Abstract
Preterm birth is a major health concern that affects 10% of all worldwide deliveries. Many preterm infants are discharged from the hospital with morbidities that lead to an increased risk for neurodevelopmental impairment, recurrent hospitalizations, and life-long conditions. Unfortunately, the treatment of these conditions is palliative rather than curative, which calls for novel and innovative strategies. Progress in regenerative medicine has offered therapeutic options for many of these conditions. Specifically, human umbilical cord mesenchymal stem cells (MSCs) and cord blood (UCB) cells have shown promise in treating adult onset diseases. Unlike bone-marrow and embryonic derived stem cells, umbilical cord-derived cells are easily and humanely obtained, have low immunogenicity, and offer the potential of autologous therapy. While there are several studies to uphold the efficacy of umbilical cord MSCs in adult therapies, there remains an unmet need for the investigation of its use in treating neonates. The purpose of this review is to provide a summary of current information on the potential therapeutic benefits and clinical applicability of umbilical cord MSCs and UCB cells. Promising preclinical studies have now led to a research movement that is focusing on cell-based therapies for preterm infants.
Keywords: Mesenchymal stem cells, umbilical cord blood, bronchopulmonary dysplasia, hypoxic ischemic encephalopathy
INTRODUCTION
Preterm birth is a major worldwide concern that affects 10% of all deliveries and produces a vast economic burden.[1] Due to advances in neonatal medicine, survival rates for smaller and more preterm infants have improved. However, many of these preterm infants are discharged from the hospital with co-morbidities that lead to increased risk for neurodevelopmental impairment, recurrent hospitalizations, and life-long medical conditions. Unfortunately, current methods of treatment for these diseases are mostly palliative rather than curative. As such, there is an unmet need to establish new effective strategies that will target major neonatal diseases.
Recent developments in stem cell research may offer a new strategy to alleviate neonatal morbidity. Particularly, the literature focuses on mesenchymal stem/stromal cells (MSCs) and umbilical cord blood (UCB) as promising therapeutic agents because of their ease of isolation and release of biologic factors shown to protect and heal injured tissues.[2] Preclinical studies have now demonstrated that MSCs and UCB provide encouraging results in an array of adult morbidities, including autoimmune, neurodegenerative, and cardiovascular diseases.[3–6] Although many studies have shown the beneficial effects of umbilical cord-derived cells in the regeneration of adult disease, there is a lack of literature regarding their use in treating neonatal diseases.
Evidence suggests that the perinatal period offers a single opportunity for a non-invasive retrieval of MSCs and UCB. Precisely, the umbilical cord provides a rich source of MSCs, hematopoietic stem cells, progenitor cells, and mononuclear cells that are hallmarked for their high cellular proliferative rates, vast capacity for multi-lineage tissue differentiation, as well as their ability to negate immunologic rejection by administration of autologous cells.[7–10] Umbilical cord blood and Wharton’s jelly-derived MSCs have translated into the clinics, as currently more than 130 clinical trials are registered utilizing these agents. Thus, the aim of this work is to provide an overall review of umbilical cord-derived cells and their potential therapeutic use in neonatal medicine.
DEFINING REGENERATIVE MEDICINE AND STEM CELLS
Regenerative medicine is an emerging field that utilizes cells and/or biomaterials to repair and restore the normal function of injured cells, tissues, and organs.[11] This area of translational research has received much public and media attention owing to its unique use of primitive and highly capable cells commonly referred to as stem cells.
A stem cell is defined as a cell that has the ability to self-renew as well as differentiate into specialized tissue cells.[12] They are responsible for giving rise to all the cell types (>200) that comprise the human body. Self-renewal occurs when the parent stem cell can mitotically divide into a daughter cell that maintains the same undifferentiated state.
Stem cells are categorized according to their plasticity. Briefly, embryonic stem cells can become any tissue cell type because they are pluripotent, while adult stem cells (also known as somatic stromal cells) have multipotent capabilities. Adult stem cells can be isolated from many tissue sources and then depending on the source can be specialized into ectodermal, mesodermal, or endodermal lineages. This review article will focus on multipotent adult stem cells derived from the umbilical cord Wharton’s jelly and will close with a brief discussion of umbilical cord blood progenitor/mononuclear cells.
BENEFITS OF ISOLATING UMBILICAL CORD STEM CELLS
One of the advantages of upcycling umbilical cordsis the ability to isolate a variety of different cells (refer to Figure 1).. For instance, the umbilical cord blood can yield blood progenitor/mononuclear cells and hematopoietic/mesenchymal stromal cells, while the Wharton’s jelly (the protecting tissue surrounding the umbilical vessels) can provide mesenchymal stem cells.[7] Additionally, umbilical cord perivascular/endothelial cells can be obtained for studies focusing on angiogenesis. Several methods have been described for the isolation of mesenchymal stem cells from the umbilical cord. The two most common techniques used to collect MSCs from Wharton’s jelly includes an enzymatic digestion or explant culture.[13,14]
Figure 1.
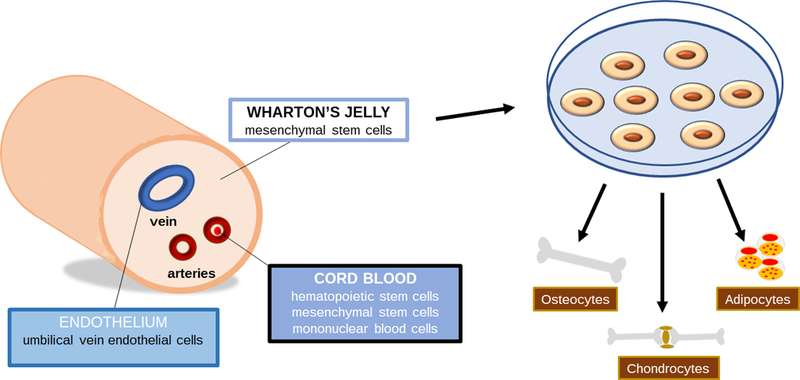
Umbilical cord derived cells
The isolation of mesenchymal stem cells from the umbilical cord blood or Wharton’s jelly is valued because of its non-invasive and non-controversial collection from tissue that traditionally has been discarded as medical waste.[2] Evidence suggests that umbilical cord-derived MSCs have higher proliferative rates and lower immunogenicity compared to adult tissue stem cells.[15] This is due in part to their lack of MHC class I and low level of MHC class II expression.[16] Unlike embryonic stem cells, transplantation of human umbilical cord-derived MSCs have not shown an increased risk for teratoma formation.[17] Most importantly, cord MSCs and UCB cells can be isolated, propagated, and in the future autologously transplanted.
