Abstract
Background and study aims Esophagogastroduodenoscopy (EGD) has utility in early detection of upper gastrointestinal (UGI) neoplasms. However, previous studies report shorter inspection times and inexperienced endoscopists contribute to overlooking gastric neoplasms. We investigated neoplasm detection rates according to inspection time and extent of EGD training.
Patients and methods In this retrospective observational study, we reviewed routine EGDs for 3,925 consecutive cases between October 2014 and March 2015. We divided the endoscopists into three groups based on median inspection time during EGD without undergoing biopsy. Using cut-off median inspection times of 7 and 10 minutes, three, five, and eight endoscopists were classified into the fast, moderate, and slow groups, respectively. We compared detection rates according to inspection time and the extent of EGD training.
Results The median inspection time among all endoscopists was 9.3 minutes (range, 6.6 – 12.0 min). The detection rate for UGI neoplasms was as follows: fast group, 3.6%; moderate group, 3.3 %; and slow group, 3.1 % ( P = 0.807). The median inspection time was significantly shorter among the intensive training ≥ 1-year group than among the < 1-year group (< 1-year: median 6.3 min; range 8.2 – 13.9 min, ≥ 1-year: median 8.9 min; range 6.4 – 11.4 min, P < 0.001). The detection rate for UGI neoplasms was significantly higher among the intensive training ≥ 1-year group than among the < 1-year group (< 1-year: 2.2 %; ≥ 1-year: 3.7 %, OR = 1.65, 95 % CI: 1.02 – 2.68, P = 0.041).
Conclusions There was no association between inspection times and neoplasm detection rates. The quality of EGD, as measured by neoplasm detection rates, may be improved by ≥ 1-year of intensive training.
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of death from cancer worldwide 1 2 . Early detection is ideal for optimum patient survival, with esophagogastroduodenoscopy (EGD) being the most sensitive method for early detection 2 3 . Several studies have demonstrated the effectiveness of endoscopy screening for gastric cancer, with a 30 % reduction in gastric cancer mortality as a result of cancer detection attributed to endoscopy screening 4 5 6 7 . Since then, in Korea and Japan, use of EGD has been allowed for gastric cancer screening in the national screening program 8 9 . Furthermore, EGD is also suggested to be effective for early detection of additional upper gastrointestinal (UGI) neoplasms, such as hypopharynx, esophageal, and duodenal tumors 10 11 . Although EGD is the standard procedure for diagnosing gastric cancer, inexperienced endoscopists tend to overlook gastric cancer due to subtle morphological changes in some early gastric cancer lesions, which are difficult to distinguish from background mucosa with atrophic change 12 13 14 . As a result, the false-negative rate for detecting gastric cancer with EGD is 4.6 % to 25.8 % 12 15 16 17 18 19 . These findings indicate the importance of quality indicators (QI) for UGI neoplasm detection in EGD.
Several studies have reported quality indicator outcome measures in EGD 20 21 22 . A few previous studies found an association between inspection time during EGD and detection rates for UGI neoplasms. Results of those studies have shown that slower endoscopists detected a higher proportion of UGI neoplasms than faster endoscopists, suggesting that inspection time may be a useful quality indicator in EGD 23 24 25 . Conversely, other previous studies found that endoscopists require sufficient training and experience to detect gastric cancer properly 12 13 14 . We thus investigated inspection time and endoscopic training and experience to clarify quality improvement for UGI neoplasm detection in EGD.
Patients and methods
Study design
In this single-center, retrospective observational study, we reviewed routine EGDs for 5,091 consecutive cases at the Cancer Institute Hospital between October 2014 and March 2015. We selected this period because there was no change in the staff of endoscopists. We excluded 464 cases with EGD history within 6 months, 369 cases with endoscopic assessment by a clinician with less than 3 years of experience, 129 cases with endoscopic assessment by a clinician who performed fewer than 50 EGDs in this period, 67 cases of total gastrectomy surgery, 55 cases lacking information on stomach examination, 46 cases with residual food in the stomach, and 36 cases with ultrathin endoscopy examinations. Before undergoing EGD, all patients provided comprehensive written informed consent . The institutional review board of the Cancer Institute Hospital of the Japanese Foundation for Cancer Research approved this study (IRB no. 2016-1158) ( Fig. 1 ).
Fig. 1.
