Abstract
Non-alcoholic fatty liver disease (NAFLD) is a major clinical concern and its treatment consumes abundant resources. While accumulation of lipids in hepatocytes initiates the disease, this in itself is not necessarily harmful; rather, initiation of inflammation and subsequent fibrosis and cirrhosis are critical steps in NAFLD pathology. Mechanisms linking lipid overload to downstream disease progression are not fully understood; however, bioactive lipid metabolism may underlie instigation of proinflammatory signaling. With the advent of high-throughput, sensitive, and quantitative mass spectrometry-based methods for assessing lipid profiles in NAFLD, several trends have emerged, including that increases in specific sphingolipids correlate with the transition from the relatively benign condition of simple fatty liver to the much more concerning inflamed state. Continued studies that implement sphingolipid profiling will enable the extrapolations of candidate enzymes and pathways involved in NAFLD, either in biopsies or plasma from human samples, and also in animal models, from which data are much more abundant. While most data thus far are derived from targeted lipidomics approaches, unbiased, semi-quantitative approaches hold additional promise for furthering our understanding of sphingolipids as markers of and players in NAFLD.
Keywords: non-alcoholic fatty liver disease, NAFLD, NASH, lipid, sphingolipids, ceramide, sphingosine-1-phosphate, lipidomics, metabolic syndrome
1. Introduction
Non-alcoholic fatty liver disease (NAFLD) is the liver component of metabolic syndrome and is associated with chronic overexposure of the liver to free fatty acid (FFA) that occurs in metabolic syndrome [1]. While a healthy hepatocyte has evolved to process, metabolize, and/or re-package free fatty acid (FFA) for storage, utilization, or distribution to other tissues, the overload of FFA can disrupt these pathways leading to aberrant FFA handling resulting in deleterious outcomes. The initiating insult involves excessive accumulation of lipid droplets, but the actual events linking lipid oversupply to hepatocyte toxicity could include disruption of lipid bilayer integrity, overloading endogenous lipid metabolic pathways, accumulation of oxidized lipids, activation of ER stress and the unfolded protein response, and/or deleterious production of bioactive lipids [2]. Hepatic injury, thought to be the major driving event behind disease progression, drives injured or dying hepatocytes to release proinflammatory factors. Subsequent activation and infiltration of immune cells, such as neutrophils and lymphocytes, lead to inflammation [3], and activation of hepatic stellate cells leads to fibrosis [4], furthering disease progression [5, 6].
The interplay between free fatty acid (FFA) toxicity to hepatocytes, signaling between hepatocytes and immune cells, and the relationship between inflammation and fibrosis are of major interest in NAFLD research [1, 7]. While the contributions of FFAs to mechanisms of hepatocyte injury and NAFLD progression are not completely understood, free palmitic acid (PA) is a particularly potent mediator of lipotoxicity [7]. PA is the product of fatty acid synthase and is the most abundant FFA in mammalian circulation. It is widely found esterified at the sn-1 position of glycerophospolipids and is also the substrate for the first step of sphingolipid synthesis [8]. Therefore, excess FFA-induced alterations to intracellular sphingolipid metabolism and sphingolipid profiles may play roles in NAFLD, as has been suggested by studies from our group and others [9–12].
Sphingolipids comprise a highly dynamic, diverse, and complex class of molecules that serve as both structural components of cellular membranes and signaling molecules in mammalian cells. The relatively recent application of sphingolipid lipidomics, or “sphingolipidomics”, has uncovered numerous potential relationships between sphingolipids and NAFLD. Instrumentation and methods technology for lipidomics analysis has been available for nearly two decades, but its direct application to disease models like NASH has become more widespread over the past five years or so. This is likely a result of increased accessibility due to instrument availability and standardization of methodology. Here we highlight some of the insights gained into NAFLD mechanisms by the application of sphingolipidomics, followed by a discussion of key technical considerations for lipidomics approaches.
2. Sphingolipid metabolism
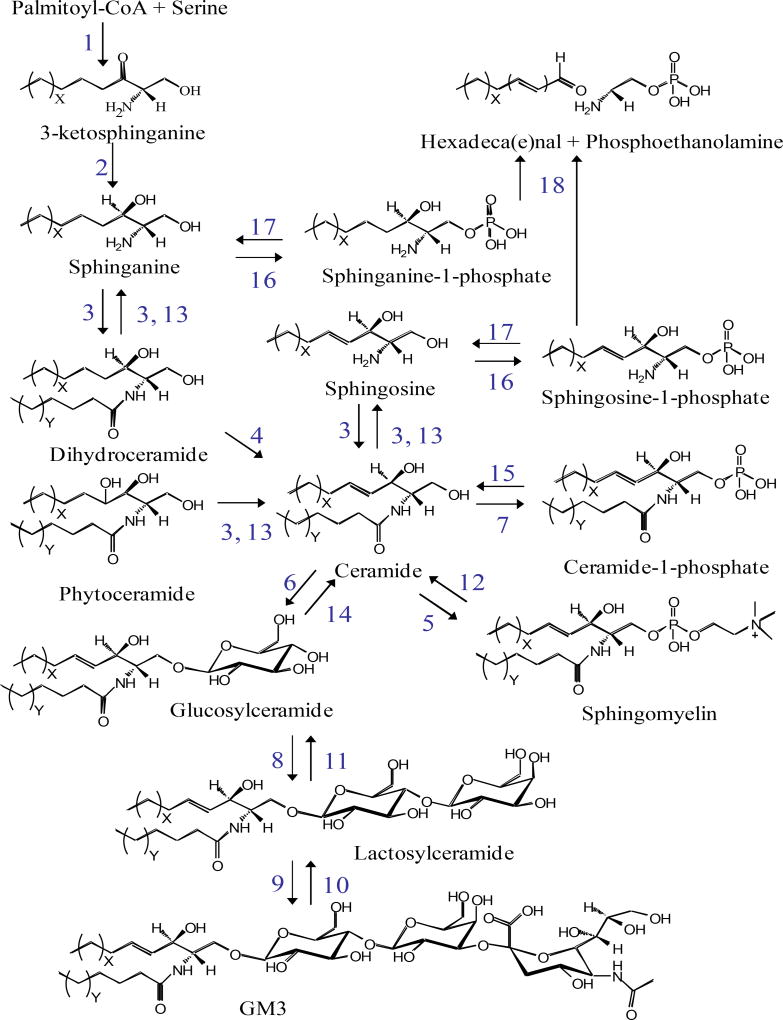
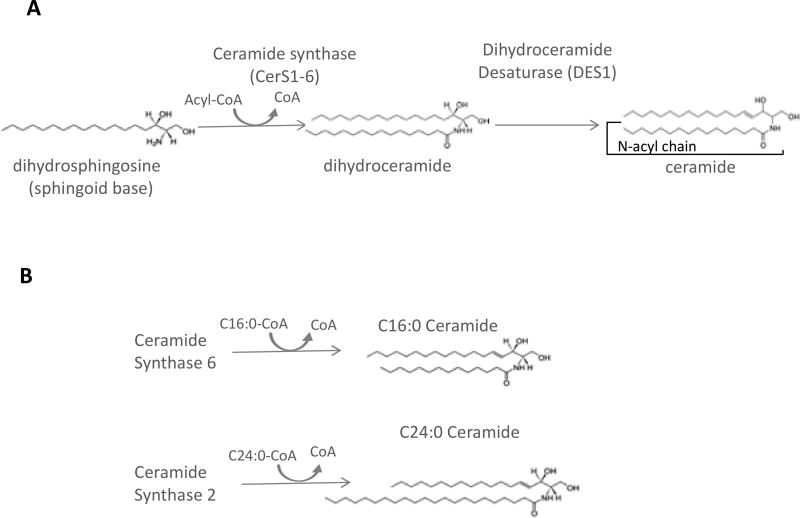
Sphingolipids arise from condensation of a fatty acyl CoA with an amino acid, giving rise to a sphingoid base, the amino alcohol upon which all sphingolipids are subsequently built (for a detailed depiction of sphingolipid pathways and structures see Figure 1). Canonical substrates for this reaction are serine and palmitoyl-CoA, though recent studies have identified aberrant lipids synthesized using glycine, alanine, and/or myristoyl- or stearoyl-CoA [13, 14], giving rise to alternative sphingolipid species whose biology is just beginning to be deciphered. However, those derived from serine and palmitoyl-CoA are by far the most abundant species. This reaction is catalyzed by serine palmitoyltransferase and is thought to be rate-limiting and highly sensitive to palmitoyl-CoA levels[15]. The product of this reaction, 3-ketodihydrosphingosine, is short lived and rapidly converted to dihydrosphingosine (also called sphinganine) by 3-ketodihydrosphingosine reductase. Dihydrosphingosine bears an amino group, which serves as the site of CoA-dependent acylation with a range of fatty acyl-CoA substrates by a family of six ceramide synthases (CerS1-6) (Fig. 2A). This enzyme family shows high homology yet also demonstrates partial acyl-CoA selectivity, which leads to a diverse and partially distinct product profiles for each CerS. For example, CerS6, implicated in liver pathology in murine high fat diet obesity models [16], generates ceramides using a long chain acyl-CoA, such as palmitoyl-CoA; in contrast, CerS2, whose depletion is implicated in liver pathology [17], utilizes acyl-CoAs of 20 carbons and longer (Fig. 2B). Therefore, determining the intracellular ceramide distribution (in terms of N-acyl chain length) provides hints as to enzymes that might underlie pathology.