THERAPEUTIC PROPERTIES OF MSCs
Homing capacity
Homing to sites of injury and inflammation are among the appealing properties of MSCs. Similar to immunologic cells, MSCs express many of the cell surface receptors that are involved in endothelial attachment and migration into tissues.[18] Although the exact mechanisms are unknown, researchers have found that MSCs can transmigrate from the bloodstream to areas of tissue injury.[19–21] Upon arrival to the target tissue, MSCs differentiate into injured cell types and release soluble factors that promote tissue regeneration.
Studies involving vascular formation after tissue injury have discovered that vascular endothelial growth factor (VEGF) plays a critical role in the migratory patterns of MSCs.[22] The upregulation of VEGF stimulates MSC homing through interactions with stromal cell-derived factor-1 (SDF-1), a chemokine expressed after inflammation/injury.[23] The chemokine receptor CXCR4 complexes with SDF-1 to signal MSC migration as well as differentiation.[24] The ability to mobilize to sites of tissue damage have driven the interest of cell-based therapy in medicine.
Paracrine release of biologic factors
MSCs communicate with nearby cells by adapting to changes in their microenvironment. Initially, the therapeutic effects of MSCs was thought to be due to cellular engraftment into the wounded tissue. Studies have now confirmed that only a small percentage of MSCs engraft.[25,26] Instead the beneficial properties of MSCs are now widely accepted as paracrine in nature.[27,28]
Secreted biologic factors have been identified in the media used to propagate the MSCs, also referred to as conditioned media.[29]. Preclinical studies in models of acute lung injury and skin injury have demonstrated similar efficacy between transplanting stem cells versus conditioned media without cells.[30,31] In addition to biologic factors, MSCs can also secrete extracellular vesicles that contain RNA or membrane proteins that function in intercellular communication and signaling.[32] Furthermore, researchers have also demonstrated mitochondrial transfer from umbilical cord MSCs to damaged cells.[33]
Antioxidant effects
Oxidative stress is characterized by a cellular imbalance between reactive oxygen species and anti-oxidants. Reactive oxygen species (ROS) present an important role in cell signaling, cell membrane and gene integrity, and more importantly they can function as regulators of cell death.[34] It has been reported that the overproduction of ROS (or underproduction of anti-oxidants) is linked to the pathophysiology of many medical conditions. Despite this, new evidence reveals that the interaction between ROS and exogenous administration of MSCs is reassuring.
Studies involving renal injury uncovered that MSCs advance the production of anti-oxidative molecules, depress renal tubular damage, and suppress renal cell apoptosis.[35] Similarly, in a cardiac study of ischemic-reperfusion MSC extracellular vesicles restored energy levels, recovered biomolecules in redox reactions, and reduced oxidative stress.[36] Arslan’s study of myocardial ischemic-reperfusion injury in mice attributed the anti-oxidant effects through the activation of the PI3k/Akt pathway.[36] These studies provide evidence that MSCs and their paracrine secretions are useful agents in oxidative stress-induced tissue damage.
Angiogenesis
Many pharmacologic therapies have targeted the formation of new blood vessels (angiogenesis) to ameliorate vascular disease. Cell-based studies have supported this concept by reinforcing the normal development, growth, and repair of blood vessels as a therapeutic mean in ischemic vascular events. Examples of angiogenic effects of MSCs have been well established in experimental studies of peripheral vascular disease as well as tissue wound healing. For instance, Liang et al demonstrated new blood formation and production of vascular growth factors after MSC administration in a rat model of diabetic peripheral vascular disease.[37] Following cell transplantation, the animals had increased blood flow, limb function, and skin wound healing. In a skin injury model comparison study between Wharton’s jelly-derived MSCs with adipose-derived MSCs, the cord-derived MSCs had a greater expression of VEGF, angiopoietin-1, and tubule formation.[38]
Zhang and associates studied the production of angiogenic factors and cardiac vascular density in a rodent model of acute myocardial infarction.[39] The investigators concluded that animals treated with MSCs had increased expression of placental growth factor, enhanced vascular density, as well as higher left ventricular fractional shortening. These findings uphold that stem cells play an important role in the growth and repair of blood vessels through the production of biologic factors that support angiogenesis.
Immune modulation
The inflammatory cascade plays a significant role in the development of many neonatal diseases. Considerable evidence suggests that MSCs mediate inflammatory pathways through direct cellular contact and indirectly through secretion of bioactive molecules. MSCs modulate key steps in both the innate and adaptive immune system through the release of anti-inflammatory factors such prostaglandin E, interleukin 10, and hepatocyte growth factor. These factors are known to regulate the activity of lymphocytes, natural killer cells, and macrophages. [40]
Exogenous MSC therapy has shown to decrease the proliferation of CD4+ and CD8+ T-cells. In an in-vitro study of umbilical cord MSCs, Vellasamy et al showed that T-cell proliferation is inhibited when exposed to direct cell-to-cell contact with MSCs.[41]. Interestingly, when compared to bone marrow derived stem cells, Wharton’s jelly-MSCs had a greater ability to suppress T-cell activation and proliferation.[42]
In addition, Spaggiairi et al have also linked the suppression of natural killer cell proliferation with MSC administration.[43] When cultured with MSCs, NK cells had a reduced ability to secrete interferon gamma. In a co-culture study of NK cells and human bone-marrow MSCs, Sotiropoulou et al showed that MSCs inhibited NK cell proliferation, function, and phenotype.[44]
CLINICAL APPLICABILITY
Mesenchymal stem cells have emerged as novel biologic agents for numerous adult and pediatric illnesses. Their multifaceted properties make them attractive therapies to promote tissue healing. Although the largest push for their use in clinical trials has been in the adult population, recent trials have begun to target neonates. Despite comprising a targeted population, advances towards novel treatments is warranted since their prolonged hospital stay and multiple morbidities make these patients among the most expensive..
