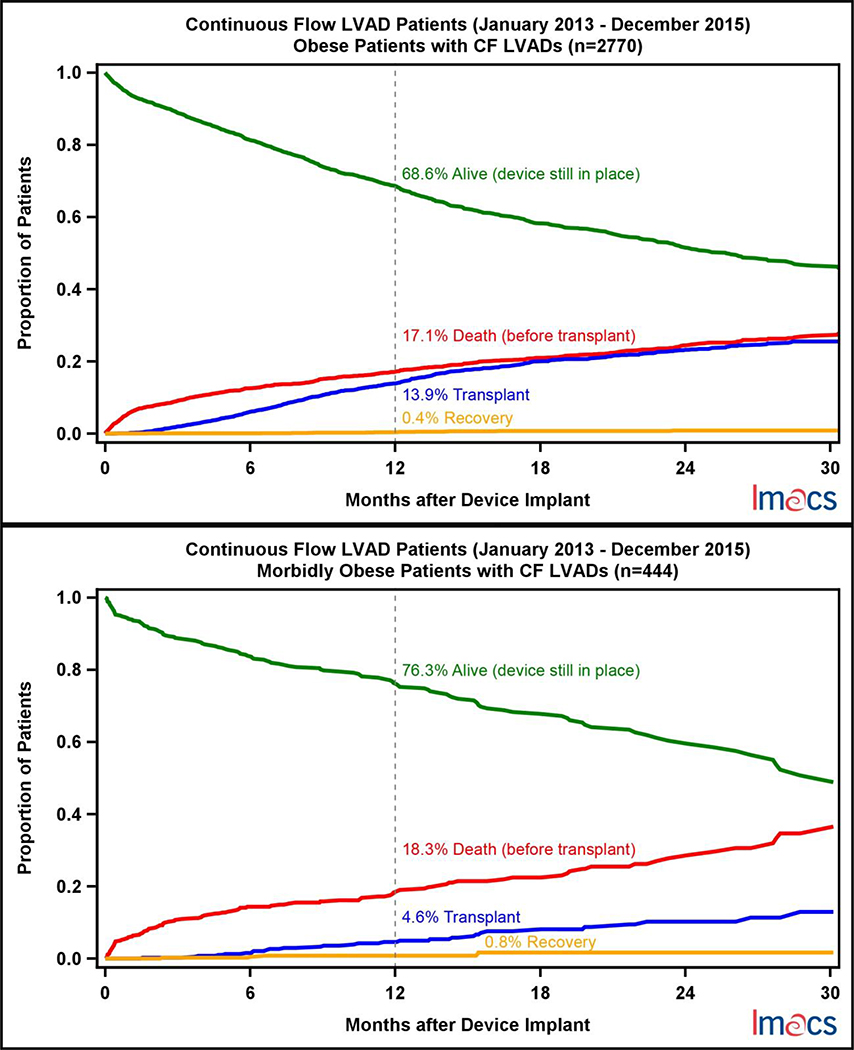
A competing outcomes analysis for each BMI cohort was performed where patients were censored for death, transplant, or cardiac recovery; otherwise they were considered alive on continued device support (Figure 6). At one year, patients who were underweight were least likely to expire (14.2%). Despite MO patients getting transplanted nearly one third as often as any other cohort at one year (4.6%), death while on device support (18.3%) was similar to the OB (17.1%) and NO (18.1%) cohort. Cardiac recovery was rare in all cohorts.
Figure 6.
Competing Outcomes by pre-implant BMI Category in continuous flow LVADs in IMACS, 2013–2015.
Discussion
In this IMACS analysis of over 9000 patients on isolated continuous flow support, we found the following: 1) Body weight does not predict survival at 2 years. 2) Recipients with a higher BMI, particularly MO patients, have increased frequencies and rates of complications after LVAD implantation. Increased morbidities include respiratory failure, infection, and device malfunction. Despite unquestionable improvements in survival and quality of life afforded by continuous flow LVAD technologies, the accompanying adverse event burden contributes to significant morbidity while on continued device support. Moreover, these adverse events incur a substantial cost to the healthcare system. Optimal medical therapy can play a significant role in improving adverse event profiles as exemplified by the use of careful anticoagulation strategies and blood pressure control.[10, 11] Technological advances have also played an integral role in decreasing adverse events with significantly lower rates of device thrombus in recipients of a fully magnetically levitated circulatory pump.[12] Additionally, patient selection is an important factor that influences outcomes in LVAD recipients and the influence that BMI has on these outcomes has not been well established. Our worldwide IMACS analysis includes the largest and most generalizable cohort to date to help answer this question.