Figure 1. Partial sphingolipid metabolic pathway with structures of selected species and numerically designated enzymatic reactions.
Enzymes correspond to serine palmitoyltransferase – 1, 3-ketosphinganine reductase – 2, dihydroceramide synthases – 3, dihydroceramide desaturases – 4, sphingomyelin synthases – 5, glucosylceramide synthase - 6, ceramide kinase – 7, lactosylceramide synthase – 8, sialyltransferase 1 – 9, sialidase – 10, galactosidase – 11, sphingomyelinases (C-type) – 12, ceramidases – 13, β-glucoceramidase – 14, ceramide-1-phosphate phosphatase (putative) – 15, sphingosine / sphinganine kinases – 16, sphingosine-1-phosphate phosphatase – 17, and sphingosine-1-phosphate lyase – 18.
Figure 2. Ceramide synthase isoenzymes generate distinct ceramide species.
(A) general ceramide synthase (CerS) reaction. (B) Different CerS isoforms show dinstinct Acyl-CoA selectivity and therefore generate distinct ceramide speciess. For example CerS6 utilizes palmitoyl-CoA, whereas CerS2 uses very long chain Acyl-CoAs, such as C24:0-CoA, shown here.
While the actual products of de novo ceramide synthesis are dihydroceramides, these are desaturated by dihydroceramide desaturase 1 to yield ceramide. Ceramide regulates numerous cell processes including apoptosis, senescence, insulin signaling, signaling from membrane receptors, and many others [18]. Ceramide also gives rise to two key proinflammatory lipid mediators: 1) it can be directly phosphorylated by ceramide kinase to yield ceramide-1-phosphate, a regulator of iPLA2 and subsequent driver of proinflammatory eicosanoid production [19]; or 2) it is catabolized to sphingosine, a single-chain molecule that is then phosphorylated by sphingosine kinase 1 or 2 to yield sphingosine-1-phosphate, a ligand for a family of G protein-coupled receptors that has known roles in proinflammatory cytokine signaling and immune cell chemotaxis [20]. Apart from these fates, ceramides are the essential building block for all complex sphingolipids, including sphingomyelin and glycosphingolipids (Fig. 1). Importantly, catabolic enzymes exist that catalyze the reverse of many of these reactions. Therefore, there is potential for continuous flux within the pathway, where total sphingolipid content may remain constant, but profiles (i.e. distribution of total content between specific sphingolipid species and pools) may change drastically.
While links between sphingolipids and NAFLD are continuing to emerge, NAFLD at various stages involves alterations in multiple biological processes including lipid metabolism, lipotoxicity, ER stress, apoptosis, inflammation, and fibrosis. Because sphingolipids are implicated in these processes in other disease contexts, it seems likely they play similar roles in NAFLD. Measuring and quantification of sphingolipids in the NAFLD context, therefore, will be essential for further research to deepen our mechanistic understanding of sphingolipids in NAFLD [21, 22].
2. Sphingolipid profiling in NAFLD
Despite the wealth of sphingolipidomics data available from patient serum and urine, there are few studies on patient liver biopsies. However, these, as well as additional insight gained from complementary approaches including animal models, studies from primary hepatocytes, and hepatocyte-like cell lines, commonly support a role for sphingolipids in the interplay between hepatic injury and inflammation, which is emerging as a central focus of lipidomics and NASH.
a) Patient Samples
To date, two studies of patient liver biopsies have provided data on multiple species of sphingolipids. In Gorden et al [23], a random group of patient biopsies were scored histologically and categorized as normal liver, livers showing steatosis, livers with nonalcoholic steatohepatitis (NASH), or cirrhotic liver, creating the opportunity to compare and contrast lipidomics trends among these patient groups. The focus of the paper was to identify circulating lipid biomarkers from among all lipid classes that correlated with NAFLD disease progression. To this end, lipid profiles of pre-classified healthy controls and NASH and cirrhosis patients were analyzed from both liver biopsies and patient serum. Lipid species were then identified that differed significantly between disease states in both liver tissue and plasma. Of the 48 species meeting this criterion, 14 were sphingolipids. Many sphingolipid classes were represented, including ceramides, 1-deoxyceramides, dihydroceramides, hexosylceramides, and sphingoid bases. Importantly, two deoxysphingolipids were identified that distinguished steatosis from NASH; these are sphingoid bases derived from glycine (rather than serine) and are increasingly implicated in toxicity in other systems [24, 25], suggesting that aberrant metabolism through serine palmitoyltransferase may play a role in the development of NASH from steatosis.
The second study using patient samples partially corroborated these findings. Using slightly different approaches, Luukkonen et al [26] collected biopsies from patients classified as low or high homeostatic assessment of insulin resistance (HOMA-IR) as an indirect readout of metabolic syndrome and also from patients carrying a mutation in the allele for the triacylglycerol lipase (PNPLA3I148M), which is associated with exacerbation of NASH. Biopsies were scored by NASH severity. The main conclusions were that NASH associated both with high HOMA-IR and PNPLA3I148M, as well as with an increase in TAGs containing polyunsaturated fatty acids. Consistent with the first study, NASH patients (but not necessarily patients with the mutant allele) also exhibited accumulations of ceramides and dihydroceramides, though other sphingolipids were not identified as correlates of disease.
