Abstract
In this research, the antimicrobial substance anti-JFL15 was partially purified using a simple two-step extraction process from the cell-free supernatants of Bacillus siamensis JFL15. Anti-JFL15 exhibited a strong antibacterial activity against various multidrug-resistant aquatic bacterial pathogens, including Escherichia coli, Edwardsiella tarda, Pseudomonas aeruginosa, Aeromonas hydrophila, and Vibrio. Liquid chromatography–mass spectrometry revealed that anti-JFL15 contained eight cyclic lipopeptides belonging to two families: bacillomycin F (m/z 1056.56–1084.59) and surfactin (m/z 1007.65–1049.70) analogs. PCR analysis showed the presence of genes (i.e., sfp gene, surfactin synthetase D, fengycin synthetase B, iturin synthetase A, iturin synthetase C and bacillomycin synthetase D) involved in the biosynthesis of cyclic lipopeptides. This study is the first to identify cyclic lipopeptides from B. siamensis and use them to suppress the growth of various multidrug-resistant aquatic bacterial pathogens. Results indicated that B. siamensis JFL15 is a promising biocontrol agent for the effective and environmentally friendly control of various multidrug-resistant aquatic bacterial pathogens.
Keywords: Bacillus siamensis, Cyclic lipopeptide, Multidrug-resistant aquatic bacterial pathogen, Pathogenic fungi, Antimicrobial activity
Introduction
Seafood safety concerns have increased in recent years with the increase in seafood consumption worldwide (Liu et al. 2015). In addition, the high demand of aquaculture has led to numerous disease outbreaks caused by various pathogens, resulting in serious economic losses (Gupta et al. 2016). Vibrio, which causes acute gastroenteritis, is an important pathogen of reared aquatic organisms (Xu et al. 2013). Antibiotic treatment of bacterial diseases in fish culture has been applied for many years (Guo et al. 2017). However, the massive use of antibiotics encourages the natural emergence of antibiotic-resistant bacteria, which can transfer their resistance genes to other bacteria that have never been exposed to the antibiotics; this phenomenon exerts negative impacts on environment and human health (Ravindran et al. 2016). The occurrence of antibiotic-resistant bacteria associated with fish diseases is a worsening worldwide problem in aquaculture because of the absence of effective and safe antibiotics (Kim et al. 2014). Therefore, the development of effective and environmentally friendly alternative strategies for the control of aquatic pathogenic bacteria is highly important. Natural products with a broad spectrum of antibacterial activities, such as against multidrug-resistant pathogenic bacteria, are the best alternative to commercial antimicrobial substances.
The use of probiotics is an environmentally suitable alternative to prevent bacterial infections in aquaculture. Gram-positive spore-forming Bacillus spp. are commonly used probiotics in aquaculture (Franco et al. 2017; Perez-Sanchez et al. 2014; Silva et al. 2012). Bacillus can form spores and thus can tolerate the harsh conditions, such as acidic and bile environment, of the animal gastrointestinal tract when administered as a diet additive (Chen et al. 2016). Species from this genus can also produce more than 20 types of antimicrobial compounds (Stein 2005; Sumi et al. 2015). Gene sequencing has revealed that more than 4% of the genes in the genome of Bacillus are involved in the production of antimicrobial compounds (Baltz 2017). Generally, antimicrobial substances are formed through ribosomal or nonribosomal synthesis. Those substances formed by ribosomal synthesis include antimicrobial proteins (Zhao et al. 2016), bacteriocin (Lim et al. 2011), bacteriocin-like inhibitory substance (Abriouel et al. 2011), and subtilin (Barbosa et al. 2015), whereas those formed by nonribosomal peptide synthetases (Niazi et al. 2014) and polyketide synthases (Baltz 2017) include cyclic lipopeptides (CLPs) from the surfactin, iturin, and fengycin families and polyketides from the bacillaene, difficidin, and microlactin families, respectively. Among these antimicrobial substances, CLPs produced by Bacillus have received increasing attention because of their stronger biological activities, including wide-spectrum antimicrobial, antitumor, antimycoplasma, and antiviral activities, and greater thermostability and resistance to degradation by proteolytic enzymes (Lee et al. 2016) when compared with other ribosomally synthesized antimicrobial compounds. These CLPs also cause less bacterial resistance compared with traditional antibiotics (Chen et al. 2017). These substances have a common amphipathic structure with a hydrophilic peptide portion and a hydrophobic fatty acid portion (Ramarathnam et al. 2007). In recent years, many scholars have investigated the antagonistic activities of CLPs against aquatic pathogens. Xu et al. (2014) determined that the CLPs produced by Bacillus amyloliquefaciens M1 showed strong antimicrobial properties against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Xiu et al. (2017) illustrated that CLPs from Bacillus sp. 176 exhibited antimicrobial activity against Vibrio alginolyticus 178 by significantly suppressing its motility. CLPs have been applied in many fields, such as agriculture, medicine, food, and environmental protection, and have received increasing attention as promising new antibiotic candidates (Lee et al. 2016). However, the lack of sources and the high production cost of CLPs limit their commercial application. In biotechnological processes, the downstream processing of CLPs is responsible for 60% of the total cost of the product because their purification require multiple steps involving salt precipitation followed by various combinations of ion-exchange, reverse-phase, affinity, and gel-exclusion chromatography (Touraki et al. 2012; Lee et al. 2016). In recent decades, numerous efforts in the bioprocess engineering field have been devoted to improving CLP production; these efforts included increasing productivity by using mutant strains and simplifying downstream processing by developing integrative processes (Jauregi et al. 2013).
In the present research, we identified and characterized the antimicrobial compounds produced by B. siamensis JFL15. The antimicrobial compounds exhibited stronger antimicrobial activity and broader antimicrobial spectrum than nisin and polymyxin B against fish pathogens with multidrug-resistant profiles. HPLC–MS analysis revealed that the antimicrobial substances are composed of multiple CLPs of the bacillomycin and surfactin families. The antibacterial effects and mechanisms of anti-JFL15 were further evaluated. The two-step separation and purification methods used in this research can provide a reference for the large-scale and efficient preparation of other antimicrobial compounds. Results suggested that B. siamensis JFL15 could be used as an environmentally friendly agent for the control of fish pathogens. To the best of our knowledge, this study is the first to purify and identify CLPs against various multidrug-resistant aquatic bacterial pathogens from Bacillus siamensis.