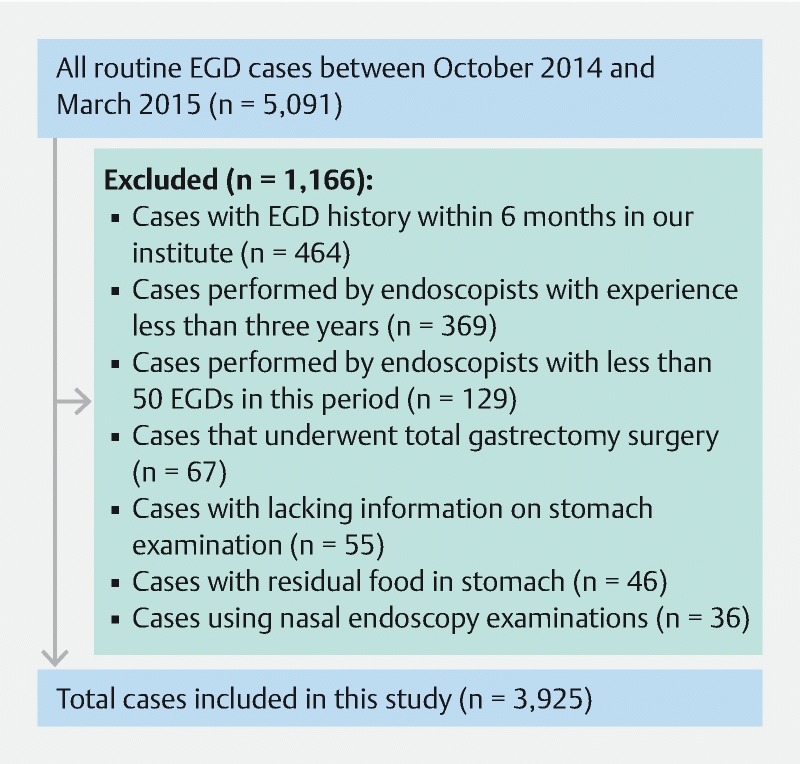
Flowchart of routine EGD cases.
Routine EGD
Routine EGDs were performed from the hypopharynx to the horizontal part of the duodenum. Approximately 60 images were captured, including about 20 images taken in the hypopharynx and esophagus, and about 40 taken in the stomach and duodenum. Digital images were automatically saved in the endoscopic filing system. Two imaging modalities were alternated with conventional white-light imaging (C-WLI) and narrow-band imaging (NBI) when using the endoscope. NBI was used during withdrawal from the esophagus. The video processor was consistently set as follows. The structure enhancement function was set at the A5 or B8 level for C-WLI, with the color mode fixed at level 0. All procedures were carried out using an endoscope manufactured by Olympus Corporation (GIF-H260 or GIF-H290Z, Olympus Medical Systems, Tokyo, Japan) and a standard endoscopic video system (EVIS LUCERA ELITE or EVIS LUCERA SPECTRUM, Olympus Medical Systems).
As a preliminary measure prior to endoscopy screening, pharyngeal anesthesia was administered using a lidocaine viscous solution. Almost all patients underwent endoscopy under intravenous (IV) sedation, except for those with no preference for anesthesia. In accordance with the guidelines regarding sedation for gastroenterological endoscopy 26 , midazolam (2.0 – 5.0 mg) was administered IV, occasionally with pethidine hydrochloride (35 mg) added for patients who were insufficiently sedated. When gastric or duodenal neoplasms were detected, a biopsy was performed after observation following NBI and dye spraying endoscopy with the application of indigo carmine. When esophageal neoplasms were detected, a biopsy was performed after observation following NBI and iodine spraying.
Procedure and outcome parameters
Inspection time was defined as the time from first image capture in the pharynx to scope removal. We divided endoscopists into three groups based on median inspection time during EGD without undergoing biopsy. The cut-off median inspection times were 7 and 10 minutes (min). The three groups were as follows: fast speed endoscopists (fast group: median inspection time, < 7 min), moderate speed endoscopists (moderate group: median inspection time, ≥ 7 min and < 10 min), and slow speed endoscopists (slow group: median inspection time, ≥ 10 min).
The reason for the EGD was divided into screening and surveillance endoscopy. Screening endoscopy was defined as an inspection for cases without history of UGI neoplasms, such as examination of symptoms and a double cancer check before cancer treatment in another organ. Surveillance endoscopy was defined as periodic examination of cases with surgical and endoscopic treatment for UGI neoplasms. The Kimura-Takemoto classification is generally used for evaluating gastric mucosal atrophy 27 . We classified patients judged to have C-1 atrophic change or higher as atrophic gastritis regardless of presence of Helicobacter pylori .
UGI neoplasms were defined as hypopharyngeal cancer, esophageal cancer, gastric adenoma/cancer, gastric carcinoid, duodenal adenoma/cancer, and duodenal carcinoid. Gastric neoplasms were defined as gastric adenoma, cancer, and carcinoid. We analyzed the characteristics, biopsy rate, and detection rate in the three groups. The detection rate was calculated as: Detection rate = number of cases in which neoplasms were detected / number of routine EGDs. Furthermore, we compared the detection rate by duration of intensive training at the Cancer Institute Hospital.