While the methods of disease scoring were similar between these two studies, Gordon et al presented absolute levels of a variety of lipid species while Luukkonen et al. reported relative values. Thus, it is difficult to directly compare lipid accumulation across the two studies. The lipidomics methodologies differed between the studies as well: the former employed reverse phase LC-MS/MS and multiple reaction monitoring, while the latter used LC-MS with a total ion scan. Importantly, despite these differences, both studies showed a general increase in ceramides and dihydroceramides. Also, due to application of mass spectrometry, N-acyl chain lengths of ceramides were determined to be specific. This is important because data increasingly support mechanistically distinct roles for sphingolipids of different chain lengths and thus, the CerS isoforms that synthesize them (Fig. 2). Both studies also showed a general increase in C18, 20, 22, 24:1 ceramides, as well as C18 and C24:1 dihydroceramides, which suggests that changes in CerS1, 2, or 4 could potentially occur in NAFLD progression in humans. It is important to note that unbiased lipidomics approaches may not be sensitive enough to detect less abundant species including sphingosine-1-phosphate (S1P), a sphingolipid metabolite implicated in NASH by our group and others [9, 11, 27].
b) Animal Models
Due to the paucity of lipidomics-focused studies on human liver biopsies in the NAFLD context, data from studies in rodent NAFLD models, which are more abundant, may provide the best information on sphingolipids in NAFLD. Among studies where NAFLD sphingolipidomes can be compared to controls, there are to date about nine performed in murine models [10, 11, 17, 28–33], three on rat [34–36], and one on rabbit [37]. Of the mouse studies, eight presented sphingolipidomics contrasting a control diet with those on a NAFLD-inducing diet, and one utilized the leptin receptor deficient ob/ob mouse [33]. The rat studies employed high fat, high sugar/high fat, or high sugar/exercise models. The high fat diet models differed slightly in fat composition and proportion in some cases, and in some cases included high sucrose in addition to fat. Lipidome coverage, i.e., the number of distinct species detected, was highly variable among the studies, but all report on multiple individual ceramide species. As with the human studies discussed above, despite differences in experimental design, there are a few surprisingly consistent trends. Comparison of patterns of ceramide species across studies revealed that NAFLD diet animals relative to control diet animals had elevated C16, 18, 20 and 22 ceramides and lower levels of C24 and 24:1 ceramides. While the magnitude and significance of the changes varied between studies, the relationship was consistent. In the future, more consistent breadth of sphingolipidome coverage might reveal the point in the metabolic/catabolic pathway where this selection takes place. It could be a manifestation of substrate preference from among the six CerS isoforms, or it could arise from selective catabolism of complex SLs by sphingomyelinases or glucosylceramidases.
Of these rodent studies, four reported on levels of sphingoid bases and/or sphingoid base phosphates [10, 11, 17, 29], but no consensus emerged as to whether the specific species increase or decrease with NASH progression. Data on sphingomyelin levels is a bit more consistent, as six studies reported association of sphingomyelin and/or dihydrosphingomyelin levels with NASH [10, 17, 29–32], and five of these reported values for individual sphingomyelin species. The consensus is that, while total sphingomyelin levels are elevated in NASH, only Montgomery et al show a specific trend for individual sphingomyelin species, specifically C24 and C24:1 sphingomyelin decrease with NAFLD progression, mirroring the similar decrease in ceramide species. Additional complex lipids, including globotriaosylceramide, glucosylceramide, galactoceramide and lactosylceramide, were quantified in some cases, but the only specific trend noted was a decrease in glucosylceramide [17].
c) Studies in cultured cells
A complete in vitro model of NAFLD presents a challenge due to the multiple cell types involved; however, mechanisms of hepatic injury and death in NASH have been recapitulated with high concentrations of exogenous fatty acids, which presumably recreates the condition of high levels of circulating FFA in metabolic disease in vivo. This approach does not take into account changes in other circulating factors in metabolic disease, such as other nutrients, metabolites, or cytokines. However, one advantage to this approach is that the specific effects of the lipotoxic component of NAFLD (e.g. from elevated circulating FFA) can be dissected out. While many studies of lipotoxicity induced with exogenous fatty acids, typically PA, have implicated sphingolipid pathways, there are only four thus far where comprehensive sphingolipid profiles were determined. Three of the studies use hepatoblastoma (HepG2 or Huh7) [38–40] cell culture models and one used HeLa-derived Chang cells [10]. In each of these studies, cells were treated with sufficient exogenous PA to induce lipotoxic outcomes, e.g. apoptosis, over 18 to 48 hours. Free PA is taken up and converted C16-dihydroceramide and downstream C16 ceramide-based SLs (e.g. C16:0 sphingomyelin). Among the in vitro studies discussed here, ceramides with C14 and C16 side chains were consistently shown to increase when palmitate was used to induce lipotoxicity, [40] and it was additionally shown that C18, C22 and C24:1 ceramide all increase. Therefore, there is high overlap between these findings and those from humans and animal models described above.
Ceramide is converted directly into the complex SLs including glucosyl-/galactosylceramides (together termed “hexosylceramides”, as few methods can distinguish between the isomers), more complex glycosphingolipids, for example gangliosides, and sphingomyelins. Thus, one might expect chain length-specific changes in ceramides to be mirrored in these more complex sphingolipids built by addition of functional groups to the ceramide moiety. Interestingly, however, the chain-length-specific increase in ceramide species only translated to C16 and C18 hexosylceramides, while in contrast, C22 and C24 hexosylceramides trended toward a decrease with treatment. This pattern implies a chain length substrate preference of glucosylceramide synthase for C16 and C18 ceramides, or potentially differential routing of some ceramides toward or away from glucosylceramide synthesis. No changes were reported for sphingomyelin or dihydrosphingomyelin species, implying that the increased availability of the ceramide substrate is not utilized for sphingomyelin synthesis, perhaps due to intracellular compartmentalization. This could be a specific property of the cell lines studied, or could hint at a specific responsiveness of C24/24.1 ceramide and complex sphingolipid levels to certain inflammatory signals in vivo. Potentially supporting this concept is the observation that glucosylceramide has been linked to binding and recruitment of inflammatory cells [41].
A pair of studies on loss of CerS2 and CerS6 function in mice revealed a relationship between C16 ceramide and glucose tolerance. The liver-specific knockout of CerS6 (Cers6ΔLIVER) had decreased production of C16 ceramide, as expected, which was associated with increased oxidation of palmitate and improved glucose tolerance [16]. In contrast, a Cers2 haploinsufficient mouse showed the expected decrease in the very long chain C22–C24 ceramides, but a compensatory increase in C16 ceramide [17]. Interestingly CerS2 mutant mice had increased liver weight and storage of triacylglycerol as well as glucose intolerance. The striking contrast between the long chain versus very long chain profiles along with the disease outcomes for the two types of CerS mutant animals implies that differential expression or activity of specific CerS isoforms is an important factor in metabolic disease progression, most likely including NAFLD, and together these studies implicate long chain, but not very long chain ceramides in hepatic pathology and systemic insulin resistance.
