Abstract
The objective of this study was to isolate the lactic acid bacteria from fermented silage sample and analyze their antibacterial activities, probiotic properties, and fermentation potential in silage. Eleven lactic acid bacteria (LAB) were selected based on distinct morphologies and preliminary studies. Cell-free supernatant (CFS) was then prepared from the selected strains for antibacterial analysis. L-30 strain and its CFS showed highest inhibition (> 10 mm) against tested foodborne pathogens as compared to other strains. Hereafter, the strain L-30 was named as KCC-30 and used for further studies. KCC-30 can survive in the harsh conditions of GIT such as low pH ( 2) and bile salt environment (oxgal) than standard L. plantarum KACC-91016 (pH 2: 27.2% vs 20.5%; oxgal: 72.3% vs 57.7%, both p < 0.05). In addition, KCC-30 exhibited strong auto-aggregation (68.3% vs 51.5%) and co-aggregation (33% vs 23.9%) properties. For silage experiment, KCC-30 treatment did not alter the nutrient profiles of silage. At the same time, KCC-30 treatment increased the lactic acid content of silage as compared to untreated silage (5.55 DM% vs 3.11 DM%). An increase of lactic acid content in the silage is due to higher lactic acid bacteria population in KCC-30 treated silage (15.33 × 107 CFU/g vs 7.66 × 107 CFU/g) than untreated silage (p < 0.05). Overall data suggested that KCC-30 exhibited strong probiotic potential and improved the quality of Lolium multiflorum silage by increasing the lactic acid level. Therefore, KCC-30 could be considered as potential strain to improve the fermentation quality of L. multiflorum silage.
Electronic supplementary material
The online version of this article (10.1007/s13205-018-1449-y) contains supplementary material, which is available to authorized users.
Keywords: Lactic acid bacteria, Low pH, Bile salt, Co-aggregation, Auto-aggregation, Silage fermentation
Introduction
Probiotic research has been mainly focused on lactic acid bacteria over the past decade; most attention has been giving to the Lactobacillus species (Wasko et al. 2012). Notably, the lactic acid bacteria (LAB) have the potential food and pharmaceutical industry applications due to their widespread health benefits. For example, the amylolytic LAB strain is used for lactic acid production directly from starch and improves the nutritional characteristics of cereal sourdoughs (Wasko et al. 2011). LAB strains such as Lactobacillus plantarum can survive in the diverse stress conditions such as bile salt environment, high temperatures, low pH, and oxygen tension. These are important criteria for choosing good probiotic strains. Recently, advanced molecular biology and analytical techniques are being used for the selection of appropriate probiotic strain (Yadav and Shukla 2017).
Recently, the practices of adding lactic acid bacteria to the fermentation process have been increased instead of chemical usage. Beneficial microbes and their secondary metabolites could be considered as alternates of toxic chemicals in food fermentation (Altay et al. 2013). Among diverse bacterial strains that produce bacteriocins and organic acids, Lactobacillus plantarum is a suitable candidate for such purpose because L. plantarum can produce antimicrobial agents such as hydrogen peroxide, organic acids, bacteriocins, diacetyl, and antimicrobial peptides (Arena et al. 2016) which suppress growth of pathogenic microorganisms in the gut (Yadav et al. 2016b). Furthermore, it can co-aggregate with pathogens that prevent the colonization of pathogens in the gut (Yadav et al. 2016a).
Animal feeds show a demanding role in the global food industry to confirm the availability of quality animal proteins for millions of people around the world. Most of the livestock animals consume crop-bedding material such as straw or wood shavings as feed ingredients (Bajagai et al. 2016). On the other hand, animals consuming crop-bedding material in the form of silage is an effective method to compensate feed demand. Silage inoculated with lactic acid bacteria had higher levels of dry-matter intake, feed efficiency, and weight gain compared with animals fed with untreated silage(Ali et al. 2017). Consumption of LAB as a probiotic supplement improves ruminal health by balancing its intestinal microbes. Similarly, the lactic acid bacteria L. buchneri as an inoculant alters fermentation patterns of maize silage leading to an increase in feed efficiency (Rabelo et al. 2018). The purpose of this study was to isolate and characterize potential bacterial strains from fermented silage (FS) and investigate their probiotic properties and fermentation potential in Lolium multiflorum silage for ruminant feed preparation.
Materials and methods
Sample collection and bacterial isolation
Fermented silage samples were collected from the National Institute of Animal Science, Cheonan, South Korea. One gram of sample was serially diluted (107) using sterile distilled water. Isolation of bacteria was processed as described previously (Ilavenil et al. 2015).
Cell-free supernatant (CFS) preparation
The pure bacterial colony was inoculated into MRS broth and incubated at 37 °C for 24 h. After 24 h, the sample was centrifuged at 8000g for 10 min at 4 °C. Cell-free supernatant (CFS) was collected and filtered through a sterilized 0.22 µm filter (Millipore, USA) and the sterile CFS was stored at 4 °C for further study.
Details of purchased bacterial strains and antagonistic screening
Food-spoiling bacterial strains such as Escherichia coli, Enterococcus faecalis, Staphylococcus aureus, Pseudomonas aeruginosa, and the standard Lactobacillus plantarum 91016 were obtained from the Korean agricultural culture collection (KACC). E. coli, S. aureus, and P. aeruginosa were cultured in tryptone soy broth (TSB) at 37 °C for 20 h while bacterial strains of E. faecalis and L. plantarum were grown in brain heart infusion broth (BHI) and MRS broth, respectively, at 37 °C for 20 h. All strains were standardized to an optical density of 0.3 at wavelength of 600 nm except Lactobacillus plantarum 91016. The optical density of Lactobacillus plantarum was adjusted to 1.0 at 600 nm. Antagonistic activity was examined by agar spot test and well diffusion method as described previously (Gaudana et al. 2010; Adeniyi et al. 2015).
Scanning electron microscopy (SEM) and molecular identification using 16s rRNA sequencing
SEM was performed as described in our previous paper (Arasu et al. 2014b) to identify the shape of KCC-30. Gene sequencing of 16s rRNA was carried out at Solgent Co (Seoul. South Korea). Genomic DNA of the selected bacterial strain was purified using QIAquick® kit (Qiagen Ltd, Crawley, UK). Amplicons were sequenced using universal primers 27 F (5′ AGA GTT TGA TCG TGG CTC AG 3′) and 1492 R (3′ GCT TAC CTT GTT ACG ACT T 5′). Aligned 16s rRNA sequence of KCC-30 was BLAST searched with non-redundant sequence database of NCBI GenBank. After BLAST search and species identification, the 16s rRNA sequences were trimmed using NCBI tools. The sequences were then submitted to NCBI according to their procedure and obtained the accession number KP091746.1.