This analysis includes all patients entered in the IMACS registry during the three-year period from 2013–2015 who had a continuous flow, left ventricular assist device implanted. While a majority of the patients were in the “normal” BMI range (18.5 ≤ BMI ≤ 30 kg/m2), there was approximately 5% in each of the cohorts that defined the extremes of BMI - underweight and morbidly obese. Infection was the most common adverse event occurring in 37% of all patients in this analysis. At 2 years, 11.5% and 26.2% of all patients suffered a VAD-related and non-VAD related infection (Figure 3 and 4). Higher pre-implant BMI was a risk factor for the development of both VAD and non-VAD related infections. This important finding was underscored by Zahr et al. who identified morbid obesity as a risk factor for the development of septic complications and need for reoperation for infection complications.[6]
In patients who were implanted with an LVAD as a bridge to transplantation, Clerkin et al. utilized the United Network for Organ Sharing (UNOS) database to show that patients with a BMI ≥ 35 kg/m2 were more likely to require a status listing upgrade for infectious complications while awaiting heart transplant.[3] In patients who were enrolled in the HeartMate II BTT and DT trials, sepsis and device related complications were significantly more common, however, non-device related complications were similar regardless of patient size.[5] Interestingly, while one would assume that morbid obesity puts patients at an increased risk for the development of perioperative infectious complications, i.e. pneumonia, urinary tract infection, or surgical site infection, the divergence of the curves in Figure 4 suggests that risk for non-VAD related infection not only persists, but is accentuated with prolonged device support. VAD related infection includes driveline, pump or cannula, and pump pocket infections. While Raymond et al. identified obesity as a risk factor for the development of driveline infection; a larger INTERMACS registry analysis by Goldstein et al. showed that patient size was not.[13, 14] A more granular investigation into the etiology of both VAD-related and non-VAD related infectious complications utilizing this database is warranted.
Survival at two years was 70.8–75.8% with no statistically significant difference amongst the 4 cohorts. These findings are consistent with prior studies.[6–8] Interestingly, in cohorts of patients who were implanted with non-contemporary devices, the extremes of BMI were a risk factor for mortality after device implantation.[15–17] Technological improvements in devices, improved patient selection with continued experience, and better long-term management of VAD recipients may all contribute to this observation. Findings from Brewer et al, supports the latter hypothesis in that two-year survival in patients from the initial series of patients implanted with HeartMate II in the BTT and DT trials ranged from 59–68%, which is inferior to the findings in this analysis.[5]
MO patients are implanted as destination therapy twice as often when compared to the underweight population. This is not surprising given the common practice of disallowing transplant candidacy for obese patients in light of the undesirability of further weight gain associated with chronic steroid therapy as well as the expected higher morbidity associated with obesity. Current guidelines from the ISHLT recommend that patients who have a BMI >=35 lose weight prior to listing.[4] Surprisingly, 39% of patients with a BMI greater than 40 kg/m2 and 56% of patients with a BMI greater than 30 kg/m2 were implanted as bridge to transplant. Despite intention to bridge to transplant, a major discrepancy amongst the BMI cohorts is exemplified in the competing outcomes analysis where only 4.6% of MO received a heart, which is far less than the obese (13.9%), non-obese (15.3%), and the underweight cohorts (14.1%). As a result of low transplantation, the MO cohort had the highest proportion of patients still alive on continued device support when compared to the other 3 groups, 76.3% vs 65.7–70.9%. Obesity predisposes patients to longer wait times while on the transplant waitlist when compared to all other sized recipients, despite equivalent survival outcomes after transplant.[1] An obese recipient can be problematic with scarce organ availability compounded by the challenge of appropriate recipient-donor size matching. Moreover, reoperative surgery and device explantation are technically more challenging in obese patients and therefore, patient selection requires an even more stringent approach to the utilization of limited organs. In a group of patients with limited options for transplant, longer duration of support and accumulated event morbidity can be anticipated. Weight loss and exercise programs, as well as nutritional support for these patients is essential. In select patients, bariatric surgery has been utilized for weight loss. In exceptional cases, concomitant bariatric surgery with VAD implantation has been successful.[18]
With regards to the type of pump implanted, morbidly obese patients were implanted with a centrifugal pump only 17% of the time, while underweight patients were 38% of the time. We suspect this difference may be attributed to technical aspects for device implantation as well as the perception that smaller devices are not able to supply higher pump outputs needed for support of larger BMIs. Regardless of device type, BMI is a significant predictor of device malfunction. We hypothesize that geometric considerations and weight fluctuations can alter inflow cannula positioning and its relationship to the pump body. Such alterations can adversely impact device function over time and hypothetically may contribute to the development of pump thrombus.[19] Furthermore, the device malfunction variable captured in the IMACS dataset has a broad definition and includes battery, controller, driveline, inflow cannula, outflow graft, and pump malfunction amongst other possibilities. Further investigation to examine patients who underwent pump exchange or surgical intervention for device malfunction is warranted to better elucidate the clinical relevance of this analysis.