The sphingolipid metabolites most directly implicated in inflammation, both generally and specific to NAFLD, are S1P and C1P. The clearest connection between NAFLD inflammation and S1P comes from the SphK1 knockout mouse. On a high saturated fat diet, this knockout had lower levels of inflammatory markers as well as reduced immune cell infiltration [9]. In other studies, S1P was shown to be a potent chemotactic agent for immune cells [42] and mesenchymal stem cells in the context of fibrosis [43]. S1P was also implicated in fibrosis in humans induced by general liver injury [44], as well as in a cholestasis-induced fibrosis model [45].
Although there are few direct links between NAFLD and C1P, it has been shown to mediate inflammatory responses in adipose tissues, in the context of a diet-induced obesity model [46], and was shown to regulate lipid droplet formation in vitro [47]. The proinflammatory action of C1P is mainly through regulation of eicosanoid biosynthesis via a direct activating interaction with cPLA2, making it a key point of crosstalk between the sphingolipid and eicosanoid pathways [48]. These studies demonstrate that sphingolipidomics is not essential to decipher individual roles of key molecules like S1P and C1P, the ability to determine total sphingolipid profiles can provide hints at potential regulation of sphingolipid pathway enzymes and roles of other lipids, sometimes several steps removed, and can therefore lead to more detailed information.
3. Sphingolipidomics considerations
Mass spectrometry-based approaches to the identification and quantification of sphingolipids are essential to continue progress in understanding of the links between NAFLD with specific sphingolipids and the enzymes that catalyze their formation. Thus, sphingolipids with minor structural differences can arise from distinct enzymes and have widely variable biological functions. For example, ceramides can vary in sphingoid base length and N-acyl chain length and arise from partially distinct metabolic pathways (Fig. 2). Moreover, the structural differences between ceramides and their precursors, the dihydroceramides, which have distinct biological functions, arise from the presence of a single double bond in the sphingoid base (Fig. 2). Additionally, because of the large range of variation of tissue contents of various sphingolipid species, with complex sphingolipids being orders of magnitude more abundant than key sphingolipid signaling molecules (e.g. S1P and C1P), a high degree of sensitivity is needed for their quantitation [49]. Given the numerous structurally related species (Fig 1), comprehensive sphingolipid analysis would ideally provide a means to distinguish very structurally similar species. To address these challenges, many classic separation strategies have been combined with mass spectrometry, yielding robust LC-MS/MS approaches that provide a high degree of coverage (i.e. number of species detected), are highly sensitive, and also quantitative. While lipidomics methodologies are now widely available and standardized, simultaneous accurate quantification of multiple lipid species with varying hydrophobicity and charge is still a challenging endeavor. When designing a study, attention to a few specific details of experimental design can have a large impact on the quality of the data. Specifically, aspects of the lipid extraction, homogenization, chromatographic separation, MS or MS/MS methodology, and normalization all contribute to data quality.
a) Sample preparation
Sample homogenization and normalization are non-trivial when preparing tissue samples. Various means of normalization have been used, including tissue wet weight, dry weight, total protein, and total phospholipid. Among 23 tissue lipidomics studies cited here, four normalized to tissue weight, two normalized to total protein using a homogenate aliquot, two normalized to total phospholipid post-extraction, and two were inferred but not specified to have been normalized to total protein. The 12 remaining studies did not specify any normalization.
The choice of lipid extraction methodology is also of high importance because the ability to quantify a wide range of lipid species assumes a relatively constant yield of each species. For sphingolipids, the Bligh Dyer extraction has the longest and widest track record [50], and of the 27 tissue and cell lipidomics studies cited here, 16 used variations on the Bligh Dyer. Four used the Folch extraction, which gives a good yield of charged lipid species at the expense of neutral lipids, one used a methyl tert-butyl ether-based extraction that yields a very similar profile to the Bligh Dyer [51]. Six studies did not give sufficient detail to identify the extraction method. While all studies specified MS, the instrument model employed was variable and there was less consistency in the reporting of methods. Inclusion of internal standards is an essential consideration for targeted lipidomics. At least one internal standard is indispensable for normalizing the lipid extraction. For sphingolipids, which occur in mammalian systems almost exclusively with even number carbon chains of at least 14 carbons, an advantage of sphingolipid analysis is that synthetic odd carbon chain sphingoid base and/or short Nacyl chain variants can be used as internal standards. This provides significant cost reductions compared to deuterated standards required for eicosanoid or steroid analyses. One such example is the use of d18:1/C12:0 ceramide as an internal standard. The fragmentation and ionization of ceramide depends mainly on the cleavage of the N-acyl chain without regard to its length. Therefore, the characteristic peak of a C12:0 synthetic ceramide standard will be identical to that of a natural, e.g. C16:0 ceramide analyte.
b) Quantitative sphingolipidomics
Lipidomics methodologies utilizing mass spectrometry have long been paired with prior chromatographic resolution including high performance liquid chromatography (HPLC), given the obvious benefits of using a mass spectrometer as a detector. Additionally, this coupling allows for lipid samples to be chromatographically focused and sequentially eluted while the sample is de-salted. Resolving species temporally provides enhanced sensitivity by reducing the overall complexity of the lipid sample being ionized at any one time, thus lowering ionization suppression (which is a loss of ionization efficiency due to presence of other endogenous or exogenous contaminants, compounds that compete for ionization or otherwise decrease ionization of the target analyte) and thereby enhancing signals. These techniques are particularly beneficial for isobaric species such as glucosylceramide and galactosylceramide, which would be difficult to distinguish otherwise. Traditionally, HPLC has been used in both reverse phase (hydrophobic column) and normal phase (hydrophilic column) separations of sphingolipids. Reverse phase allows separation of species by both length and degree of saturation of the sphingoid base and N-acyl chain, whereas, normal phase has been primarily used to resolve species by their 1-position constituents, such as the polar headgroups of phosphorylated sphingolipids. The importance of chromatographic resolution is exemplified by its requirement for resolution of sphingoid bases. For example, while sphingosine and sphinganine (also called dihydrosphingosine) have unique precursor and product ion m/z values, their mass differs by only 2 AMU. Because the typical biological abundance of sphingosine is significantly higher than sphinganine, the naturally occurring [M + 2] isotope of sphingosine (arising from ~ 2.0% natural isotopic abundance) has the potential to interfere with sphinganine quantitation if the species are not chromatographically resolved prior to mass determination [52]. Additional complexities arise when species have extremely similar compositions such as glucosyl- vs. galactosylceramides, which are stereoisomers and therefore show no differences in mass. These species normally are not resolved in standard HPLC methods; thus, they require specialized HPLC conditions, e.g. use of a chiral column, in order to be correctly resolved, identified, and quantitated.
The type of data produced can depend greatly on the type of ionization utilized with high resolution lipidomics. Commonly, electrospray ionization (ESI) [52, 60–67], atmospheric pressure chemical ionization (APCI) [68–70], and recently, atmospheric pressure photoionization (APPI) [71, 72] have been used in both positive and negative modes. Ionization techniques for general sphingolipidomics are handled in the positive ion mode given the inherent ability of most sphingolipid headgroups to form [M+H]+ ions. In these analyses, close attention should be paid towards source temperature and solvent flow rate as in-source degradation of headgroups, particularly carbohydrate moieties, is a concern. ESI with negative mode ionization is primarily used for acidic glycosphingolipids such as sulfatides [62, 65] and gangliosides[52]. APCI has been mainly used for neutral gangliosides [68]; whereas, APPI is emerging as a technique to provide structural information on difficult species such as globotriaosylceramides [73].