Bronchopulmonary Dysplasia
Bronchopulmonary dysplasia (BPD) is a chronic lung disease in premature neonates that is a result of prolonged oxygen and ventilatory support. This condition is characterized by impaired alveolar development and lung vascular growth. Infants diagnosed with BPD are at increased risk for neurodevelopmental impairment, recurrent hospitalizations, and long-term cardio-pulmonary morbidity.[45] Despite advances in neonatal medicine, few therapies have shown clinical efficacy in preventing or treating BPD.
Origins of BPD are often multifactorial involving triggers such as mechanical ventilation, inflammation, oxygen toxicity, infections, and genetics. After birth, extremely preterm infant lungs are exposed to inflammation from positive pressure ventilation, antioxidant stress from chronic hyperoxia, and poor lung growth and development secondary to abnormal angiogenesis. Of all the neonatal conditions, BPD is at the forefront of translational studies incorporating regenerative research.
Preclinical studies
Cord MSC therapy is effective in improving alveolar development and vascular development in animal models of hyperoxia-induced BPD (Refer to Table 1). Intratracheal injection of 5 × 105 MSCs improved survival, weight gain, and decreased markers of inflammation in a rodent model of BPD.[46] Most studies attribute the positive findings to paracrine release of VEGF or hepatocyte growth factor, as engraftment of cells to lung tissue is low. Thebaud’s research group showed long-term improvement in exercise tolerance and lung architecture after a single dose of MSCs in rats with BPD.[47]
Table 1.
Summary of preclinical studies of umbilical cord-derived MSCs to treat BPD
| Source | Animal characteristics | Objective | MSC characteristics | Outcome |
|---|---|---|---|---|
|
Author: Chang et al[46] Year: 2011 |
Animal model: Sprague-Dawley rat pups BPD model: 95% oxygen from birth to 14 days of age |
Optimize dose of cord blood MSCs in attenuating BPD |
Source: human umbilical cord blood Dose: Low: 5 × 103 MSCs; High: 5 × 104, or 5 × 105 MSCs Delivery: intratracheal at postnatal day 5 |
Improved lung architecture (per mean linear intercept), survival, and body weight gain in higher doses of MSCs Higher doses of MSCs decreased inflammatory cytokines: TNF-α, IL-1β, IL-6, and TGF-β Reduced oxidative stress in higher doses of MSCs |
|
Author: Ahn et al[68] Year: 2015 |
Animal model: Sprague-Dawley rat pups BPD model: 90% oxygen from birth to 14 days of age |
Determine the optimal cell type to protect against BPD |
Source: human umbilical cord blood Dose: 5 × 105 MSCs Delivery: intratracheal at postnatal day 5 |
Decreased mean linear intercept in MSC group Inflammatory cytokines IL-1α, IL-1β, IL-6, and TNF-α Increased lung VEGF and HGF in MSC group |
|
Author: Liu et al[69] Year: 2014 |
Animal model: Severe combined immunodeficient mice BPD model: 90% oxygen from birth to 7 days of age |
Studied the morphologic and functional effects of intranasal vs. intraperitoneal administration of MSCs in BPD |
Source: cord tissue MSCs Dose: Low: 1 × 105 MSCs; Mid: 5 × 105 MSCs; High: 5 × 106 MSCs Delivery: intratracheal at postnatal day 5 |
No significant changes in lung mechanics after MSC administration Histologic evaluation: • reduction in mean septal wall thickness in intraperitoneal administration of high dose MSCs • mean cord length was similarly reduced in high dose MSCs given via intranasal and intraperitoneal route |
|
Author: Sung et al[70] Year: 2015 |
Animal model: Sprague Dawley rat pups BPD model: 90% oxygen from birth to 14 days of age |
Determine the optimal route of MSC transplantation for BPD |
Source: human umbilical cord blood MSCs Dose: Intratracheal: 5 × 105 MSCs; Intravenous: 2 × 106 MSCs Delivery: intratracheal vs. intravenous at postnatal day 5 |
MSC transplantation via both routes improved survival Greater decrease in mean alveolar volume in intratracheal delivery of MSCs; while mean linear intercept was similar between both experimental groups Genetic expression of inflammatory mediators was down-regulated in intratracheal MSCs but not intravenous Greater engraftment in intratracheal administration |
|
Author: Pierro et al[47] Year: 2012 |
Animal model: Sprague Dawley rat pups BPD model: 95% oxygen from birth to 14 days of age |
Determine if cell-based therapy is efficient and safe for treating BPD |
Source: human umbilical cord blood MSCs, perivascular cells, conditioned media from both cell types Dose: 3 × 105 MSCs; 6 × 105 MSCs Delivery: intratracheal at postnatal day 4 (prevention) or day 14 (regeneration) |
Short-term: MSCs, perivascular cells, as well as conditioned media from both cell types improved lung compliance and decreased mean linear intercept Conditioned media from MSCs and perivascular cells improved angiogenesis, but greater in perivascular cells Long-term: Exercise capacity improved after the following were given: MSCs, perivascular cells, and conditioned media of both cell types |
|
Author: Chang et al[71] Year: 2013 |
Animal model: Sprague Dawley rat pups BPD model: 90% oxygen from birth to 14 days of age followed by 60% for 7 days |
Determine the optimal timing for MSC transplantation to improve efficacy in BPD |
Source: human umbilical cord blood MSCs Dose: 5 × 105 MSCs Delivery: intratracheal at postnatal day 3 (early), day 10 (late), or combination (day 13) |
Survival rates were similar between control groups and hyperoxic animals that received MSCs at day 3 Mean alveolar volume was best maintained in early MSC delivery group Marker of oxidative stress was reduced in early, late, and combination therapy of MSCs Hyperoxia-induced decrease in lung HGF was upregulated in early and combination therapy |
|
Author: Chang et al[72] Year: 2014 |
Animal model: Sprague Dawley rat pups BPD model: 90% oxygen from birth to 14 days of age |
Examined whether VEGF secreted by MSCs plays a pivotal role in protecting against BPD |
Source: human umbilical cord blood MSCs Dose: 1 × 105 MSCs, with/without VEGF Delivery: intratracheal at postnatal day 5 |
MSCs deceased mean linear intercept and mean alveolar volume BPD+MSCs demonstrated improved lung vascular formation VEGF knockdown decreased the regenerative properties of MSCs |
MSC-mesenchymal stem cell; BPD-bronchopulmonary dysplasia; TNF-tumor necrosis factor; IL-interleukin; TGF-transforming growth factor; VEGF-vascular endothelial growth factor; HGF-hepatocyte growth factor
As previously described, multiple studies show that the benefits of MSCs depend on the release of soluble factors that protect and heal alveoli and pulmonary vessels from injury. Most studies attribute the benefits of MSCs to treat neonatal chronic lung disease through its role in improved vascular growth and development. Studies using contrast imaging have clearly associated improved lung alveolarization secondary to the release of VEGF into the cellular environment.