Materials and methods
Bacterial strains and culture conditions
Bacillus siamensis JFL15, which exhibits antimicrobial activity to a number of multidrug-resistant aquatic bacterial pathogens, was isolated from the gastrointestinal tract of hairtail and the genome sequenced (GenBank genome accession number LFWQ00000000). The indicator bacteria applied in this study were provided by Professor Zaiguang Fang of Hainan University Institute of Marine (Hainan, China). The indicator fungi Magnaporthe grisea and Rhizoctonia solani were provided by Professor Erxun Zhou at South China Agricultural University (Guangzhou, China), and the indicator fungi Colletotrichum gloeosporioides and Peronophythora litchii were from the laboratory storage. Luria–Bertani (LB) broth medium (containing 10 g/L tryptone, 5 g/L yeast extract, and 5 g/L NaCl in distilled water) was used as the growth medium for the strain JFL15 and indicator bacteria, and mineral salt medium (MSM) (containing 20 g/L sucrose, 2 g/L NH4NO3, 3 g/L KH2PO4, 10 g/L Na2HPO4, 0.2 g/L MgSO4, 0.2 g/L yeast extract, 0.7 µg/L CaCl2, and 1 µg/L MnSO4 in distilled water) was used for the production of antimicrobial compounds by culturing at 30 °C for 3 days with continuous shaking at 200 rpm. The indicator of pathogenic fungi was incubated on a PDA plate at 28 °C for 7 days.
Measurement of drug resistance of fish pathogens
Fifteen indicator bacteria were aerobically cultivated overnight in an LB liquid medium at 37 °C for 24 h. Sterile Oxford cups (6 × 10 mm) were placed on LB plates seeded with the different bacterial cultures. Each cup was added with 150 µL of commonly used antibiotics (100 µg/mL) (Table 2) and then incubated overnight at 37 °C. The clear zones around the Oxford cups were observed. All experiments were conducted in triplicate.
Table 2.
Drug resistance of different aquatic bacterial pathogens
| Number | Bacterial strain | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|---|
| 4001 | Aeromonas hydrophila | − | + | − | − | + | + |
| 4002 | Photobacterium damsela | − | − | + | − | + | + |
| 4003 | Vibrio harveyi | − | + | + | − | + | + |
| 4004 | Vibrio alginolyticus | − | + | + | − | + | + |
| 5101 | Aeromonas hydrophila | − | + | + | − | + | + |
| 5102 | Aeromonas hydrophila | − | + | + | + | + | + |
| 5103 | Aeromonas hydrophila | − | + | + | − | + | + |
| 5105 | Escherichia coli | − | − | − | − | + | + |
| 5111 | Edwardsiella tarda | − | + | − | + | + | + |
| 5112 | Pseudomonas aeruginosa | + | + | + | + | + | + |
| 5201 | Vibrio alginolyticus | − | + | + | − | + | + |
| 5203 | Vibrio harveyi | − | + | + | − | + | + |
| 5205 | Vibrio parahaemolyticus | − | + | + | + | + | + |
| 5206 | Vibrio vulnificus | − | − | + | − | + | + |
| 5209 | Vibrio chagasii | − | − | + | + | + | + |
The concentration of each antibiotic was 100 µg/mL. 1 chloramphenicol, 2 ampicillin, 3 tetracycline, 4 kanamycin, 5 hygromycin, 6 erythromycin; + resistance to the antibiotic, − sensitive to the antibiotic
Production and partial purification of antimicrobial substance from JFL15
The strain JFL15 was incubated in MSM medium for the production of CLPs at 180 rpm and 30 °C for 72 h. The cell-free supernatant was obtained by centrifuging the cultures at 8000×g for 20 min, and the antimicrobial substance was concentrated through ammonium sulfate precipitation at 4 °C storage overnight. The cultured supernatant was then precipitated by different concentrations of ammonium sulfate (10–80%), and the antimicrobial activities of these precipitates were examined to identify the concentration of ammonium sulfate with the highest activity. The precipitate was collected by centrifugation (8000×g, 10 min) at 4 °C, and crude lipopeptide was obtained by vacuum freeze drying before extraction with phosphate-buffered saline (PBS, pH 7.4) for four times. The insoluble precipitate was extracted with methanol thrice to obtain the last residual antimicrobial substance. The methanol extract was concentrated using a rotary evaporator under reduced pressure, and the partially purified antimicrobial substance from B. siamensis JFL15 was designated anti-JFL15.
Determination of antimicrobial activity of anti-JFL15
The antimicrobial activity of anti-JFL15 was determined by using the Oxford cup method (Moyne et al. 2001): hyphae discs of phytopathogens (M. grisea, R. solani, and C. gloeosporioides) were placed in the center of each PDA plate with the sterilized Oxford cup, which was 3 cm away from the edge of the mycelial colony. Anti-JFL15 (150 µL) was then added into the Oxford cup and then incubated at 28 °C for 7 days. The antifungal effect of anti-JFL15 was determined based on the semidiameter of the inhibition zone. For the analysis of antibacterial activity, 30 µL of indicator bacteria was mixed with 30 mL of LB broth, which was cooled to approximately 55 °C and poured into sterile plates placed with sterile Oxford cups in advance. When the plates solidified, the Oxford cups were removed, and 150 µL of anti-JFL15 was added into the hole. The plates were incubated overnight at 37 °C, and the diameter of the clear zone was used as a measure of antibacterial activity. The same volume of methanol was used as control in all antimicrobial experiments.
The zones of inhibition were manually measured with accuracy ± 1 mm. All experiments were conducted in triplicate.
PCR amplification of lipopeptide biosynthetic genes from genomic DNA
Total genomic DNA was extracted from JFL15 using the TIANamp Bacteria DNA Kit [Tiangen Biotech (Beijing) Co., Ltd.] in accordance with the manufacturer’s instructions. Lipopeptide biosynthetic genes, namely surfactin (sfp and srfD), iturin (ituA and ituC), bacillomycin (bmyA), and fengycin (fenB) were identified in the genome sequence of B. siamensis JFL15, primers synthesized and the genes detected in the genomic DNA extract using PCR. PCR was conducted in 50-µL volumes with varying annealing temperatures and times for each specific primer (Table 1) in PCR amplifier (BIORAD, USA).
Table 1.