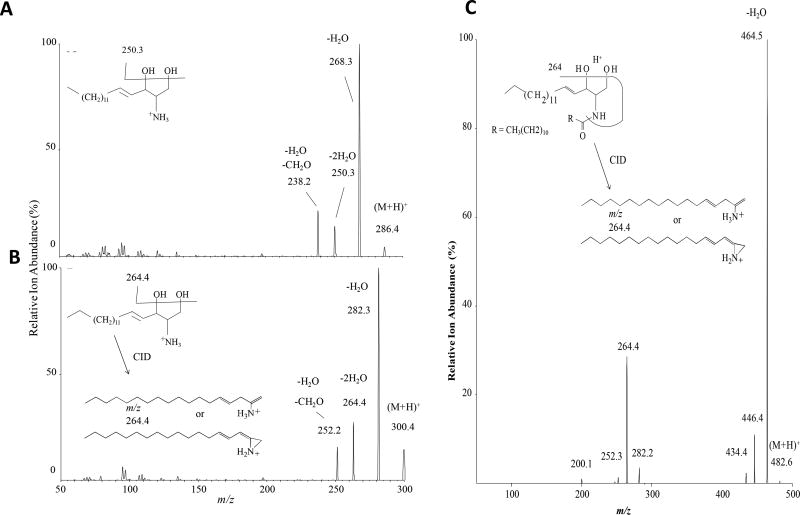
As discussed above, in the last several years, it has become apparent that ceramides exist as numerous species distinguished by chain length and saturation of the N-acyl chain (Fig. 2). Moreover, these species not only have distinct biological functions, but are generated by distinct CerS isoforms. Therefore, studies into the biology of individual CerS and sphingolipids have flourished and revealed highly specialized functions of both CerS and the ceramides produced. Mass spectrometric approaches have largely enabled progress in this area, as utilizing structure-specific fragmentation allows distinction of sphingoid base and N-acyl components. For example, sphingosine fragments via a double dehydration, yielding a conjugated carbocation of 264 AMU (Fig 3A–B). Similarly, ceramide fragments via cleavage of its hydroxl headgroup from the 1 position, dehydration at the 3-position, and cleavage of the N-acyl chain to m/z =64.4 for d18:1 species (Fig 3C). Some complex sphingolipids would fragment via loss of the 1 position constituent; for example, carbohydrate headgroups. In contrast, sphingomyelin fragments in such a way that the charge is maintained in the headgroup, yielding a characteristic ion fragment of m/z=184.
Figure 3. Product ion scans by ABSciex 5500 Qtrap.
(A) d17:1 sphingosine, an odd-chain synthetic sphingoid base, which is a commonly used sphingolipid standard. (B) d18:1 dihydrosphingosine/sphinganine, and (C) d18:1/12:0 ceramide.
c) Qualitative sphingolipidomics
HPLC-MS/MS methodologies have been used across a wide range of sphingolipids, from sphingoid bases and their 1-phosphates to ceramides, hexosylceramides, lactosylceramides, sphingomyelins[53, 54], and even higher order glycosphingolipids [55, 56]. While separation of all species via HPLC would be ideal, it is not necessarily required as long as the species in question have distinct precursor/production pairs. Utilization of high resolution MS and MS/MS analyses with no prior chromatographic resolution via quadrupole-time-of-flight (Q-TOF) mass spectrometers with infusion-based analyses are producing increasingly thorough profiles of the lipidome [57–59]. In these methodologies, very small volumes of sample are infused into the mass spectrometer for extended periods of time at either microliter per minute or nanoliter per minute flow rates. This provides extensive coverage of a given mass range coupled with the ability to perform MS/MS analyses on desired peaks for structural identification via structure specific fragment ions or neutral losses corresponding to unique lipid fragment ions or neutral losses and comparison to existing databases. Here it should be noted that a time-of-flight (TOF) mass analyzer is vastly superior to a quadrupole due to the resolution of the instrument, which provides much higher resolution for ions, thereby enabling clearer distinction between the target analyte and other molecules.
While very structurally informative, it is important to address the potential limitations of accurate mass (i.e. TOF) techniques. Without accompanying chromatographic separation, differentiation of isobaric species (i.e., those with the same molecular mass) remains a challenge. In addition, these methods are extremely prone to ionization suppression due to the extreme variance of lipid concentrations in a biological sample with a wide range of gas phase acidity or basicity. The widely disparate energetics of fragmentation across lipid species also presents a challenge for quantitation of lipids via these methods. Because chain length, levels of desaturation, hydroxylation, and headgroups can vary significantly amongst species, and these then impact then energy required for ionization, simply increasing collision energies within a given mass range will differentially impact different sphingolipid species, with the result that ratios between species are not necessarily constant at distinct collision energies, and this limits the quantitative capacity of these approaches. Nonetheless, approaches that do not rely on an initial chromatographic separation are valuable for rapid screening to determine pattern differences between samples and have enabled many advances in our understanding.
5. Concluding remarks
The recent improvements in accessibility and sophistication of lipidomics approaches and their applications in NAFLD have revealed relationships between bioactive sphingolipids and their pathology. Additionally, despite wide variations in model systems and approaches used in the literature, commonalities continue to emerge, such as the distinct response of C16–C22 versus C24 and C24:1 ceramides observed in human studies, in several rodent models, and in cultured cells. Conflicting data arising from in vivo vs in vitro models may be due to the contribution from nonparenchymal cells such as immune cells and fibroblasts. The known roles of S1P, C1P and glucosylceramide in regulating immune cell recruitment and action imply that understanding of the metabolic interplay between these and other species will be essential to accurately understand changes during NASH progression.
The fast-advancing area of lipidomics methodology, specifically the use of TOF to greatly improve mass resolution, and the use of nanoliter per minute chromatography are pushing the frontiers of resolution and quantitative accuracy for sphingolipidomics. Ideally, experimental design should take into account the method of chromatographic separation, factors that may lead to ion suppression, selecton of the molecular ion and ionization modes that are compatible with target species, and application of high resolution or tandem mass spectrometry to yield reliable species identifications. Improvements in technology, methodology, and sophistication on the part of investigators will allow the interplay among sphingolipids and their metabolic pathways to be linked to specific molecular species and pathways and contribute to increased understanding of their roles in NAFLD pathology.