Clinical studies
Just recently, scientists in South Korea completed the first phase I dose-escalation clinical trial of MSC therapy for preterm neonates at risk for developing BPD.[48] This study included 9 neonates between gestational ages 23 to 29 weeks and a birthweight of 500–1250 grams. Patients either received a low dose, defined as 1 × 107 cells/kg, or high dose (2 × 107 cells/kg) of allogeneic cord blood-derived MSCs. Administration of MSCs did not have any acute cardiopulmonary complications and although not powered to show efficacy did demonstrate a trend towards improving BPD severity as well as markers of systemic inflammation.
Despite these favorable findings, the molecular mechanisms that MSCs impact to regulate normal alveolar development in premature infants remains incompletely understood. Evidence does favor the theory that exogenous MSCs most likely provide benefit in BPD by rescuing endogenous lung stem cells. Collectively, use of MSCs for attenuating changes associated with BPD seems encouraging and open new prospects for regenerative medicine research. Further studies are required in this area to address key questions (optimal route, dose, frequency, timing) before undertaking any assertive measures.
UMBILICAL CORD BLOOD CELLS
Human UCB is rich in stem, progenitor, and mononuclear cells of hematopoietic lineage. Similar to adult stem cells, progenitor cells can differentiate into several cell types, but they are more lineage committed and have limited self-renewal.[55] The umbilical cord blood also contains a plentiful supply of mononuclear cells (T cells, B cells, and monocytes). Like Wharton’s derived-MSCs, UCB cells have several advantages when compared to other sources of hematopoietic/progenitor cells: i) rapid proliferation, ii) decreased incidence of graft versus host disease, iii) simple retrieval, iv) can be easily processed and cryopreserved, and v) contain higher number of immunologic cells.[10,56,57] However, one significant drawback to UCB is that the number of cells retrieved is low and often cord banks must pool multiple umbilical cords to obtain the desired yield.[58]
Hypoxic Ischemic Encephalopathy
Hypoxic ischemic encephalopathy (HIE), also known as perinatal asphyxia, is distinguished by neuronal cell death after a period of decreased/lack of blood flow and oxygen to the brain. In developed countries HIE affects approximately 1–5 neonates per 1000 live births and is the most common cause of neonatal seizures at birth.[49] Survivors from severe asphyxia are at increased risk for devastating outcomes including mental deficiency, life-long seizures, and cerebral palsy.[50] Even with rapid advances in perinatal care, the incidence of HIE has not changed.
Since most cases of HIE occur in-utero, treatment options are limited and focus on reducing the extent of initial brain injury. Infants with moderate to severe asphyxia undergo hypothermic therapy to decrease brain oxygen and energy requirements, reduce free radical expression, and diminish excitatory neurotransmitter release.[50] Nowadays, cell-based therapies have surfaced as future treatment options for infants with HIE.
Preclinical studies
In addition to improving lung injury, umbilical cord MSCs mediate improvements in cognition, motor-sensory ability, as well as infarct size in animal models of HIE. For instance, a single intraventricular dose of MSCs into a rat with a cerebral artery occlusion attenuated the lesion volume and improved survival rates after the brain injury.[51] Further supporting these findings, Zhang and Zhu have shown restoration of sensorimotor capacity and increase in precursor neuronal cells in rodents that received MSC administration.[52,53] Although the mechanism that underlies the restorative properties of MSCs for HIE treatment is still unknown, both reports attribute the homing properties, differentiation capabilities, and most importantly the expression of trophic factors that lessen inflammation as the source for organ recovery.
Clinical studies
Data for the first phase I clinical trial (NCT00593242) to evaluate the feasibility and safety of autologous cord blood cell infusions for moderate to severe HIE has been released.[54] Twenty-three infants received non-cryopreserved, volume- and red blood cell-reduced UCB and were compared to 82 infants who underwent conventional medical management. The study dose was substantially higher (5 × 107 cells/kg) than those used for the BPD trial and the majority of subjects received multiple infusions. This study showed that autologous cord blood transfusion is feasible, has a high recovery of viable cells, and did not result in significant adverse reactions. Preliminary neurodevelopmental outcomes in the subset of patients who received UCB therapy seemed propitious.
Hypoplastic left heart syndrome
Hypoplastic left heart syndrome (HLHS) is one of the most complex and deadly congenital heart diseases.[59,60] Typically, HLHS will require multiple staged surgical procedures to correct the underdeveloped left side of the heart.[61] Even patients who survive the three major surgeries, have a significantly lower life expectancy.[60,62]
In the United States, five sites are currently conducting human clinical trials with regenerative cells for HLHS (clinicaltrials.gov). Mayo Clinic, University of Oklahoma, and Duke University are utilizing autologous umbilical cord blood cells. Most of the studies will be administering the cells directly to the right side of the heart during the first surgical repair. In Japan, the APOLLON clinical trial is in Phase III testing of autologous cardiac stem/progenitor cells for single ventricle disease. Furthermore, the TICAP (Transcoronary Infusion of Cardiac Progenitor Cells in Hypoplastic Left Heart Syndrome) trial in Japan demonstrated safety, improved right ventricle ejection fraction and decreased heart failure in a small cohort of neonates who received autologous heart progenitor cells. [63]
Intraventricular hemorrhage
Twenty five percent of very preterm infants develop spontaneous intraventricular hemorrhages (IVH). [64] The etiology of IVH is multifactorial and is potentiated by an immature vascular development in the germinal matrix, as well as rapid changes in cerebral blood flow. [65] Using an experimental model of severe IVH, Ahn et al demonstrate that administration of umbilical cord blood stem cells reduced hydrocephalus and improved behavioral testing.[66] The same team of investigators showed that only early transplantation of MSCs (two days after IVH induction) showed neuroprotection. [67] Recruitment is underway for patients with severe IVH ( phase II trial)and intraventricular administration of UCB MSCs. The outcomes focus on death,ventricular shunt, as well as ventricular dilatation, and will incorporate magnetic resonance imaging at term equivalent.
