Abstract
Hepatic encephalopathy (HE) is a complex neuropsychiatric syndrome that occurs during chronic liver disease (CLD). While ammonia and other precipitating factors in liver disease including inflammation, bile acids, oxidative stress, and lactate play a role in the pathogenesis of HE, the exact mechanism that leads to HE is not fully understood. Notably, accumulating evidence points toward a synergic effect rather than independent actions among precipitating factors that contributes to the development and severity of HE in CLD. Hence, this review is aimed to briefly discuss the single and synergic interplay of pathological factors in the progression and severity of HE.
Keywords: hepatic encephalopathy, astrocyte swelling, brain edema, cirrhosis, ammonia, inflammation, neurotransmission, oxidative stress, bile acids, lactate
Abbreviations: AQP4, Aquaporin 4; BAs, Bile Acids; BBB, Blood-Brain Barrier; BDL, Bile Duct Ligation; cGMP, Cyclic Guanosine Monophosphate; CLD, Chronic Liver Disease; CSF, Cerebrospinal Fluid; GABA, Gamma-Aminobutyric Acid; GAMSAs, GABAA Receptor Modulating Steroid Antagonists; GFAP, Glial Fibrillary Acid Protein; GLAST, Glial Glutamate-Aspartate Transporter; GS, Glutamine Synthetase; GPR81, G-Protein-Coupled Receptor 81; HE, Hepatic Encephalopathy; ICP, Intracranial Pressure; ILs, Interleukins; mGluR, Metabotropic Glutamate Receptor; MRI, Magnetic Resonance Imaging; NMDA, N-Methyl-d-Aspartate Glutamate Receptor; NO, Nitric Oxide; NF-?B, Nuclear Factor Kappa B; PCA, Portacaval Anastomosis; ROS, Reactive Oxygen Species; TJ, Tight Junction; TNF-a, Tumor Necrosis Alpha
Hepatic encephalopathy (HE) is a common and debilitating neuropsychiatric complication observed in patients with chronic liver disease (CLD, cirrhosis). HE is classified into two primary forms: covert and overt HE. Covert HE is characterized by neurological alterations and subclinical symptoms including personality changes, poor memory, sleep problems, decreased concentration, reduced speed of information processing and motor incoordination that significantly affect the quality of life and daily functioning activities including working or driving a car. Covert HE can progress to overt HE with clinical symptoms such as lethargy, gross disorientation, asterixis and coma, with a negative impact on patient survival.1 It is reported that >80% of cirrhotic patients develop covert HE and, 30% of patients with end-stage liver disease develop overt HE2, a major cause of hospitalizations. Thus, HE is a burden on the socioeconomic status of the patient and healthcare systems.3
Pathogenesis of HE in CLD
The pathological basis of HE in CLD is complex and multifactorial. The exact mechanisms responsible for HE are not fully characterized; nevertheless, it is recognized that blood-derived precipitating factors are responsible for the development of neurological decline in patients with cirrhosis. In particular, hyperammonemia leading to increased cerebral ammonia has been shown to be associated with astrocyte swelling and the onset of brain edema, two key features observed in cirrhotic patients suffering from HE, as well as, in animal models of HE.4 Overall, astrocyte swelling and brain edema cause alterations in chemical homeostasis and neurotransmission impairments, believed to be responsible for neurological decline in CLD. Aside ammonia, other systemic factors such as inflammation, oxidative stress, and increased bile acids (BAs) and lactate contribute to the progression and severity of HE.5 This review focuses on the pathogenesis of HE and on the single and synergic interplay of pathological factors in the progression and severity of HE.
Blood-Brain Barrier Alterations in CLD Lead to Brain Edema and HE
Brain edema can arise because of two pathophysiological processes, cytotoxic and vasogenic, both which result in an increased entry of water into the brain causing swelling. Vasogenic brain edema develops as a result of blood-brain barrier (BBB) breakdown that initiates osmotic changes across the BBB, leading to brain hypertonicity and subsequently an increased influx of water into the brain.4, 6 Cytotoxic brain edema involves cellular alterations related to osmotic gradient functions without a physical breakdown in the BBB.7
In the past, the presence of brain edema was believed to be restricted to cases of acute liver failure, primarily because of the fact that patients with acute liver failure developed increased intracranial pressure (ICP). Because increased ICP is rarely observed in CLD, brain edema was less suspected.4 However, advanced and sensitive magnetic resonance imaging (MRI) techniques have detected water changes in brains of patients with CLD (without an increase in ICP), which has been found to be associated with HE.8 Interestingly, HE in CLD is also associated with lower brain volume (loss of neurons),8 which might explain why elevated ICP is rarely observed in CLD. Moreover, magnetic transfer ratio analysis from MRI has been used to define mild diffuse brain edema, called low-grade brain edema, in CLD patients with HE.9 This implies that despite the absence of increased ICP, low-grade brain edema promotes neurological deficits. In accord, bile duct ligated (BDL) rats, a recognized model of CLD and HE, also develop brain edema.10
Brain edema in CLD is due to water increase in the interstitial compartment, which suggests vasogenic edema secondary to BBB breakdown.11, 12 Indeed, interstitial water progressively increased along with severity of HE in cirrhotic patients.12 Positron emission tomography studies in cirrhotic patients with minimal/covert HE demonstrated an increased BBB permeability versus patients without HE and healthy controls,13, 14 implying that HE may result from increased permeability-surface area that allows neurotoxic substances to enter easily from circulation.13 However, other reports have shown no difference on BBB permeability between patients with HE, without HE, and controls.15 Moreover, diffusion tensor imaging studies in cirrhotic patients reported the presence of both cytotoxic and vasogenic mechanisms with no difference between patients with and without HE.11 In accord, cirrhotic BDL rats showed increased BBB permeability accompanied by brain edema and impaired water channel AQP4 and tight junction (TJ) proteins,16 suggesting vasogenic edema, whereas others showed no changes in BBB structure,17 expression of TJ proteins, or brain extravasation.4
Astrocyte Swelling in CLD Leads to Brain Edema and HE
Astrocytes constitute a key component of the BBB and protect neurons against excitotoxicity by the maintenance of brain solute-water homeostasis; therefore, astrocyte disturbances represent a crucial feature in brain edema and neuronal alterations.6 Postmortem brain tissue from HE patients showed morphological changes (including swelling) called Alzheimer type II astrocytosis, while neuronal morphology was not altered.18 Using electron microscopy, astrocyte swelling has been reported in BDL rats.17 Therefore, morphological and functional deterioration of astrocytes impairs neuronal function and plays a central role in the etiology of brain edema and HE in CLD. The mechanisms that might explain astrocyte swelling-mediated brain edema include dysregulation of AQP4 channel, altered brain sodium and potassium homeostasis,6 accumulated intracellular astrocytic glutamine,19 and increased extracellular glutamate.6
