Abstract
Q fever caused by the gram negative bacteria, Coxiella burnetii, is an occupational hazard for those who live and work in rural settings and those who are in contact with animals, especially abattoir and slaughterhouse workers. Australia is the only country to register a vaccine to prevent Q fever (Q-vax®, Seqirus, Australia) that is used in high risk populations. Seroprevalence studies conducted to determine the burden of Q fever (C. burnetii infection) in different settings have demonstrated high levels of heterogeneity with estimates of the percent positive ranging from 30% to 70%. There is a need for a more systematic evaluation of the findings of these studies in order to provide summary estimates of the seroprevalence in different settings.
We searched for published articles using PubMed, MEDLINE-EMBASE, and Scopus databases using search terms obtained from an initial review of published reports of recent Q fever outbreaks. Data on the seroprevalence of C. burnetii infection (Q fever) was extracted from the selected studies and a random effects meta-analysis was performed with stratification by outbreak status, year, country and serological techniques used. Results were visualised with a forest plot with 95% CI and measures of heterogeneity (I2) for the random effects model.
A total of 19 articles that met the search criteria were included. The reported seroprevalence rate ranged from 4.7% to 91.7% among abattoir and slaughterhouse workers. No inter-group heterogeneity was observed (p = 0.956), supporting the pooling of all studies into one pooled measure. The pooled estimate of seropositivity for C. burnetii infection in people working in abattoirs and slaughterhouses was 26% (95% CI: 18–35%) regardless of the evidence of an “outbreak”, the time of year or country. Seropositivity for C burnetii was independent of a person's age and years of occupational experience. Within abattoirs and slaughterhouses, slaughtering of cattle, sheep and goats are the most important risk factors associated with seropositivity and for those who showed over symptoms upon infection.
We recommend that vaccination programmes are directed towards people employed in the meat processing industry to mitigate the significant health and economic impacts of Q fever.
Keywords: Q fever, Coxiella burnetii, Abattoir, Slaughterhouse, Butcher, Meta-analysis
1. Background
Q fever (Q stands for query), caused by the highly pathogenic bacteria called Coxiella burnetii, is a zoonotic disease [15] and has worldwide distribution [28]. Since its discovery and description in Australia in 1937 [10,13] there have been several Q fever outbreaks reported internationally and the disease is considered endemic in most regions of the world [12,22,28,43]. The Netherlands is the country which experienced the highest ever reported Q fever outbreak [35]. Intensive farming of dairy goats and dairy sheep was the main reason for the outbreaks that occurred in The Netherlands [34].
Domestic ruminants and pets and in Australia, native marsupials, are the main reservoirs of infection [8,9,37,40]. Transmission to humans occurs mainly through inhalation of contaminated aerosols originating from parturient animals and their birth products [15,29]. Humans are considered accidental and dead-end hosts as there is no evidence of human-to-human transmission [15]. The seroprevalence of C. burnetii infection can range from 30% to 70% in people working in high-risk occupations such as farmers, veterinarians and abattoir workers [4].
Prevention of Q fever in Australia is through targeted immunization especially in those working in, or associated with the meat industry using the locally produced Q Vax vaccine (Seqirus, Australia), which has high efficacy in adults [25,26]. Q Vax is reported to provide up to 93% immune protection [32] with long-lived immune responses to C. burnetii [23]. However, the incidence of Q fever in people working in the red meat industry remains relatively high. Therefore, the current review provides information on the variability of the prevalence of Q fever exposure and risk factors for exposure in this occupational group.
2. Materials and methods
A search for published articles was conducted using several strategies (see details in Table 1, Table 2, Table 3 and supporting files 1–2 and Fig. 2): an online search of PubMed, MEDLINE-EMBASE, and Scopus databases was conducted using the terms Q fever, Coxiella burnetii, seroprevalence, sero-epidemiology, serology, incidence, prevalence, abattoir, abattoir workers, slaughterhouse, slaughterhouse workers, and butcher and meat workers. Further key words were then obtained from an initial review of reports of outbreak investigations in various countries.
Table 1.