Intensive endoscopic training in the Cancer Institute Hospital
The Cancer Institute Hospital is a famous high-volume center in Japan, which carries out 12,000 routine EGDs, approximately 450 gastric endoscopic submucosal dissections (ESD), and approximately 200 esophageal ESDs per year. Among the total 12,000 routine EGDs, each trainee can carry out more than 1000 routine EGDs. All patients planned for ESD and surgery undergo preoperative EGD in our hospital as well as a preoperative conference before treatment. Therefore, all trainees have opportunities to learn the endoscopic characteristics and features of approximately 550 surgeries and 450 ESD cases of gastric neoplasm, and approximately 100 surgeries and 200 ESD cases of esophageal neoplasm each year. Thus, intensive training in performing an EGD, endoscopic diagnosis, and treatment of UGI neoplasms are possible among the trainees.
Statistical analysis
Statistical analysis was carried out using SPSS software, version 24.0 (SPSS, Chicago, Illinois, United States). We compared categorical parameters using the chi-squared test and continuous parameters using the Mann-Whitney U-test and the Kruskal-Wallis test. P values < 0.05 were determined to be statistically significant.
Results
Characteristics of endoscopists
All included routine EGDs were carried out by 16 endoscopists. Each endoscopist had carried out a minimum of 1000 EGDs and had ≥ 3 years of experience before initiation of this study. Eleven were certified by the Japan Gastroenterological Endoscopy Society. A total of 1,931 of 3,925 EGDs (49.2 %) were normal without undergoing biopsy. Median inspection time during EGD without undergoing biopsy was 9.3 minutes (interquartile range, 6.6 – 12.0 min). Three endoscopists were classified into the fast group (median 6.3 min; range, 5.0 – 7.5 min), five into the moderate group (median 9.0 min; range, 7.0 – 11.1 min), and eight into the slow group (median 11.8 min; range, 9.0 – 14.7 min), based on the aforementioned cut-off median inspection times. All endoscopists in the fast group were experts who had intensive training for ≥ 5 years. Other endoscopist characteristics are shown in Table 1 .
Table 1. Number of EGDs, training period, inspection time, and neoplasm detection rates by endoscopists.
| Detection rate (%) | |||||||||
| Endoscopist | Certified 1 | Intensive training (year) | Median time of group (min) 2 | Median time of endoscopist (min) 2 | No. of EGDs | Biopsy rate (%) | UGI neoplasms | Gastric neoplasms | |
| Fast-speed endoscopists group (n = 1,052) | A | + | ≥ 5 | 6.3 (5.0 – 7.5) | 6.2 (5.1 – 7.2) | 406 | 42.6 | 11 (2.7) | 9 (2.2) |
| B | + | ≥ 5 | 6.3 (4.7 – 7.9) | 435 | 55.6 | 16 (3.7) | 12 (2.8) | ||
| C | + | ≥ 5 | 6.9 (4.7 – 9.1) | 211 | 69.2 | 11 (5.2) | 10 (4.7) | ||
| Moderate-speed endoscopists group (n = 1,314) | D | + | 0.5 | 7.9 (5.3 – 10.5) | 132 | 43.9 | 3 (2.3) | 0 (0) | |
| E | – | 1 | 8.4 (6.9 – 10.0) | 587 | 52.0 | 22 (3.7) | 15 (2.6) | ||
| F | + | 2 | 9.0 (7.1 – 11.0) | 9.3 (6.7 – 12.0) | 185 | 42.2 | 5 (2.7) | 4 (2.2) | |
| G | – | 0.5 | 9.8 (7.9 – 11.7) | 173 | 46.2 | 5 (2.9) | 3 (1.7) | ||
| H | + | ≥ 5 | 9.9 (8.3 – 11.7) | 236 | 49.6 | 9 (3.8) | 5 (2.1) | ||
| Slow-speed endoscopists group (n = 1,559) | I | + | ≥ 5 | 11.8 (9.0 – 14.7) | 10.1 (7.5 – 12.8) | 170 | 35.9 | 6 (3.5) | 5 (2.9) |
| J | + | 2 | 10.9 (9.1 – 12.7) | 169 | 51.5 | 7 (4.1) | 5 (3.0) | ||
| K | + | ≥ 5 | 11.2 (8.8 – 13.6) | 148 | 55.4 | 6 (4.1) | 3 (2.0) | ||
| L | – | 0.5 | 11.7 (8.2 – 15.1) | 278 | 53.6 | 5 (1.8) | 4 (1.4) | ||
| M | + | 1.5 | 11.7 (9.5 – 13.9) | 200 | 56.5 | 11 (5.5) | 8 (4.0) | ||
| N | – | 0.5 | 12.3 (9.3 – 14.9) | 160 | 70.0 | 4 (2.5) | 4 (2.5) | ||
| O | – | 0.5 | 12.7 (8.8 – 15.2) | 148 | 45.9 | 3 (2.0) | 2 (1.4) | ||
| P | + | 1 | 13.3 (10.0 – 16.5) | 287 | 42.9 | 7 (2.4) | 4 (1.4) | ||
| Total or Median | 16 | 11 | 9.3 (6.6 – 12.0) | 3925 | 50.8 | 131 (3.3) | 93 (2.4) | ||
EGD, esophagogastroduodenoscopy; UGI, upper gastrointestinal neoplasms.
