Abstract
Although the use of vaccines has controlled enteric diseases in dogs in many developed countries, vaccine coverage is still under optimal situation in Brazil. There is a large population of nonimmunized dogs and few studies about the identification of the viruses associated with diarrhea. To address this situation, stool samples from 325 dogs were analyzed by polymerase chain reaction for the detection of common enteric viruses such as Canine adenovirus (CAdV), Canine coronavirus (CCoV), Canine distemper virus (CDV), Canine rotavirus (CRV) and Carnivorous protoparvovirus 1 (canine parvovirus 2; CPV-2). At least one of these species was detected in 56.6% (184/325) of the samples. The viruses detected most frequently in either diarrheic or nondiarrheic dog feces were CPV-2 (54.3% of the positive samples), CDV (45.1%) and CCoV (30.4%), followed by CRV (8.2%) and CAdV (4.9%). Only one agent was detected in the majority of the positive samples (63%), but co-infections were present in 37% of the positive samples and mainly included CDV and CPV-2. The data presented herein can improve the clinical knowledge in regions with low vaccine coverage and highlight the need to improve the methods used to control these infectious diseases in domestic dogs.
Keywords: Distemper, Parvovirus, Diarrhea, Dog, Co-infection
Introduction
Gastrointestinal disorders are frequently reported in companion animal clinics as leading to severe dehydration and death in South America.1, 2 They can have bacterial, parasitic or viral etiologies.3 Viruses associated with enteric illnesses in dogs are an important cause of mortality in nonprotected populations.2 Among these, canine parvovirus (CPV-2) and canine coronavirus (CCoV) are considered the most common viral enteric pathogens in dogs worldwide.4, 5, 6, 7, 8 Canine distemper virus (CDV) is endemic to South America and is frequently associated with enteric disorders.8, 9, 10, 11 Canine adenovirus type 1 (CAdV-1) is commonly linked to hepatitis but was also associated to severe gastroenteritis including vomiting and diarrhea.12 The canine rotavirus (CRV) is an unusual enteric pathogen in dogs but is important due to its zoonotic potential.13, 14
The search for multiple pathogens in fecal samples from dogs can mirror common exposure but can also show interactions between pathogens determining or aggravating disease.15, 16 Moreover, there is a lack of research searching for multiple viral pathogens in dogs, which could uncover the real etiology of canine gastroenteritis. Therefore, the present study aimed to verify the frequency of canine enteric viruses (CPV-2, CCoV, CDV, CAdV1 and CRV) in stool samples from dogs.
Materials and methods
Ethics and sample collection
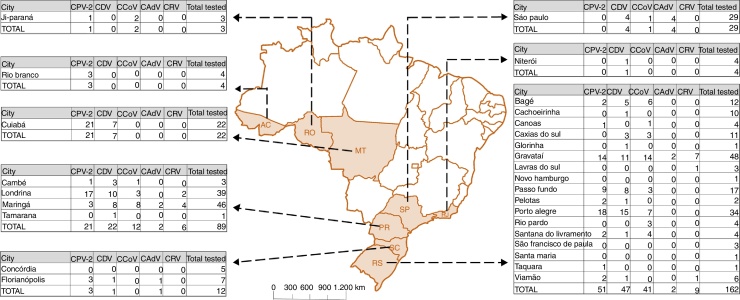
Fecal samples were collected from the rectal ampullae of 325 dogs at veterinary clinics during 2008 and 2014 and stored at −20 °C. The animals sampled were from eight of the federal States of Brazil (Rio Grande do Sul, Santa Catarina, Paraná, Rio de Janeiro, São Paulo, Mato Grosso do Sul, Rondônia and Acre) (Fig. 1). The majority of the analyzed dog population was unvaccinated (282/325), 24 animals were vaccinated but with incomplete vaccine protocols and 19 received complete protocols (Table 1). Dogs defined as vaccinated received the complete vaccination protocol using three doses of polyvalent vaccine, while the ones defined as vaccinated with incomplete protocols received one or two doses. Polyvalent vaccines used in dogs in Brazil are constituted by CPV-2, CDV, CCoV, CAdV2, canine parainfluenza virus and Leptospira spp. with minor differences according the different manufacturers. The sanitary status was defined by the clinic during sample collection where 82/325 were apparently healthy, 77/325 presented enteric disease-associated signs and 166/325 had this information not determined. Table S1 describes associated-information about all dog samples analyzed in the present study.