Probiotic screening of KCC-30
In this study, the probiotic parameters such as acid tolerance, bile salt tolerance, auto- and co-aggregation and cell surface hydrophobicity properties (Srigopalram et al. 2017).
Growth and fermentation potential of KCC-30 in Lolium multiflorum and Medicago sativa soup at different aerobic conditions
Soup preparation
Lolium multiflorum and Medicago sativa were collected at National Institute of Animal Science, South Korea and cut into small pieces of about 5–10 cm in length. These forage samples were then ground and sterilized using ethylene oxide. Each sterile forage powder sample (300 g) was then mixed with 1000 ml of sterile distilled water separately and kept in a shaker (150 rpm) at room temperature for 24 h. Forage soups were then sequentially subjected to filtration through muslin cloth (15 layers), Whatman No. 1 filter paper, 0.4 µM membrane filter, and 0.22 µM membrane filter for sterilization. Sterile filtrate was then stored at 4 °C for further analysis.
Lactobacilli inoculation to forage soup and growth profile measurement
L. plantarum KCC-30 and KACC-91016 were inoculated in MRS broth at optimal conditions (37 °C) for 20 h and standardized to an optical density of 1.0 (600 nm). From this, 100 µl of each culture was inoculated into 250 ml conical flask containing different forage soups (50 ml) and incubated under different aerobic conditions (aerobic, microaerobic, and anaerobic) at 37 °C for 48 h. The growth of Lactobacilli was measured using Bio-Rad iMark microplate reader (USA). Then pH values of respective forage soups at different conditions were noted after 24 h of inoculation using an InoLab pH meter.
Estimation of organic acids
The production of organic acid (lactic acid and acetic acid) was estimated using HPLC equipped with a UV detector (HPLC auto sampler, Agilent 1100 Series. USA). The sample filtrate was prepared using Whatman no 0.6 and 0.22 µM syringe filter. After that, the filtrate was injected to HPLC. Organic acids were separated on Zorbax XDB C-18 column (LC Column 250 × 4.6 mm. Agilent Technologies. USA) utilizing 0.005 M sulphuric acid (pH 2.4) under isocratic elution. Aliquots of 10 µl injection volume were chromatographically separated at flow rate of 0.7 ml/min and column temperature of 45 °C. The total run time was 30 min and the wavelength used for organic acid detection was 210 nm (Arasu et al. 2014a).
Effect of KCC-30 as inoculant on Lolium multiflorum silage quality enhancement and nutrient profile
Young Lolium multiflorum samples were collected at National Institute of Animal Science, South Korea. About 250 g of Lolium multiflorum were weighed and cut into small pieces (5–10 cm in length). The experiment setup had two groups: non-inoculated and KCC-30 (1 × 106 CFU/g) inoculated group. The air inside the silage bag was sucked out and anaerobic condition was maintained. In the same way, triplicate samples were prepared and stored at room temperature for 45 days. Silage nutrient profile such as crude protein (CP), neutral detergent fiber (NDF), acid detergent fiber (ADF), and total digestible nutrient (TDN) was estimated using standard protocols as described in our previous study (Srigopalram et al. 2017).
Silage microbial profile analyses
Ten grams of silage sample was soaked in 90 ml of sterile distilled water and placed in an orbital shaker (150 rpm) at 37 °C for 90 min. Tenfold serial dilution was made using sterile distilled water. Serially diluted (107) silage sample of 100 µl was spread onto MRS agar media and incubated at 37 ± 1 °C for 48 h. For counting yeasts and molds, 3M petrifilm (3M Microbiology products, St. Paul, USA) was used. Potato dextrose agar (Difco) was used for fungal enumeration.
Statistical analysis
All numerical data of this experiment were obtained from triplicate analysis. All statistical analyses were carried out using SPSS software (SPSS-16 Inc, Chicago, IL, USA). One-way analysis of variance (ANOVA) was performed to analyze significant difference between samples. Student’s t test was used to determine treatment mean difference at 5% probability level. Results are presented as mean ± SD.
Results
Preliminary screening
Thirty bacterial strains were isolated from fermented silage using MRS agar media specific for lactic acid-producing bacterial growth. Based on distinct morphologies and preliminary studies, 11 strains and their CFSs were used for screening antagonistic activity against food-spoiling bacteria.
Antibacterial activity of isolated bacteria
Agar spot method
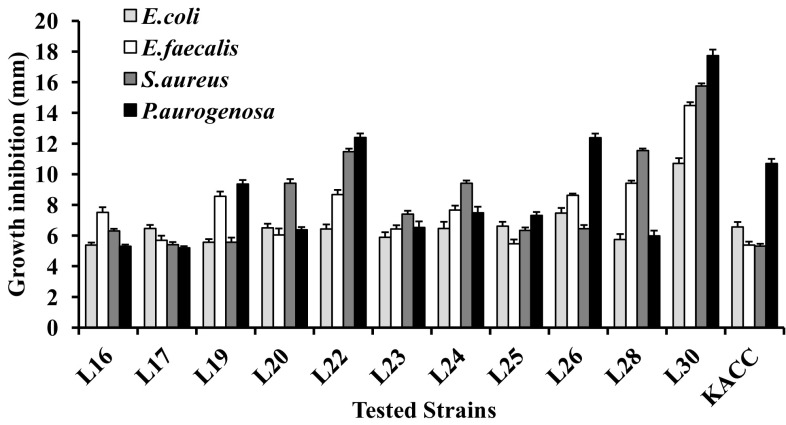
In agar spot assay, most of these isolated bacterial strains and the standard KACC-91016 strain showed significant inhibitory activity (> 5 mm) against foodborne pathogens tested. Among these isolated strain, L30 showed a more intensive antagonistic pattern (> 14 mm inhibition) against E. faecalis, S. aureus, and P. aeruginosa than other isolated strains. It exhibited the maximum inhibition against P. aeruginosa (17.74 mm). Furthermore, strain L30 overtook the inhibition potential of standard L. plantarum KACC-91016 against all tested pathogens (Fig. 1).
Fig. 1.
