Abstract
Bovine viral diarrhea virus can cause acute disease in livestock, leading to economic losses. We show that Prostaglandin A1 inhibits bovine viral diarrhea virus replication in Madin-Darby bovine kidney cells (94% inhibition using 5 μg/mL). Light and electron microscopy of infected cells shows that Prostaglandin A1 also prevents virus-induced vacuolization, but at higher concentrations (10 μg/mL).
Keywords: Bovine viral diarrhea virus, BVDV, Prostaglandins, Prostaglandin A1, Virus-induced vacuolization
Bovine viral diarrhea virus (BVDV) is an insidious, complex and ubiquitous pathogen that can affect cattle of all breeds and all ages, as well as others domestic and wild ruminants.1 The disease caused by BVDV ranges in severity from asymptomatic to acute infection and fatal mucosal disease, with a mortality index of approximately 5%, which leads to an economic impact of BVDV infections of up to £993 per cow per year.2
In pregnant cows, BVDV can reach the fetus and cause abortion, stillbirth or teratogenic defects, depending on the gestational period when infection was established.1 When the infection is established in the final period of gestation, calves can give birth to immunotolerant and persistently infected calves that are considered reservoirs of the virus.3 Also, the serum of infected animals may contain active BVDV viral particles, which may result in permanent infection in primary cultures and established cell lines that are routinely cultivated using bovine serum, in the laboratory.
BVDV is the type-species of the Pestivirus genus (from the Flaviviridae family) that also includes the border disease virus (BDV) and the classical swine fever virus (CSFV), responsible for significant economic losses worldwide.4 The pestivirus capsid has an icosahedral symmetry formed by a single protein (capsid protein C), and is enveloped by a lipoprotein membrane. Its genome consists of a positive sense, single-stranded RNA of about 12,000 nucleotides, containing a large open reading frame coding for a single polyprotein with about 4000 amino acids. This large polypeptide is cleaved (by host and viral proteases) to generate the capsid protein (C), the glycoproteins Erns, E1 and E2, a non-structural protease (Npro) and a number of other non-structural proteins (p7, NS2/NS3, NS4A, NS4B, NS5A and NS5B). The E2 protein appears to be of major importance for pestivirus neutralization.5
BVDV isolates are divided into the genetic groups BVDV 1 and 2, which are genetically related to a separate species, the BVDV-3 or Hobi group,6 which was initially identified in fetal bovine serum from Brazil.7 BVDV isolates are also classified into cytopathogenic (cpBVDV) or non-cytopathogenic (ncpBVDV) depending on their ability to cause cytopathic effect in cell culture.3 Birk et al.8 showed that cpBVDV induced extensive vacuolization in Madin-Darby bovine kidney (MDBK) cells; however, this phenomenon appeared morphologically different from that observed in apoptosis and necrosis.
Prostaglandins are natural eicosanoids secreted by a variety of human tissues and capable of causing profound and diverse physiological effects at very low concentrations. All eicosanoids function locally at the site of synthesis, through receptor-mediated G-protein linked signaling pathways. Prostaglandin A1 (PGA1) is capable of blocking the replication of a wide variety of RNA and DNA viruses, including CSFV.9, 10, 11 Despite the importance of BVDV for animal health, the effect of PGA1 on BVDV replication has not been examined. Therefore, we evaluate the effect of PGA1 on the replication of cpBVDV in MDBK cells, focusing on changes in BVDV-induced vacuolization.
MDBK cells were cultured in vitro in Dulbecco Modified Eagle Medium (DMEM), supplemented with 10% BVDV-free fetal bovine serum (FBS, Gibco), penicillin (500 U/mL), streptomycin (100 μg/mL) and amphotericin B (fungizone, at 2.5 μg/mL).
Monolayers of MDBK cells were infected with cpBVDV-1 (NADL strain) at a multiplicity of infection of 0.1. Viral particles were allowed to interact with host cells for 1 h in the absence of FBS, and then the supernatant was replaced with complete growth medium containing 0.1, 1, 2.5, 5 or 10 μg/mL Prostaglandin A1 (PGA1, Sigma Chemical Co.; stored as a 1 mg/mL solution in 100% ethanol). After treatment of monolayers with PGA1 for 36 h, culture supernatants were tested for the presence of virus by 50% tissue culture infective dose (TCID50) titration, and cell monolayers were fixed and stained with the Panoptic Solution Kit® (Laborclin Ltda. Pinhais/PR, Brazil). Panoptic-stained monolayers were observed in a Zeiss-Observer D1 light microscope equipped with a Nomarski differential interferential contrast system.