Certified by the Japan Gastroenterological Endoscopy Society
Inspection time of EGD without biopsy cases, median (interquartile range)
Characteristics of routine EGD between the fast- vs moderate- vs slow-speed endoscopist groups
Among 5,091 consecutive routine EGDs, 3,925 EGDs satisfying the inclusion criteria were analyzed in this study. Characteristics of routine EGDs in three groups are shown in Table 2 . There was no significant difference in age and sex among the three groups. The interval between EGDs was ≤ 1.5 years in approximately 75 % of cases. Approximately half of EGDs were conducted for screening purposes, and half for surveillance purposes in each group. The prevalence of atrophic gastritis was > 70 % in each group. Approximately 35 % of cases had a history of gastric cancer; approximately 10 % of cases had a history of esophageal cancer. No significant differences were identified among the three groups in terms of endoscopic system used. The total UGI biopsy rate showed no significant difference among the fast group (53 %), the moderate group (49 %), and the slow group (51 %) ( P = 0.075). The proportion of endoscopists with ≥ 1-year intensive training in the Fast group was significantly higher than those in the other two groups (fast group: 100 %; moderate group: 77 %; slow group: 63 %, P < 0.001).
Table 2. Characteristics of routine EGDs between the fast- vs moderate- vs slow-speed endoscopist groups.
| Fast-speed endoscopists group (n = 1,052) | Moderate-speed endoscopists group (n = 1,314) | Slow-speed endoscopists group (n = 1,559) | P value | |
| Age, median (range) | 67 (18 – 92) | 67 (20 – 93) | 68 (18 – 92) | 0.321 |
| Sex (%) male/female | 649 (62)/403 (38) | 833 (63)/481 (37) | 649 (62)/403 (38) | 0.460 |
| Interval between EGDs (%) | 0.215 | |||
|
827 (79) | 1010 (77) | 1230 (79) | |
|
162 (15) | 195 (15) | 226 (14) | |
|
63 (6) | 109 (8) | 103 (7) | |
| Purpose of EGD (%) | ||||
|
530 (50)/522 (50) | 600 (46)/714 (54) | 762 (49)/797 (51) | 0.107 |
| Gastric mucosal atrophy (%) | ||||
|
733 (70)/319 (30) | 970 (74)/344 (26) | 1113 (71)/446 (29) | 0.078 |
| Past history of gastric cancer (%) | ||||
|
352 (33)/700 (67) | 471 (36)/ 843 (64) | 569 (36)/990 (64) | 0.265 |
| Past history of esophageal cancer (%) | ||||
|
123 (12)/929 (88) | 135 (10)/1179 (90) | 194 (12)/1365 (88) | 0.188 |
| Endoscope model (%) | ||||
|
487 (46)/565 (54) | 571 (43)/743 (57) | 741 (47)/818 (53) | 0.087 |
| Light source model (%) | ||||
|
487 (46)/565 (54) | 571 (43)/743 (57) | 718 (46)/841 (54) | 0.275 |
| UGI biopsy cases (%) Done / Not done | 561 (53)/491 (47) | 639 (49)/675 (51) | 794 (51)/765 (49) | 0.075 |
| Intensive training (%) | ||||
| < 1-year/ ≥ 1-year | 0 (0)/1052 (100) | 306 (23)/1008 (77) | 585 (37)/974 (63) | < 0.001 |
EGD, esophagogastroduodenoscopy
Neoplasm detection rate for fast vs moderate vs slow groups
Neoplasm detection rates are shown in Table 3 . The neoplasm detection rate showed no significant difference among the three groups. The detection rate for UGI neoplasms was as follows: fast group, 3.6 %; moderate group, 3.3 %; slow group, 3.1 % ( P = 0.807). The detection rate for gastric neoplasms was as follows: fast group, 2.9 %; moderate group, 2.1 %; slow group, 2.2 % ( P = 0.336).