Antibacterial activity of selected L. plantarum by agar spot assay. Antibacterial activity was determined by zone of inhibition (mm) around the bacterial spot. The zone of inhibition was expressed as mean ± SD of three replicates
Well diffusion assay and molecular identification of bacteria
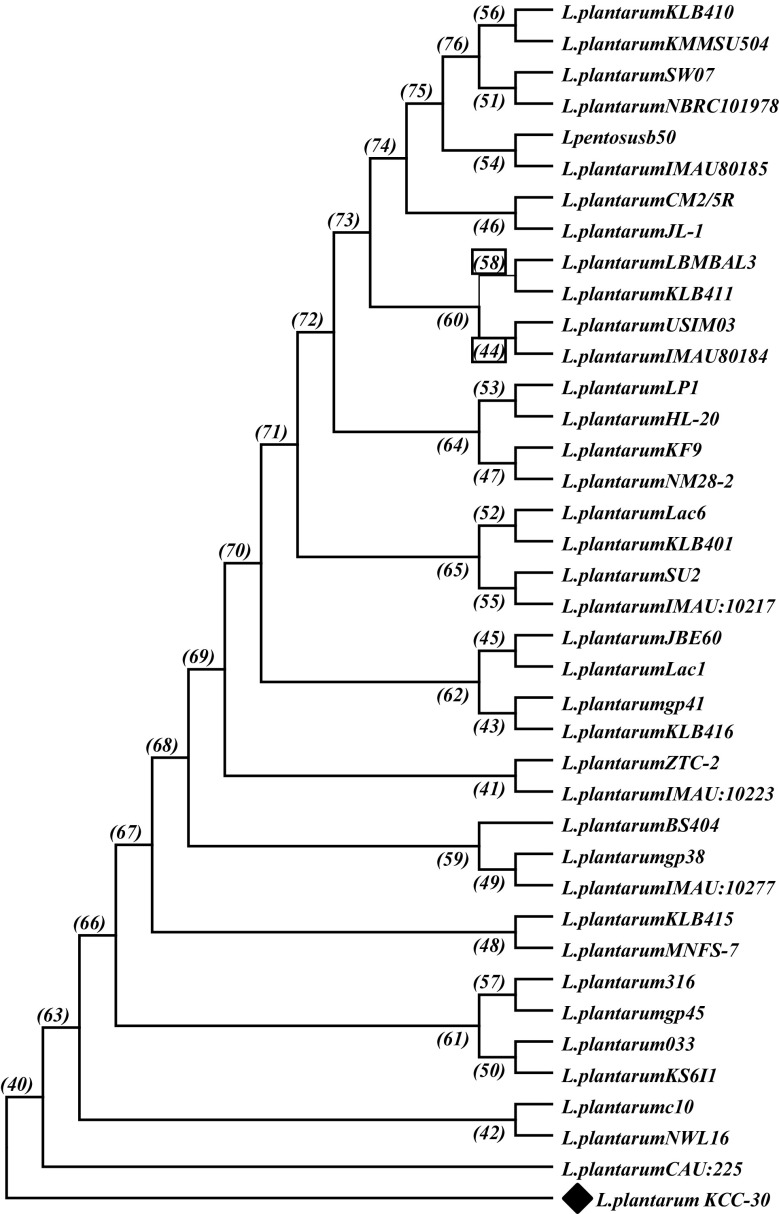
The antagonistic activity of CFS of isolated strains was tested by well diffusion method. CFSs of L23, L30, and KACC-91016 exhibited the maximum inhibitory activity against S. aureus (11.3 mm), P. aeruginosa (16.3 mm), and E. faecalis (8.8 mm), respectively. However, L-30 CFS maintained its higher inhibition pattern (> 10 mm) in zone size against all tested pathogens than other strains (Table 1). Based on antagonistic results, strain L30 selected for further study. Hereafter, it is called as KCC-30. SEM analysis (Supplementary Fig. 1) and gene sequencing of 16s rRNA of KCC-30 shared > 99% similarities with Lactobacillus plantarum. Hence, we constructed a phylogenic tree to demonstrate the relationship of L. plantarum KCC-30 with other closely related L. plantarum using 16S rRNA gene sequences (Fig. 2).
Table 1.
Antibacterial activity of CFS of Lactobacillus plantarum determined by well diffusion assay and expressed as size of inhibition zone around wells (mm)
| Strains name | E. coli a | E. faecalis a | S. aureus a | P. aeruginosa a |
|---|---|---|---|---|
| L16 | 4 | 4.3 | 4.9 | 4.2 |
| L17 | 4.1 | 4.3 | 4.3 | 5.2 |
| L19 | 7 | 8.3 | 6 | 5.3 |
| L20 | 6.1 | 9.2 | 7.2 | 6.7 |
| L22 | 5.3 | 10.6 | 9.4 | 12.3 |
| L23 | 5.3 | 9.5 | 11.3 | 6.2 |
| L24 | 8.3 | 8.2 | 9.7 | 7.9 |
| L25 | 9.6 | 6.6 | 10.5 | 9.4 |
| L26 | 6.8 | 12.5 | 7.5 | 9.3 |
| L28 | 7.5 | 9.5 | 7.4 | 5.6 |
| L30 | 10.3 | 12.5 | 14.4 | 16.3 |
| KACC-91016 | 7.7 | 8.8 | 7.3 | 8.7 |
aMean values of inhibition zone of respective bacterial strains against tested pathogens in mm
Fig. 2.