Primer and PCR information
| Lipopeptide | Target gene | Primer name | Sequence (5ʹ–3ʹ) | Annealing temp. (°C) | Product size (bp) |
|---|---|---|---|---|---|
| Surfactin | sfp gene | Sfp-F Sfp-R |
ATGAAGATTTACGGAATTTA TTATAAAAGCTCTTCGTACG |
46 | 675 |
| Surfactin | SrfD | SrfAD-F SrfAD-R |
CCGTTCGCAGGAGGCTATTCC CGCCCATCCTGCTGAAAAAGCG |
60 | 1300 |
| Fengycin | FenB | FenB-R FenB-R |
CTATAGTTTGTTGACGGCTC CAGCACTGGTTCTTGTCGCA |
53 | 1600 |
| Iturin | ItuA | ItuA-F ItuA-R |
ATGTATACCAGTCAATTCC GATCCGAAGCTGACAATAG |
46 | 1047 |
| Iturin | ItuC | ItuC-F ItuC-R |
CCCCCTCGGTCAA GTGAATA TTGGTTAAGCCCTGATGCTC |
54 | 594 |
| Bacillomycin | BamA | BamA-F | AAAGCGGCTCAAGAAGCGAAACCC | 63 | 1200 |
| BamA-R | CGATTCAGCTCATCGACCAGGTAGGC |
PCR primers were determined from the genome sequence of B. siamensis JFL15 (GenBank genome accession number LFWQ00000000)
LC–MS/MS analysis
LC–MS analysis was performed to identify the partially purified bioactive substance anti-JFL15. The substance was separated using UHPLC on an Agilent system equipped with a C18 column (250 mm × 4.6 mm, 5 µm, Agilent, USA). The mobile phase consisted of solvent A (acetonitrile) and solvent B (0.1% formic acid in water). A linear gradient was used for elution at a flow rate of 1 mL/min as follows: 0–30 min, from 10 to 50% B (linear gradient); 30–50 min, from 50 to 93% B (linear gradient); and 50–70 min, 93% B (isocratic). Elution was monitored by determining absorbance at 214 nm. The column temperature was 28 °C, and the injection volume was 2 µL. The peaks were then analyzed in two (positive and negative) ion modes from m/z 200 to 2000 using quadrupole time-of-flight mass spectrometry (Q-TOF-MS) (Agilent Technologies 6540B, USA). The Q-TOF-MS system was equipped with an ESI interface, a collision cell, and two mass analyzers. The operating parameters included a capillary voltage of 800 V, a cone voltage of 40 V, a fragmentor voltage of 175 V, and a capillary temperature of 27 °C.
Statistical analysis
Data were collected from three independent experiments and expressed as the mean ± standard deviation of three replicates. Statistical analysis was performed using ANOVA in SPSS 19.0 software. Statistical significance was considered at P < 0.05 or < 0.01.
Results
Measurement of drug resistance of the fish pathogens
To determine the resistance of fish pathogens to common antibiotics, the effects of different antibiotics on the growth of fish pathogens were examined. As shown in Table 2, all fish pathogens used in this study were resistant to different antibiotics. Pseudomonas aeruginosa was resistant to all six antibiotics, suggesting that all fish pathogens in this study are multidrug-resistant strains.
PCR amplification of lipopeptide biosynthetic genes from genomic DNA
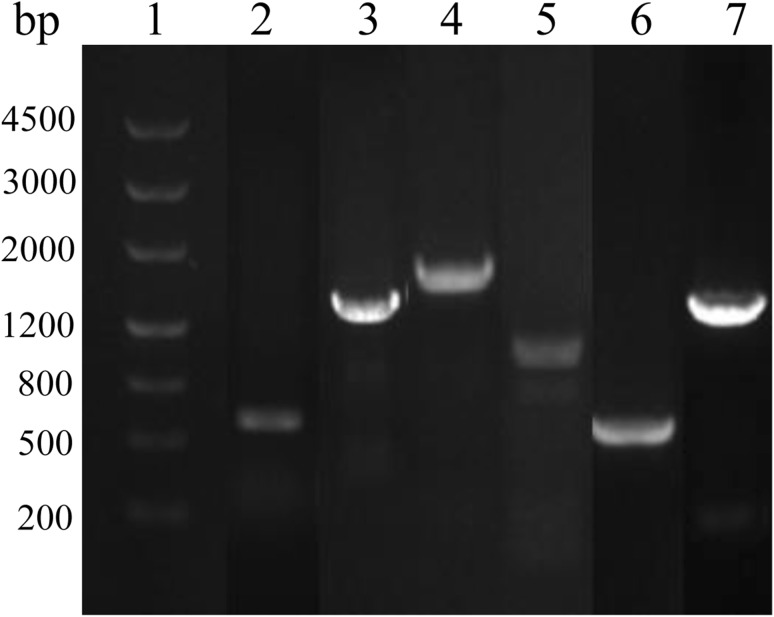
All mentioned PCR products, such sfp (surfactin regulatory gene), srfD (surfactin synthetase D), fenB (fengycin synthetase B), ituA (iturin synthetase A), ituC (iturin synthetase C), and bamD (bacillomycin synthetase D), were amplified with PCR. Results showed that all products exhibited a single thick band that was the size of the expected product (Fig. 1). The presence of all these genes in B. siamensis JFL15 facilitated the production of various CLPs, such as surfactin, fengycin, iturin, and bacillomycin.
Fig. 1.
PCR amplification of lipopeptide biosynthetic genes in B. siamensis JFL15. 1: 4500-bp marker (TaKaRa); 2–7: PCR products of sfp, srfD, fenB, ituA, ituC, and bamD, respectively
Production and partial purification of antimicrobial substance
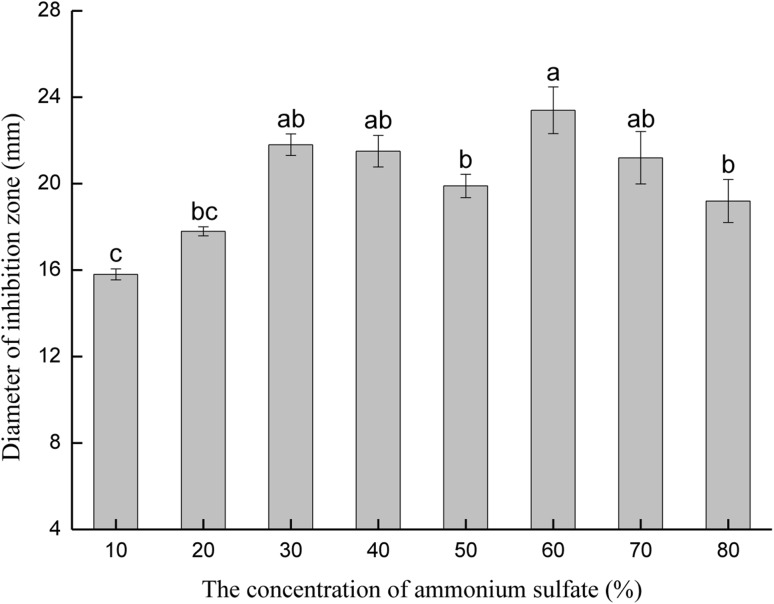
For mass production of the antimicrobial substance, B. siamensis JFL15 was incubated in 5 L of MSM medium at 30 °C for 3 days, and the cell-free supernatant was concentrated using the ammonium sulfate precipitation method. All components of ammonium sulfate precipitation demonstrated antibacterial activity against the multidrug-resistant Aeromonas hydrophila, and the antibacterial activity increased with the rise of ammonium sulfate saturation. The largest antibacterial activity was obtained when the saturation of ammonium sulfate reached 60%, which was the optimal concentration for the purification of the bioactive substance (Fig. 2). Results indicated that 60% saturation of ammonium sulfate could precipitate most of the antimicrobial substances in the cell-free supernatant.