Table 3. Comparison of neoplasm detection rates between the fast- vs moderate- vs slow-speed endoscopist groups.
| Fast-speed endoscopists group (n = 1,052) | Moderate-speed endoscopists group (n = 1,314) | Slow-speed endoscopists group (n = 1,559) | P value | |
| Detection rate for UGI neoplasms (%) | 38 (3.6) | 44 (3.3) | 49 (3.1) | 0.807 |
| Detection rate for gastric neoplasms (%) | 31 (2.9) | 27 (2.1) | 35 (2.2) | 0.336 |
UGI, upper gastrointestinal neoplasms
As shown in Table 4 , 133 neoplasms (131 cases; two cases had both esophageal cancer and gastric cancer) (hypopharynx cancer: seven cases; esophageal cancer: 24 cases; early gastric cancer: 68 cases; gastric adenoma: 24 cases; gastric carcinoid: one case; duodenal cancer: two cases; duodenal adenoma: six cases; duodenal carcinoid: one case) were detected in this study.
Table 4. Characteristics of the all detected neoplasms in the fast- vs moderate- vs Slow-speed endoscopist groups.
| Fast-speed endoscopists group (n = 1,052) | Moderate-speed endoscopists group (n = 1,314) | Slow-speed endoscopists group (n = 1,559) | Total (n = 3,925) | |
| UGI neoplasms (%) | 38 (3.6) 1 | 44 (3.3) | 49 (3.1) | 131 (3.3) |
| Hypopharynx cancer (%) | 0 (0) | 4 (0.30) | 3 (0.19) | 7 (0.18) |
| Esophageal cancer (%) | 8 (0.76) | 9 (0.67) | 7 (0.45) | 24 (0.61) |
| Gastric neoplasm (%) | 31 (2.9) | 27 (2.1) | 35 (2.2) | 93 (2.4) |
| Gastric cancer (%) | 24 (2.3) | 20 (1.5) | 24 (1.5) | 68 (1.7) |
| Gastric adenoma (%) | 7 (0.67) | 6 (0.46) | 11 (0.71) | 24 (0.61) |
| Gastric carcinoid (%) | 0 (0) | 1 (0.08) | 0 (0) | 1 (0.03) |
| Duodenal cancer (%) | 0 (0) | 1 (0.08) | 1 (0.06) | 2 (0.05) |
| Duodenal adenoma (%) | 1 (0.10) | 2 (0.15) | 3 (0.19) | 6 (0.15) |
| Duodenal carcinoid (%) | 0 (0) | 1 (0.08) | 0 (0) | 1 (0.03) |
UGI, upper gastrointestinal
Two cases in the fast-speed endoscopists group had double cancers of esophageal cancer and gastric cancer.
Characteristics of routine EGD between the intensive training < 1-year vs ≥ 1-year groups
Characteristics of routine EGDs are shown in Table 5 . Five endoscopists were classified as having intensive training < 1 year and 11 as having ≥ 1 year, based on duration of intensive training at the Cancer Institute Hospital. The proportion of history of gastric cancer among the Intensive training < 1-year group was significantly higher than that among the Intensive training ≥ 1-year group (< 1-year group: 40 %; ≥ 1-year: 34 %; P = 0.001). The median inspection times among the Intensive training ≥ 1-year group were significantly shorter than that among the Intensive training < 1-year group (< 1-year group: median 6.3 min; range 8.2 – 13.9 min; ≥ 1-year group: median 8.9 min; range 6.4 – 11.4 min, P < 0.001). The other parameters were not different between the two groups.
Table 5. Characteristics of routine EGD between the Intensive training < 1-year vs ≥ 1-year groups.
| Intensive training < 1-year group (n = 891) | Intensive training ≥ 1-year group (n = 3,034) | P value | |
| Age, median (range) | 67 (21 – 88) | 67 (18 – 93) | 0.521 |
| Sex (%)Male/female | 542 (61)/349 (39) | 1894 (62)/1140 (38) | 0.388 |
| Interval between EGDs (%) | 0.606 | ||
|
699 (79) | 2368 (78) | |
|
135 (15) | 447 (15) | |
|
56 (6) | 219 (7) | |
| Purpose of EGD (%) | |||
|
405 (46)/486 (54) | 1479 (49)/1555 (51) | 0.084 |
| Gastric mucosal atrophy (%) | |||
|
647 (73)/244 (27) | 2169 (72)/865 (28) | 0.512 |
| Past history of gastric cancer (%) | |||
|
359 (40)/532 (60) | 1033 (34)/2001 (66) | 0.001 |
| Past history of esophageal cancer (%) | |||
|
91 (10)/800 (90) | 361 (12)/2673 (88) | 0.166 |
| Endoscope model (%) | |||
|
432 (49)/459 (51) | 1367 (45)/1667 (55) | 0.071 |
| Light source model (%) | |||
|
425 (48)/466 (52) | 1351 (44)/1683 (56) | 0.095 |
| UGI biopsy cases (%) Done/Not done | 467 (52)/424 (48) | 1527 (50)/1507 (50) | 0.274 |
| Inspection time, min, median (range 1 ) | 11.0 (8.2 – 13.9) | 8.9 (6.4 – 11.4) | < 0.001 |
EGD, esophagogastroduodenoscopy
Interquartile range
Comparison of neoplasm detection rates between Intensive training < 1-year vs ≥ 1-year group
As shown in Table 6 , detection rates for UGI neoplasms and gastric neoplasms in the Intensive training ≥ 1-year group were significantly higher than that in the Intensive training < 1-year group (UGI neoplasms; < 1-year: 2.2 %; ≥ 1-year: 3.7 %, odds ratio = 1.65, 95 % CI: 1.02 – 2.68, P = 0.041; gastric neoplasms; < 1-year: 1.5 %; ≥ 1-year: 2.6 %, odds ratio = 1.83, 95 % CI: 1.01 – 3.30, P = 0.045).