Phylogenetic tree construction using neighbor-joining method based on 16S rRNA sequences of strain KCC-30 and other closely related Lactobacillus plantarum. Bullet indicates isolate of the present study
Probiotic properties of KCC-30
Acid tolerance assay
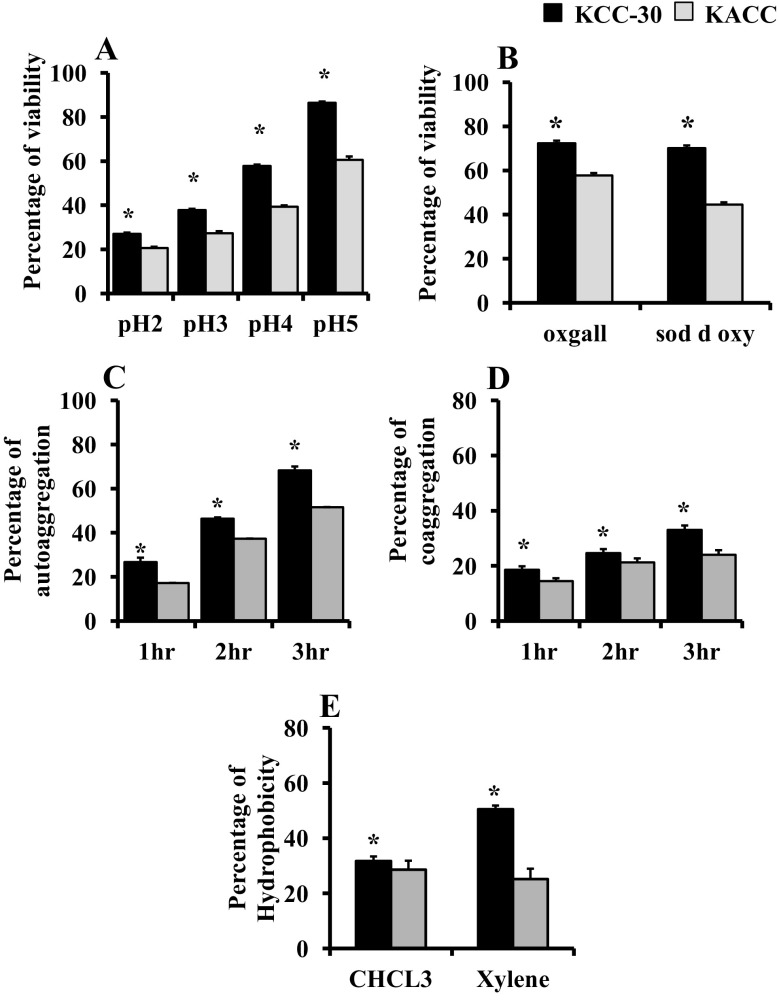
Acid tolerance is considered as a significant feature for probiotic strain selection. Hence, we prepared MRS broth with various pH conditions (pH 2 to pH 5) and inoculated KCC-30. The results demonstrated that KCC-30 significantly survived in the harsh pH condition (pH-2; 27%) than KACC-91016 (Fig. 3a). KCC-30 produced different types of industrially important enzymes. KCC-30 possesses more sensitivity against commonly used antibiotics (Supplementary Table 1 and Table 2).
Fig. 3.
Stress tolerance and adhesion properties of KCC-30. a Acid tolerance analysis; b bile tolerance analysis; c auto-aggregation analysis; d co-aggregation analysis; e hydrophobicity of Lactobacillus plantarum (KCC-30 and KACC-91016). *Statistical significance at p < 0.05
Bile salt tolerance assay
KCC-30 tolerated bile salt environment than the KACC-91016. Bile salt tolerance rate of KCC-30 and KACC-91016 were 72.3% and 57.75%, respectively, in MRS broth supplemented with 0.3% oxgall. Also, KCC-30 showed significant survival ability in bile salt sodium deoxycholate-supplemented MRS broth (Fig. 3b).
Aggregation and hydrophobicity analysis
KCC-30 exhibited significant auto-aggregation properties. The percentage of auto- aggregation was increased in a time dependent manner. Percentages of auto-aggregation of KCC-30 at 1, 2, and 3 h were 26.69 ± 1.98, 46.30 ± 0.59, and 68.30 ± 1.74, respectively (Fig. 3c). Co-aggregation of KCC-30 showed significant increases with increasing incubation time than KACC-91,016 (Fig. 3d). In addition, KCC-30 showed significant adhesion properties to hydrocarbons such as chloroform and xylene than KACC-91016. KCC-30 showed the maximum adhesion towards hydrocarbon xylene (Fig. 3e).
Fermentation potential of KCC-30
Growth of KCC-30 in Lolium multiflorum and Medicago sativa forage soup under different conditions
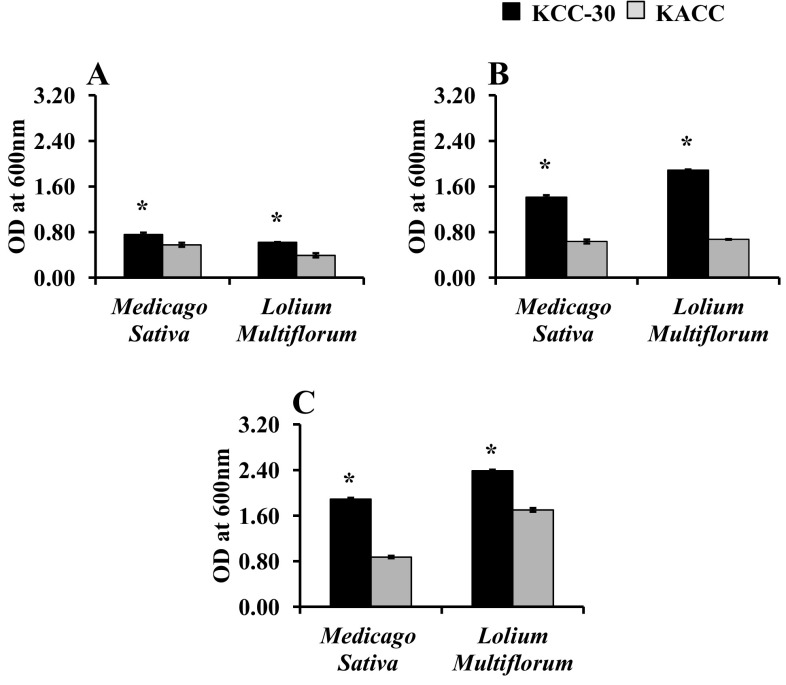
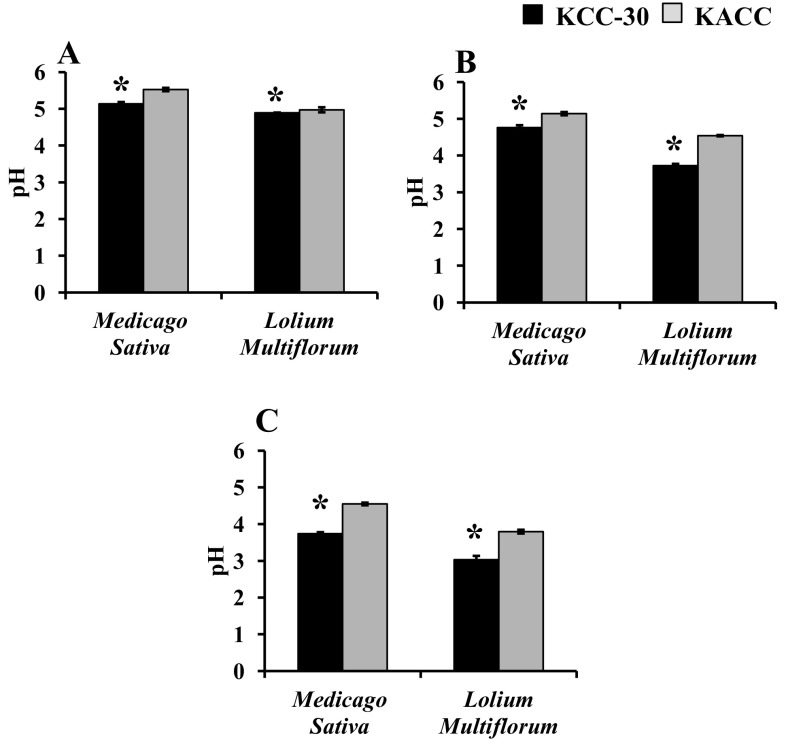
Medicago sativa and L. multiflorum forage soups were used to ferment with KCC-30 or KACC-91016 for 48 h under different conditions. As a result, KCC-30 achieved faster growth under anaerobic condition than aerobic conditions (Fig. 4a–c). In addition, KCC-30 overtook the growth rate of KACC-91016 in both forage soups. However, KCC-30 achieved faster growth in L. multiflorum soup than that in M. sativa forage soup under anaerobic condition (Fig. 4c). Furthermore, KCC-30 significantly decreased the pH of both forage soups under anaerobic condition than KACC-91016 (Fig. 5a–c). The maximum pH reduction was noted in KCC-30-inoculated L. multiflorum soup under anaerobic condition (Fig. 5c).