CONCLUSION
The umbilical cord offers a vast supply of mesenchymal stem cells that can be aimed to treat neonatal diseases. Preclinical studies provide evidence for the remarkable properties these cells possess. Aside from improving inflammation and angiogenesis, umbilical cord cells can migrate and abate oxidant stress. Further research entailing safety and long-term effects are necessary before stem cell therapy becomes a clinical option for neonates. Some of the more important questions that need to be addressed include:
WHO: Which patients are eligible for stem cell therapy?
WHAT: Which cell population/cell derivative will provide the best clinical outcomes?
WHEN: Should cells be given prophylactically or as rescue therapy?
HOW: Which route and dose is most efficacious?
WHY: What are the molecular mechanisms that cell-based therapies are targeting in neonatal disease?
Table 2.
Summary of preclinical studies of umbilical cord-derived MSCs to treat HIE
| Source | Animal characteristics | Objective | MSC characteristics | Outcome |
|---|---|---|---|---|
|
Author: Zhu et al[53] Year: 2014 |
Animal model: 3-day old Sprague-Dawley rat pups HIE model: left common carotid artery ligation, followed by a 4-hour period of hypoxia with 6% oxygen |
Effects of MSCs on behavioral function and glial cell function |
Source: human umbilical cord blood Dose: 1 × 106 MSCs Delivery: intraperitoneal injection immediately after HIE, and then once/day for 3 days |
Improved exploratory behavior and mental stress in HIE+MSC group Reduced asymmetry of forepaw preference in HIE+MSC group HIE+MSC group showed: • decreased loss of oligodendrocytes • decreased astrocyte proliferation |
|
Author: Xia et al[73] Year: 2010 |
Animal model: 7-day old Sprague-Dawley rat pups HIE model: left common carotid artery ligation, followed by a 3-hour recovery period and 2.5-hour period of hypoxia with 8% oxygen |
Investigate the effect of intracerebral transplantation of human cord blood-derived MSCs on HIE in rat neonates |
Source: huma numbilical cord blood Dose: 1 × 105 MSCs Delivery: left cortical parenchymal injection 3 days after HIE |
Nerve function improved after HIE+MSC injection |
|
Author: Zhang et al[52] Year: 2014 |
Animal model: 7-day old Sprague Dawley rat pups HIE model: right common carotid artery ligation, followed by hypoxia for 2-hours at 8% oxygen |
Potential therapeutic effect of Wharton’s jelly MSCs in a rat model of HIE |
Source: human Wharton’s jelly MSCs Dose: 5 × 105 MSCs Delivery: 24 hour vs. 72 hour, jugular vein |
afterE to evaluate neuroloical deficits HIE+MSC group had decreased escape latency in water maze test, higher rotarod latency, and Longa scoring (24 hour delivery with better results) |
|
Author: Kim et al[51] Year: 2012 |
Animal model: 10-day old male Sprague Dawley rat pups HIE model: right middle cerebral artery occlusion at day 10 |
Determine the therapeutic efficacy of human umbilical cord-derived MSC transplantation in attenuating the severe brain injury induced by MCAO in newborn rats |
Source: human umbilical cord blood MSCs Dose: 1 × 105 MSCs Delivery: intraventricular 6-hrs after MCAO |
Longa scoring was performed at 6 hr, 7 days, 20 days afterHIE to evaluate neurolocal defi Mild improvement in sensorimotor testing Lesion size was smaller in HIE+MSC Double survival rates at one month in HIE+MSC group |
MSC-mesenchymal stem cell; HIE-hypoxic ischemic encephalopathy; MCAO-middle cerebral artery occlusion
Acknowledgments
FUNDING
The project described was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant KL2 TR001118. This study was also supported by The University of Texas Health San Antonio School of Medicine Clinical Investigator Kickstart Pilot Grant.
DISCLOSURE STATEMENT
In accordance with Taylor & Francis policy and my ethical obligation as a researcher, I am reporting that A. Moreira was supported by a grant from the NIH KL2 TR001118. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
REFERENCES
- [1].WHO | The worldwide incidence of preterm birth: a systematic review of maternal mortality and morbidity. WHO. 2011; [DOI] [PMC free article] [PubMed]
- [2].Batsali AK, Kastrinaki M-C, Papadaki HA, et al. Mesenchymal stem cells derived from Wharton’s Jelly of the umbilical cord: biological properties and emerging clinical applications. Curr Stem Cell Res Ther [Internet]. 2013. [cited 2016 Oct 19];8:144–155. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23279098. [DOI] [PubMed] [Google Scholar]
- [3].Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med [Internet]. 2013. [cited 2016 Nov 21];45:e54 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24232253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Walker PA, Shah SK, Harting MT, et al. Progenitor cell therapies for traumatic brain injury: barriers and opportunities in translation. Dis Model Mech [Internet]. 2009. [cited 2017 Apr 4];2:23–38. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19132123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Yu G, Borlongan CV, Stahl CE, et al. Systemic delivery of umbilical cord blood cells for stroke therapy: a review. Restor Neurol Neurosci [Internet]. 2009. [cited 2017 Apr 4];27:41–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19164852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Copeland N, Harris D, Gaballa MA. Human umbilical cord blood stem cells, myocardial infarction and stroke. Clin Med [Internet]. 2009. [cited 2017 Apr 4];9:342–345. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19728507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].El Omar R, Beroud J, Stoltz J-F, et al. Umbilical cord mesenchymal stem cells: the new gold standard for mesenchymal stem cell-based therapies? Tissue Eng Part B Rev [Internet]. 2014. [cited 2016 Oct 17];20:523–544. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24552279. [DOI] [PubMed] [Google Scholar]