Fig. 1.
Map of Brazil. The map indicates the Brazilian federative state of origin of each sample describing the cities collected and number of positive samples for each evaluated virus. RS: Rio Grande do Sul; SC: Santa Catarina; PR: Paraná; SP: São Paulo; RJ: Rio de Janeiro; MT: Mato Grosso; RO: Rondônia; AC: Acre.
Table 1.
Detection of enteric viruses in dogs with different health and immunization status.
| Virus | Health status |
Vaccination status |
|||
|---|---|---|---|---|---|
| Enteric disease | Healthy | Vaccinated | Incomplete | Non-vaccinated | |
| CPV-2 | 36 (46.8%) | 10 (12.2%) | 5 (26.3%) | 13 (54.2) | 82 (29.1%) |
| CDV | 32 (41.6%) | 18 (22%) | 0 (0%) | 7 (29.2%) | 76 (27%) |
| CCoV | 12 (15.6%) | 20 (25.4%) | 0 (0%) | 12 (50%) | 44 (15.6%) |
| CRV | 2 (2.6%) | 1 (1.2%) | 1 (5.3%) | 0 (0%) | 14 (5%) |
| CAdV | 0 (0%) | 1 (1.2%) | 0 (0%) | 0 (0%) | 9 (3.2%) |
| Total | 77 | 82 | 19 | 24 | 282 |
The project was registered with the Ethics Committee on the Use of Animals (CEUA) of Universidade Federal do Rio Grande do Sul under protocol number #24984.
DNA/RNA isolation and reverse transcription (RT)
Fecal samples were diluted to 20% (w/v) in phosphate-buffered saline (PBS, pH 7.4) and stored at −80 °C for further analysis.
DNA was isolated from the supernatant using guanidine-isothiocyanate and a silica-based17 commercial kit (NewGene Preamp, Simbios Biotecnologia, Cachoeirinha, RS, Brazil).
Total RNA was extracted using TRIzol® LS Reagent (Life Technologies®, Carlsbad, CA, USA). The cDNA was synthesized with SuperScript®III Reverse Transcriptase Kit (Life Technologies®, Carlsbad, CA, USA) using reverse primers for each target9, 18, 19, 20, 21 in a total volume of 20 μL.
PCR
PCR and RT-PCR were performed using previously published protocols. The CPV-2 PCR protocol aimed to amplify 583 bp of the VP2 gene,18 while the CAdV assay targeted 509 bp of the E3 coding region.19 For CDV detection, RT-PCR was performed to amplify a fragment of 479 bp of the N gene, followed by nested PCR that aims to amplify a 286 bp fragment.9 The CCoV protocol amplified 410 bp of the M gene,20 and the CRV protocol targeted 1062 bp of the VP7 gene.21 The PCR products were electrophoresed in 2% agarose gels, visualized under UV light and compared with a 100 bp molecular weight ladder (Ludwig Biotecnologia, Cotia, SP, Brazil).
PCR and RT-PCR positive samples were submitted for DNA sequencing (ACTGene Análises Moleculares Ltda., Alvorada, RS, Brazil) in order to confirm the identities of amplified viruses. PCR amplification products were purified using the NucleoSpin Extract II Kit (Macherey-Nagel, Düren, Germany). After, both strands were sequenced with an ABI PRISM 3100 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) using a BigDye Terminator v.3.1 cycle sequencing kit (Applied Biosystems).
The commercial vaccine Recombitek® (Boehringer Ingelheim, Ingelheim am Rhein, Germany) was used as a positive control for the CPV-2 and CDV assays, while Vanguard Plus® (Pfizer, Wayne, PA, USA) was used for CCoV and CAdV and RotaTeq (Merck Sharp & Dohme Corp., Kenilworth, NJ, USA) was used for the CRV protocol.