Fig. 4.
Growth profile of Lactobacilli strains (KCC-30 and KACC-91016) on forage soups (Medicago Sativa and Lolium Multiflorum) under different growth conditions. a Aerobic condition; b microaerobic condition; c anaerobic condition. *Statistical significance at p < 0.05
Fig. 5.
pH changes in Lactobacilli (KCC-30 and KACC-91016)-inoculated and control forage soups under different growth conditions. a Aerobic condition; b microaerobic condition; c anaerobic condition. *Statistical significance at p < 0.05
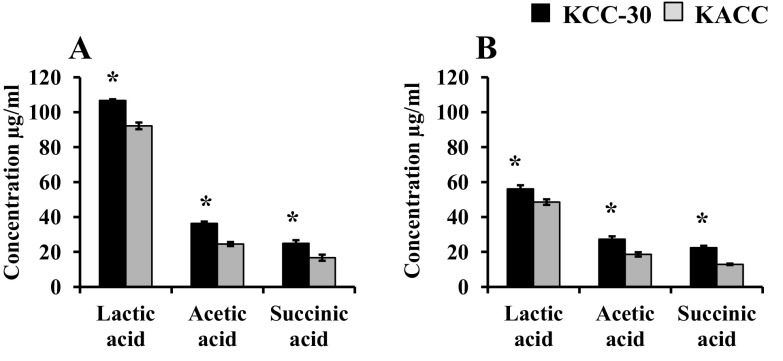
KCC-30 showed predominant growth under all conditions tested. However, the maximum growth rate of KCC-30 was exhibited under an anaerobic condition. Hence, we estimated the organic acid content of anaerobic forage soups. KCC-30 achieved higher lactic acid production in L. multiflorum soup (106.7 µg/ml; Fig. 6a) than that in M. sativa soup (56.07 µg/ml; Fig. 6b). At the same time, KCC-30 produced low amounts of acetic acid and succinic acid in both soups.
Fig. 6.
Estimation of organic acid production in Lactobacilli (KCC-30 and KACC-91016)-inoculated and un-inoculated forage soups under aerobic growth condition. a Lolium multiflorum; b Medicago sativa. *Statistical significance at p < 0.05
Application of KCC-30 as inoculant for improving L. multiflorum silage quality
Based on anaerobic fermentation characteristics of KCC-30 in L. multiflorum soup, L. multiflorum silage was prepared using KCC-30. L. multiflorum forage was supplemented with KCC-30 and then kept under anaerobic condition. The silage quality was examined after 45 days. Silage nutrient profiles such as CP, ADF, NDF, and TDN levels were not altered by the addition of KCC-30 as compared to those of the non-inoculated control group (Table 2).It indicates that addition of KCC-30 did not alter the native form of plant nutrient profiles. KCC-30-inoculated group showed the significant increase in LAB population as compared to non-inoculated control group (Table 3). Furthermore, no yeast or mold growth was noted in KCC-30-inoculated group whereas the non-inoculated control group showed significant yeast growth (23.66 × 103 CFU/g). Subsequently, KCC-30-inoculated group resulted in significant increase in lactic acid production compared to the non-inoculated control group. In addition, the non-inoculated silage control group showed the highest butyric acid production while the KCC-30-inoculated group showed almost no butyric acid production (Table 4).
Table 2.
Nutritive value of Lolium multiflorum silage according to inoculation of lactic acid bacteria
| Treatment | CP (%) | ADF (%) | NDF (%) | TDN (%) |
|---|---|---|---|---|
| Non-inoculated | 9.61 | 33.29 | 52.28 | 62.60 |
| LP KCC-30-inoculated | 9.22 | 30.82 | 49.71 | 65.52 |
LP Lactobacillus plantarum, CP crude protein, ADF acid detergent fiber, NDF neutral detergent fiber, TDN total digestible nutrient
Table 3.
Changes of microbes on Lolium multiflorum silage according to inoculation of lactic acid bacteria
| Treatment | LAB (× 107 CFU/g) | Yeast (× 103 CFU/g) | Fungi (× 103 CFU/g) |
|---|---|---|---|
| Non-inoculated | 7.66b | 23.66 | 0 |
| LP KCC-30-inoculated | 15.33a | 0 | 0 |
LP Lactobacillus plantarum, CFU colony forming unit
a,bValues with different letters within a column are significantly different at 5% level
Table 4.
pH changes in experimental Lolium multiflorum silages according to inoculation of lactic acid bacteria
| Treatment | pH | Lactic acid (DM%) | Acetic acid (DM%) | Butyric acid (DM%) | Flieg’s score |
|---|---|---|---|---|---|
| Non-inoculated | 4.82a | 3.11a | 0.33 | 0.97a | 58 |
| LP KCC-30-inoculated | 3.69b | 5.55b | 0.39 | 0.09b | 100 |
LP Lactobacillus plantarum, DM dry matter
a,bValues with different letters within a column are significantly different at 5% level
Discussion
L. Plantarum is a well-known beneficial microbe for its involvement in fermentation and preservation of food products by organic acid production (Haghshenas et al. 2015). Consequently, L. Plantarum strains have contributed to the improvement of dairy products, sausage, fish, fermented vegetables, as well as silage to improve the flavor, texture, and nutritional value of food products (Giraffa et al. 2010). Hence, we need to identify the new wild-type L. plantarum strains from a natural source is increased in recent decades (Hursting and Dunlap 2012). Based on the present study, 30 lactic acid bacterial strains were isolated from FS sample. Among these, 11 strains were selected based on their distinct morphologies to test their antagonistic potential against food spoiling bacteria. Of these 11 strains, KCC-30 demonstrated remarkable antagonistic activity against food-spoiling bacteria. It could strengthen the usage of L. plantarum KCC-30 as perseverant in food material against bacterial spoilage through extracellular bacteriocins production (Yadav and Shukla 2017).