- [8].Waller-Wise R Umbilical cord blood: information for childbirth educators. J Perinat Educ [Internet]. 2011. [cited 2017 Apr 4];20:54–60. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22211060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Acosta SA, Franzese N, Staples M, et al. Human Umbilical Cord Blood for Transplantation Therapy in Myocardial Infarction. J Stem Cell Res Ther [Internet]. 2013. [cited 2017 Apr 4]; Available from: http://www.ncbi.nlm.nih.gov/pubmed/24307973. [PMC free article] [PubMed] [Google Scholar]
- [10].Roura S, Pujal J-M, Gálvez-Montón C, et al. The role and potential of umbilical cord blood in an era of new therapies: a review. Stem Cell Res Ther [Internet]. 2015. [cited 2016 Oct 25];6:123 Available from: http://stemcellres.com/content/6/1/123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Heidary Rouchi A, Mahdavi-Mazdeh M. Regenerative Medicine in Organ and Tissue Transplantation: Shortly and Practically Achievable? Int J organ Transplant Med [Internet]. 2015. [cited 2016 Oct 24];6:93–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26306154. [PMC free article] [PubMed] [Google Scholar]
- [12].Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. [DOI] [PubMed] [Google Scholar]
- [13].Overview G. T RANSLATIONAL AND C LINICAL R ESEARCH : M ESENCHYMAL S TEM C ELLS S ERIES Concise Review : Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. 2007;2886–2895. [DOI] [PubMed] [Google Scholar]
- [14].Moreira A, Kahlenberg S, Hornsby P. Therapeutic potential of mesenchymal stem cells for diabetes. J Mol Endocrinol [Internet]. 2017. [cited 2017 Nov 9];59:R109–R120. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28739632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Weiss ML, Troyer DL. Stem cells in the umbilical cord. Stem Cell Rev [Internet]. 2006. [cited 2016 Nov 21];2:155–162. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17237554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stavropoulos-Giokas C, Dinou A, Papassavas A, et al. The Role of HLA in Cord Blood Transplantation. Bone Marrow Res [Internet]. 2012. [cited 2016 Nov 21];2012:1–9. Available from: http://www.hindawi.com/journals/bmr/2012/485160/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Wakao S, Kuroda Y, Ogura F, et al. Regenerative Effects of Mesenchymal Stem Cells: Contribution of Muse Cells, a Novel Pluripotent Stem Cell Type that Resides in Mesenchymal Cells. Cells [Internet]. 2012. [cited 2017 Jan 16];1:1045–1060. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24710542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Sohni A, Verfaillie CM, Sohni A, et al. Mesenchymal stem cells migration homing and tracking. Stem Cells Int [Internet]. 2013. [cited 2016 Nov 21];2013:130763 Available from: http://www.ncbi.nlm.nih.gov/pubmed/24194766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Karp JM, Sock G, Teo L. Cell Stem Cell Mesenchymal Stem Cell Homing: The Devil Is in the Details. Stem Cell. 4:206–216. [DOI] [PubMed] [Google Scholar]
- [20].Caplan AI, Correa D. The MSC: an injury drugstore. Cell Stem Cell [Internet]. 2011. [cited 2017 Feb 16];9:11–15. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21726829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Caplan AI, Dennis JE. Mesenchymal Stem Cells as Trophic Mediators. J Cell Biochem J Cell Biochem. 2006;98:1076–1084. [DOI] [PubMed] [Google Scholar]
- [22].Arora S, Saha S, Roy S, et al. Role of Nonmuscle Myosin II in Migration of Wharton’s Jelly-Derived Mesenchymal Stem Cells. Stem Cells Dev [Internet]. 2015. [cited 2016 Nov 30];24:2065–2077. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25923805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jin F, Hagemann N, Schäfer ST, et al. SDF-1 restores angiogenesis synergistically with VEGF upon LDL exposure despite CXCR4 internalization and degradation. Cardiovasc Res. 2013;100. [DOI] [PubMed] [Google Scholar]
- [24].Cencioni C, Capogrossi MC, Napolitano M. The SDF-1/CXCR4 axis in stem cell preconditioning. Cardiovasc Res. 2012;94. [DOI] [PubMed] [Google Scholar]
- [25].Chen L, Tredget EE, Wu PYG, et al. Paracrine factors of mesenchymal stem cells recruit macrophages and endothelial lineage cells and enhance wound healing. PLoS One [Internet]. 2008. [cited 2016 Nov 21];3:e1886 Available from: http://www.ncbi.nlm.nih.gov/pubmed/18382669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Baraniak PR, McDevitt TC. Stem cell paracrine actions and tissue regeneration. Regen Med [Internet]. 2010. [cited 2016 Nov 21];5:121–143. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20017699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Huang S, Wu Y, Gao D, et al. Paracrine action of mesenchymal stromal cells delivered by microspheres contributes to cutaneous wound healing and prevents scar formation in mice. Cytotherapy [Internet]. 2015. [cited 2016 Oct 18];17:922–931. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25939802. [DOI] [PubMed] [Google Scholar]
- [28].Lee JW, Fang X, Krasnodembskaya A, et al. Concise Review: Mesenchymal Stem Cells for Acute Lung Injury: Role of Paracrine Soluble Factors. Stem Cells. 2011;29:913–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pires AO, Neves-Carvalho A, Sousa N, et al. The Secretome of Bone Marrow and Wharton Jelly Derived Mesenchymal Stem Cells Induces Differentiation and Neurite Outgrowth in SH-SY5Y Cells. Stem Cells Int [Internet]. 2014. [cited 2016 Nov 30];2014:438352 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25132857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Li D, Liu X, et al. Human umbilical cord mesenchymal stem cells reduce systemic inflammation and attenuate LPS-induced acute lung injury in rats. J Inflamm [Internet]. 2012. [cited 2016 Oct 19];9:33 Available from: http://journal-inflammation.biomedcentral.com/articles/10.1186/1476-9255-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fong C-Y, Tam K, Cheyyatraivendran S, et al. Human Wharton’s jelly stem cells and its conditioned medium enhance healing of excisional and diabetic wounds. J Cell Biochem [Internet]. 2014. [cited 2016 Nov 30];115:290–302. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24038311. [DOI] [PubMed] [Google Scholar]