Results
Genomic amplification products of at least one virus were detected in 56.6% (184/325) of the samples. CPV-2 was the most commonly detected amplicon in 54.3% (100/184) of the positive samples, followed by CDV (45.1%, 83/184), CCoV (30.4%, 56/184), CRV (8.2%, 15/184) and CAdV (4.9%, 9/184). Fig. 1 describes the results for each analyzed virus per Brazilian location.
Regarding the sanitary status, CPV-2 was the most frequent virus in dogs displaying signs of enteric disease, followed by CDV, CCoV and CRV, with no detection of CAdV-positive animals (Table 1). CCoV was the most frequent in apparently healthy dogs, followed by CDV, CDV, CPV-2, CRV and CAdV. No significant difference was observed between groups.
The vaccination status was also evaluated in the present study. In vaccinated dogs, only CPV-2 and CRV were detected (Table 1). In dogs that received the incomplete vaccine protocol, CPV-2 was the most frequent, followed by CCoV, CDV and no detection of CRV and CAdV-positive animals. In unvaccinated dogs, CPV-2 was also the most frequent detected virus, followed by CDV, CCoV, CRV and CAdV. No significant difference was observed between groups.
Single infections were observed in the majority of the positive samples (63.0%, 116/184), where CPV-2 was the most frequently detected (44.0%, 51/116), followed by CDV (28.4%, 33/116), CCoV (19.8%, 23/116), CRV (4.3%, 5/116) and CAdV (3.4%, 4/116) (Table 2). Co-infections (samples positive for two or more viruses) were observed in 37.0% (68/184) of the positive samples. Among them, dual infections were the most common (86.8%, 59/68), although triple (10.3%, 7/68) and quadruple (2.9%, 2/68) infections were also found. Regarding the sanitary status, co-infections were observed in 31.2% (24/77) of the dogs presenting enteric-associated clinical signs and in 15.9% (13/82) of apparently healthy dogs (Table 3). When the vaccination status was evaluated (Table 3), co-infections were observed in 5.3% (1/59) of the vaccinated dogs, in 45.8% of dogs that received incomplete protocols of vaccination and in 19.9% of the unvaccinated dogs. No significant difference was observed between groups.
Table 2.
Single infection and co-infection rates in dogs with positive PCR detection of common diarrhea-associated viral agents.
| CDV | CPV-2 | CCoV | CRV | CAdV | |
|---|---|---|---|---|---|
| Single infection | 30/113 (26.5%) | 51/113 (45.1%) | 23/113 (20.3%) | 5/113 (4.4%) | 4/113 (3.5%) |
| CDV + CPV-2 | 30/67 (44.7%) | 30/67 (44.7%) | |||
| CPV-2 + CCoV | 10/67 (14.9%) | 10/67 (14.9%) | |||
| CDV + CCoV | 9/67 (13.4%) | 9/67 (13.4%) | |||
| CCoV + CRV | 4/67 (5.9%) | 4/67 (5.9%) | |||
| CDV + CAdV | 2/67 (2.9%) | 2/67 (2.9%) | |||
| CPV-2 + CRV | 1/67 (1.5%) | 1/67 (1.5%) | |||
| CPV-2 + CAdV | 1/67 (1.5%) | 1/67 (1.5%) | |||
| CCoV + CAdV | 1/67 (1.5%) | 1/67 (1.5%) | |||
| CRV + CAdV | 0 | 0 | |||
| CDV + CPV-2 + CCoV | 3/67 (4.4%) | 3/67 (4.4%) | 3/67 (4.4%) | ||
| CDV + CCoV + CRV | 2/67 (2.9%) | 2/67 (2.9%) | 2/67 (2.9%) | ||
| CDV + CPV-2 + CAdV | |||||
| CDV + CPV-2 + CRV | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | ||
| CDV + CCoV + CAdV | |||||
| CPV-2 + CCoV + CAdV | |||||
| CPV-2 + CCoV + CRV | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | ||
| CCoV + CRV + CAdV | |||||
| CDV + CPV-2 + CCoV + CRV | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | |
| CDV + CPV-2 + CCoV + CAdV | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | 1/67 (1.5%) | |
| Total | 83/184 | 100/184 | 23/184 | 15/184 | 9/184 |
Table 3.