Interaction of lactic acid bacteria with the pathogen is considered an important criterion to delay or control pathogenic growth because pathogen like S. aureus conveys pathogenesis through colonization of the infection site (Yadav et al. 2016a). Pathogenic growth control by KCC-30 seems reasonable to confirm that the contact between KCC-30 and the tested pathogen is good during antimicrobial activities. Fermented food that contains lactic acid bacteria, mainly L. plantarum, could protect the host against pathogenic microbes by ingestion, stimulate the metabolism, and increase vitamin production in the intestine (Al Kassaa et al. 2014). CFS of bacterial origin may include diverse molecules that are useful for screening antagonistic potential against pathogenic microbes (Wang et al. 2014). In the present study, CFSs of KCC-30 and KACC-91016 showed potent inhibition against four pathogens tested in agar well diffusion assay. However, KCC-30 showed stronger inhibition than the standard L. plantarum KACC-91016. Growth inhibition of pathogens by lactic acid bacteria demonstrates the production of antimicrobial compounds and its synergistic effect against undesirable bacterial growth (Zhang et al. 2016). Similarly, our findings revealed the involvement of extracellular secretion in the antagonistic potential of KCC-30 against tested pathogens.
Tolerance of lactic acid bacteria at high concentrations of bile salts and low pH is an important prerequisite for probiotic screening. Stress-tolerant lactic acid bacteria can afford beneficial properties to the host (Valan Arasu et al. 2015). The survival rate of Lactobacilli in low-pH condition mainly depends on the activity of the enzyme. Furthermore, constant gradient extracellular and cytoplasmic pH plays a significant role in acid tolerance of Lactobacilli. Sugars like glucose play significant role in increasing intracellular pH of Lactobacilli, thereby enhancing the survival of lactic acid bacteria at low extracellular pH harsh condition (Park and Lim 2015). Similarly, KCC-30 showed significantly survival in the acidic environment (pH 2 and pH 3) than KACC-91016, in agreement with the notion that acid tolerance of lactic acid bacteria depends on outer membrane potential to survive under stress conditions (Yadav et al. 2016a). KCC-30 exhibited positive Bsh activity. It suggests that bile salt resisting genes might be expressed in KCC-30. These genes conserve cell wall and cell membrane integrity of L. plantarum against high-bile salt environment (Alcantara and Zuniga 2012). Bsh-positive nature of bacterial strain is an important probiotic feature since it can remove cholesterol. Bile salt-tolerant strains can deconjugate the bile salts, thus providing defense against the host immune system (Yadav et al. 2016b). Subsequently, it changes the hydrophobicity and fluidity of the bacterial outer membrane (Ilavenil et al. 2016). Our results revealed that KCC-30 could be considered as a tolerant strain under harsh GIT conditions.
Adhesion is an important property of probiotic strains. It helps the probiotic strains to interact with membranes of other microorganisms (Nejati et al. 2016). Additionally, high surface hydrophobicity and aggregation are needed for colonization of probiotics in the intestinal tract. In this study, KCC-30 strain showed stronger adhesion to hydrocarbons chloroform and xylene than KACC-91016. Particularly, KCC-30 strain exhibited more adhesion towards hydrocarbon xylene. Auto-aggregation can also prevent pathogenic bacteria from forming biofilm so that pathogenic bacteria could be removed from the GI tract. KCC-30 demonstrated substantial auto-aggregation property. In addition, its rate of auto-aggregation was increased with increasing time (Ng et al. 2015). Co-aggregation of beneficial bacterial strain with pathogen can remove pathogen by producing antimicrobial substances. Furthermore, co-aggregation with the pathogens could prevent colonization of pathogen in the gut (Ilavenil et al. 2016). The co-aggregation property of KCC-30 suggests that it might have the potential to interact with pathogens.
Although bacterial-CFS contains various antagonistic components, organic acids (mainly lactic acid) are its potential antimicrobial components (Park and Lim 2015). Generally, lactic acid bacterial strains produce higher amount of lactic acid than acetic acid. The amount of organic acid produced by LAB strain directly decides its fermentation quality (Chen et al. 2017). Similarly, in this study, KCC-30 fermented L. multiflorum forage soup under anaerobic condition better than that under aerobic conditions. Subsequently, HPLC analysis revealed that KCC-30 produced high level of lactic acid with less amount of acetic acid in the anaerobically fermented L. multiflorum forage soups. These results suggested that organic acid increases, mainly lactic acid, could be the reason for the strong antagonistic activity of KCC-30.
As described above, KCC-30 showed significant fermentation property of L. multiflorum soup that initiated us to analyze the effect of KCC-30 on L. multiflorum silage quality enhancement by its fermentation. Ensilation is the best preservation method for preserving forage crops for ruminant animals. Silage nutrient profile and quality mainly depend on the technological factor used (Sucu et al. 2016). Chemical additives in silage can enhance aerobic spoilage without producing enough volatile fatty acids (Filya 2003b). However using L. plantarum as additives has various advantages, such as easy to use, eco-friendly, and non-corrosive to farm equipment (Filya 2003a). Nutrient profiles in the experimental silage such as CP, ADF, NDF, and TDN levels were not significantly altered. It indicates the addition of KCC-30 could not affect the native form of nutrients in the plant. These results are consistent with the previous report (Zanine et al. 2016), showing that improved fermentation does not affect chemical compositions or reduce the nutritional value of the silage.