Fig. 2.
Effect of different saturations of ammonium sulfate on the antibacterial activity of crude precipitates. Values (mean percentage cumulative mortality ± SE, n = 3) containing different superscripts denote significant difference among the treatments (p < 0.05). a, b, c indicated a significant level of antimicrobial activity of each experimental group
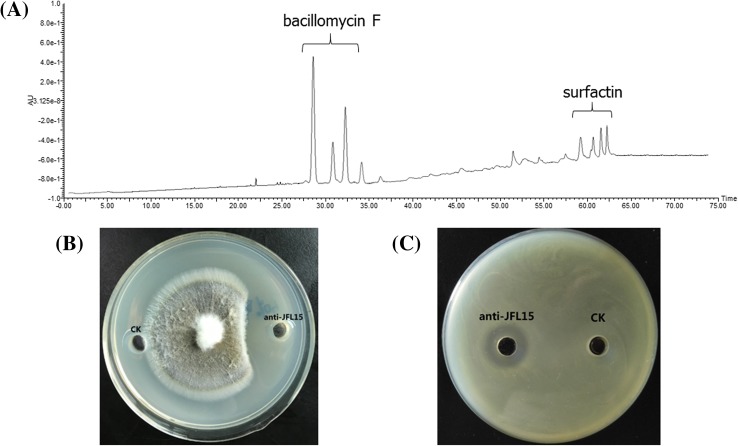
The precipitate was extracted by a simple two-step purification process. CLPs such as surfactin, iturin, and fengycin are common amphipathic compounds. Therefore, the concentrated substance was extracted by PBS buffer solution (pH 7.4) for four times, and the insoluble precipitate was extracted by methanol to obtain the last residual antimicrobial substance (anti-JFL15). This two-step extraction–purification process significantly improved the purity of anti-JFL15, which only contained two clusters of peaks: the first between 28.49 and 34.68 min and the second between 60.54 and 63.35 min. Anti-JFL15 exhibited strong antibacterial and antifungal activities against A. hydrophila and C. gloeosporioides, respectively (Fig. 3).
Fig. 3.
RP-HPLC and antimicrobial activity analyses of anti-JFL15 from B. siamensis JFL15. a RP-HPLC activity analyses of anti-JFL15. b The antifungal activity against C. gloeosporioides of anti-JFL15. c The antibacterial activity against A. hydrophila of anti-JFL15. CK: positive control with methanol
LC–MS/MS analysis CLPs
Comprehensive MS analysis of anti-JFL15 revealed the presence of two types of CLPs belonging to the bacillomycin and surfactin families. The protonated molecular ion [M+H]+ weights of the bacillomycin and surfactin families ranged from m/z 1057.57 to 1085.59 and m/z 1008.65 to 1050.71, respectively (Table 3). All molecules within each family had a 14 Da difference in molecular weight, suggesting that several lipopeptide analogs containing distinct lengths of fatty acid chains (CH2 = 14 Da) were present in anti-JFL15.
Table 3.
m/z value of CLPs detected by ESI-CID-MS
| Compound no. | m/z [M+H]+ | m/z [M−H]− | M | Identified |
|---|---|---|---|---|
| 1 | 1057.5714 | 1055.5516 | 1056.56 | C14Bacillomycin F |
| 2 | 1071.5813 | 1069.5716 | 1070.58 | C15Bacillomycin F |
| 3 | 1071.5831 | 1069.5697 | 1070.57 | C15Bacillomycin F |
| 4 | 1085.5959 | 1083.5845 | 1084.59 | C16Bacillomycin F |
| 5 | 1008.6574 | 1006.6458 | 1007.65 | C13Surfactin |
| 6 | 1022.6717 | 1020.6603 | 1021.67 | C14Surfactin |
| 7 | 1036.6887 | 1034.6779 | 1035.68 | C15Surfactin |
| 8 | 1050.7052 | 1048.6918 | 1049.70 | C16Surfactin |
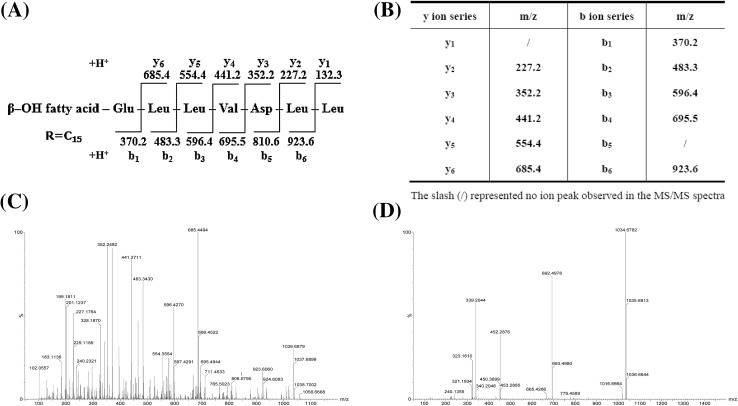
The fragmentation data of the specific masses based on LC–MS/MS were analyzed to determine the presence of the fingerprint masses of each compound related to a structure previously reported. Comparison of the high-resolution mass and MS/MS spectral characteristics with published data revealed that two types of CLPs with strong antimicrobial activity were identical to bacillomycin F and surfactin. In this study, component 7 (Table 3) was selected as an example. ESI-CID-MS analysis was performed using [M+H]+ (m/z 1036.69) as precursor ions to further confirm whether this substance is C15 surfactin. The detailed LC–MS/MS-based fragmentation analysis of the mass fragment ions showed b-type fragment ions with m/z 370.2, 483.3, 596.4, 695.5, and 923.6, and y-type fragment ions with m/z 227.2, 352.2, 441.2, 554.4, and 685.4. The b- and y-type fragment ions were generated by broken precursor ions (Fig. 4). In comparison with literature data, results illustrate that the substance with a molecular weight at m/z 1036.69 is C15 surfactin (Yang et al. 2015; Jasim et al. 2016).