Glutamine Metabolism Impairment in CLD Induces Astrocyte Swelling, Brain Edema and HE
The amino acid glutamine is one of the end-products of ammonia detoxification, and about 85% of ammonia in astrocytes is transformed into glutamine. Glutamine has also been involved in the mechanism that explains the neurophysiology of HE and brain edema. Interestingly, biochemical evaluations of cerebrospinal fluid (CSF) of HE show a positive correlation between the severity of HE and glutamine levels.19 Similarly, in vivo proton-MR glutamine signal increases along with HE severity in CLD.19 High-performance liquid chromatography fluorescence studies in postmortem brain tissue from cirrhotic patients with HE revealed that brain glutamine was increased in different brain structures, with the highest increase in the prefrontal cortex, a brain region involved in neurological deficits of HE.19
The mechanisms that explain the role of glutamine in astrocyte swelling and brain edema in CLD are associated with hyperammonemia that leads to increased astrocytic glutamine. In this context, brain edema results from osmotic actions of glutamine in astrocytes which cause hypertonicity along with cytotoxic astrocyte swelling.19 Additionally, brain edema involves detrimental effects of cytoplasm glutamine on astrocyte mitochondria including excessive oxidative stress, mitochondrial permeability transition, and other mitochondrial deteriorations known to be involved in astrocyte swelling.20
Glutamate Metabolism Impairment in CLD Promotes Brain Edema and HE
The amino acid glutamate is the main excitatory neurotransmitter in the central nervous system, and consequently, its alterations are associated with neurological problems including HE in CLD.7 The maintenance of glutamate homeostasis depends on the novo synthesis of glutamine in astrocytes. Particularly, when extracellular glutamate is elevated, glutamate acts as a toxic substance leading to astrocyte and neuronal dysfunction.7 Extracellular glutamate in brain tissue and CSF is increased in cirrhotic patients with HE but not in those without HE.21
The cytotoxic effects of glutamate results from its accumulation at the synaptic cleft due to altered glutamate transporters that reduces astrocytic glutamate uptake.7 Moreover, alterations in astrocyte glutamate transporters are associated with motor and cognitive deficits in HE patients.22 Glial glutamate-aspartate transporter (GLAST) is upregulated in brain tissue from cirrhotic patients with HE but not in patients without HE against controls.23 Experiments in brain tissue of portacaval anastomosis (PCA)-shunted rats, a chronic model of hyperammonemia and HE without liver disease, suggests a transitory downregulation of GLAST, which increases extracellular glutamate, followed by upregulation mechanism to counteract cytotoxic glutamate levels.23
Furthermore, extracellular glutamate accumulation is associated with brain edema in cirrhotic patients.7, 24 Cultured astrocytes swell when exposed to glutamate by a mechanism associated with metabotropic glutamate receptor (mGluR) activation6 and increased AQP4 channel expression.25 Hence, glutamate-induced cell swelling in cirrhosis may alter excitatory neurotransmission, which leads to neurological deficiencies.26 For instance, increased expression of N-methyl-d-aspartate glutamate receptor (NMDA) in CLD is associated with motor and cognitive deficits in HE patients.22 In accord, the expression of NMDA is increased in brain cortex of cirrhotic animals.22 Moreover, the function of glutamate-nitric oxide (NO)-cyclic guanosine monophosphate (cGMP), a pathway involved in cognitive processes, is reduced in animal models of CLD along with cognitive/learning deficits, suggesting that cGMP alterations is associated with HE.27 Additionally, enhanced glutamatergic neurotransmission may influence other neurotransmitters involved in the pathogenesis of HE such as gamma-aminobutyric acid (GABA), the major inhibitory neurotransmitter.28
GABA Alterations in CLD Promotes Brain Edema and HE
The inhibitory neurotransmitter GABA is also implicated in the pathogenesis of HE, and it has been proposed that enhanced GABA neurotransmission mediated by the activation of GABAA receptor leads to neurological deficiencies. Indeed, GABAA activation is associated with sedative, motor, and cognitive deficits in HE. Besides GABA, other substances that are elevated in CLD, such as cerebral endogenous benzodiazepines and neurosteroids, activate GABAA and increase GABAergic tone. Interestingly, HE enhances neuronal sensibility to drugs that increase GABAergic tone.28 In agreement, the GABAA antagonist flumazenil transiently improves HE in cirrhotic patients, and HE worsens again when stopping the treatment.29 Still, flumazenil remains a choice when HE is triggered by exogenous benzodiazepines or in patients with overt HE who do not respond to the standard therapeutic care.30 Consistent with increased GABAergic tone in HE, the synthesis of GABA is elevated in BDL rats,31 and pharmacological activation of GABAA in naive animals leads to visual evoked responses similar to those observed in animals with HE.28
Additionally, GABA has been involved in osmotic regulation and brain water homeostasis in various disease conditions causing brain edema. Astrocytes regulate anion fluxes via GABAA, thereby participating in osmotic tension. Thus, GABAA activation may affect cell volume and regulate neuronal activation. Moreover, GABAA is coexpressed with AQP4 in astrocytes, and it has been demonstrated that the activation of GABAA also mediates osmotic swelling.32 Furthermore, postmortem analysis of brain tissue from patients with HE revealed increased neurosteroids, molecules that promote the activation of GABAergic system via GABAA. Thus, the use of GABAA receptor modulating steroid antagonists (GAMSAs) that attenuate GABAergic tone has been shown to improve cognition in patients with HE. In agreement, GAMSAs improve learning, memory, and motor coordination impairments in experimental HE. GAMSAs are currently under clinical research in patients with HE.33
Interestingly, alterations within the interplay between glutamatergic and GABAergic neurotransmission are believed to be causal in cognitive and motor impairment in HE. For instance, the activation of NMDA and GABAA receptors impairs the glutamate-NO-cGMP pathway, leading to neurological deficits in animal models of HE.27
Precipitating factors of HE in CLD
Ammonia, oxidative stress, inflammation, BAs, and lactate are pathogenic factors that are elevated in circulation as a result of CLD. These systemic factors can precipitate HE and initiate a cascade of pathophysiological pathways which ultimately lead to astrocyte and neuronal dysfunction (as mentioned previously) and hence neurological decline (HE) in patients with CLD.