Cell viability was determined by incubating monolayers in a solution containing 5 μg/mL Neutral red (diluted in PBS) for 3 h at 37 °C, in wells of 96-well plates. Then, the solution was discarded, and cells were incubated with 4% formaldehyde (in PBS) for 1 min, and 100% methanol for 20 min. Samples were analyzed by absorbance at 490 nm, in an ELISA plate reader (SpectraMax M2).
For transmission electron microscopy (TEM) analysis, treated and untreated monolayers in 25 cm2 plastic culture flasks were fixed in 2.5% glutaraldehyde in 0.1 M cacodylate buffer (pH 7.2), post-fixed for 1 h in 1% OsO4/0.8% potassium ferrocyanide in the same buffer, dehydrated in ethanol and ‘flat-embedded’ in Polybed resin (Polysciences1), to preserve the cell architecture. Ultrathin sections (both routine, and en face) were stained with uranyl acetate and lead citrate and observed in a FEI Tecnai T20 transmission electron microscope.
For the visualization of extracellular lamellae by TEM, 0.5 mg/mL ruthenium red was added to the TEM fixation and post-fixation solutions (as well as to the intermediate washes in cacodylate buffer), and cells were processed for TEM as described above. This procedure was also performed during en face sectioning, in order to avoid removal of the cells from the substratum, which could disrupt and disorient their architecture.
Treatment of BVDV-infected MDBK monolayers with PGA1 at concentrations of 0.1 and 0.5 μg/mL reduced the virus yield in culture supernatants by 50%, and treatment with the highest concentration of PGA1 tested in this assay (5 μg/mL) reduced the viral yield by 94% (Table 1). Thus, PGA1 treatment resulted in strong inhibition of BVDV replication in MDBK cells.
Table 1.
Effect of prostaglandin A1 (PGA1) on bovine viral diarrhea virus (BVDV) replication in MDBK cells, expressed as the TCID50 and the percentage of replication inhibition.
| PGA1 concentration (μg/mL) | TCID50/mL | % Inhibition |
|---|---|---|
| 0 | 1 × 105 | 0 |
| 0.5 | 5.6 × 104 | 44 |
| 1.0 | 5.0 × 104 | 50 |
| 2.5 | 1.9 × 104 | 81 |
| 5 | 6.3 × 103 | 94 |
MDBK cells were infected with BVDV and treated with different concentrations of PGA1. The percentage of BVDV replication inhibition was calculated by measuring the virus yield in the supernatant of infected cells 36 h after PGA1 treatment. While 1.0 μg/mL PGA1 inhibited 50% of BDVD production, the highest concentration tested in this assay (5 μg/mL PGA1) blocked replication by >90%.