- [32].Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol [Internet]. 2002. [cited 2016 Nov 22];2:569–579. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12154376. [DOI] [PubMed] [Google Scholar]
- [33].Hsu Y-C, Wu Y-T, Yu T-H, et al. Mitochondria in mesenchymal stem cell biology and cell therapy: From cellular differentiation to mitochondrial transfer. Semin Cell Dev Biol [Internet]. 2016. [cited 2016 Oct 18];52:119–131. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26868759. [DOI] [PubMed] [Google Scholar]
- [34].Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol [Internet]. 2014. [cited 2016 Nov 15];24:R453-62. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24845678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Zhang G, Zou X, Miao S, et al. The Anti-Oxidative Role of Micro-Vesicles Derived from Human Wharton-Jelly Mesenchymal Stromal Cells through NOX2/gp91(phox) Suppression in Alleviating Renal Ischemia-Reperfusion Injury in Rats. Camussi G, editor. PLoS One [Internet]. 2014. [cited 2016 Dec 1];9:e92129 Available from: http://dx.plos.org/10.1371/journal.pone.0092129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Arslan F, Lai RC, Smeets MB, et al. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013;10:301–312. [DOI] [PubMed] [Google Scholar]
- [37].Transplantation of human placenta derived mesenchymal stem cell alleviates critical limb ischemia in diabetic nude rat. Cell Transplant [Internet]. 2016 [cited 2016 Nov 29]; Available from: http://www.ingentaconnect.com/content/cog/ct/pre-prints/content-ct-1594_liang_et_al. [DOI] [PMC free article] [PubMed]
- [38].Edwards SS, Zavala G, Prieto CP, et al. Functional analysis reveals angiogenic potential of human mesenchymal stem cells from Wharton’s jelly in dermal regeneration. Angiogenesis [Internet]. 2014. [cited 2016 Oct 19];17:851–866. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24728929. [DOI] [PubMed] [Google Scholar]
- [39].Zhang J, Wu Y, Chen A, et al. Mesenchymal stem cells promote cardiac muscle repair via enhanced neovascularization. Cell Physiol Biochem [Internet]. 2015. [cited 2016 Nov 29];35:1219–1229. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25766532. [DOI] [PubMed] [Google Scholar]
- [40].Zhao Q, Ren H, Han Z. Mesenchymal stem cells: Immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother. 2016;2:3–20. [Google Scholar]
- [41].Vellasamy S, Tong CK, Azhar NA, et al. Human mesenchymal stromal cells modulate T-cell immune response via transcriptomic regulation. Cytotherapy [Internet]. 2016. [cited 2016 Nov 23];18:1270–1283. Available from: http://linkinghub.elsevier.com/retrieve/pii/S1465324916304510. [DOI] [PubMed] [Google Scholar]
- [42].Prasanna SJ, Gopalakrishnan D, Shankar SR, et al. Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One [Internet]. 2010. [cited 2016 Dec 1];5:e9016 Available from: http://www.ncbi.nlm.nih.gov/pubmed/20126406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Leibacher J, Henschler R, Friedenstein A, et al. Biodistribution, migration and homing of systemically applied mesenchymal stem/stromal cells. Stem Cell Res Ther [Internet]. 2016. [cited 2016 Nov 21];7:7 Available from: http://stemcellres.com/content/7/1/7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sotiropoulou PA, Perez SA, Gritzapis AD, et al. Interactions Between Human Mesenchymal Stem Cells and Natural Killer Cells. Stem Cells [Internet]. 2006. [cited 2016 Dec 1];24:74–85. Available from: http://doi.wiley.com/10.1634/stemcells.2004-0359. [DOI] [PubMed] [Google Scholar]
- [45].Jacob SV, Coates AL, Lands LC, et al. Long-term pulmonary sequelae of severe bronchopulmonary dysplasia. J Pediatr [Internet]. 1998. [cited 2017 Nov 9];133:193–200. Available from: http://www.ncbi.nlm.nih.gov/pubmed/9709705. [DOI] [PubMed] [Google Scholar]
- [46].Chang YS, Choi SJ, Sung DK, et al. Intratracheal Transplantation of Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Dose-Dependently Attenuates Hyperoxia-Induced Lung Injury in Neonatal Rats. 2011;20:1843–1854. [DOI] [PubMed] [Google Scholar]
- [47].Pierro M, Ionescu L, Montemurro T, et al. Short-term, long-term and paracrine effect of human umbilical cord-derived stem cells in lung injury prevention and repair in experimental bronchopulmonary dysplasia. Thorax [Internet]. 2013. [cited 2016 Dec 1];68:475–484. Available from: http://www.ncbi.nlm.nih.gov/pubmed/23212278. [DOI] [PubMed] [Google Scholar]
- [48].Chang YS, Ahn SY, Yoo HS, et al. Mesenchymal stem cells for bronchopulmonary dysplasia: phase 1 dose-escalation clinical trial. J Pediatr [Internet]. 2014. [cited 2017 Jan 16];164:966–972.e6. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24508444. [DOI] [PubMed] [Google Scholar]
- [49].Douglas-Escobar M, Weiss MD. Hypoxic-Ischemic Encephalopathy. JAMA Pediatr [Internet]. 2015. [cited 2017 Jan 26];169:397 Available from: http://www.ncbi.nlm.nih.gov/pubmed/25685948. [DOI] [PubMed] [Google Scholar]
- [50].Perlman JM. Pathogenesis of hypoxic-ischemic brain injury. J Perinatol [Internet]. 2007. [cited 2016 Oct 19];27:S39–S46. Available from: http://www.nature.com/doifinder/10.1038/sj.jp.7211716. [Google Scholar]
- [51].Kim ES, Ahn SY, Im GH, et al. Human umbilical cord blood–derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr Res [Internet]. 2012. [cited 2017 Jan 16];72:277–284. Available from: http://www.nature.com/doifinder/10.1038/pr.2012.71. [DOI] [PubMed] [Google Scholar]
- [52].Zhang X, Zhang Q, Li W, et al. Therapeutic effect of human umbilical cord mesenchymal stem cells on neonatal rat hypoxic-ischemic encephalopathy. J Neurosci Res [Internet]. 2014. [cited 2017 Jan 16];92:35–45. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24265136. [DOI] [PubMed] [Google Scholar]
- [53].Zhu L, Bai X, Zhang N, et al. Improvement of human umbilical cord mesenchymal stem cell transplantation on glial cell and behavioral function in a neonatal model of periventricular white matter damage. Brain Res [Internet]. 2014. [cited 2017 Jan 16];1563:13–21. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24680746. [DOI] [PubMed] [Google Scholar]