Ammonia as a Precipitating Factor for HE in CLD
Ammonia is a by-product of nitrogen metabolism produced from deamination of dietary protein (amino acids) by glutaminase in gut enterocytes and by urease-containing bacteria that break down urea to ammonia. Primarily produced within the gut, ammonia is then absorbed and transported to the liver via the portal vein. In the liver, ammonia is metabolized via urea cycle pathway and subsequently excreted through the kidneys. However, in liver disease, the capacity to remove ammonia is decreased and as a result blood ammonia accumulates and hyperammonemia arises.34 Ammonia is considered the major neurotoxin involved in HE, and lowering ammonia remains the primary therapeutic strategy.35 Indeed, systemic ammonia levels correlate positively with HE severity36 and negatively with neurological performance.37 Particularly, plasma ammonia is higher in cirrhotic patients with HE than in patients without HE.9, 37 Furthermore, effective ammonia-lowering agents lead to improvement of the mental status in cirrhotic patients with HE.37
Ammonia Induces Brain Edema and Astrocyte Swelling
Brain MRI (with magnetization transfer ratio) assessments showed that hyperammonemia-induced neurological impairments in cirrhotic patients are associated with the presence of brain edema.9 Indeed, induced hyperammonemia in cirrhotic patients directly drives changes in brain water distribution, producing brain edema.38 Similarly, hyperammonemia in BDL rats is associated with brain edema and poor neurological performance,17 which are normalized after attenuation in rising blood ammonia.39
Hyperammonemia-induced brain edema in CLD patients results from detrimental actions on astrocytes and subsequent swelling; such actions include glutamine accumulation,19 reduction of glial fibrillary acid protein (GFAP), Alzheimer type II astrocytosis,40 and increased AQP4 expression.6 In BDL rats, hyperammonemic diet induces Alzheimer type II astrocytosis along with brain edema.41
In the brain, astrocytes are the only cells that can remove ammonia because of the fact that astrocytes express glutamine synthetase (GS). Particularly, GS metabolizes ammonia and glutamate to generate the osmotically active glutamine. However, as stated previously, in the setting of hyperammonemia, the increased conversion into glutamine leads to hypertonicity and hence cytotoxic astrocyte swelling.19 Biochemical analysis of CSF of cirrhotic patients with HE show a positive correlation between CSF glutamine and blood ammonia.21 Moreover, induced hyperammonemia in cirrhotic patients leads to elevated brain glutamine accompanied by brain edema.38
In BDL rats, hyperammonemia is associated with increased brain glutamine, brain edema, and motor activity impairment.10, 41 In addition, ammonia infusion into PCA rats increases CSF glutamine and induces brain edema,42 an effect likely involved in HE.43 Conversely, the attenuation of glutamine accumulation (GS inhibition) prevents brain edema likely by canceling ammonia-induced astrocyte swelling.42 These findings suggest that brain swelling in CLD is mediated by osmotic effects of accumulated glutamine in astrocytes secondary to ammonia detoxification. However, others reported that raised brain glutamine in CLD patients is not correlated with blood ammonia levels,44 and studies in BDL rats showed that glutamine is not central in developing brain edema.10 Despite controversy, neurological deficits associated with induced hyperammonemia in CLD may be determined by the ability of the brain to buffer ammonia-induced increase in glutamine, by counteracting with osmolyte release.43
In line with the role of glutamine as a mediator of ammonia swelling effects, it has been shown that in hyperammonemic conditions, glutamine is transported in excess from the cytoplasm into mitochondria which results in high levels of ammonia and subsequently excessive oxidative stress and mitochondrial permeability transition that leads to astrocyte swelling.19, 20 Moreover, rat cerebral mitochondria exposed to glutamine showed mitochondrial swelling and subsequent activation of mitochondrial permeability transition in a dose-dependent manner, whereas the exposure to neurotoxic levels of ammonium neither enhanced the effects of glutamine nor produced the effects when added alone, suggesting that the conversion to glutamine in cytoplasm is required to produce ammonia detrimental effects.45
Ammonia Impairs Glutamatergic and GABAergic Neurotransmission
Extracellular glutamate and glutamatergic neurotransmission are influenced by ammonia in CLD.26 Chronic hyperammonemia affects signal transduction related to NMDA receptors. In particular, ammonia impairs glutamate-NO-cGMP pathway, associated with NMDA receptor, as observed in brain tissue of cirrhotic patients and animal models of chronic HE. Interestingly, such effects are observed in neurons but not in astrocytes. Considering that the glutamate-NO-cGMP pathway is necessary for learning and cognitive processes, its alterations in CLD may explain cognitive deficits in HE.46 Accordingly, cognitive and learning deficits in animal models of CLD and hyperammonemia are restored after pharmacological normalization of cGMP using sildenafil, an inhibitor of phosphodiesterase 5, which increases cGMP.27 In vivo brain microdialysis in rats showed that ammonia intoxication activates NMDA receptors and extracellular cGMP, which correlates with neurological symptoms of hyperammonemia.47 Moreover, increased mGluR in hyperammonemic rats promotes memory and learning deficits.26
Additionally, in rat brain, high-affinity glutamate uptake is reduced when exposed to ammonia, leading to high extracellular glutamate.48 In agreement, cultured astrocytes exposed to ammonia increase extracellular glutamate through NMDA receptor.49 Although, the glutamate-clearing efficacy in swollen astrocytes has not been explored, glutamate clearance function might be nullified, enhancing extracellular glutamate accumulation and its toxic effects. Overall, these findings suggest that toxicity, brain edema, and neurological deficits caused by ammonia in CLD are mediated by enhancing extracellular glutamate and NMDA receptor activation.
Detrimental actions of ammonia on the brain are also mediated by enhanced GABAergic neurotransmission through GABAA receptor. For example, hyperammonemia enhanced GABAergic tone along with motor and cognitive deficits, while higher ammonia further increases GABAergic tone, leading to clinical signs of HE.28 Studies in hyperammonemic rats showed that memory impairments result from increased activation of GABAA in the cerebellum and decreased activation in cortex, whereas the blockage of GABAA normalizes memory.50 In addition, ammonia enhances the neuroinhibitory effects of GABA by increasing GABA affinity to GABAA51 and synergistically increases natural benzodiazepines actions.28 Moreover, ammonia decreases GABA uptake in astrocytes which results in enhanced availability of GABA at the synaptic cleft.28 Besides, benzodiazepine agonists exacerbate ammonia-induced astrocyte swelling, whereas benzodiazepine antagonist attenuates ammonia-induced swelling.52
Inflammation as a Precipitating Factor for HE in CLD
Systemic inflammation arises in the setting of liver injury and is characterized by the detection of proinflammatory cytokines in circulation including interleukins interleukins (ILs) such as IL-1߬ IL-6, IL-8, IL-12, and tumor necrosis factor alpha (TNF-a).34 Inflammation and proinflammatory cytokines are associated with worse cognitive performance in cirrhotic patients with HE against those without HE.9 Indeed, the highest inflammatory response is observed in patients with poor prognosis and overt HE.53 Particularly, serum IL-6 and TNF-a correlate well with the severity of HE in CLD patients,36, 37, 53 whereas their reduction improves HE.9 In PCA and BDL rats, anti-inflammatory drugs (ibuprofen) reduce proinflammatory markers in brain and blood, while restoring learning and motor coordination deficits.54 Similarly, the normalization of serum and brain TNF-a using a TNF-a inhibitor restores memory deficits in BDL rats.55
Furthermore, postmortem analysis of microglia marker Iba-1 and gene expression in brain cortex of cirrhotic patients with HE showed an upregulation when compared with patients without HE.56 Particularly, genes related to microglia activation, inflammatory pathways, cell proliferation, and apoptosis are upregulated in HE patients.57 Therefore, these findings suggest that neuroinflammation is involved in HE pathogenesis.