Single infection and co-infection rates in dogs with different health and immunization status.
| Status of infection | Health status |
Vaccination status |
|||
|---|---|---|---|---|---|
| Enteric disease | Healthy | Vaccinated | Incomplete | Non-vaccinated | |
| Negativea | 22 (28.6%) | 45 (54.9%) | 14 (73.7%) | 5 (20.9%) | 119 (42.2%) |
| Single infectionb | 31 (40.3%) | 24 (29.3%) | 4 (21.1%) | 8 (33.3%) | 104 (36.9%) |
| Dual infectionc | 21 (22.3%) | 13 (15.9%) | 1 (5.3%) | 9 (37.5%) | 49 (17.4%) |
| Triple infectiond | 3 (3.9%) | 0 (0%) | 0 (0%) | 2 (8.3%) | 5 (1.8%) |
| Quadruple infectione | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0.7%) |
| Quintuple infectionf | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total | 77 | 82 | 19 | 24 | 282 |
Samples negative for all the tested viruses.
Positive for at least one of the tested viruses.
Positive for at least two of the tested viruses.
Positive for at least three of the tested viruses.
Positive for at least four of the tested viruses.
Positive for all the tested viruses.
The most frequent viruses involved in co-infections were CDV and CPV-2, with each one detected in 73.5% (50/68) and 72.1% (49/68) of the samples positive for more than one virus, respectively, followed by CCoV (48.5%, 33/68), CRV (14.7%, 10/68) and CAdV (7.4%, 5/68). The combination of CDV and CPV-2 was the most frequently observed (52.9%, 36/68 CDV + CPV-2 positive samples), although other viruses were also detected in six of these samples (Table 1).
PCR and RT-PCR positive samples were submitted for DNA sequencing and confirmed amplification of the wild strains and no contamination with positive controls (data not shown).
Discussion
In the present study, we verified the frequency of five canine viral enteropathogens in a dog population with low vaccination coverage where CPV-2 was the most frequently detected virus (Table 2). The data presented herein agree with previous studies where CPV-2 is reported as the most common cause of severe diarrhea in puppies.7, 15, 16, 22 However, the frequency of CPV-2 found in our study (54.3%) was higher when compared with the ranges reported in other studies (16–48.7%).7, 15, 16, 22 The CPV-2 prevalence varies between studies, depending on the inclusion criteria for participation. However, since vaccination against canine pathogens other than rabies is not mandatory in Brazil, the vaccine coverage for these viruses must be drastically lower, which contributes to the high rate of detection observed in our study.
CCoV is generally the second most common viral agent detected in diarrheic dogs,7, 15, 16, 22 but in the present study, CDV had a higher frequency of detection (Table 2). However, the frequency of detection observed for CCoV was still higher than that reported other studies.7, 15, 16, 22 Some CCoV strains can cause severe diarrhea and intestinal damage indistinguishable from those caused by CPV-2.6
CDV is endemic to Brazil8, 23 but not to the regions sampled in previous studies.7, 16, 22 This could explain the high rates of CDV infection detected in the present study. Moreover, CDV causes a multisystemic disease with immunodepression that can favor infection by other pathogens including other diarrhea-associated viruses.24
CRV and CAdV were also identified in 8.2 and 4.9% of the dogs tested, respectively. Both viral agents are linked to diarrhea and vomiting13 and should not be excluded in the diagnosis of canine diarrhea. Moreover, CRV is a zoonotic pathogen, and its frequency in dogs needs to be determined to better assess the risk of infection in humans.
The samples analyzed in the present study were obtained by convenience from veterinary clinics which could bias information regarding sanity. Nevertheless, CPV-2 remained as the most frequent viral agent in the different dog groups (Table 1). The only exception was in dogs apparently healthy where CCoV and CDV were more frequent than CPV-2 but with no statistical significance.