Fig. 4.
Structure of C15 surfactin. Main cleavage site in MS/MS fragments and amino acid composition of C15 surfactin (a). Predicted b- and y-ion fragment lists of C15 surfactin (b). Positive ion MS/MS spectrum of C15 surfactin (m/z 1036.69) (c) and negative ion MS/MS spectrum of C15 surfactin (m/z 1034.68) (d)
Inhibitory spectrum of anti-JFL15
The inhibition spectrum of anti-JFL15 against various bacteria and fungi was determined using the agar disk diffusion method. The antimicrobial activity of anti-JFL15 was compared with those of commercially available nisin and polymyxin B (Sigma), which served as bacteriocin and lipopeptide representatives, respectively (Table 4). Nisin did not inhibit the growth of Gram-negative and fungi indicators, whereas polymyxin B inhibited only Gram-negative bacteria. The bioactive substances of anti-JFL15 inhibited all indicators, including Gram-negative bacteria and fungi. The antibacterial activity of anti-JFL15 against Gram-negative bacteria was stronger than that of polymyxin B. These results suggested that anti-JFL15 has a strong and wide spectrum of antimicrobial activity against multidrug-resistant fish pathogens.
Table 4.
Inhibition spectrum of anti-JFL15
| Number | Indicator strain | Antibacterial activity (diameter of inhibition zone/mm) | ||
|---|---|---|---|---|
| Nisin | Anti-JFL15 | Polymyxin B | ||
| Bacteria | ||||
| 4001 | Aeromonas hydrophila | − | 14.2 ± 0.3 | 13.6 ± 0.4 |
| 4002 | Photobacterium damsela | − | 20.2 ± 0.9 | 12.7 ± 0.3 |
| 4003 | Vibrio harveyi | − | 20.0 ± 0.5 | 12.8 ± 0.2 |
| 4004 | Vibrio alginolyticus | − | 19.2 ± 0.1 | 14.4 ± 0.4 |
| 5101 | Aeromonas hydrophila | − | 14.8 ± 0.3 | 13.4 ± 0.3 |
| 5102 | Aeromonas hydrophila | − | 19.4 ± 0.7 | 14.5 ± 0.5 |
| 5103 | Aeromonas hydrophila | − | 14.7 ± 0.3 | 13.5 ± 0.5 |
| 5105 | Escherichia coli | − | 19.2 ± 0.4 | 13.3 ± 0.3 |
| 5111 | Edwardsiella tarda | − | 15.2 ± 0.3 | 12.9 ± 0.4 |
| 5112 | Pseudomonas aeruginosa | − | 20.7 ± 0.5 | 12.3 ± 0.3 |
| 5201 | Vibrio alginolyticus | − | 16.2 ± 0.4 | 13.7 ± 0.4 |
| 5203 | Vibrio harveyi | − | 21.2 ± 0.4 | 13.2 ± 0.2 |
| 5205 | Vibrio parahaemolyticus | − | 17.2 ± 0.4 | 11.2 ± 0.1 |
| 5206 | Vibrio vulnificus | − | 20.0 ± 0.3 | 11.1 ± 0.2 |
| 5209 | Vibrio chagasii | − | 16.9 ± 0.3 | 11.6 ± 0.3 |
| Fungi | ||||
| Magnaporthe grisea | − | 28.5 ± 0.5 | − | |
| Rhizoctonia solani | − | 36.7 ± 0.4 | − | |
| Colletotrichum gloeosporioides | − | 34.4 ± 0.6 | − | |
| Peronophythora litchii | − | 35.3 ± 0.3 | − | |
Data are given as means ± SD, n = 3. Symbols: −, no inhibition
Discussion
Severe water pollution with the rapid development of large-scale intensive aquaculture over the past few years has caused various aquatic animal disease outbreaks. These outbreaks have restricted the healthy development of the aquaculture industry by causing economic losses and food safety concerns (Zhang et al. 2014). According to the recent report by the Center for Disease Control and Prevention Food-borne Surveillance Network (FoodNet), more than 24,000 people in 2016 were affected by food-borne illnesses mainly caused by E. coli, Edwardsiella tarda, P. aeruginosa, A. hydrophila, and Vibrio (Abdullah et al. 2017). To avoid such high economic losses in fish, several veterinary drugs are applied in aquaculture to prevent or treat disease outbreaks. However, the application of antibiotics and chemo-therapeutics to control these diseases causes the rapid development of multidrug-resistant pathogens, which pose risks to the environment and consumers. Table 2 shows that all the bacterial pathogens used in this research are multidrug-resistant strains, which are consistent with the experimental results of Xu et al. (2014). Within Vibrionaceae, more than 50% of the 134 isolates that were examined showed multidrug resistance to several different antibiotics, including chloramphenicol (Xu et al. 2014). In addition, out of the 56 isolates of A. hydrophila that were examined, five showed a high frequency of multiple (20) drug resistance (Ali et al. 2016). Therefore, alternatives for antibiotics should immediately be discovered with the increasing demand for environmentally friendly aquaculture.
These CLPs, which are the main CLP families in Bacillus, have a common amphipathic structure with a hydrophilic peptide portion and a hydrophobic fatty acid portion. Screening for the presence of biosynthetic genes is essential for identifying organisms with biosynthetic potential and targeting compounds responsible for the bioactivity. Active compounds can then be easily identified and purified with the presence of these biosynthetic genes.
The cell-free supernatant was concentrated by ammonium sulfate precipitation. As shown in Fig. 2, the antibacterial activity varied with ammonium sulfate saturation. One reason for this finding is that Bacillus can produce a variety of antibacterial substances, such as ribosomal antibiotics (e.g., bacteriocin, antibacterial peptide, and antibacterial protein) and nonribosomal antibiotics (e.g., CLPs and polyketides) (Sumi et al. 2015). Another reason is that different concentrations of ammonium sulfate can precipitate different antibacterial active substances. For example, An et al. (2015) suggested the purification of a novel bacteriocin CAMT2 by ammonium sulfate precipitation at 60% saturation. In addition, Shi et al. (2015) have successfully purified a thermostable antibacterial protein from B. subtilis FB123 using 50% saturated ammonium sulfate. Meanwhile, Lee et al. (2016) have purified two types of antimicrobial lipopeptides, bacillomycin and surfactin, by using 80% saturation of ammonium sulfate.