KCC-30 significantly lowers the pH of the silage than the non-inoculated control group. These findings are consistent with our previous report (Srigopalram et al. 2017) that greater lactic acid bacteria growth can result in high lactic acid production and facilitate rapid silage fermentation. Furthermore, there was no butyric acid production in KCC-30 inoculated group, confirming the decrease in NH3-N formation (Cezário et al. 2015). Since microbial activities inhibit NH3-N, proteolysis of amino acids by Clostridia can also deactivate lactic acid bacteria (Ferreira et al. 2013). Both experimental silages showed lower acetic acid production. However, the non-inoculated group showed significantly higher amount of butyric acid production than KCC-30 inoculated group. Butyric acid production can lead to undesirable fermentation characteristics of silage. Undesirable fermentation affects the acceptability of silage by ruminants because of decreased palatability (Tabacco et al. 2009). However, KCC-30-inoculated silage group showed almost no butyric acid production, thus favoring desirable fermentation characteristics.
Conclusion
Our finding indicates that L. plantarum KCC-30 isolated from fermented silage sample possesses favorable anti-pathogenic activity, desirable tolerance to harsh GIT environment and acceptable probiotic skills. The requirement of quality animal proteins is escalating rapidly for millions of people around the world. Hence, increasing the feed intake of the ruminant is of primary need. In this study, L. plantarum KCC-30 improved silage fermentation with good smell without altering nutrient profiles in the L. multiflorum forage via increasing lactic acid and reducing acetic and butyric acid level. Hence, this strain could be considered as a better inoculant for quality silage preparation.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This work was carried out with the support of Cooperative Research Program for Agriculture Science & Technology Development (Project title: Development of Quality Improvement and Standardization Technique for Low Moisture Storage Forage, Project no. “PJ010916012017”) funded by Rural Development Administration, Republic of Korea. This study was also supported by 2014 Postdoctoral Fellowship Program of National Institute of Animal Science funded by Rural Development Administration, Republic of Korea.
Compliance with ethical standards
Conflict of interest
The authors have no conflicts of interest related to this study to disclose.
References
- Adeniyi BA, Adetoye A, Ayeni FA. Antibacterial activities of lactic acid bacteria isolated from cow faeces against potential enteric pathogens. Afr Health Sci. 2015;15(3):888–895. doi: 10.4314/ahs.v15i3.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Kassaa I, Hamze M, Hober D, Chihib NE, Drider D. Identification of vaginal lactobacilli with potential probiotic properties isolated from women in North Lebanon. Microb Ecol. 2014;67(3):722–734. doi: 10.1007/s00248-014-0384-7. [DOI] [PubMed] [Google Scholar]
- Alcantara C, Zuniga M. Proteomic and transcriptomic analysis of the response to bile stress of Lactobacillus casei BL23. Microbiology. 2012;158(Pt 5):1206–1218. doi: 10.1099/mic.0.055657-0. [DOI] [PubMed] [Google Scholar]
- Ali G, Liu Q, Yuan X, Dong Z, Desta ST, Li J, Bai X, Shah AA, Shao T. Characteristics of lactic acid bacteria isolates and their effects on the fermentation quality of acacia (Sophora japonica L.) leaf silage at low temperatures. Grassl Sci. 2017;63(3):141–149. doi: 10.1111/grs.12162. [DOI] [Google Scholar]
- Altay F, Karbancıoglu-Güler F, Daskaya-Dikmen C, Heperkan D. A review on traditional Turkish fermented non-alcoholic beverages: microbiota, fermentation process and quality characteristics. Int J Food Microbiol. 2013;167(1):44–56. doi: 10.1016/j.ijfoodmicro.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Arasu MV, Jung M-W, Kim DH, Ilavenil S, Jane M, Park HS, Al-Dhabi NA, Jeon BT, Choi KC. Enhancing nutritional quality of silage by fermentation with Lactobacillus plantarum. Indian J Microbiol. 2014;54(4):396–402. doi: 10.1007/s12088-014-0473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arasu MV, Jung MW, Ilavenil S, Kim DH, Park HS, Park JW, Al-Dhabi NA, Choi KC. Characterization, phylogenetic affiliation and probiotic properties of high cell density Lactobacillus strains recovered from silage. J Sci Food Agric. 2014;94(12):2429–2440. doi: 10.1002/jsfa.6573. [DOI] [PubMed] [Google Scholar]
- Arena MP, Silvain A, Normanno G, Grieco F, Drider D, Spano G, Fiocco D. Use of Lactobacillus plantarum strains as a bio-control strategy against food-borne pathogenic microorganisms. Front Microbiol. 2016 doi: 10.3389/fmicb.2016.00464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajagai YS, Klieve AV, Dart PJ, Bryden WL. Probiotics in animal nutrition—production, impact and regulation. Food Agric Organ U N. 2016;179(1):1–108. [Google Scholar]
- Cezário AS, Ribeiro KG, Santos SA, Valadares Filho SdC, Pereira OG. Silages of Brachiaria brizantha cv. Marandu harvested at two regrowth ages: microbial inoculant responses in silage fermentation, ruminant digestion and beef cattle performance. Anim Feed Sci Technol. 2015;208:33–43. doi: 10.1016/j.anifeedsci.2015.06.025. [DOI] [Google Scholar]
- Chen X, Zhuang Y, Dong Z, Zhang J. Factors influencing the distribution of lactic acid bacteria on Pennisetum grasses. Grassl Sci. 2017;63(3):150–158. doi: 10.1111/grs.12161. [DOI] [Google Scholar]
- Ferreira DdJ, Lana RdP, Zanine AdM, Santos EM, Veloso CM, Ribeiro GA. Silage fermentation and chemical composition of elephant grass inoculated with rumen strains of Streptococcus bovis. Anim Feed Sci Technol. 2013;183(1–2):22–28. doi: 10.1016/j.anifeedsci.2013.04.020. [DOI] [Google Scholar]
- Filya I. The effect of Lactobacillus buchneri and Lactobacillus plantarum on the fermentation, aerobic stability, and ruminal degradability of low dry matter corn and sorghum silages. J Dairy Sci. 2003;86(11):3575–3581. doi: 10.3168/jds.S0022-0302(03)73963-0. [DOI] [PubMed] [Google Scholar]