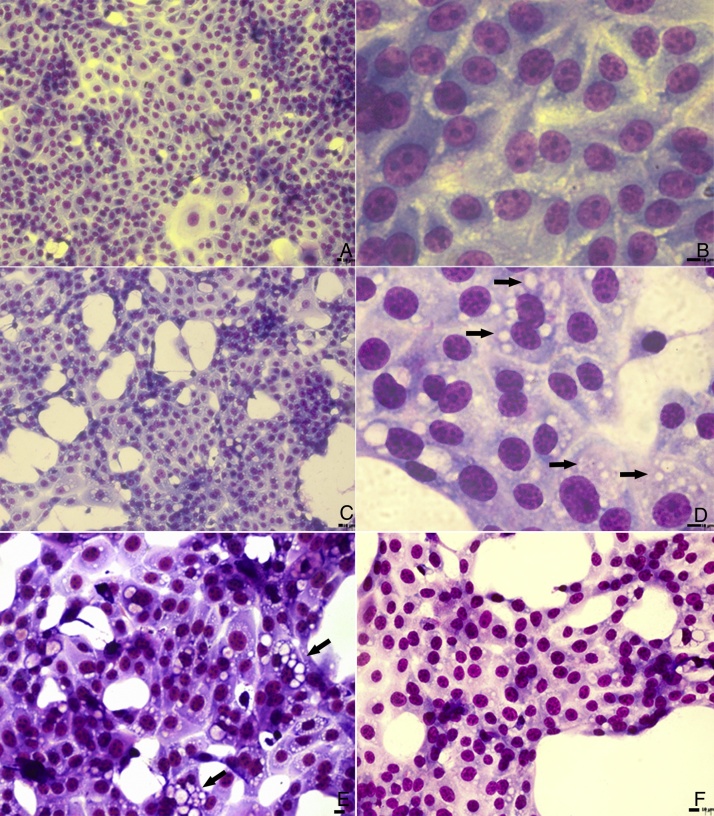
In light microscopy images, similar cytoplasmic alterations were observed in untreated and PGA1-treated cells (Fig. 1). Although treatment with 5 μg/mL decreased by almost 2 logs the virus titration in the supernatant of infected cells (Table 1), vacuolization was not inhibited by PGA1 at this concentration (Fig. 1E). A reduction in vacuolization was only detectable when cells were treated with 10 μg/mL PGA1 (Fig. 1F). At this concentration, treatment of MDBK cells with PGA1 for 36 h did not result in cytotoxicity, as determined by microscopic examination or vital dye exclusion (data not shown).
Fig. 1.
Light microscopy analysis of bovine viral diarrhea virus (BVDV) infected cells treated with prostaglandin A1 (PGA1). MDBK cells were treated with PGA1 for 36 h, and then subjected to panoptic staining. Low and high magnification images of untreated mock-infected cells (A, B), and of infected cells kept untreated (C, D) or treated with 5 (E) and 10 (F) μg/mL PGA1. Although 5 μg/mL PGA1 strongly blocked BVDV replication (E), it did not inhibit the vacuolization process (arrows). Vacuolization inhibition was only achieved after treatment with 10 μg/mL PGA1 (F). Scale bars: 8 μm.
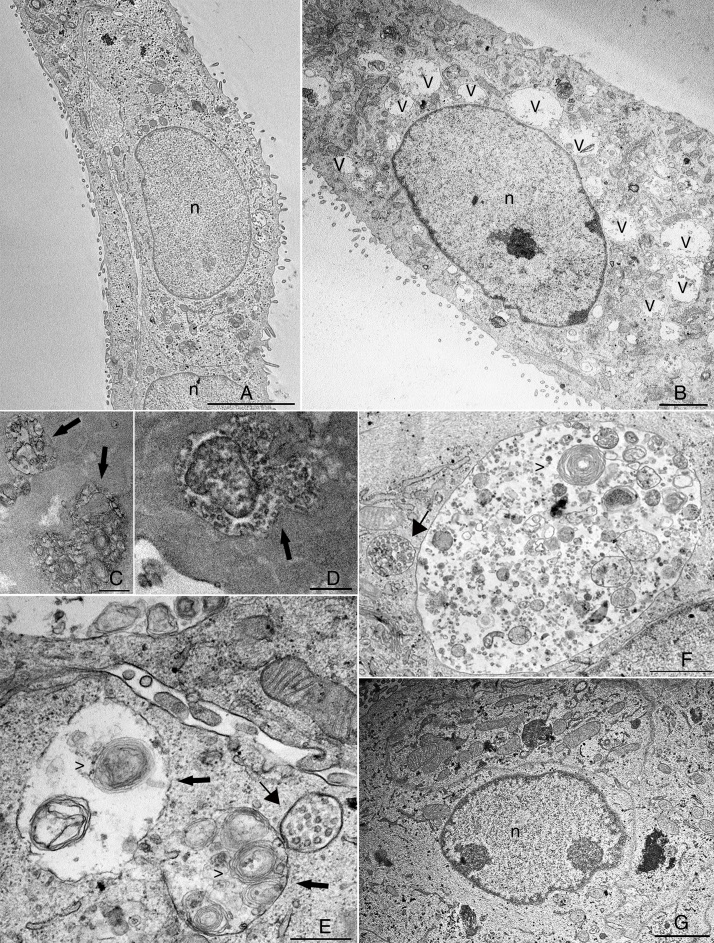
The cytosolic vacuoles induced by BVDV infection were further investigated by transmission electron microscopy analysis (Fig. 2). BVDV infection induced a dramatic increase in vacuolar structures in MDBK cells (Fig. 2B). When viewed in both traditional and en face sections, many of the vesicles in infected cells were positive for ruthenium red and, thus, contained extracellular material (Fig. 2C and D). One of the main features that we observed in BVDV-infected cells was the presence of multilamellar structures inside vacuoles (Fig. 2C, E and F), while smaller vacuoles with internal vesicles surrounded those with multilamellar structures (Fig. 2E, F). Multilamellar structures were previously reported by Sanz-Sanchez and Risco, where they were linked to a cellular response to detachment from substratum due to bunyavirus infection.12 Despite the strong reduction in BVDV replication achieved by treatment with 5 μg/mL PGA1 (Table 1), this PGA1 concentration did not prevent vacuolization in BVDV-infected cells (Fig. 1E). A strong reduction of virus-induced vacuolization was only achieved after treatment with 10 μg/mL PGA1 (Fig. 2G).