Interestingly, systemic and central inflammation is associated with astrocyte swelling and brain edema.17, 56 Neurological deficits promoted by inflammation are associated with the presence of brain edema in cirrhotic patients with HE; in fact, the attenuation of inflammation restores brain edema and neurological performance.9 Consistent, inflammatory response in BDL rats is also accompanied by brain edema.41 Moreover, inflammatory response induced by lipopolysaccharide-endotoxin shock increases cytotoxic brain edema in both hyperammonemic and nonhyperammonemic (sham) rats, while exacerbates brain edema and induces precoma in BDL rats.17 Inflammation-induced brain edema is characterized by BBB breakdown that results from cytotoxicity in brain endothelial cells, TJ protein disruption, and facilitating entry of leukocytes.6 Particularly, IL-158 and TNF-a are associated with increased BBB permeability and brain edema.59 Additionally, cultured astrocytes swell when exposed to TNF-a, IL-1߬ IL-6, or interferon gamma,60 while such effects are related to detrimental actions on GFAP and AQP4 channels and oxidative stress.61
Furthermore, the nuclear factor kappa B (NF-?B), a pathway involved in inflammation and astrocyte swelling,60 is activated in cirrhotic patients with HE.57 Conversely, the nonabsorbable antibiotic rifaximin inhibits NF-?B and prevents toxicity of apoptotic factors in cultured cells.62 Additionally, other brain neuroinflammatory factors involved in brain edema, such as inducible NO synthase, prostaglandin E2,54 are associated with neurological deficits in CLD. Besides astrocyte alterations, neuroinflammation impairs neurotransmission which, in turn, leads to HE.63 For instance, neuroinflammation reduces glutamate uptake, increases extracellular glutamate, stimulates glutamate receptors, and promotes glutamate release from astrocytes which enhance neurotransmission.63 Neuroinflammation also increases GABAergic tone and motor coordination deficits in PCA rats, whereas the anti-inflammatory sildenafil restores GABA neurotransmission and motor impairments.64
Oxidative Stress as a Precipitating Factor for HE in CLD
Another factor involved in the pathogenesis of HE is oxidative stress due to reactive oxygen species (ROS) including peroxides, free radicals, and lipid peroxides. In CLD, the synthesis of proteins associated with antioxidant effects including albumin and glutathione is reduced, while the generation of ROS is increased. Thus, there is an imbalance between the production and neutralization of ROS in CLD that leads to oxidative stress and cellular dysfunction.65 Clinical studies demonstrated systemic oxidative stress (3-nitrotyrosine markers) in cirrhotic patients with HE but not in patients without HE.66 In agreement, oxidative stress markers were increased in postmortem cortical brain tissue from cirrhotic patients with HE but not in those without HE, suggesting that brain oxidative stress is a feature of HE.23 The generation of ROS in CLD patients is also associated with the release of liver oxidant enzymes in plasma such as xanthine oxidase. In animal models, we previously demonstrated that cirrhotic BDL rats with brain edema and HE also develop systemic oxidative stress but not brain oxidative stress. Conversely, the antioxidant treatment using a xanthine oxidase inhibitor, allopurinol, attenuates oxidative stress and brain edema in BDL rats.65
Although the pathogenic association between ROS formation and astrocyte swelling in HE has not been totally elucidated, in vitro studies with brain cells show that astrocyte swelling induced by ammonium chloride, TNF-a, or diazepam (GABAA agonist) is inhibited by the antioxidant epigallocatechin gallate, suggesting that the pathways that trigger oxidative stress in astrocytes induce astrocyte swelling.67 Therefore, these findings indicate that oxidative stress plays a role in the development of astrocyte swelling, brain edema, and HE in CLD.
BAs as Precipitating Factors for HE in CLD
Elevated blood levels of BAs are also implicated in HE pathogenesis in CLD.5 BAs are synthesized from cholesterol in the liver, then BAs reach intestines, and they are metabolized by microbiota. The majority of BAs are reabsorbed by BA transporters and return to the liver to be recycled.68 However, in liver disease, BAs are not recycled in liver and accumulate in circulation. Thus, BAs enter the brain through BA transporters on BBB, which effects are associated with HE.5 Serum BAs are higher in cirrhotic patients than in controls because of reduced hepatic BA clearance.69 Moreover, serum, CSF, and brain BAs were enhanced in patients who died of fulminant hepatic failure, while such effects were related to coma duration and brain edema in 50% of patients.68 Nevertheless, clinical trials to explore the role of BAs in cirrhotic patients with and without HE are needed to elucidate the role of BAs in the development of HE.
In BDL rats, plasma and brain BAs are also increased, and such effect is related to increased BBB permeability.5 Besides BBB permeability, acute intracarotid injection of BAs in rats leads to astrocyte swelling and brain edema.70 In addition, BAs impair neurotransmission systems involved in the pathogenesis of HE such as GABAergic, glutamatergic, noradrenergic, and serotonergic effects which might be associated with memory and motor coordination impairments in HE. Particularly, upregulation of brain BA transporters and activation of farnesoid X nuclear receptor in neurons has been implicated.68
Lactate
Lactate derived from astrocytes may be crucial to ensure a supply of substrates for brain metabolism. Lactate is an organic molecule specifically synthesized from glucose and metabolized by lactate dehydrogenase, in both neurons and astrocytes. Lactate is transported into the extracellular space to be taken and used as an energy substrate by neurons. Traditionally, increased cerebral lactate has been considered a marker of energy failure/impairment, but recently alterations in lactate homeostasis were associated with neuronal dysfunction and HE.71 Increased concentrations of lactate in systemic circulation and in brain are reported in HE patients.71 Experiments in BDL rats show that brain lactate and glutamine are increased along with brain edema, whereas the inhibition of lactate synthesis attenuates brain lactate and brain edema.10
Besides lactate's role as an energy substrate, lactate also acts as a signaling molecule by activating G-protein-coupled receptor 81 (GPR81). GPR81 activation by lactate stimulates the inhibitory G-protein, which inhibits adenylyl cyclase and leads to a decrease in cAMP. GPR81 is present and active in mouse brain including hippocampal, cortex, and cerebellar neurons, as well as, in astrocytes.72 Nevertheless, the function of GPR81 in brain and its role in HE remain to be determined.