Acknowledgments
This work was supported by National Institutes of Health grant HL117233 and Veterans’ Affairs grant I01BX0002000 to L.A.C., National Institutes of Health grant R01GM043880 to S.S. J.C.A. was supported by P30 CA016059 to Virginia Commonwealth University Lipidomics Core.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Arab JP, Arrese M, Trauner M. Recent Insights into the Pathogenesis of Nonalcoholic Fatty Liver Disease. Annu Rev Pathol. 2018;13:321–350. doi: 10.1146/annurev-pathol-020117-043617. [DOI] [PubMed] [Google Scholar]
- 2.Bellanti F, Villani R, Facciorusso A, Vendemiale G, Serviddio G. Lipid oxidation products in the pathogenesis of non-alcoholic steatohepatitis. Free Radic Biol Med. 2017;111:173–185. doi: 10.1016/j.freeradbiomed.2017.01.023. [DOI] [PubMed] [Google Scholar]
- 3.Chiurchiu V, Leuti A, Maccarrone M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front Immunol. 2018;9:38. doi: 10.3389/fimmu.2018.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kleuser B. Divergent Role of Sphingosine 1-Phosphate in Liver Health and Disease. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Del Campo JA, Gallego P, Grande L. Role of inflammatory response in liver diseases: Therapeutic strategies. World J Hepatol. 2018;10:1–7. doi: 10.4254/wjh.v10.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grosch S, Alessenko AV, Albi E. The Many Facets of Sphingolipids in the Specific Phases of Acute Inflammatory Response. Mediators Inflamm. 2018;2018:5378284. doi: 10.1155/2018/5378284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hirsova P, Ibrahim SH, Gores GJ, Malhi H. Lipotoxic lethal and sublethal stress signaling in hepatocytes: relevance to NASH pathogenesis. J Lipid Res. 2016;57:1758–1770. doi: 10.1194/jlr.R066357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hannun YA, Obeid LM. Many ceramides. J Biol Chem. 2011;286:27855–27862. doi: 10.1074/jbc.R111.254359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Geng T, Sutter A, Harland MD, Law BA, Ross JS, Lewin D, Palanisamy A, Russo SB, Chavin KD, Cowart LA. SphK1 mediates hepatic inflammation in a mouse model of NASH induced by high saturated fat feeding and initiates proinflammatory signaling in hepatocytes. J Lipid Res. 2015;56:2359–2371. doi: 10.1194/jlr.M063511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AY, Lee JW, Kim JE, Mock HJ, Park S, Kim S, Hong SH, Kim JY, Park EJ, Kang KS, Kim KP, Cho MH. Dihydroceramide is a key metabolite that regulates autophagy and promotes fibrosis in hepatic steatosis model. Biochem Biophys Res Commun. 2017;494:460–469. doi: 10.1016/j.bbrc.2017.10.110. [DOI] [PubMed] [Google Scholar]
- 11.Mauer AS, Hirsova P, Maiers JL, Shah VH, Malhi H. Inhibition of sphingosine 1-phosphate signaling ameliorates murine nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2017;312:G300–G313. doi: 10.1152/ajpgi.00222.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez L, Torres S, Baulies A, Alarcon-Vila C, Elena M, Fabrias G, Casas J, Caballeria J, Fernandez-Checa JC, Garcia-Ruiz C. Myristic acid potentiates palmitic acid-induced lipotoxicity and steatohepatitis associated with lipodystrophy by sustaning de novo ceramide synthesis. Oncotarget. 2015;6:41479–41496. doi: 10.18632/oncotarget.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hornemann T, Penno A, Rutti MF, Ernst D, Kivrak-Pfiffner F, Rohrer L, von Eckardstein A. The SPTLC3 subunit of serine palmitoyltransferase generates short chain sphingoid bases. J Biol Chem. 2009;284:26322–26330. doi: 10.1074/jbc.M109.023192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gable K, Gupta SD, Han G, Niranjanakumari S, Harmon JM, Dunn TM. A disease-causing mutation in the active site of serine palmitoyltransferase causes catalytic promiscuity. J Biol Chem. 2010;285:22846–22852. doi: 10.1074/jbc.M110.122259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Merrill AH., Jr Characterization of serine palmitoyltransferase activity in Chinese hamster ovary cells. Biochim Biophys Acta. 1983;754:284–291. doi: 10.1016/0005-2760(83)90144-3. [DOI] [PubMed] [Google Scholar]
- 16.Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, Mauer J, Xu E, Hammerschmidt P, Bronneke HS, Trifunovic A, LoSasso G, Wunderlich FT, Kornfeld JW, Bluher M, Kronke M, Bruning JC. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–686. doi: 10.1016/j.cmet.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Raichur S, Wang ST, Chan PW, Li Y, Ching J, Chaurasia B, Dogra S, Ohman MK, Takeda K, Sugii S, Pewzner-Jung Y, Futerman AH, Summers SA. CerS2 haploinsufficiency inhibits beta-oxidation and confers susceptibility to diet-induced steatohepatitis and insulin resistance. Cell Metab. 2014;20:687–695. doi: 10.1016/j.cmet.2014.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pettus BJ, Bielawska A, Spiegel S, Roddy P, Hannun YA, Chalfant CE. Ceramide kinase mediates cytokine- and calcium ionophore-induced arachidonic acid release. J Biol Chem. 2003;278:38206–38213. doi: 10.1074/jbc.M304816200. [DOI] [PubMed] [Google Scholar]
- 20.Maceyka M, Spiegel S. Sphingolipid metabolites in inflammatory disease. Nature. 2014;510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu FPS, Dworski S, Medin JA. Deletion of MCP-1 Impedes Pathogenesis of Acid Ceramidase Deficiency. Sci Rep. 2018;8:1808. doi: 10.1038/s41598-018-20052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Helke K, Angel P, Lu P, Garrett-Mayer E, Ogretmen B, Drake R, Voelkel-Johnson C. Ceramide Synthase 6 Deficiency Enhances Inflammation in the DSS model of Colitis. Sci Rep. 2018;8:1627. doi: 10.1038/s41598-018-20102-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorden DL, Myers DS, Ivanova PT, Fahy E, Maurya MR, Gupta S, Min J, Spann NJ, McDonald JG, Kelly SL, Duan J, Sullards MC, Leiker TJ, Barkley RM, Quehenberger O, Armando AM, Milne SB, Mathews TP, Armstrong MD, Li C, Melvin WV, Clements RH, Washington MK, Mendonsa AM, Witztum JL, Guan Z, Glass CK, Murphy RC, Dennis EA, Merrill AH, Jr, Russell DW, Subramaniam S, Brown HA. Biomarkers of NAFLD progression: a lipidomics approach to an epidemic. J Lipid Res. 2015;56:722–736. doi: 10.1194/jlr.P056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Penno A, Reilly MM, Houlden H, Laura M, Rentsch K, Niederkofler V, Stoeckli ET, Nicholson G, Eichler F, Brown RH, Jr, von Eckardstein A, Hornemann T. Hereditary sensory neuropathy type 1 is caused by the accumulation of two neurotoxic sphingolipids. J Biol Chem. 2010;285:11178–11187. doi: 10.1074/jbc.M109.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertea M, Rutti MF, Othman A, Marti-Jaun J, Hersberger M, von Eckardstein A, Hornemann T. Deoxysphingoid bases as plasma markers in diabetes mellitus. Lipids Health Dis. 2010;9:84. doi: 10.1186/1476-511X-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luukkonen PK, Zhou Y, Sadevirta S, Leivonen M, Arola J, Oresic M, Hyotylainen T, Yki-Jarvinen H. Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol. 2016;64:1167–1175. doi: 10.1016/j.jhep.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Al Fadel F, Fayyaz S, Japtok L, Kleuser B. Involvement of Sphingosine 1-Phosphate in Palmitate-Induced Non-Alcoholic Fatty Liver Disease. Cell Physiol Biochem. 2016;40:1637–1645. doi: 10.1159/000453213. [DOI] [PubMed] [Google Scholar]