Fig. 2.
Transmission electron microscopy (TEM) analysis of bovine viral diarrhea virus (BVDV)-infected cells treated with prostaglandin A1 (PGA1). Infected and non-infected MDBK cells were treated with PGA1 for 36 h, and then processed for routine TEM. Vacuoles (v) were rare or absent in the majority of sections from non-infected cells (A), but were abundant in BVDV-infected cells not treated with PGA1 (B). Many of these structures exhibited extracellular content, as shown by ruthenium red labeling (arrows) in traditional (C) and en face (D) sections. Treatment with 1 μg/mL (E) or 2.5 μg/mL (F) PGA1 could not prevent BVDV-induced vacuolization in infected cells, which had large vacuoles (arrows) with multilamellar structures (arrowheads), and often surrounded by smaller vesicles-containing vacuoles (thin arrows). (G) Treatment with 10 g/mL PGA1, however, dramatically reduced virus-induced vacuolization. n, nucleus. Scale bars: (A) 5 μm; (B, G) 2 μm; (C, E) 500 nm; (D, F) 1 μm.
Although prostaglandins (PGs) are capable of inhibiting the replication of a variety of RNA and DNA viruses, their mode of action is not entirely clear.9, 10, 11 In some systems, PGAs exert potent antiviral activity by inducing the synthesis of proteins from the heat shock protein 70 (HSP70) family, through cycloheximide-sensitive activation of a heat-shock trigger factor.13
The reduction of virus-induced vacuolization after PGA1 treatment (Fig. 2G) is in agreement with the widely described antiviral effect of this drug.10 This activity, initially attributed to the induction of HSP-70 proteins, can also be triggered by other pathways, such as nuclear factor-κ B regulation13, 14 and, more recently, by a mechanism targeting the small ribosomal subunit (40S) and the eukaryotic initiation factors eIF3s.11 This virus-induced vacuolization seems not to be crucial for viral replication and is probably caused by the interference of BVDV NS3 protein on the smooth endoplasmic reticulum.15 Furthermore, the targeting of viral proteins by cyclopentenone prostaglandins was already reported by Kalantari et al.16
The fact that treatment with 5 μg/mL PGA1 inhibited BVDV production 94%, but did not block virus-induced vacuolization in host cells suggests that the vacuoles are, indeed, not involved in viral replication and that PGA1 probably reached its saturation point. Our data suggest that the antiviral activity of PGA1 is not associated with the inhibition of vacuole production, although the viral-induced vacuoles almost disappeared when higher concentrations of PGA1 were used.
Our work shows that the antiviral activity of PGA1 during BVDV interaction with MDBK cells does not rely on a significant reduction in virus-induced cytoplasmic vacuolization, but in the viral titers reduction. Since the antiviral effects of PGs on BVDV had not been described, this is the first report of the parameters involved in the protection of MDBK cells from BVDV infection.