More than one-third of the dogs tested for CPV-2, CCoV, CDV, CRV and CAdV were positive for co-infections (Table 2). No significant difference in co-infection rates were observed between the groups regarding clinical signs and vaccination status (Table 3). The occurrence of co-infection can increase the pathogenicity of the disease since these viral agents can act as immunosuppressants.12, 24, 25 There is a lack of studies searching for multiple viral pathogens in dogs, which could uncover the real etiology and interactions in the clinics. Moreover, a single search for the more common pathogens can reveal the real epidemiology of less common viral enteropathogens. Canine diarrhea can have other etiologies such as bacteria and parasites that are frequently reported.15, 16 Despite CPV-2 being the most frequently detected diarrhea-associated viral pathogen in the present study, a high degree of co-infections was observed. These co-infection rates reinforce that the search for more common individual pathogens could uncover the real epidemiology of less common viral enteropathogens.
In the present study, stool samples from dogs were evaluated for the presence of viral pathogens, and CPV-2 was found to be the most common. In contrast with previous studies, CDV was the second most common viral agent detected, probably since it is endemic to Brazil and not to the regions sampled in other studies. Moreover, the high frequency of co-infected dogs that was observed can increase the pathogenicity of the disease. The data presented herein can improve the clinical knowledge in regions with low vaccine coverage and highlight the need to improve the methods of controlling infectious diseases in domestic dogs.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgements
We are thankful to Simbios Biotecnologia Ltda. for kindly supplying the DNA extraction kits, and we thank the veterinarians who collected the samples. Financial support was provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível (CAPES/Brazil), Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul (FAPERGS) and Propesq/UFRGS.
Associate Editor: Fernando Spilki
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.bjm.2018.02.006.
Appendix A. Supplementary data
The following are the supplementary data to this article:
References
- 1.Hubbard K., Skelly B.J., McKelvie J., Wood J.L.N. Risk of vomiting and diarrhoea in dogs. Vet Rec. 2007;161:755–757. doi: 10.1136/vr.161.22.755. [DOI] [PubMed] [Google Scholar]
- 2.Greene C.E., Decaro N. Infectious Diseases of the Dog and Cat. 2012. Canine viral enteritis; pp. 67–80. [Google Scholar]
- 3.Adam F. Infectious diseases of the dog and cat. In: Greene C.E., Vandevelde M., editors. 4th ed. Vol 55. Elsevier; St Louis: 2014. pp. 25–42. (Journal of Small Animal Practice). [Google Scholar]
- 4.Decaro N., Desario C., Billi M. Western European epidemiological survey for parvovirus and coronavirus infections in dogs. Vet J. 2011;187:195–199. doi: 10.1016/j.tvjl.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto L.D., Streck A.F., Gonçalves K.R. Typing of canine parvovirus strains circulating in Brazil between 2008 and 2010. Virus Res. 2012;165:29–33. doi: 10.1016/j.virusres.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinto L.D., Barros I.N., Budaszewski R.F. Vol 202. 2014. (Characterization of Pantropic Canine Coronavirus from Brazil). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decaro N., Buonavoglia C. Canine parvovirus – a review of epidemiological and diagnostic aspects, with emphasis on type 2c. Vet Microbiol. 2012;155:1–12. doi: 10.1016/j.vetmic.2011.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Budaszewski R.d.F., Pinto L.D., Weber M.N. Genotyping of canine distemper virus strains circulating in Brazil from 2008 to 2012. Virus Res. 2014;180:76–83. doi: 10.1016/j.virusres.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Fischer C.D.B., Ikuta N., Canal C.W. Detection and differentiation of field and vaccine strains of canine distemper virus using reverse transcription followed by nested real time PCR (RT-nqPCR) and RFLP analysis. J Virol Methods. 2013;194:39–45. doi: 10.1016/j.jviromet.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calderon M.G., Remorini P., Periolo O., Iglesias M., Mattion N., La Torre J. Detection by RT-PCR and genetic characterization of canine distemper virus from vaccinated and non-vaccinated dogs in Argentina. Vet Microbiol. 2007;125:341–349. doi: 10.1016/j.vetmic.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Headley S.A.A., Amude A.M., Alfieri A.F.A.A., Bracarense A.P.F.R.L., Alfieri A.F.A.A. Epidemiological features and the neuropathological manifestations of canine distemper virus-induced infections in Brazil: a review. Semin Ciências Agrárias. 2012;33:1945–1978. [Google Scholar]