Limitations
The IMACS registry is an international registry that enrolls and prospectively follows patients who are implanted with a durable device. The registry is comprised of data including pre-implant patient characteristics, device information and post implant clinical events. This study is limited by its retrospective analysis and as with any large registry analysis, granularity in the data is the tradeoff for a large sample size. Importantly, patient selection and selection bias must be accounted for when considering this analysis. Furthermore, this analysis is based on BMI at the time of device implantation and is unable to account for weight change while on continued device support.
Conclusion
Implantation of a durable device in a patient of any size has excellent outcomes and equivalent survival. However, increased body mass presents an increased risk for the development of infectious complications and device malfunction while on continued device support. A strategy for weight loss and nutritional support must be provided to obese patients in an attempt to reduce adverse events. Further studies are needed to help elucidate factors that can improve weight loss in obese LVAD patients.
Table 4.
Multi-phase parametric multivariable risk factors for time to first Non-VAD– related Infection. Early hazard indicates an initially high and rapidly declining hazard phase, and a constant hazard stays the same across time.
| Risk Factors | Early Hazard | Constant Hazard | ||
|---|---|---|---|---|
| HR | P-value | HR | P-value | |
| Demographics | ||||
| Younger Age at Implant (10 years) | 1.22 | <0.0001 | ||
| Female | 1.45 | <0.0001 | ||
| Higher Pre-implant BMI (10 kg/m2) | 1.47 | <0.0001 | ||
| Clinical Status | ||||
| Chronic Pulmonary Disease | 1.44 | <0.0001 | ||
| Pulmonary Hypertension | 1.24 | <0.01 | ||
| Pre-implant Ventilator/Intubation | 1.43 | 0.001 | ||
| Pre-implant Major Infection | 1.74 | <0.0001 | ||
| Pre-implant IABP | 1.19 | 0.009 | ||
| Higher Pre-implant Creatinine | 1.12 | <0.01 | ||
| IMACS Level 1 | 1.55 | <0.0001 | ||
| IMACS Level 2 | 1.31 | <0.0001 | ||
| Implantation | ||||
| Concomitant Surgery | 1.36 | <0.0001 | ||
| Re-intubation | 2.17 | <0.0001 | ||
Variables used in the analysis are listed in Table 1 and only predictors with p-value < 0.05 are listed in this table
Acknowledgement
The authors would like to acknowledge all contributors worldwide and thank them for their data collection and enabling us to write this manuscript.
Data collection for this work was funded in whole or in part with Federal Funds from the National Heart, Lung and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract No. HHSN268201100025C.