synergic relationship between precipitating factors in CLD induces HE
Although, ammonia plays a major role in the pathogenesis of HE in CLD, it has been demonstrated that ammonia levels are not always correlated with the severity of HE in all patients, suggesting a major interpersonal variability.73 This may highlight the importance of different pathophysiological factors that contribute to the development of HE.5
Interestingly, clinical and fundamental research implies a cooperative interaction between inflammation and ammonia.9, 41 Indeed, inflammation is associated with hyperammonemia, brain edema, and poor neurological performance in cirrhotic patients with HE against those without HE.9 Accordingly, the coattenuation of systemic ammonia and inflammation by the sugar-like laxative lactulose and the antibiotic rifaximin, respectively, improves brain edema and HE in CLD patients,9 with better efficacy than single actions on ammonia.74 Similarly, inflammation and hyperammonemia in BDL rats lead to brain edema and HE.41 Moreover, chronic hyperammonemia in rats increases proinflammatory cytokines along with astrocyte and microglia activation.50 In vitro experiments showed that ammonia induces the release of glutamate, prostaglandins, and IL-1߮56 Additionally, astrocyte swelling induced by proinflammatory cytokines is exacerbated when pre-exposed to ammonia.60 Thus, it is suggested that ammonia sensibilizes astrocytes to the cytotoxic effects of inflammatory cytokines by activation of NF-?B pathway.60
Hyperammonemia-induced neuroinflammation increases GABA release and extracellular GABA by increasing membrane expression of GABA transporter (GAT-3) in activated astrocytes, which leads to memory and motor coordination deficiencies in hyperammonemic rats,50 whereas sulforaphane, an anti-inflammatory/antioxidant compound with actions on a target against neuroinflammation (Nrf2 system), restores neurotransmission and neurological performance in hyperammonemic rats.50 Additionally, ammonia and inflammation coactions in brain induce oxidative/nitrosative stress resulting from activation of glutamate receptors and consequent nitration of key brain proteins such as GS. Overall, these findings support that inflammation and ammonia interaction in CLD aggravates astrocyte swelling, brain edema, and HE.75
Data from the literature also suggest a synergic relationship between oxidative stress and ammonia in the development of astrocyte swelling, brain edema, and HE. Clinical studies show that the combination of oxidative stress and hyperammonemia in CLD patients leads to HE rather than the single present of hyperammonemia.65 In vitro studies using cultured astrocytes show that high doses (>500 'M) but not low doses (<200 'M) of ammonia promotes ROS generation. In BDL rats, the attenuation of systemic ammonia using oral carbon microspheres, AST-120, reduces brain edema but not systemic oxidative stress. Moreover, as stated previously, the attenuation of systemic oxidative stress using allopurinol reduces brain edema in BDL rats.65 Furthermore, ammonia-induced oxidative stress is associated with astrocytic and neuronal RNA oxidation that affects neurotransmitter system and gene expression, which contribute to cognition deficits in HE.76 These results suggest a synergic effect of ammonia and systemic oxidative stress which promotes brain edema and HE in CLD.
Interestingly, it has been suggested that hyperammonemia, inflammation, and BAs play a synergic effect that exacerbates BBB permeability, astrocyte swelling, and brain edema in liver disease.70 Moreover, BAs stimulate proinflammatory cytokine expression in the liver, which indirectly might affect brain function.77
Regarding lactate, whether lactate is influenced by other biomarkers of HE is not clear, but brain lactate might be modulated by ammonia, since, cultured astrocytes increase lactate production when exposed to a high dose of ammonia.78 Moreover, neuroinflammatory conditions on BBB in vitro model decrease the expression of GPR81, whereas increase IL-1߬ BBB permeability, and extracellular lactate.79
Conclusion
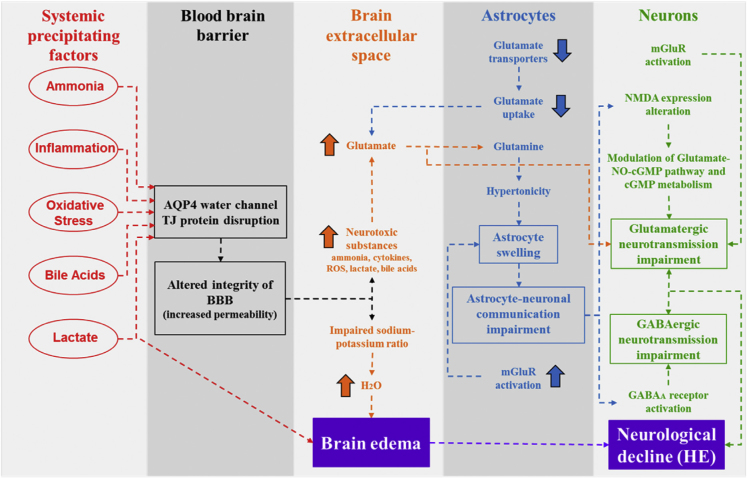
HE is a complex neuropsychiatric disease caused by different neurotoxic precipitating factors that accumulate in circulation and brain in the setting of CLD. Thus, hyperammonemia, systemic inflammation, oxidative stress, hyperlactatemia, and BA impairments are known to participate in the pathogenesis of HE. Particularly, precipitating factors progressively impair BBB integrity, which further facilitates their entry into the brain. BBB impairment in CLD is associated with astrocyte swelling and brain edema. Astrocyte swelling and brain edema in CLD also result from homeostasis dysregulation of astrocyte glutamine and extracellular glutamate, which influences neurotransmission systems. In particular, detrimental effects on glutamatergic and GABAergic systems disrupt brain cell communication, resulting in neurological deficits in CLD (Figure 1). Interestingly, in addition to their independent actions, the synergic interaction between precipitating factors may better explain the complexity involved in the development of HE in cirrhotic patients. Further research focusing on this interaction is needed to better understand the precise mechanisms that lead to HE, which in turn will lead to better diagnostic and treatment strategies for HE.
Figure 1.
Blood-brain barrier (BBB) impairment, astrocyte swelling, and brain edema in chronic liver disease promotes hepatic encephalopathy (HE). Precipitating factors affect the BBB permeability, which facilitates the entry of neurotoxic substances into brain extracellular space resulting in brain edema. Additionally, neurotoxic substances exert detrimental actions on astrocytes, which disturb glutamate and glutamine metabolism, leading to astrocyte swelling. Thus, astrocyte swelling contributes to brain edema and affects astrocyte-neuronal communication, resulting in a disturbance of glutamatergic and GABAergic neurotransmission systems, which finally accounts for HE. AQP4, Aquaporin 4; TJ, Tight Junction; ROS, Reactive Oxygen Species; mGluR, Metabotropic Glutamate Receptor; NMDA, N-Methyl-d-Aspartate Glutamate Receptor; NO, Nitric Oxide; cGMP, Cyclic Guanosine Monophosphate; GABA, Gamma-Aminobutyric Acid.
Conflicts of interest
The authors have no conflicts of interest to declare.
Financial support
Canadian Institutes of Health Research (CIHR): MOP-130556.