- 28.Patterson RE, Kalavalapalli S, Williams CM, Nautiyal M, Mathew JT, Martinez J, Reinhard MK, McDougall DJ, Rocca JR, Yost RA, Cusi K, Garrett TJ, Sunny NE. Lipotoxicity in steatohepatitis occurs despite an increase in tricarboxylic acid cycle activity. Am J Physiol Endocrinol Metab. 2016;310:E484–494. doi: 10.1152/ajpendo.00492.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanyal AJ, Pacana T. A Lipidomic Readout of Disease Progression in A Diet-Induced Mouse Model of Nonalcoholic Fatty Liver Disease. Trans Am Clin Climatol Assoc. 2015;126:271–288. [PMC free article] [PubMed] [Google Scholar]
- 30.Montgomery MK, Brown SH, Lim XY, Fiveash CE, Osborne B, Bentley NL, Braude JP, Mitchell TW, Coster AC, Don AS, Cooney GJ, Schmitz-Peiffer C, Turner N. Regulation of glucose homeostasis and insulin action by ceramide acyl-chain length: A beneficial role for very long-chain sphingolipid species. Biochim Biophys Acta. 2016;1861:1828–1839. doi: 10.1016/j.bbalip.2016.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Chaurasia B, Kaddai VA, Lancaster GI, Henstridge DC, Sriram S, Galam DL, Gopalan V, Prakash KN, Velan SS, Bulchand S, Tsong TJ, Wang M, Siddique MM, Yuguang G, Sigmundsson K, Mellet NA, Weir JM, Meikle PJ, Bin MYMS, Shabbir A, Shayman JA, Hirabayashi Y, Shiow ST, Sugii S, Summers SA. Adipocyte Ceramides Regulate Subcutaneous Adipose Browning, Inflammation, and Metabolism. Cell Metab. 2016;24:820–834. doi: 10.1016/j.cmet.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Kasumov T, Li L, Li M, Gulshan K, Kirwan JP, Liu X, Previs S, Willard B, Smith JD, McCullough A. Ceramide as a mediator of non-alcoholic Fatty liver disease and associated atherosclerosis. PLoS One. 2015;10:e0126910. doi: 10.1371/journal.pone.0126910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sajan MP, Ivey RA, Lee MC, Farese RV. Hepatic insulin resistance in ob/ob mice involves increases in ceramide, aPKC activity, and selective impairment of Akt-dependent FoxO1 phosphorylation. J Lipid Res. 2015;56:70–80. doi: 10.1194/jlr.M052977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Skop V, Malinska H, Trnovska J, Huttl M, Cahova M, Blachnio-Zabielska A, Baranowski M, Burian M, Oliyarnyk O, Kazdova L. Positive effects of voluntary running on metabolic syndrome-related disorders in non-obese hereditary hypertriacylglycerolemic rats. PLoS One. 2015;10:e0122768. doi: 10.1371/journal.pone.0122768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Iannucci LF, Cioffi F, Senese R, Goglia F, Lanni A, Yen PM, Sinha RA. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci Rep. 2017;7:2023. doi: 10.1038/s41598-017-02205-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taltavull N, Ras R, Marine S, Romeu M, Giralt M, Mendez L, Medina I, Ramos-Romero S, Torres JL, Nogues MR. Protective effects of fish oil on pre-diabetes: a lipidomic analysis of liver ceramides in rats. Food Funct. 2016;7:3981–3988. doi: 10.1039/c6fo00589f. [DOI] [PubMed] [Google Scholar]
- 37.Byeon SK, Lee JC, Chung BC, Seo HS, Moon MH. High-throughput and rapid quantification of lipids by nanoflow UPLC-ESI-MS/MS: application to the hepatic lipids of rabbits with nonalcoholic fatty liver disease. Anal Bioanal Chem. 2016;408:4975–4985. doi: 10.1007/s00216-016-9592-y. [DOI] [PubMed] [Google Scholar]
- 38.Zhu Q, Yang J, Zhu R, Jiang X, Li W, He S, Jin J. Dihydroceramide-desaturase-1-mediated caspase 9 activation through ceramide plays a pivotal role in palmitic acid-induced HepG2 cell apoptosis. Apoptosis. 2016;21:1033–1044. doi: 10.1007/s10495-016-1267-9. [DOI] [PubMed] [Google Scholar]
- 39.Seessle J, Liebisch G, Schmitz G, Stremmel W, Chamulitrat W. Palmitate activation by fatty acid transport protein 4 as a model system for hepatocellular apoptosis and steatosis. Biochim Biophys Acta. 2015;1851:549–565. doi: 10.1016/j.bbalip.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 40.Deevska GM, Dotson PP, 2nd, Karakashian AA, Isaac G, Wrona M, Kelly SB, Merrill AH, Jr, Nikolova-Karakashian MN. Novel Interconnections in Lipid Metabolism Revealed by Overexpression of Sphingomyelin Synthase-1. J Biol Chem. 2017;292:5110–5122. doi: 10.1074/jbc.M116.751602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mondal N, Stolfa G, Antonopoulos A, Zhu Y, Wang SS, Buffone A, Jr, Atilla-Gokcumen GE, Haslam SM, Dell A, Neelamegham S. Glycosphingolipids on Human Myeloid Cells Stabilize E-Selectin-Dependent Rolling in the Multistep Leukocyte Adhesion Cascade. Arterioscler Thromb Vasc Biol. 2016;36:718–727. doi: 10.1161/ATVBAHA.115.306748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kakazu E, Mauer AS, Yin M, Malhi H. Hepatocytes release ceramide-enriched pro-inflammatory extracellular vesicles in an IRE1alpha-dependent manner. J Lipid Res. 2016;57:233–245. doi: 10.1194/jlr.M063412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kong Y, Wang H, Wang S, Tang N. FTY720, a sphingosine-1 phosphate receptor modulator, improves liver fibrosis in a mouse model by impairing the motility of bone marrow-derived mesenchymal stem cells. Inflammation. 2014;37:1326–1336. doi: 10.1007/s10753-014-9877-2. [DOI] [PubMed] [Google Scholar]
- 44.Sato M, Ikeda H, Uranbileg B, Kurano M, Saigusa D, Aoki J, Maki H, Kudo H, Hasegawa K, Kokudo N, Yatomi Y. Sphingosine kinase-1, S1P transporter spinster homolog 2 and S1P2 mRNA expressions are increased in liver with advanced fibrosis in human. Sci Rep. 2016;6:32119. doi: 10.1038/srep32119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li C, Jiang X, Yang L, Liu X, Yue S, Li L. Involvement of sphingosine 1-phosphate (SIP)/S1P3 signaling in cholestasis-induced liver fibrosis. Am J Pathol. 2009;175:1464–1472. doi: 10.2353/ajpath.2009.090037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitsutake S, Date T, Yokota H, Sugiura M, Kohama T, Igarashi Y. Ceramide kinase deficiency improves diet-induced obesity and insulin resistance. FEBS Lett. 2012;586:1300–1305. doi: 10.1016/j.febslet.2012.03.032. [DOI] [PubMed] [Google Scholar]
- 47.Gubern A, Barcelo-Torns M, Barneda D, Lopez JM, Masgrau R, Picatoste F, Chalfant CE, Balsinde J, Balboa MA, Claro E. JNK and ceramide kinase govern the biogenesis of lipid droplets through activation of group IVA phospholipase A2. J Biol Chem. 2009;284:32359–32369. doi: 10.1074/jbc.M109.061515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hait NC, Maiti A. The Role of Sphingosine-1-Phosphate and Ceramide-1-Phosphate in Inflammation and Cancer. Mediators Inflamm. 2017;2017:4806541. doi: 10.1155/2017/4806541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hannun YA, Obeid LM. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat Rev Mol Cell Biol. 2008;9:139–150. doi: 10.1038/nrm2329. [DOI] [PubMed] [Google Scholar]