Footnotes
Disclosures
Dr. Kirklin is the PI of the INTERMACS registry and receives institutional support through INTERMACS funds. Dr. Cowger reports institutional grant support from Medtronic/Heartware and Abbott/Thoratec. She is a on the Medtronic steering committee and has received researchrelated travel funds from Abbott. Dr. Lund reports consulting for Novartis, Merck, Boehringer Ingelheim, Sanofi, Vifoir Pharma, and AstraZeneca with grants from Novartis, Boehringer Ingelheim, Vifoir Pharma and AstraZeneca outside the submitted the work. Dr. Goldstein is a national PI for the MOMENTUM 3 clinical trial and consultant for Ethicon. He has received research-related travel funds from Abbott.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weiss ES, et al. , Impact of recipient body mass index on organ allocation and mortality in orthotopic heart transplantation. J Heart Lung Transplant, 2009. 28(11): p. 1150–7. [DOI] [PubMed] [Google Scholar]
- 2.Lietz K, et al. , Pretransplant cachexia and morbid obesity are predictors of increased mortality after heart transplantation. Transplantation, 2001. 72(2): p. 277–83. [DOI] [PubMed] [Google Scholar]
- 3.Clerkin KJ, et al. , The Impact of Obesity on Patients Bridged to Transplantation With Continuous-Flow Left Ventricular Assist Devices. JACC Heart Fail, 2016. 4(10): p. 761–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehra MR, et al. , The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J Heart Lung Transplant, 2016. 35(1): p. 1–23. [DOI] [PubMed] [Google Scholar]
- 5.Brewer RJ, et al. , Extremes of body mass index do not impact mid-term survival after continuous-flow left ventricular assist device implantation. J Heart Lung Transplant, 2012. 31(2): p. 167–72. [DOI] [PubMed] [Google Scholar]
- 6.Zahr F, et al. , Obese patients and mechanical circulatory support: weight loss, adverse events, and outcomes. Ann Thorac Surg, 2011. 92(4): p. 1420–6. [DOI] [PubMed] [Google Scholar]
- 7.Mohamedali B, Yost G, and Bhat G, Obesity as a Risk Factor for Consideration for Left Ventricular Assist Devices. J Card Fail, 2015. 21(10): p. 800–5. [DOI] [PubMed] [Google Scholar]
- 8.Go PH, et al. , Effect of Body Mass Index on Outcomes in Left Ventricular Assist Device Recipients. J Card Surg, 2016. 31(4): p. 242–7. [DOI] [PubMed] [Google Scholar]
- 9.Website I; Available from: http://www.ishlt.org/ContentDocuments/IMACS_Appendix_DAdverse_Event_Definitions_122112.pdf.
- 10.Saeed O, et al. , Blood pressure and adverse events during continuous flow left ventricular assist device support. Circ Heart Fail, 2015. 8(3): p. 551–6. [DOI] [PubMed] [Google Scholar]
- 11.Nassif ME, et al. , Relationship Between Anticoagulation Intensity and Thrombotic or Bleeding Outcomes Among Outpatients With Continuous-Flow Left Ventricular Assist Devices. Circ Heart Fail, 2016. 9(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehra MR, et al. , A Fully Magnetically Levitated Circulatory Pump for Advanced Heart Failure. N Engl J Med, 2017. 376(5): p. 440–450. [DOI] [PubMed] [Google Scholar]
- 13.Raymond AL, et al. , Obesity and left ventricular assist device driveline exit site infection. ASAIO J, 2010. 56(1): p. 57–60. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein DJ, et al. , Continuous-flow devices and percutaneous site infections: clinical outcomes. J Heart Lung Transplant, 2012. 31(11): p. 1151–7. [DOI] [PubMed] [Google Scholar]
- 15.Mano A, et al. , Body mass index is a useful predictor of prognosis after left ventricular assist system implantation. J Heart Lung Transplant, 2009. 28(5): p. 428–33. [DOI] [PubMed] [Google Scholar]
- 16.Butler J, et al. , Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg, 2005. 79(1): p. 66–73. [DOI] [PubMed] [Google Scholar]
- 17.Musci M, et al. , Body mass index and outcome after ventricular assist device placement. Ann Thorac Surg, 2008. 86(4): p. 1236–42. [DOI] [PubMed] [Google Scholar]
- 18.Shah SK, et al. , Simultaneous left ventricular assist device placement and laparoscopic sleeve gastrectomy as a bridge to transplant for morbidly obese patients with severe heart failure. J Heart Lung Transplant, 2015. 34(11): p. 1489–91. [DOI] [PubMed] [Google Scholar]
- 19.Kazui T, et al. , Left Ventricular Assist Device Inflow Angle and Pump Positional Change Over Time Adverse Impact on Left Ventricular Assist Device Function. Ann Thorac Surg, 2016. 102(6): p. 1933–1940. [DOI] [PubMed] [Google Scholar]