References
- 1.Stewart C.A., Smith G.E. Minimal hepatic encephalopathy. Nat Rev Gastroenterol Hepatol. 2007;4(12):677–685. doi: 10.1038/ncpgasthep0999. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gómez M., Boza F., García-Valdecasas M.S. Subclinical hepatic encephalopathy predicts the development of overt hepatic encephalopathy. Am J Gastroenterol. 2001;96(9):2718–2723. doi: 10.1111/j.1572-0241.2001.04130.x. [DOI] [PubMed] [Google Scholar]
- 3.Neff G. Pharmacoeconomics of hepatic encephalopathy. Pharmacotherapy. 2010;30(5 part 2):28S–32S. doi: 10.1592/phco.30.pt2.28S. [DOI] [PubMed] [Google Scholar]
- 4.Bosoi C.R., Rose C.F. Brain edema in acute liver failure and chronic liver disease: similarities and differences. Neurochem Int. 2013 Mar;62(4):446–457. doi: 10.1016/j.neuint.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 5.Liere V., Sandhu G., DeMorrow S. Recent advances in hepatic encephalopathy. F1000Res. 2017;6:1637. doi: 10.12688/f1000research.11938.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stokum J.A., Kurland D.B., Gerzanich V., Simard J.M. Mechanisms of astrocyte-mediated cerebral edema. Neurochem Res. 2015;40(2):317–328. doi: 10.1007/s11064-014-1374-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemberg A., Fernández M.A. Hepatic encephalopathy, ammonia, glutamate, glutamine and oxidative stress. Ann Hepatol. 2009 Apr-Jun;8(2):95–102. [PubMed] [Google Scholar]
- 8.Chavarria L., Cordoba J. Magnetic resonance imaging and spectroscopy in hepatic encephalopathy. J Clin Exp Hepatol. 2015;5(suppl 1):S69–S74. doi: 10.1016/j.jceh.2013.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rai R., Ahuja C.K., Agrawal S. Reversal of low-grade cerebral edema after lactulose/rifaximin therapy in patients with cirrhosis and minimal hepatic encephalopathy. Clin Transl Gastroenterol. 2015;6(9):e111. doi: 10.1038/ctg.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bosoi C.R., Zwingmann C., Marin H. Increased brain lactate is central to the development of brain edema in rats with chronic liver disease. J Hepatol. 2014;60(3):554–560. doi: 10.1016/j.jhep.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Chavarria L., Alonso J., Garcí-Martínez R. Biexponential analysis of diffusion-tensor imaging of the brain in patients with cirrhosis before and after liver transplantation. AJNR Am J Neuroradiol. 2011;32(8):1510–1517. doi: 10.3174/ajnr.A2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kale R.A., Gupta R.K., Saraswat V.A. Demonstration of interstitial cerebral edema with diffusion tensor MR imaging in type C hepatic encephalopathy. Hepatology. 2006;43(4):698–706. doi: 10.1002/hep.21114. [DOI] [PubMed] [Google Scholar]
- 13.Lockwood A.H., Yap E.W., Wong W.H. Cerebral ammonia metabolism in patients with severe liver disease and minimal hepatic encephalopathy. Cereb Blood Flow Metab. 1991;11(2):337–341. doi: 10.1038/jcbfm.1991.67. [DOI] [PubMed] [Google Scholar]
- 14.Ahl B., Weissenborn K., van den Hoff J. Regional differences in cerebral blood flow and cerebral ammonia metabolism in patients with cirrhosis. Hepatology. 2004 Jul;40(1):73–79. doi: 10.1002/hep.20290. [DOI] [PubMed] [Google Scholar]
- 15.Keiding S., Sùrensen M., Bender D., Munk O.L., Ott P., Vilstrup H. Brain metabolism of 13N-ammonia during acute hepatic encephalopathy in cirrhosis measured by positron emission tomography. Hepatology. 2006 Jan;43(1):42–50. doi: 10.1002/hep.21001. [DOI] [PubMed] [Google Scholar]
- 16.Dhanda S., Sandhir R. Blood-brain barrier permeability is exacerbated in experimental model of hepatic encephalopathy via MMP-9 activation and downregulation of tight junction proteins. Mol Neurobiol. 2018;55(5):3642–3659. doi: 10.1007/s12035-017-0521-7. [DOI] [PubMed] [Google Scholar]
- 17.Wright G., Davies N.A., Shawcross D.L. Endotoxemia produces coma and brain swelling in bile duct ligated rats. Hepatology. 2007;45(6):1517–1526. doi: 10.1002/hep.21599. [DOI] [PubMed] [Google Scholar]
- 18.Norenberg M.D., Rao K.V., Jayakumar A.R. Mechanisms of ammonia-induced astrocyte swelling. Metab Brain Dis. 2005;20(4):303–318. doi: 10.1007/s11011-005-7911-7. [DOI] [PubMed] [Google Scholar]
- 19.Albrecht J., Norenberg M.D. Glutamine: a Trojan horse in ammonia neurotoxicity. Hepatology. 2006;44(4):788–794. doi: 10.1002/hep.21357. [DOI] [PubMed] [Google Scholar]
- 20.Rama Rao K.V., Norenberg M.D. Glutamine in the pathogenesis of hepatic encephalopathy: the trojan horse hypothesis revisited. Neurochem Res. 2014;39(3):593–598. doi: 10.1007/s11064-012-0955-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watanabe A., Takei N., Higashi T. Glutamic acid and glutamine levels in serum and cerebrospinal fluid in hepatic encephalopathy. Biochem Med. 1984;32(2):225–231. doi: 10.1016/0006-2944(84)90076-0. [DOI] [PubMed] [Google Scholar]
- 22.Soria Fregozo C., Beltrán M.L., Flores Soto M.E., Pérez Vega M.I., Beas Zárate C., Huacuja Ruiz L. Expression of NMDA receptor subunits in rat prefrontal cortex with CCL4-induced hepatic damage after a treatment with Rosmarinus officinalis L. Neurologia. 2012;27(5):261–267. doi: 10.1016/j.nrl.2011.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Görg B., Qvartskhava N., Bidmon H.J. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52(1):256–265. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poveda M.J., Bernabeu A., Concepción L. Brain edema dynamics in patients with overt hepatic encephalopathy A magnetic resonance imaging study. Neuroimage. 2010;52(2):481–487. doi: 10.1016/j.neuroimage.2010.04.260. [DOI] [PubMed] [Google Scholar]
- 25.Shi Z., Zhang W., Lu Y. Aquaporin 4-mediated glutamate-induced astrocyte swelling is partially mediated through metabotropic glutamate receptor 5 activation. Front Cell Neurosci. 2017;11:116. doi: 10.3389/fncel.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cauli O., Rodrigo R., Llansola M. Glutamatergic and gabaergic neurotransmission and neuronal circuits in hepatic encephalopathy. Metab Brain Dis. 2009;24(1):69–80. doi: 10.1007/s11011-008-9115-4. [DOI] [PubMed] [Google Scholar]
- 27.Llansola M., Montoliu C., Agusti A. Interplay between glutamatergic and GABAergic neurotransmission alterations in cognitive and motor impairment in minimal hepatic encephalopathy. Neurochem Int. 2015;88:15–19. doi: 10.1016/j.neuint.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 28.Jones E.A. Ammonia, the GABA neurotransmitter system, and hepatic encephalopathy. Metab Brain Dis. 2002;17(4):275–281. doi: 10.1023/a:1021949616422. [DOI] [PubMed] [Google Scholar]