As biosurfactants, CLPs are amphipathic molecules with hydrophilic and hydrophobic moieties. The precipitate was purified by a simple two-step extraction process (PBS and methanol), which substantial increased the purity of anti-JFL15 that only contained two clusters of peaks (Fig. 3). A possible reason for this result may be most of the water-soluble impurities are dissolved in PBS. The two-step extraction method can be applied to purify CLPs or other antimicrobial substances to save time, effort, and cost relative to the traditional downstream extraction process, which is laborious, provides low yields, and takes up 60% of the total cost of the product. Different from our two-step extraction method, the traditional extraction process requires multiple steps involving salt precipitation followed by various combinations of ion-exchange, reverse-phase, affinity, gel-exclusion chromatography, and HPLC.
ESI-CID-MS/MS analyses indicated that the CLP was broken and therefore generate a series of specific b- and y-type ion fragments, which can be observed as a “fingerprint” of the MS/MS spectrum of a unique compound. For example, C15 surfactin was identified by the typical b- and y-type fragment ions generated by broken precursor ions [M+H]+ (m/z 1036.69) at m/z 370.2, 483.3, 596.4, 695.5, 923.6 and 227.2, 352.2, 441.2, 554.4, 685.4, respectively (Fig. 4). These results are consistent with the mass fragments reported in a previous study (Pathak and Keharia 2014). As shown in Fig. 3, anti-JFL15 exhibited high antibacterial and antifungal activities mainly because surfactin has strong antibacterial activity and bacillomycins (D, F, L, and LC) have significant antifungal activities (Zhang et al. 2013). In addition, distinct lipopeptide families co-produced by a single strain exert a synergistic effect and mutually enhance their respective activities. For example, the antifungal activity of bacillomycin D against gray mold disease is stimulated by surfactin homologs produced by the same strain of B. amyloliquefaciens SD-32 (Tanaka et al. 2015). Thus, anti-JFL15 may potentially be used in some biotechnological industries because it contains a heterogeneous lipopeptide mixture comprising eight species from two CLP families. Moreover, the commercial surfactin standard (purity: ≥ 98%, Sigma) contains four homologs (C12–C15) (Lee et al. 2016) and no bacillomycin F standard is on the market. So, anti-JFL15 can be further used to produce commercial surfactin and bacillomycin F standard by HPLC purification. As shown in Fig. 1, B. siamensis JFL15 can produce three types of CLPs (surfactin, fengycin and iturin), but only surfactin and iturin were identified in anti-JFL15. This result can be attributed to the fact that at least six fengycin homologs were identified in the PBS extraction component by their strong water solubility (data not shown).
Many Bacillus species, such as B. subtilis, B. amyloliquefaciens, B. licheniformis, B. pumilus, and B. mojavensis, have been used as probiotics in aquaculture due to their strong antibacterial activities against various multidrug-resistant aquatic bacterial pathogens (Liu et al. 2015; Chakraborty et al. 2017a, b; Chen et al. 2017; Cheng et al. 2017). However, only a few studies have reported the use of B. siamensis in aquaculture (Meidong et al. 2017). CLPs have been previously characterized from Bacillus, such as Bacillus subtilis (Farace et al. 2015) and Bacillus amyloliquefaciens (Kim et al. 2017), but this study is the first to purify and identify CLPs from the species of B. siamensis and use them to suppress the growth of various multidrug-resistant aquatic bacterial pathogens.
In conclusion, the antimicrobial substance (named anti-JFL15) from cell-free supernatants of B. siamensis JFL15 was partially purified using a simple two-step extraction process, i.e., PBS and methanol extraction after ammonium sulfate precipitation at 60% saturation, which saved time, effort, and cost. Anti-JFL15 exhibited a stronger and wider spectrum of antimicrobial activity than commercially available nisin and polymyxin B. Moreover, anti-JFL15 was identified as a lipopeptide mixture of four bacillomycin F and four surfactin analogs, which may potentially be used in some biotechnological industries, such as aquaculture protection, environmental bioremediation, pharmaceuticals, and cosmetics. These results indicated that B. siamensis JFL15 was a promising biocontrol agent for an effective and environmentally friendly control of various multidrug-resistant aquatic bacterial pathogens. Future studies will focus on other antibacterial mechanisms of anti-JFL15 against aquatic bacterial pathogens and apply proteomics and transcriptomics methods to investigate the signaling pathways involved in the antibacterial effects.
Acknowledgements
We would like to thank the Projects of Science and Technology of Guangdong Province (Grant numbers 2015A020209121, 2015A030313425 and 2015A030310225), and the Project of Science and Technology of Guangzhou City (Grant number 201607010197) supported this work. We are grateful to Professor Erxun Zhou for his kind providing of indicator fungi strains M. grisea and R. solani, and Professor Zaiguang Fang of Hainan University Institute of Marine for his kind providing of indicator bacteria.
Compliance with ethical standards
Conflict of interest
The authors declare that there is no conflict of interest about this research.
Contributor Information
Jun-Fang Lin, Phone: +86-20-85285382, Email: linjf@scau.edu.cn.
Li-Qiong Guo, Phone: +86-20-85285382, Email: guolq@scau.edu.cn.