Funding
This work was supported by Programa Nacional de Apoio ao Desenvolvimento da Metrologia, Qualidade e Tecnologia (Pronametro) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Associate Editor: Fernando Spilki
References
- 1.Nettleton P. Bovine viral diarrhoea virus: biology, diagnosis and control. Vet Rec. 2013;172(17):447–448. doi: 10.1136/vr.f2526. [DOI] [PubMed] [Google Scholar]
- 2.Yarnall M.J., Thrusfield M.V. Engaging veterinarians and farmers in eradicating bovine viral diarrhoea: a systematic review of economic impact. Vet Rec. 2017 doi: 10.1136/vr.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meyling A., Houe H., Jensen A.M. Epidemiology of bovine virus diarrhoea virus. Rev Sci Tech. 1990;9(1):75–93. doi: 10.20506/rst.9.1.489. [DOI] [PubMed] [Google Scholar]
- 4.Freitas T.R., Caldas L.A., Rebello M.A. Effect of prostaglandin A1 in porcine cells persistently infected with classical swine fever virus. J Basic Microbiol. 2003;43(6):468–472. doi: 10.1002/jobm.200310295. [DOI] [PubMed] [Google Scholar]
- 5.Weiland E., Stark R., Haas B., Rümenapf T., Meyers G., Thiel H.J. Pestivirus glycoprotein which induces neutralizing antibodies forms part of a disulfide-linked heterodimer. J Virol. 1990;64(8):3563–3569. doi: 10.1128/jvi.64.8.3563-3569.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu L., Larska M., Xia H. Atypical pestivirus and severe respiratory disease in Calves, Europe. Emerg Infect Dis. 2012;18(11):1917–1918. doi: 10.3201/eid1811.111298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schirrmeier H., Depner K., Hoffman B., Beer M. Genetic and antigenic characterization of an atypical pestivirus isolate, a putative member of a novel pestivirus species. J Gen Virol. 2004;85(12):3647–3652. doi: 10.1099/vir.0.80238-0. [DOI] [PubMed] [Google Scholar]
- 8.Birk A.V., Dubovi E.J., Cohen-Gould L., Donis R., Szeto H.H. Cytoplasmic vacuolization responses to cytopathic bovine viral diarrhoea virus. Virus Res. 2008;132(1–2):76–85. doi: 10.1016/j.virusres.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Freitas T.R., Caldas L.A., Rebello M.A. Prostaglandin A1 inhibits replication of classical swine fever virus. Mem Inst Oswaldo Cruz. 1998;93(6):815–818. doi: 10.1590/s0074-02761998000600021. [DOI] [PubMed] [Google Scholar]
- 10.Santoro M.G. Antiviral activity of cyclopentenone prostanoids. Trends Microbiol. 1997;5(7):276–281. doi: 10.1016/S0966-842X(97)01066-4. [DOI] [PubMed] [Google Scholar]
- 11.Tsukimoto A., Sugiyama R., Abe M. A new role for PGA1 in inhibiting hepatitis C virus-IRES-mediated translation by targeting viral translation factors. Antiviral Res. 2015;117:1–9. doi: 10.1016/j.antiviral.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 12.Sanz-Sanchez L., Risco C. Multilamellar structures and filament bundles are found in the cell surface during bunyavirus egress. PLoS ONE. 2013;8(6):e65526. doi: 10.1371/journal.pone.0065526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amici C., Santoro M.G. Suppression of virus replication by prostaglandin A is associated with heat shock protein synthesis. J Gen Virol. 1991;72:1877–1885. doi: 10.1099/0022-1317-72-8-1877. [DOI] [PubMed] [Google Scholar]
- 14.Rossi A., Elia G., Santoro M.G. Inhibition of nuclear factor kappa β by prostaglandin A1: an effect associated with heat shock transcription factor activation. Proc Natl Acad Sci U S A. 1997;94(2):746–750. doi: 10.1073/pnas.94.2.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang G., Flick-Smith H., McCauley J.W. Differences in membrane association and sub-cellular distribution between NS2-3 and NS3 of bovine viral diarrhoea virus. Virus Res. 2003;97:89–102. doi: 10.1016/s0168-1702(03)00223-5. [DOI] [PubMed] [Google Scholar]
- 16.Kalantari P., Narayan V., Henderson A.J., Prabhu K.S. 15-Deoxy-delta12, 14-prostaglandin J2 inhibits HIV-1 transactivating protein, Tat, through covalent modification. FASEB J. 2009;23(8):2366–2373. doi: 10.1096/fj.08-124982. [DOI] [PMC free article] [PubMed] [Google Scholar]