Table 6. Comparison of UGI neoplasm detection rates between the Intensive training < 1-year vs ≥ 1-year groups.
| Detection rate (%) | Odds ratios (95 % CI) | P value | |
| Detection for UGI neoplasms (%) | |||
| Intensive training < 1-year group | 20 (2.2) | 1.0 (Reference) | |
| Intensive training ≥ 1-year group | 111 (3.7) | 1.65 (1.02 – 2.68) | 0.041 |
| Detection for gastric neoplasms (%) | |||
| Intensive training < 1-year group | 13 (1.5) | 1.0 (Reference) | |
| Intensive training ≥ 1-year group | 80 (2.6) | 1.83 (1.01 – 3.30) | 0.045 |
UGI, upper gastrointestinal
Discussion
Early detection of UGI neoplasms is ideal for ensuring optimum patient survival. Furthermore, EGD is the most sensitive method for early detection. Although quality control of EGD is necessary in early detection of UGI neoplasms, few studies have assessed quality indicators in EGD. Several quality indicators have been identified in colonoscopy for colorectal cancer screening, such as adenoma detection rate (ADR), inspection time, and surveillance intervals 28 29 30 . Measuring the ADR of individual colonoscopists is a priority in the quality improvement process for colonoscopy 30 ; the same is also true in EGD. However, the detection rate can be affected by prevalence, so simply comparing the detection rate in each hospital is not a good method. Prevalence also varies widely based on geographic location, race, and socioeconomic status 31 . Especially, the predominant risk factor for gastric cancer is Helicobacter pylori infection, and major risk factors for esophageal squamous cell cancer include consumption of alcohol and smoking. Thus, in the current study, we compared the detection rate in the same hospital, as there is no associated bias with prevalence.
Previous studies have shown that slow-speed endoscopists had a higher detection rate for UGI neoplasms than fas- speed endoscopists 23 24 25 . In this study, the neoplasm detection rates showed no significant difference among the fast, moderate, and slow groups. There was no association between inspection time and neoplasm detection rates. However, median inspection time in EGD without undergoing biopsy was 9.3 minutes, which was longer than that in previous reports. Moreover, median inspection time in the fast group was 6.3 minutes, and even the fastest endoscopist had a median inspection time ≥ 6 minutes. Meanwhile, previous reports have indicated that inspection time is a quality indicator for EGD; Kawamura et al. 23 (fast-speed endoscopist group mean inspection time, 4.4 min), Teh et al. 24 (fast-speed endoscopist group mean inspection time, 5.5 min), and Park et al. 25 (fast-speed endoscopist group mean inspection time, 2.38 minutes, defined as the time from when the endoscope reached the duodenum to the time it was withdrawn) have suggested that EGD be carried out with a shorter inspection time than that seen in this study. Thus, according to the cut-off inspection times shown in previous reports, the endoscopists classified as fast in this study are actually moderate or slow. This may indicate that the fast endoscopists in this study (median inspection time ≥ 6 min) had a suitable inspection time to decrease the likelihood of overlooking UGI neoplasms. However, all endoscopists in the fast group in this study were expert endoscopists who had sufficient experience (intensive training at our hospital for ≥ 5-years).