References
- Abdullah, Asghar A, Butt MS, Shahid M, Huang Q. Evaluating the antimicrobial potential of green cardamom essential oil focusing on quorum sensing inhibition of Chromobacterium violaceum. J Food Sci Technol. 2017;54(8):2306–2315. doi: 10.1007/s13197-017-2668-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abriouel H, Franz CMAP, Omar NB, Galvez A. Diversity and applications of Bacillus bacteriocins. FEMS Microbiol Rev. 2011;35:201–232. doi: 10.1111/j.1574-6976.2010.00244.x. [DOI] [PubMed] [Google Scholar]
- Ali SS, Shaaban MT, Abomohra AE-F, El-Safity K. Macroalgal activity against multiple drug resistant Aeromonas hydrophila: a novel treatment study towards enhancement of fish growth performance. Microb Pathog. 2016;101:89–95. doi: 10.1016/j.micpath.2016.10.026. [DOI] [PubMed] [Google Scholar]
- An J, Zhu W, Liu Y, Zhang XM, Sun LJ, Hong PZ, Wang YL, Xu CH, Xu DF, Liu HM. Purification and characterization of a novel bacteriocin CAMT2 produced by Bacillus amyloliquefaciens isolated from marine fish Epinephelus areolatus. Food Control. 2015;51:278–282. doi: 10.1016/j.foodcont.2014.11.038. [DOI] [Google Scholar]
- Baltz RH. Gifted microbes for genome mining and natural product discovery. J Ind Microbiol Biotechnol. 2017;44:573–588. doi: 10.1007/s10295-016-1815-x. [DOI] [PubMed] [Google Scholar]
- Barbosa J, Caetano T, Mendo S. Class I and Class II lanthipeptides produced by Bacillus spp. J Nat Prod. 2015;78:2850–2866. doi: 10.1021/np500424y. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Thilakan B, Kizhakkekalam VK. Antibacterial aryl-crowned polyketide from Bacillus subtilis associated with seaweed Anthophycus longifolius. ARPN J Eng Appl Sci. 2017;12:3218–3221. doi: 10.1111/ijlh.12426. [DOI] [PubMed] [Google Scholar]
- Chakraborty K, Thilakan B, Raola VK. Previously undescribed antibacterial polyketides from heterotrophic Bacillus amyloliquefaciens associated with seaweed Padina gymnospora. Appl Biochem Biotechnol. 2017;184:1–17. doi: 10.1007/s12010-017-2562-9. [DOI] [PubMed] [Google Scholar]
- Chen Y, Li J, Xiao P, Li GY, Yue S, Huang J, Zhu WY, Mo ZL. Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquac Nutr. 2016;22:374–381. doi: 10.1111/anu.12259. [DOI] [Google Scholar]
- Chen YL, Liu SA, Mou HJ, Ma YX, Li M, Hu XK. Characterization of lipopeptide biosurfactants produced by Bacillus licheniformis MB01 from marine sediments. Front Microbiol. 2017;8:1–11. doi: 10.3389/fmicb.2017.00871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng AC, Lin HL, Shiu YL, Tyan YC, Liu CH. Isolation and characterization of antimicrobial peptides derived from Bacillus subtilis E20-fermented soybean meal and its use for preventing Vibrio infection in shrimp aquaculture. Fish Shellfish Immunol. 2017;67:270–279. doi: 10.1016/j.fsi.2017.06.006. [DOI] [PubMed] [Google Scholar]
- Farace G, Fernandez O, Jacquens L, Coutte F, Krier F, Jacques P, Clement C, Barka EA, Jacquard C, Dorey S. Cyclic lipopeptides from Bacillus subtilis activate distinct patterns of defence responses in grapevine. Mol Plant Pathol. 2015;16:177–187. doi: 10.1111/mpp.12170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco R, Martín L, Arenal A, Santiesteban D, Sotolongo J, Cabrera H, Mejías J, Rodríguez G, Moreno AG, Pimentel E, Castillo NM. Evaluation of two probiotics used during farm production of white shrimp Litopenaeus vannamei (Crustacea: Decapoda) Aquac Res. 2017;48:1936–1950. doi: 10.1111/are.13031. [DOI] [Google Scholar]
- Guo L, Guo J, Xu F. Optimized extraction process and identification of antibacterial substances from Rhubarb against aquatic pathogenic Vibrio harveyi. 3 Biotech. 2017;7:377. doi: 10.1007/s13205-017-1012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Geetika V, Gupta P. Growth performance, feed utilization, digestive enzyme activity, innate immunity and protection against Vibrio harveyi of freshwater prawn, Macrobrachium rosenbergii fed diets supplemented with Bacillus coagulans. Aquac Int. 2016;24:1379–1392. doi: 10.1007/s10499-016-9996-x. [DOI] [Google Scholar]
- Jasim B, Sreelakshmi KS, Mathew J, Radhakrishnan EK. Surfactin, iturin, and fengycin biosynthesis by endophytic Bacillus sp. from Bacopa monnieri. Microb Ecol. 2016;72:106–119. doi: 10.1007/s00248-016-0753-5. [DOI] [PubMed] [Google Scholar]
- Jauregi P, Coutte F, Catiau L, Lecouturier D, Jacques P. Micelle size characterization of lipopeptides produced by B. subtilis and their recovery by the two-step ultrafiltration process. Sep Purif Technol. 2013;104:175–182. doi: 10.1016/j.seppur.2012.11.017. [DOI] [Google Scholar]
- Kim YO, Park IS, Kim DJ, Nam BH, Kim DG, Jee YJ, An CM. Identification and characterization of a bacteriocin produced by an isolated Bacillus sp. SW1-1 that exhibits antibacterial activity against fish pathogens. J Korean Soc Appl Biol Chem. 2014;57:605–612. doi: 10.1007/s13765-014-4174-1. [DOI] [Google Scholar]
- Kim K, Lee Y, Ha A, Kim JI, Park AR, Yu NH, Son H, Choi GJ, Park HW, Lee CW, Lee T, Lee YW, Kim JC. Chemosensitization of Fusarium graminearum to chemical fungicides using cyclic lipopeptides produced by Bacillus amyloliquefaciens strain JCK-12. Front Plant Sci. 2017;8:1–16. doi: 10.3389/fpls.2017.02010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MH, Lee J, Nam YD, Lee JS, Seo MJ, Yi SH. Characterization of antimicrobial lipopeptides produced by Bacillus sp. LM7 isolated from chungkookjang, a Korean traditional fermented soybean food. Int J Food Microbiol. 2016;221:12–18. doi: 10.1016/j.ijfoodmicro.2015.12.010. [DOI] [PubMed] [Google Scholar]
- Lim JH, Jeong HY, Kim SD. Characterization of the bacteriocin J4 produced by Bacillus amyloliquefaciens j4 isolated from Korean traditional fermented soybean paste. J Appl Biol Chem. 2011;54:468–474. doi: 10.3839/jksabc.2011.072. [DOI] [Google Scholar]