- Filya I. The effect of Lactobacillus buchneri, with or without homofermentative lactic acid bacteria, on the fermentation, aerobic stability and ruminal degradability of wheat, sorghum and maize silages. J Appl Microbiol. 2003;95(5):1080–1086. doi: 10.1046/j.1365-2672.2003.02081.x. [DOI] [PubMed] [Google Scholar]
- Gaudana SB, Dhanani AS, Bagchi T. Probiotic attributes of Lactobacillus strains isolated from food and of human origin. Br J Nutr. 2010;103(11):1620–1628. doi: 10.1017/s0007114509993643. [DOI] [PubMed] [Google Scholar]
- Giraffa G, Chanishvili N, Widyastuti Y. Importance of lactobacilli in food and feed biotechnology. Res Microbiol. 2010;161(6):480–487. doi: 10.1016/j.resmic.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Haghshenas B, Nami Y, Haghshenas M, Abdullah N, Rosli R, Radiah D, Yari Khosroushahi A. Bioactivity characterization of Lactobacillus strains isolated from dairy products. Microbiol Open. 2015;4(5):803–813. doi: 10.1002/mbo3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann N Y Acad Sci. 2012;1271(1):82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S, Park HS, Vijayakumar M, Arasu MV, Kim DH, Ravikumar S, Choi KC. Probiotic potential of lactobacillus strains with antifungal activity isolated from animal manure. Sci World J. 2015;2015:1–10. doi: 10.1155/2015/802570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilavenil S, Vijayakumar M, Kim daH, Valan Arasu M, Park HS, Ravikumar S, Choi KC. Assessment of probiotic, antifungal and cholesterol lowering properties of Pediococcus pentosaceus KCC-23 isolated from Italian ryegrass. J Sci Food Agric. 2016;96(2):593–601. doi: 10.1002/jsfa.7128. [DOI] [PubMed] [Google Scholar]
- Nejati F, Babaei M, Taghi-Zadeh A. Characterisation of Lactobacillus helveticus strains isolated from home-made dairy products in Iran. Int J Dairy Technol. 2016;69(1):89–95. doi: 10.1111/1471-0307.12223. [DOI] [Google Scholar]
- Ng SY, Koon SS, Padam BS, Chye FY. Evaluation of probiotic potential of lactic acid bacteria isolated from traditional Malaysian fermented Bambangan (Mangifera pajang) CyTA J Food. 2015;13(4):563–572. doi: 10.1080/19476337.2015.1020342. [DOI] [Google Scholar]
- Park S-Y, Lim S-D. Probiotic characteristics of Lactobacillus plantarum FH185 isolated from human feces. Korean J Food Sci Anim Resour. 2015;35(5):615–621. doi: 10.5851/kosfa.2015.35.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabelo CHS, Basso FC, Lara EC, Jorge LGO, Härter CJ, Mesquita LG, Silva LFP, Reis RA. Effects of Lactobacillus buchneri as a silage inoculant and as a probiotic on feed intake, apparent digestibility and ruminal fermentation and microbiology in wethers fed low-dry-matter whole-crop maize silage. Grass Forage Sci. 2018;73(1):67–77. doi: 10.1111/gfs.12303. [DOI] [Google Scholar]
- Srigopalram S, Park HS, Ilavenil S, Kim DH, Arasu MV, Kuppusamy P, Lee KD, Choi KC. Isolation, in vitro probiotic characterization of Lactobacillus plantarum and its role on italian ryegrass silage quality enhancement. Int J Agric Biol. 2017;19:164–170. doi: 10.17957/IJAB/15.0260. [DOI] [Google Scholar]
- Sucu E, Kalkan H, Canbolat O, Filya I. Effects of ensiling density on nutritive value of maize and sorghum silages. Rev Bras Zoot. 2016;45:596–603. doi: 10.1590/S1806-92902016001000003. [DOI] [Google Scholar]
- Tabacco E, Piano S, Cavallarin L, Bernardes TF, Borreani G. Clostridia spore formation during aerobic deterioration of maize and sorghum silages as influenced by Lactobacillus buchneri and Lactobacillus plantarum inoculants. J Appl Microbiol. 2009;107(5):1632–1641. doi: 10.1111/j.1365-2672.2009.04344.x. [DOI] [PubMed] [Google Scholar]
- Valan Arasu M, Jung MW, Kim DH, Park HS, Ilavenil S, Al-Dhabi NA, Choon Choi K. Identification and phylogenetic characterization of novel Lactobacillus plantarum species and their metabolite profiles in grass silage. Ann Microbiol. 2015;65(1):15–25. doi: 10.1007/s13213-014-0830-2. [DOI] [Google Scholar]
- Wang G, Zhao Y, Tian F, Jin X, Chen H, Liu X, Zhang Q, Zhao J, Chen Y, Zhang H, Chen W. Screening of adhesive lactobacilli with antagonistic activity against Campylobacter jejuni. Food Control. 2014;44:49–57. doi: 10.1016/j.foodcont.2014.03.042. [DOI] [Google Scholar]
- Wasko A, Polak-Berecka M, Targonski Z. Purification and characterization of pullulanase from Lactococcus lactis. Prep Biochem Biotechnol. 2011;41(3):252–261. doi: 10.1080/10826068.2011.575316. [DOI] [PubMed] [Google Scholar]
- Wasko A, Kieliszek M, Targonski Z. Purification and characterization of a proteinase from the probiotic Lactobacillus rhamnosus OXY. Prep Biochem Biotechnol. 2012;42(5):476–488. doi: 10.1080/10826068.2012.656869. [DOI] [PubMed] [Google Scholar]
- Yadav R, Shukla P. An overview of advanced technologies for selection of probiotics and their expediency: a review. Crit Rev Food Sci Nutr. 2017;57(15):3233–3242. doi: 10.1080/10408398.2015.1108957. [DOI] [PubMed] [Google Scholar]
- Yadav R, Puniya AK, Shukla P. Probiotic properties of Lactobacillus plantarum RYPR1 from an indigenous fermented beverage Raabadi. Front Microbiol. 2016;7:1683. doi: 10.3389/fmicb.2016.01683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R, Singh PK, Puniya AK, Shukla P. catalytic interactions and molecular docking of bile salt hydrolase (BSH) from L. plantarum RYPR1 and its prebiotic utilization. Front Microbiol. 2016;7:2116. doi: 10.3389/fmicb.2016.02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanine AdM, Bonelli EA, de Souza AL, Ferreira DdJ, Santos EM, Ribeiro MD, Geron LJV, Pinho RMA. Effects of Streptococcus bovis isolated from bovine rumen on the fermentation characteristics and nutritive value of tanzania grass silage. Sci World J. 2016;2016:8517698, 8512016. doi: 10.1155/2016/8517698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Xiang X, Lu Q, Zhang L, Ma F, Wang L. Adhesions of extracellular surface-layer associated proteins in Lactobacillus M5-L and Q8-L. J Dairy Sci. 2016;99(2):1011–1018. doi: 10.3168/jds.2015-10020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.