In terms of endoscopic training and experience, previous studies reported that inexperienced endoscopists tend to overlook gastric cancer 12 13 14 . This means that endoscopists require sufficient training to detect gastric cancer properly. In this study, although all trainees had experienced more than 1000 EGDs in another hospital, the detection rate for UGI and gastric neoplasms by endoscopists with ≥ 1-year intensive training was significantly higher than that by endoscopists with < 1-year intensive training. In addition, endoscopists with ≥ 1-year intensive training have significantly shorter inspection times than endoscopists with < 1-year intensive training. Thus, intensive training for ≥ 1 year made it possible to detect more neoplasms within a shorter examination time. This indicates that the quality of EGD, as measured by neoplasm detection rates, may be improved by ≥ 1-year intensive training. Each trainee at our hospital can carry out approximately 1000 EGDs each year. Moreover, each trainee has opportunities to learn from the endoscopic findings of approximately 1000 cases of gastric neoplasm, and 300 cases of esophageal cancer each year. To improve the detection rate, it is also necessary to gain experience identifying many tumor characteristics and features in addition to technical training in EGD.
In this study, the biopsy rate (50.8 %) was slightly high, which may be because we tend to perform many biopsies, as it is a cancer specialty hospital visited by many patients with a high cancer risk. Approximately 35 % of cases showed gastric cancer history, approximately 10 % of cases showed esophageal cancer history, and more than 70 % of cases showed atrophic gastritis, which has a high risk of progressing to gastric cancer. In these patients, we usually perform more careful examinations to avoid overlooking small lesions. Accordingly, nearly all neoplasms found in this study were determined to be early-stage cancer.
The current study had some limitations. First, it was a non-randomized, retrospective, single-center study. Second, this study included a small number of cases. Third, a patient-selection bias might have been present. To reduce selection bias, we included all consecutive EGDs performed in a period where the staff of endoscopists was unchanged. Further, we planned the study after the case selection period. Therefore, we believe information bias from the endoscopists is relatively low. However, future prospective study with randomization in multiple centers is necessary to validate our findings.
Conclusion
In conclusion, there was no association between inspection time and UGI neoplasm detection rates. Endoscopists with ≥ 1-year intensive training have significantly higher UGI neoplasm detection rates than endoscopists with < 1 year of intensive training, although inspection times were significantly shorter. Accordingly, our results demonstrated that the quality of EGD, as measured by UGI neoplasm detection rates, may be improved by intensive training.
Footnotes
Competing interests None
References
- 1.Ferlay J, Soerjomataram I, Dikshit R et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Can. 2015;135:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Sano T, Coit D G, Kim H H et al. Proposal of a new stage grouping of gastric cancer for TNM classification: International Gastric Cancer Association staging project. Gastric Cancer. 2017;20:217–225. doi: 10.1007/s10120-016-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katai H, Ishikawa T, Akazawa K et al. Five-year survival analysis of surgically resected gastric cancer cases in Japan: a retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001–2007) Gastric Cancer. 2018;21:144–154. doi: 10.1007/s10120-017-0716-7. [DOI] [PubMed] [Google Scholar]
- 4.Hamashima C, Shabana M, Okada K et al. Mortality reduction from gastric cancer by endoscopic and radiographic screening. Cancer Sci. 2015;106:1744–1749. doi: 10.1111/cas.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamashima C, Ogoshi K, Narisawa R et al. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol. 2015;21:2460–2466. doi: 10.3748/wjg.v21.i8.2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsumoto S, Yoshida Y. Efficacy of endoscopic screening in an isolated island: a case-control study. Indian J Gastroenterol. 2014;33:46–49. doi: 10.1007/s12664-013-0378-2. [DOI] [PubMed] [Google Scholar]
- 7.Hamashima C, Ogoshi K, Okamoto M et al. A community-based, case-control study evaluating mortality reduction from gastric cancer by endoscopic screening in Japan. PLoS ONE. 2013;8:E79088. doi: 10.1371/journal.pone.0079088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hamashima C, Shibuya D, Yamazaki H et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38:259–267. doi: 10.1093/jjco/hyn017. [DOI] [PubMed] [Google Scholar]