- 12.Pratelli A., Martella V., Elia G. Severe enteric disease in an animal shelter associated with dual infections by canine adenovirus type 1 and canine coronavirus. J Vet Med. 2001;48:385–392. doi: 10.1046/j.1439-0450.2001.00466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Luchs A., Cilli A., Morillo S.G., Carmona R.d.C.C., Timenetsky M.d.C.S.T. Rare G3P[3] rotavirus strain detected in Brazil: possible human–canine interspecies transmission. J Clin Virol. 2012;54:89–92. doi: 10.1016/j.jcv.2012.01.025. [DOI] [PubMed] [Google Scholar]
- 14.Khamrin P., Maneekarn N., Peerakome S., Yagyu F., Okitsu S., Ushijima H. Molecular characterization of a rare G3P[3] human rotavirus reassortant strain reveals evidence for multiple human-animal interspecies transmissions. J Med Virol. 2006;78:986–994. doi: 10.1002/jmv.20651. [DOI] [PubMed] [Google Scholar]
- 15.Gizzi A.B.d.R., Oliveira S.T., Leutenegger C.M. Presence of infectious agents and co-infections in diarrheic dogs determined with a real-time polymerase chain reaction-based panel. BMC Vet Res. 2014;10:23. doi: 10.1186/1746-6148-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Duijvestijn M., Mughini-Gras L., Schuurman N., Schijf W., Wagenaar J.A., Egberink H. Enteropathogen infections in canine puppies: (co-)occurrence, clinical relevance and risk factors. Vet Microbiol. 2016;195:115–122. doi: 10.1016/j.vetmic.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boom R., Sol C.J., Salimans M.M., Jansen C.L., Wertheim-van Dillen P.M., van der Noordaa J. Rapid and simple method for purification of nucleic acids. J Clin Microbiol. 1990;28:495–503. doi: 10.1128/jcm.28.3.495-503.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buonavoglia C., Martella V., Pratella A. Evidence for evolution of canine parvovirus type 2 in Italy. J Gen Virol. 2001;82:3021–3025. doi: 10.1099/0022-1317-82-12-3021. [DOI] [PubMed] [Google Scholar]
- 19.Linné T. Differences in the E3 regions of the canine adenovirus type 1 and type 2. Virus Res. 1992;23:119–133. doi: 10.1016/0168-1702(92)90072-h. [DOI] [PubMed] [Google Scholar]
- 20.Herrewegh A.A., Smeenk I., Horzinek M.C., Rottier P.J., de Groot R.J. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J Virol. 1998;72:4508–4514. doi: 10.1128/jvi.72.5.4508-4514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gouvea V., Glass R.I., Woods P. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schulz B.S., Strauch C., Mueller R.S., Eichhorn W., Hartmann K. Comparison of the prevalence of enteric viruses in healthy dogs and those with acute haemorrhagic diarrhoea by electron microscopy. J Small Anim Pract. 2008;49:84–88. doi: 10.1111/j.1748-5827.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Headley S.A., Amude A.M., Alfieri A.F., Bracarense A.P.F.R.L., Alfieri A.A., Summers B.A. Molecular detection of Canine distemper virus and the immunohistochemical characterization of the neurologic lesions in naturally occurring old dog encephalitis. J Vet Diagn Invest. 2009;21:588–597. doi: 10.1177/104063870902100502. [DOI] [PubMed] [Google Scholar]
- 24.Viana M., Cleaveland S., Matthiopoulos J. Dynamics of a morbillivirus at the domestic–wildlife interface: canine distemper virus in domestic dogs and lions. Proc Natl Acad Sci U S A. 2015;112:1464–1469. doi: 10.1073/pnas.1411623112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pratelli A., Tempesta M., Roperto F.P., Sagazio P., Carmichael L., Buonavoglia C. Fatal coronavirus infection in puppies following canine parvovirus 2b infection. J Vet Diagn Invest. 1999;11:550–553. doi: 10.1177/104063879901100615. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.