- 29.Ferenci P., Grimm G. Benzodiazepine antagonist in the treatment of human hepatic encephalopathy. Adv Exp Med Biol. 1990;272:255–265. doi: 10.1007/978-1-4684-5826-8_17. [DOI] [PubMed] [Google Scholar]
- 30.Romero-Gómez M. Pharmacotherapy of hepatic encephalopathy in cirrhosis. Expert Opin Pharmacother. 2010;11(8):1317–1327. doi: 10.1517/14656561003724721. [DOI] [PubMed] [Google Scholar]
- 31.Leke R., Bak L.K., Iversen P. Synthesis of neurotransmitter GABA via the neuronal tricarboxylic acid cycle is elevated in rats with liver cirrhosis consistent with a high GABAergic tone in chronic hepatic encephalopathy. J Neurochem. 2011;117(5):824–832. doi: 10.1111/j.1471-4159.2011.07244.x. [DOI] [PubMed] [Google Scholar]
- 32.Cesetti T., Ciccolini F., Li Y. GABA not only a neurotransmitter: osmotic regulation by GABAAR signaling. Front Cell Neurosci. 2012;6:3. doi: 10.3389/fncel.2012.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kornerup L.S., Gluud L.L., Vilstrup H., Dam G. Update on the therapeutic management of hepatic encephalopathy. Curr Gastroenterol Rep. 2018;20(5):21. doi: 10.1007/s11894-018-0627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aldridge D.R., Tranah E.J., Shawcross D.L. Pathogenesis of hepatic encephalopathy: role of ammonia and systemic inflammation. J Clin Exp Hepatol. 2015;5(suppl 1):S7–S20. doi: 10.1016/j.jceh.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rose C.F. Ammonia-lowering strategies for the treatment of hepatic encephalopathy. Clin Pharmacol Ther. 2012;92(3):321–331. doi: 10.1038/clpt.2012.112. [DOI] [PubMed] [Google Scholar]
- 36.Odeh M., Sabo E., Srugo I., Oliven A. Relationship between tumor necrosis factor-alpha and ammonia in patients with hepatic encephalopathy due to chronic liver failure. Ann Med. 2005;37(8):603–612. doi: 10.1080/07853890500317414. [DOI] [PubMed] [Google Scholar]
- 37.Jain L., Sharma B.C., Srivastava S., Puri S.K., Sharma P., Sarin S. Serum endotoxin, inflammatory mediators, and magnetic resonance spectroscopy before and after treatment in patients with minimal hepatic encephalopathy. J Gastroenterol Hepatol. 2013;28(7):1187–1193. doi: 10.1111/jgh.12160. [DOI] [PubMed] [Google Scholar]
- 38.Mardini H., Smith F.E., Record C.O., Blamire A.M. Magnetic resonance quantification of water and metabolites in the brain of cirrhotics following induced hyperammonaemia. J Hepatol. 2011;54(6):1154–1160. doi: 10.1016/j.jhep.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 39.Bosoi C.R., Parent-Robitaille C., Anderson K., Tremblay M., Rose C.F. AST-120 (spherical carbon adsorbent) lowers ammonia levels and attenuates brain edema in bile duct-ligated rats. Hepatology. 2011;53(6):1995–2002. doi: 10.1002/hep.24273. [DOI] [PubMed] [Google Scholar]
- 40.Sobel R.A., DeArmond S.J., Forno L.S., Eng L.F. Glial fibrillary acidic protein in hepatic encephalopathy. An immunohistochemical study. J Neuropathol Exp Neurol. 1981;40(6):625–632. doi: 10.1097/00005072-198111000-00004. [DOI] [PubMed] [Google Scholar]
- 41.Jover R., Rodrigo R., Felipo V. Brain edema and inflammatory activation in bile duct ligated rats with diet-induced hyperammonemia: a model of hepatic encephalopathy in cirrhosis. Hepatology. 2006;43(6):1257–1266. doi: 10.1002/hep.21180. [DOI] [PubMed] [Google Scholar]
- 42.Master S., Gottstein J., Blei A.T. Cerebral blood flow and the development of ammonia-induced brain edema in rats after portacaval anastomosis. Hepatology. 1999;30(4):876–880. doi: 10.1002/hep.510300428. [DOI] [PubMed] [Google Scholar]
- 43.Shawcross D.L., Balata S., Olde Damink S.W. Low myo-inositol and high glutamine levels in brain are associated with neuropsychological deterioration after induced hyperammonemia. Am J Physiol Gastrointest Liver Physiol. 2004;287(3):G503–G509. doi: 10.1152/ajpgi.00104.2004. [DOI] [PubMed] [Google Scholar]
- 44.Sawara K., Kato A., Yoshioka Y., Suzuki K. Brain glutamine and glutamate levels in patients with liver cirrhosis: assessed by 3.0-T MRS. Hepatol Res. 2004;30(1):18–23. doi: 10.1016/j.hepres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 45.Zieminska E., Dolinska M., Lazarewicz J.W., Albrecht J. Induction of permeability transition and swelling of rat brain mitochondria by glutamine. Neurotoxicology. 2000;21(3):295–300. [PubMed] [Google Scholar]
- 46.Rodrigo R., Felipo V. Brain regional alterations in the modulation of the glutamate-nitric oxide-cGMP pathway in liver cirrhosis. Role of hyperammonemia and cell types involved. Neurochem Int. 2006;48(6-7):472–477. doi: 10.1016/j.neuint.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 47.Hermenegildo C., Monfort P., Felipo V. Activation of N-methyl-D-aspartate receptors in rat brain in vivo following acute ammonia intoxication: characterization by in vivo brain microdialysis. Hepatology. 2000;31(3):709–715. doi: 10.1002/hep.510310322. [DOI] [PubMed] [Google Scholar]
- 48.Bender A.S., Norenberg M.D. Effects of ammonia on L-glutamate uptake in cultured astrocytes. Neurochem Res. 1996;21(5):567–573. doi: 10.1007/BF02527755. [DOI] [PubMed] [Google Scholar]
- 49.Ohara K., Aoyama M., Fujita M., Sobue K., Asai K. Prolonged exposure to ammonia increases extracellular glutamate in cultured rat astrocytes. Neurosci Lett. 2009;462(2):109–112. doi: 10.1016/j.neulet.2009.06.090. [DOI] [PubMed] [Google Scholar]
- 50.Hernández-Rabaza V., Cabrera-Pastor A., Taoro-Gonzalez L. Neuroinflammation increases GABAergic tone and impairs cognitive and motor function in hyperammonemia by increasing GAT-3 membrane expression. Reversal by sulforaphane by promoting M2 polarization of microglia. J Neuroinflammation. 2016;13(1):83. doi: 10.1186/s12974-016-0549-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takahashi K., Kameda H., Kataoka M., Sanjou K., Harata N., Akaike N. Ammonia potentiates GABAA response in dissociated rat cortical neurons. Neurosci Lett. 1993;151(1):51–54. doi: 10.1016/0304-3940(93)90043-k. [DOI] [PubMed] [Google Scholar]
- 52.Norenberg M.D., Bender A.S. Astrocyte swelling in liver failure: role of glutamine and benzodiazepines. Acta Neurochir Suppl (Wien) 1994;60:24–27. doi: 10.1007/978-3-7091-9334-1_6. [DOI] [PubMed] [Google Scholar]
- 53.Goral V., Atayan Y., Kaplan A. The relation between pathogenesis of liver cirrhosis, hepatic encephalopathy and serum cytokine levels: what is the role of tumor necrosis factor a? Hepatogastroenterology. 2011;58(107-108):943–948. [PubMed] [Google Scholar]
- 54.Rodrigo R., Cauli O., Gomez-Pinedo U. Hyperammonemia induces neuroinflammation that contributes to cognitive impairment in rats with hepatic encephalopathy. Gastroenterology. 2010;139(2):675–684. doi: 10.1053/j.gastro.2010.03.040. [DOI] [PubMed] [Google Scholar]