- Liu XF, Li Y, Li JR, Cai LY, Li XX, Chen JR, Lyu SX. Isolation and characterisation of Bacillus spp. antagonistic to Vibrio parahaemolyticus for use as probiotics in aquaculture. World J Microbiol Biotechnol. 2015;31:795–803. doi: 10.1007/s11274-015-1833-2. [DOI] [PubMed] [Google Scholar]
- Meidong R, Doolgindachbaporn S, Jamjan W, Sakai K, Tashiro Y, Okugawa Y, Tongpim S. A novel probiotic Bacillus siamensis B44v isolated from Thai pickled vegetables (Phak-dong) for potential use as a feed supplement in aquaculture. J Gen Appl Microbiol. 2017;63:246–253. doi: 10.2323/jgam.2016.12.002. [DOI] [PubMed] [Google Scholar]
- Moyne AL, Shelby R, Cleveland TE, Tuzun S. Bacillomycin D: an iturin with antifungal activity against Aspergillus flavus. J Appl Microbiol. 2001;90:622–629. doi: 10.1046/j.1365-2672.2001.01290.x. [DOI] [PubMed] [Google Scholar]
- Niazi A, Manzoor S, Asari S, Bejai S, Meijer J, Bongcam-rudloff E. Genome analysis of Bacillus amyloliquefaciens subsp. plantarum UCMB5113: a rhizobacterium that improves plant growth and stress management. PLoS One. 2014;9:e104651. doi: 10.1371/journal.pone.0104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak KV, Keharia H. Identification of surfactins and iturins produced by potent fungal antagonist, Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) tree using mass spectrometry. 3 Biotech. 2014;4:283–295. doi: 10.1007/s13205-013-0151-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Sanchez T, Ruiz-Zarzuela I, Blas ID, Balcazar JL. Probiotics in aquaculture: a current assessment. Rev Aquac. 2014;6:133–146. doi: 10.1111/raq.12033. [DOI] [Google Scholar]
- Ramarathnam R, Bo S, Chen Y, Dilantha Fernando WG, Gao XW, Kievit TD. Molecular and biochemical detection of fengycin- and bacillomycin D-producing Bacillus spp., antagonistic to fungal pathogens of canola and wheat. Can J Microbiol. 2007;53:901–911. doi: 10.1139/W07-049. [DOI] [PubMed] [Google Scholar]
- Ravindran C, Varatharajan GR, Rajasabapathy R (2016) Antibacterial activity of marine Bacillus substances against Vibrio cholerae and Staphylococcus aureus and in vivo evaluation using embryonic zebrafish test system. 78:417–422
- Shi BH, Zheng H, Huang JZ, Luo XZ, Luo XL. Purification and partial characterization of a thermostable antimicrobial protein from Bacillus subtilis FB123. World J Microbiol Biotechnol. 2015;31:1285–1290. doi: 10.1007/s11274-015-1871-9. [DOI] [PubMed] [Google Scholar]
- Silva EF, Soares MA, Calazans NF, Vogeley JL, Valle BC, Soares R, Peixoto S. Effect of probiotic (Bacillus spp.) addition during larvae and postlarvae culture of the white shrimp Litopenaeus vannamei. Aquac Res. 2012;44:13–21. doi: 10.1111/j.1365-2109.2011.03001.x. [DOI] [Google Scholar]
- Stein T. Bacillus subtilis antibiotics: structures, syntheses and specific functions. Mol Microbiol. 2005;56:845–857. doi: 10.1111/j.1365-2958.2005.04587.x. [DOI] [PubMed] [Google Scholar]
- Sumi CD, Yang BW, Yeo I-C, Hahm YT. Antimicrobial peptides of the genus Bacillus: a new era for antibiotics. Can J Microbiol. 2015;61:93–103. doi: 10.1139/cjm-2014-0613. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Amaki Y, Ishihara A, Nakajima H. Synergistic effects of [Ile7]surfactin homologues with bacillomycin D in suppression of gray mold disease by Bacillus amyloliquefaciens biocontrol strain SD-32. J Agric Food Chem. 2015;63:5344–5353. doi: 10.1021/acs.jafc.5b01198. [DOI] [PubMed] [Google Scholar]
- Touraki M, Frydas I, Karamanlidou G, Mamara A. Partial purification and characterization of a bacteriocin produced by Bacillus subtilis NCIMB 3610 that exhibits antimicrobial activity against fish pathogens. J Biol Res. 2012;18:310–319. [Google Scholar]
- Xiu PY, Liu R, Zhang DC, Sun CM. Pumilacidin-like lipopeptides derived from marine bacterium Bacillus sp. strain 176 suppress the motility of Vibrio alginolyticus. Appl Environ Microbiol. 2017;83:e00450–e00417. doi: 10.1128/AEM.00450-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu DF, Wang YL, Sun LJ, Liu HM, Li JR. Inhibitory activity of a novel antibacterial peptide AMPNT-6 from Bacillus subtilis against Vibrio parahaemolyticus in shrimp. Food Control. 2013;30:58–61. doi: 10.1016/j.foodcont.2012.07.025. [DOI] [Google Scholar]
- Xu HM, Rong YJ, Zhao MX, Song B, Chi ZM. Antibacterial activity of the lipopetides produced by Bacillus amyloliquefaciens M1 against multidrug-resistant Vibrio spp. isolated from diseased marine animals. Appl Microbiol Biotechnol. 2014;98:127–136. doi: 10.1007/s00253-013-5291-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Li X, Li X, Yu HM, Shen ZY. Identification of lipopeptide isoforms by MALDI-TOF-MS/MS based on the simultaneous purification of iturin, fengycin, and surfactin by RP-HPLC. Anal Bioanal Chem. 2015;407:2529–2542. doi: 10.1007/s00216-015-8486-8. [DOI] [PubMed] [Google Scholar]
- Zhang B, Dong CJ, Shang QM, Cong Y, Kong WJ, Li PL. Purification and partial characterization of bacillomycin L produced by Bacillus amyloliquefaciens K103 from lemon. Appl Biochem Biotechnol. 2013;171:2262–2272. doi: 10.1007/s12010-013-0424-7. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Yu HR, Tong T, Tong WP, Dong LF, Xu MZ, Wang ZC. Dietary supplementation of Bacillus subtilis and fructooligosaccharide enhance the growth, non-specific immunity of juvenile ovate pompano, Trachinotus ovatus and its disease resistance against Vibrio vulnificus. Fish Shellfish Immunol. 2014;38:7–14. doi: 10.1016/j.fsi.2014.02.008. [DOI] [PubMed] [Google Scholar]
- Zhao J, Wu YX, Ho HH, Chen ZJ, Li XY, He YQ. PBT1, a novel antimicrobial protein from the biocontrol agent Bacillus subtilis XF-1 against Plasmodiophora brassicae. Eur J Plant Pathol. 2016;145:583–590. doi: 10.1007/s10658-016-0905-y. [DOI] [Google Scholar]