- [54].Cotten CM, Murtha AP, Goldberg RN, et al. Feasibility of autologous cord blood cells for infants with hypoxic-ischemic encephalopathy. J Pediatr [Internet]. 2014. [cited 2017 Jan 22];164:973–979.e1. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24388332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Seaberg RM, van der Kooy D. Stem and progenitor cells: the premature desertion of rigorous definitions. Trends Neurosci [Internet]. 2003. [cited 2017 Apr 24];26:125–131. Available from: http://www.sciencedirect.com/science/article/pii/S0166223603000316. [DOI] [PubMed] [Google Scholar]
- [56].Sirinoglu Demiriz I, Tekgunduz E, Altuntas F, et al. What Is the Most Appropriate Source for Hematopoietic Stem Cell Transplantation? Peripheral Stem Cell/Bone Marrow/Cord Blood. Bone Marrow Res [Internet]. 2012. [cited 2017 Apr 25];2012:1–5. Available from: http://www.hindawi.com/journals/bmr/2012/834040/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Danby R, Rocha V, Gálvez-Montón C, et al. Improving Engraftment and Immune Reconstitution in Umbilical Cord Blood Transplantation. Front Immunol [Internet]. 2014. [cited 2017 Apr 25];5:68 Available from: http://journal.frontiersin.org/article/10.3389/fimmu.2014.00068/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Gluckman E History of cord blood transplantation. Bone Marrow Transplant [Internet]. 2009. [cited 2017 Apr 25];44:621–626. Available from: http://www.nature.com/doifinder/10.1038/bmt.2009.280. [DOI] [PubMed] [Google Scholar]
- [59].Roberts G, Elza S, Ingūna L. Hypoplastic left heart syndrome: a review. Acta medica Litu [Internet]. 2016. [cited 2017 Apr 27];23:86–98. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28356795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yabrodi M, Mastropietro CW. Hypoplastic left heart syndrome: from comfort care to long-term survival . Off J Int Pediatr Res Found [Internet]. 2017. [cited 2017 Apr 27]; Available from: https://www.nature.com/pr/journal/v81/n1-2/pdf/pr2016194a.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Arnold RR, Loukanov T, Gorenflo M. Hypoplastic Left Heart Syndrome — Unresolved Issues. Front Pediatr [Internet]. 2014. [cited 2017 Apr 27];2:125 Available from: http://journal.frontiersin.org/article/10.3389/fped.2014.00125/abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Fruitman DS. Hypoplastic left heart syndrome: Prognosis and management options . Paediatr Child Health [Internet]. 2000. [cited 2017 Apr 27];5:219–225. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20177524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Ishigami S, Ohtsuki S, Tarui S, et al. Intracoronary Autologous Cardiac Progenitor Cell Transfer in Patients With Hypoplastic Left Heart Syndrome: The TICAP Prospective Phase 1 Controlled Trial. Circ Res [Internet]. 2015. [cited 2017 Apr 27];116:653–664. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25403163. [DOI] [PubMed] [Google Scholar]
- [64].Whitelaw A Core Concepts: Intraventricular Hemorrhage. Neoreviews [Internet] 2011. [cited 2017 Apr 28];12. Available from: http://neoreviews.aappublications.org/content/12/2/e94. [Google Scholar]
- [65].Ballabh P. Intraventricular hemorrhage in premature infants: mechanism of disease. Pediatr Res [Internet]. 2010. [cited 2017 Apr 28];67:1–8. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19816235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Ahn SY, Chang YS, Sung DK, et al. Mesenchymal stem cells prevent hydrocephalus after severe intraventricular hemorrhage. Stroke. 2013;44:497–504. [DOI] [PubMed] [Google Scholar]
- [67].Park WS, Sung SI, Ahn SY, et al. Optimal Timing of Mesenchymal Stem Cell Therapy for Neonatal Intraventricular Hemorrhage. Cell Transplant [Internet]. 2016. [cited 2017 Apr 25];25:1131–1144. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26440762. [DOI] [PubMed] [Google Scholar]
- [68].Ahn SY, Chang YS, Sung DK, et al. Cell type-dependent variation in paracrine potency determines therapeutic efficacy against neonatal hyperoxic lung injury. Cytotherapy [Internet]. 2015;17:1025–1035. Available from: 10.1016/j.jcyt.2015.03.008. [DOI] [PubMed] [Google Scholar]
- [69].Liu L, Mao Q, Chu S, et al. Intranasal versus Intraperitoneal Delivery of Human Umbilical Cord Tissue–Derived Cultured Mesenchymal Stromal Cells in a Murine Model of Neonatal Lung Injury. Am J Pathol [Internet] 2014. [cited 2016 Dec 1];184:3344–3358. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25455688. [DOI] [PubMed] [Google Scholar]
- [70].Sung DK, Chang YS, Ahn SY, et al. Optimal Route for Human Umbilical Cord Blood-Derived Mesenchymal Stem Cell Transplantation to Protect Against Neonatal Hyperoxic Lung Injury: Gene Expression Profiles and Histopathology. Almeida-Porada GD, editor. PLoS One [Internet]. 2015. [cited 2016 Dec 1];10:e0135574 Available from: http://www.ncbi.nlm.nih.gov/pubmed/26305093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Chang YS, Choi SJ, Ahn SY, et al. Timing of Umbilical Cord Blood Derived Mesenchymal Stem Cells Transplantation Determines Therapeutic Efficacy in the Neonatal Hyperoxic Lung Injury Covas DT, editor. PLoS One [Internet]. 2013. [cited 2016 Dec 1];8:e52419 Available from: http://www.ncbi.nlm.nih.gov/pubmed/23349686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Chang YS, Ahn SY, Jeon HB, et al. Critical Role of Vascular Endothelial Growth Factor Secreted by Mesenchymal Stem Cells in Hyperoxic Lung Injury. Am J Respir Cell Mol Biol [Internet]. 2014. [cited 2016 Dec 1];51:391–399. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24669883. [DOI] [PubMed] [Google Scholar]
- [73].Xia G, Hong X, Chen X, et al. Intracerebral transplantation of mesenchymal stem cells derived from human umbilical cord blood alleviates hypoxic ischemic brain injury in rat neonates. J Perinat Med [Internet]. 2010. [cited 2016 Dec 1];38:215–221. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20121545. [DOI] [PubMed] [Google Scholar]