- 55.Sheen J.M., Chen Y.C., Hsu M.H., Tain Y.L., Yu H.R., Huang L.T. Combined intraperitoneal and intrathecal etanercept reduce increased brain tumor necrosis factor-alpha and asymmetric dimethylarginine levels and rescues spatial deficits in young rats after bile duct ligation. Front Cell Neurosci. 2016;10:167. doi: 10.3389/fncel.2016.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zemtsova I., Görg B., Keitel V., Bidmon H.J., Schrör K., Häussinger D. Microglia activation in hepatic encephalopathy in rats and humans. Hepatology. 2011;54(1):204–215. doi: 10.1002/hep.24326. [DOI] [PubMed] [Google Scholar]
- 57.Görg B., Bidmon H.J., Häussinger D. Gene expression profiling in the cerebral cortex of patients with cirrhosis with and without hepatic encephalopathy. Hepatology. 2013;57(6):2436–2447. doi: 10.1002/hep.26265. [DOI] [PubMed] [Google Scholar]
- 58.Blamire A.M., Anthony D.C., Rajagopalan B., Sibson N.R., Perry V.H., Styles P. Interleukin-1beta -induced changes in blood-brain barrier permeability, apparent diffusion coefficient, and cerebral blood volume in the rat brain: a magnetic resonance study. J Neurosci. 2000;20(21):8153–8159. doi: 10.1523/JNEUROSCI.20-21-08153.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vakili A., Mojarrad S., Akhavan M.M., Rashidy-Pour A. Pentoxifylline attenuates TNF-a protein levels and brain edema following temporary focal cerebral ischemia in rats. Brain Res. 2011;1377:119–125. doi: 10.1016/j.brainres.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 60.Rama Rao K.V., Jayakumar A.R., Tong X., Alvarez V.M., Norenberg M.D. Marked potentiation of cell swelling by cytokines in ammonia-sensitized cultured astrocytes. J Neuroinflammation. 2010;7:66. doi: 10.1186/1742-2094-7-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chastre A., Jiang W., Desjardins P., Butterworth R.F. Ammonia and proinflammatory cytokines modify expression of genes coding for astrocytic proteins implicated in brain edema in acute liver failure. Metab Brain Dis. 2010;25(1):17–21. doi: 10.1007/s11011-010-9185-y. [DOI] [PubMed] [Google Scholar]
- 62.Esposito G., Nobile N., Gigli S. Rifaximin improves Clostridium difficile toxin a-induced toxicity in Caco-2. Front Pharmacol. 2016;7:120. doi: 10.3389/fphar.2016.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández-Rabaza V., Cabrera-Pastor A., Taoro-González L. Hyperammonemia induces glial activation, neuroinflammation and alters neurotransmitter receptors in hippocampus, impairing spatial learning: reversal by sulforaphane. J Neuroinflammation. 2016;13:41. doi: 10.1186/s12974-016-0505-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Agusti A., Hernández-Rabaza V., Balzano T. Sildenafil reduces neuroinflammation in cerebellum, restores GABAergic tone, and improves motor in-coordination in rats with hepatic encephalopathy. CNS Neurosci Ther. 2017;23(5):386–394. doi: 10.1111/cns.12688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bosoi C.R., Rose C.F. Oxidative stress: a systemic factor implicated in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2013;28(2):175–178. doi: 10.1007/s11011-012-9351-5. [DOI] [PubMed] [Google Scholar]
- 66.Montoliu C., Cauli O., Urios A. 3-nitro-tyrosine as a peripheral biomarker of minimal hepatic encephalopathy in patients with liver cirrhosis. Am J Gastroenterol. 2011;106(9):1629–1637. doi: 10.1038/ajg.2011.123. [DOI] [PubMed] [Google Scholar]
- 67.Lachmann V., Görg B., Bidmon H.J., Keitel V., Häussinger D. Precipitants of hepatic encephalopathy induce rapid astrocyte swelling in an oxidative stress dependent manner. Arch Biochem Biophys. 2013;536(2):143–151. doi: 10.1016/j.abb.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 68.McMillin M., DeMorrow S. Effects of bile acids on neurological function and disease. FASEB J. 2016;30(11):3658–3668. doi: 10.1096/fj.201600275R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Taegtmeyer A.B., Haschke M., Tchambaz L. A study of the relationship between serum bile acids and propranolol pharmacokinetics and pharmacodynamics in patients with liver cirrhosis and in healthy controls. PLoS One. 2014;9(6):e97885. doi: 10.1371/journal.pone.0097885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tominaga S., Watanabe A., Tsuji T. Synergistic effect of bile acid, endotoxin, and ammonia on brain edema. Metab Brain Dis. 1991;6(2):93–105. doi: 10.1007/BF00999907. [DOI] [PubMed] [Google Scholar]
- 71.Bosoi C.R., Rose C.F. Elevated cerebral lactate: implications in the pathogenesis of hepatic encephalopathy. Metab Brain Dis. 2014;29(4):919–925. doi: 10.1007/s11011-014-9573-9. [DOI] [PubMed] [Google Scholar]
- 72.Lauritzen K.H., Morland C., Puchades M. Lactate receptor sites link neurotransmission, neurovascular coupling, and brain energy metabolism. Cereb Cortex. 2014;24(10):2784–2795. doi: 10.1093/cercor/bht136. [DOI] [PubMed] [Google Scholar]
- 73.Mallet M., Weiss N., Thabut D., Rudler M. Why and when to measure ammonemia in cirrhosis? Clin Res Hepatol Gastroenterol. 2018;S2210-7401(18):30031–30037. doi: 10.1016/j.clinre.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 74.Sharma B.C., Sharma P., Lunia M.K., Srivastava S., Goyal R., Sarin S.K. A randomized, double-blind, controlled trial comparing rifaximin plus lactulose with lactulose alone in treatment of overt hepatic encephalopathy. Am J Gastroenterol. 2013;108(9):1458–1463. doi: 10.1038/ajg.2013.219. [DOI] [PubMed] [Google Scholar]
- 75.Jayakumar A.R., Rama Rao K.V., Norenberg M.D. Neuroinflammation in hepatic encephalopathy: mechanistic aspects. J Clin Exp Hepatol. 2015;5(suppl 1):S21–S28. doi: 10.1016/j.jceh.2014.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Häussinger D., Görg B. Interaction of oxidative stress, astrocyte swelling and cerebral ammonia toxicity. Curr Opin Clin Nutr Metab Care. 2010;13(1):87–92. doi: 10.1097/MCO.0b013e328333b829. [DOI] [PubMed] [Google Scholar]
- 77.Cai S.Y., Boyer J.L. The role of inflammation in the mechanisms of bile acid-induced liver damage. Dig Dis. 2017;35(3):232–234. doi: 10.1159/000450916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kala G., Hertz L. Ammonia effects on pyruvate/lactate production in astrocytes--interaction with glutamate. Neurochem Int. 2005;47(1-2):4–12. doi: 10.1016/j.neuint.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Boitsova E.B., Morgun A.V., Osipova E.D. The inhibitory effect of LPS on the expression of GPR81 lactate receptor in blood-brain barrier model in vitro. J Neuroinflammation. 2018;15(1):196. doi: 10.1186/s12974-018-1233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]