- 50.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 51.Matyash V, Liebisch G, Kurzchalia TV, Shevchenko A, Schwudke D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J Lipid Res. 2008;49:1137–1146. doi: 10.1194/jlr.D700041-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merrill AH, Sullards MC, Allegood JC, Kelly S, Wang E. Sphingolipidomics: High-throughput, structure-specific, and quantitative analysis of sphingolipids by liquid chromatography tandem mass spectrometry. Methods. 2005;36:207–224. doi: 10.1016/j.ymeth.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 53.Sullards MC, Merrill AH., Jr Analysis of sphingosine 1-phosphate, ceramides, and other bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Science's STKE : signal transduction knowledge environment. 2001;2001:pl1. doi: 10.1126/stke.2001.67.pl1. [DOI] [PubMed] [Google Scholar]
- 54.Pettus BJ, Bielawska A, Kroesen BJ, Moeller PD, Szulc ZM, Hannun YA, Busman M. Observation of different ceramide species from crude cellular extracts by normal-phase high-performance liquid chromatography coupled to atmospheric pressure chemical ionization mass spectrometry. Rapid communications in mass spectrometry : RCM. 2003;17:1203–1211. doi: 10.1002/rcm.1043. [DOI] [PubMed] [Google Scholar]
- 55.Kushi Y, Rokukawa C, Numajir Y, Kato Y, Handa S. Analysis of underivatized glycosphingolipids by high-performance liquid chromatography/atmospheric pressure ionization mass spectrometry. Analytical biochemistry. 1989;182:405–410. doi: 10.1016/0003-2697(89)90615-5. [DOI] [PubMed] [Google Scholar]
- 56.Suzuki M, Yamakawa T, Suzuki A. A micro method involving micro high-performance liquid chromatography-mass spectrometry for the structural characterization of neutral glycosphingolipids and monosialogangliosides. Journal of biochemistry. 1991;109:503–506. doi: 10.1093/oxfordjournals.jbchem.a123411. [DOI] [PubMed] [Google Scholar]
- 57.Ekroos K, Chernushevich IV, Simons K, Shevchenko A. Quantitative profiling of phospholipids by multiple precursor ion scanning on a hybrid quadrupole time-of-flight mass spectrometer. Analytical chemistry. 2002;74:941–949. doi: 10.1021/ac015655c. [DOI] [PubMed] [Google Scholar]
- 58.Trzeciecka A, Arbelo U, Barron A, Edwards G, Sampathkumar S, Toris C, Bhattacharya SK. Shotgun Sphingolipid Analysis of Human Aqueous Humor. Methods in molecular biology (Clifton, N.J.) 2018;1695:97–107. doi: 10.1007/978-1-4939-7407-8_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefanko A, Thiede C, Ehninger G, Simons K, Grzybek M. Lipidomic approach for stratification of acute myeloid leukemia patients. PLoS One. 2017;12:e0168781. doi: 10.1371/journal.pone.0168781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bielawski J, Szulc ZM, Hannun YA, Bielawska A. Simultaneous quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods. 2006;39:82–91. doi: 10.1016/j.ymeth.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Han X, Gross RW. Shotgun lipidomics: electrospray ionization mass spectrometric analysis and quantitation of cellular lipidomes directly from crude extracts of biological samples. Mass Spectrom Rev. 2005;24:367–412. doi: 10.1002/mas.20023. [DOI] [PubMed] [Google Scholar]
- 62.Hsu FF, Turk J. Studies on sulfatides by quadrupole ion-trap mass spectrometry with electrospray ionization: structural characterization and the fragmentation processes that include an unusual internal galactose residue loss and the classical charge-remote fragmentation. J Am Soc Mass Spectrom. 2004;15:536–546. doi: 10.1016/j.jasms.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 63.Murphy RC, Axelsen PH. Mass spectrometric analysis of long-chain lipids. Mass Spectrom Rev. 2011;30:579–599. doi: 10.1002/mas.20284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Scherer M, Leuthauser-Jaschinski K, Ecker J, Schmitz G, Liebisch G. A rapid and quantitative LC-MS/MS method to profile sphingolipids. J Lipid Res. 2010;51:2001–2011. doi: 10.1194/jlr.D005322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shaner RL, Allegood JC, Park H, Wang E, Kelly S, Haynes CA, Sullards MC, Merrill AH., Jr Quantitative analysis of sphingolipids for lipidomics using triple quadrupole and quadrupole linear ion trap mass spectrometers. J Lipid Res. 2009;50:1692–1707. doi: 10.1194/jlr.D800051-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugawara T, Aida K, Duan J, Hirata T. Analysis of glucosylceramides from various sources by liquid chromatography-ion trap mass spectrometry. J Oleo Sci. 2010;59:387–394. doi: 10.5650/jos.59.387. [DOI] [PubMed] [Google Scholar]
- 67.Sullards MC, Allegood JC, Kelly S, Wang E, Haynes CA, Park H, Chen Y, Merrill AH. Methods in Enzymology. Academic Press; 2007. Structure-Specific, Quantitative Methods for Analysis of Sphingolipids by Liquid Chromatography–Tandem Mass Spectrometry: “Inside-Out” Sphingolipidomics; pp. 83–115. [DOI] [PubMed] [Google Scholar]
- 68.Farwanah H, Kolter T, Sandhoff K. Mass spectrometric analysis of neutral sphingolipids: methods, applications, and limitations. Biochim Biophys Acta. 2011;1811:854–860. doi: 10.1016/j.bbalip.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 69.Farwanah H, Wirtz J, Kolter T, Raith K, Neubert RH, Sandhoff K. Normal phase liquid chromatography coupled to quadrupole time of flight atmospheric pressure chemical ionization mass spectrometry for separation, detection and mass spectrometric profiling of neutral sphingolipids and cholesterol. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:2976–2982. doi: 10.1016/j.jchromb.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 70.van Smeden J, Hoppel L, van der Heijden R, Hankemeier T, Vreeken RJ, Bouwstra JA. LC/MS analysis of stratum corneum lipids: ceramide profiling and discovery. J Lipid Res. 2011;52:1211–1221. doi: 10.1194/jlr.M014456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gaudin M, Imbert L, Libong D, Chaminade P, Brunelle A, Touboul D, Laprevote O. Atmospheric pressure photoionization as a powerful tool for large-scale lipidomic studies. J Am Soc Mass Spectrom. 2012;23:869–879. doi: 10.1007/s13361-012-0341-y. [DOI] [PubMed] [Google Scholar]
- 72.Munoz-Garcia A, Ro J, Brown JC, Williams JB. Identification of complex mixtures of sphingolipids in the stratum corneum by reversed-phase high-performance liquid chromatography and atmospheric pressure photospray ionization mass spectrometry. J Chromatogr A. 2006;1133:58–68. doi: 10.1016/j.chroma.2006.06.067. [DOI] [PubMed] [Google Scholar]
- 73.Delobel A, Roy S, Touboul D, Gaudin K, Germain DP, Baillet A, Brion F, Prognon P, Chaminade P, Laprevote O. Atmospheric pressure photoionization coupled to porous graphitic carbon liquid chromatography for the analysis of globotriaosylceramides. Application to Fabry disease. J Mass Spectrom. 2006;41:50–58. doi: 10.1002/jms.945. [DOI] [PubMed] [Google Scholar]