- 9.Lee K S, Oh D K, Han M A et al. Gastric cancer screening in Korea: report on the national cancer screening program in 2008. Cancer Res Treat. 2011;43:83–88. doi: 10.4143/crt.2011.43.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muto M, Minashi K, Yano T et al. Early detection of superficial squamous cell carcinoma in the head and neck region and esophagus by narrow band imaging: a multicenter randomized controlled trial. J Clin Oncol. 2010;28:1566–1572. doi: 10.1200/JCO.2009.25.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goda K, Kikuchi D, Yamamoto Y et al. Endoscopic diagnosis of superficial non-ampullary duodenal epithelial tumors in Japan: multicenter case series. Dig Endosc. 2014;26:23–29. doi: 10.1111/den.12277. [DOI] [PubMed] [Google Scholar]
- 12.Hosokawa O, Hattori M, Douden K et al. Difference in accuracy between gastroscopy and colonoscopy for detection of cancer. Hepatogastroenterology. 2007;54:442–444. [PubMed] [Google Scholar]
- 13.Yamazato T, Oyama T, Yoshida T et al. Two years' intensive training in endoscopic diagnosis facilitates detection of early gastric cancer. Intern Med. 2012;51:1461–1465. doi: 10.2169/internalmedicine.51.7414. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Chen Z Y, Chen C D et al. Training in early gastric cancer diagnosis improves the detection rate of early gastric cancer: an observational study in China. Medicine. 2015;94:E384. doi: 10.1097/MD.0000000000000384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Melo A R, Soares M, Libanio D et al. Missing rate for gastric cancer during upper gastrointestinal endoscopy: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol. 2016;28:1041–1049. doi: 10.1097/MEG.0000000000000657. [DOI] [PubMed] [Google Scholar]
- 16.Raftopoulos S C, Segarajasingam D S, Burke V et al. A cohort study of missed and new cancers after esophagogastroduodenoscopy. Am J Gastroenterol. 2010;105:1292–1297. doi: 10.1038/ajg.2009.736. [DOI] [PubMed] [Google Scholar]
- 17.Yalamarthi S, Witherspoon P, McCole D et al. Missed diagnoses in patients with upper gastrointestinal cancers. Endoscopy. 2004;36:874–879. doi: 10.1055/s-2004-825853. [DOI] [PubMed] [Google Scholar]
- 18.Vradelis S, Marchnard N, Warren B F et al. Quality control in upper gastrointestinal endoscopy: detection rates of gastric cancer in Oxford 2005 – 2008. Postgrad Med J. 2011;87:335–339. doi: 10.1136/pgmj.2010.101832. [DOI] [PubMed] [Google Scholar]
- 19.Menon S, Trudgill N. How commonly is upper gastrointestinal cancer missed at endoscopy? A 276 meta-analysis. Endosc Int Open. 2014;2:E46–E50. doi: 10.1055/s-0034-1365524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bisschops R, Areia M, Coron E et al. Performance measures for upper gastrointestinal endoscopy: a European Society of Gastrointestinal Endoscopy (ESGE) Quality Improvement Initiative. Endoscopy. 2016;48:843–864. doi: 10.1055/s-0042-113128. [DOI] [PubMed] [Google Scholar]
- 21.East J E, Vleugels J L, Roelandt P et al. Advanced endoscopic imaging: European Society of Gastrointestinal Endoscopy (ESGE) Technology Review. Endoscopy. 2016;48:1029–1045. doi: 10.1055/s-0042-118087. [DOI] [PubMed] [Google Scholar]
- 22.Rutter M D, Senore C, Bisschops R et al. The European Society of Gastrointestinal Endoscopy Quality Improvement Initiative: developing performance measures. Endoscopy. 2016;48:81–89. doi: 10.1055/s-0035-1569580. [DOI] [PubMed] [Google Scholar]
- 23.Kawamura T, Wada H, Sakiyama N et al. Inspection time as a quality indicator of screening upper gastrointestinal endoscopy for asymptomatic examinees. Dig Endosc. 2017;29:569–575. doi: 10.1111/den.12804. [DOI] [PubMed] [Google Scholar]
- 24.Teh J L, Tan J R, Lau L J et al. Longer inspection time improves detection of gastric cancer during diagnostic upper gastrointestinal endoscopy. Clin Gastroenterol Hepatol. 2015;13:480–487. doi: 10.1016/j.cgh.2014.07.059. [DOI] [PubMed] [Google Scholar]
- 25.Park J M, Huo S M, Lee H H et al. Longer observation time increases proportion of neoplasms detected by esophagogastroduodenoscopy. Gastroenterology. 2017;153:460–469. doi: 10.1053/j.gastro.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 26.Obara K, Haruma K, Irisawa A et al. Guidelines for sedation in gastroenterological endoscopy. Dig Endosc. 2015;24:435–449. doi: 10.1111/den.12464. [DOI] [PubMed] [Google Scholar]
- 27.Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87–97. [Google Scholar]
- 28.Fayad N F, Kahi C J. Quality measures for colonoscopy: a critical evaluation. Clin. Gastroenterol Hepatol. 2014;12:1973–1980. doi: 10.1016/j.cgh.2013.09.052. [DOI] [PubMed] [Google Scholar]
- 29.Lee T J, Rutter M D, Blanks R G et al. Colonoscopy quality measures: experience from the NHS Bowel Cancer Screening Programme. Gut. 2012;61:1050–1057. doi: 10.1136/gutjnl-2011-300651. [DOI] [PubMed] [Google Scholar]
- 30.Rex D K, Petrini J L, Baron T H et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. doi: 10.1111/j.1572-0241.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- 31.Crew K D, Neugut A I. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354–362. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]