Characteristics of studies that included abattoir and slaughterhouse workers.
| Author (Year) | Outbreak | Country | Setting | Size (n) | Seropositive | Prevalence (%) | Lab method |
|---|---|---|---|---|---|---|---|
| Gilroy [17] | Yes | Australia | Abattoir | 68 | 29 | 43 | CFT |
| Abebe [1] | No | Ethiopia | Abattoir | 465 | 30 | 6.5 | CFT |
| Adesiyun [2] | No | Trinidad | Abattoir | 85 | 4 | 4.7 | ELISA |
| Khalili [24] | No | Iran | Slaughterhouse | 75 | 51 | 68 | ELISA |
| Marrie [27] | No | Canada | Slaughterhouse | 96 | 12 | 12.5 | CFT |
| Esmaeili [14] | No | Iran | Slaughterhouse | 190 | 43 | 22.5 | ELISA |
| Aflatoonian [3] | No | Iran | Slaughterhouse | 64 | 5 | 7.8 | ELISA |
| Perez-Trallero [33] | No | Spain | Slaughterhouse | 36 | 33 | 91.7 | IFA |
| Berktaş [7] | No | Turkey | Slaughterhouse | 41 | 27 | 65.9 | ELISA |
| Htwe [21] | No | Japan | Abattoir | 107 | 12 | 11.2 | IFA |
| CDC [44] | Yes | USA | Abattoir | 42 | 19 | 45.2 | CFT |
| Beech [45] | Yes | Australia | Abattoir | 516 | 50 | 9.7 | CFT |
| Schnurrenberger [46] | No | USA | Abattoir | 2091 | 104 | 5 | CFT |
| Schonell [47] | No | UK | Abattoir | 96 | 21 | 28.1 | CFT |
| Riemann [48] | No | Brazil | Abattoir | 144 | 42 | 29 | AGGLUTINATION |
| McKelvie [30] | Yes | Australia | Abattoir | 139 | 22 | 15.8 | CFT |
| CDNANZ [49] | Yes | Australia | Abattoir | 100 | 18 | 18 | CFT |
| Donaghy [11] | Yes | UK | Abattoir | 228 | 49 | 21.5 | NA |
| Wilson [42] | Yes | UK | Slaughterhouse | 179 | 75 | 41.9 | IFA |
| Berktaş [7] | No | Turkey | Butcher house | 77 | 33 | 42.9 | ELISA |
Note: CFT = Complement Fixation Test, IFA = Immunofluorescence Assay, and ELISA = Enzyme-Linked Immunosorbent Assay. NA refers to Not Available.
Table 2.
PubMed search strategy: Articles search history and strategy for abattoirs and slaughterhouse workers.
| Search | Query | Items found |
|---|---|---|
| #12 | Search (((q fever) OR coxiella burnetii)) AND (((((((seroprevalence) OR seroepidemiology) OR serology) OR serological) OR prevalence) OR incidence) OR epidemiology) | 2864 |
| #11 | Search ((((((seroprevalence) OR seroepidemiology) OR serology) OR serological) OR prevalence) OR incidence) OR epidemiology | 2,745,738 |
| #10 | Search (q fever) OR coxiella burnetii | 5865 |
| #9 | Search epidemiology | 1,970,371 |
| #8 | Search incidence | 2,343,722 |
| #7 | Search prevalence | 2,189,454 |
| #6 | Search serological | 56,243 |
| #5 | Search serology | 194,892 |
| #4 | Search seroepidemiology | 1336 |
| #3 | Search seroprevalence | 25,616 |
| #2 | Search coxiella burnetii | 3157 |
| #1 | Search q fever | 4994 |
Table 3.
MEDLIN-EMBASE search history: Articles search history and strategy for abattoirs and slaughterhouse workers.
| No. | Query | Results |
|---|---|---|
| #11 | ‘q fever’/exp. OR ‘coxiella burnetii’/exp. AND (‘seroprevalence’/exp. OR ‘seroepidemiology’/exp. OR ‘serology’/exp. OR ‘prevalence’/exp. OR ‘incidence’/exp. OR ‘epidemiology’/exp) | 2246 |
| #10 | ‘seroprevalence’/exp. OR ‘seroepidemiology’/exp. OR ‘serology’/exp. OR ‘prevalence’/exp. OR ‘incidence’/exp. OR ‘epidemiology’/exp | 2,687,691 |
| #9 | ‘q fever’/exp. OR ‘coxiella burnetii’/exp | 6631 |
| #8 | ‘epidemiology’/exp | 2,514,169 |
| #7 | ‘incidence’/exp | 313,776 |
| #6 | ‘prevalence’/exp | 520,913 |
| #5 | ‘serology’/exp | 202,792 |
| #4 | ‘seroepidemiology’/exp | 2906 |
| #3 | ‘seroprevalence’/exp | 15,639 |
| #2 | ‘coxiella burnetii’/exp | 3640 |
| #1 | ‘q fever’/exp | 5152 |
Fig. 2.
Search strategy decision tree.
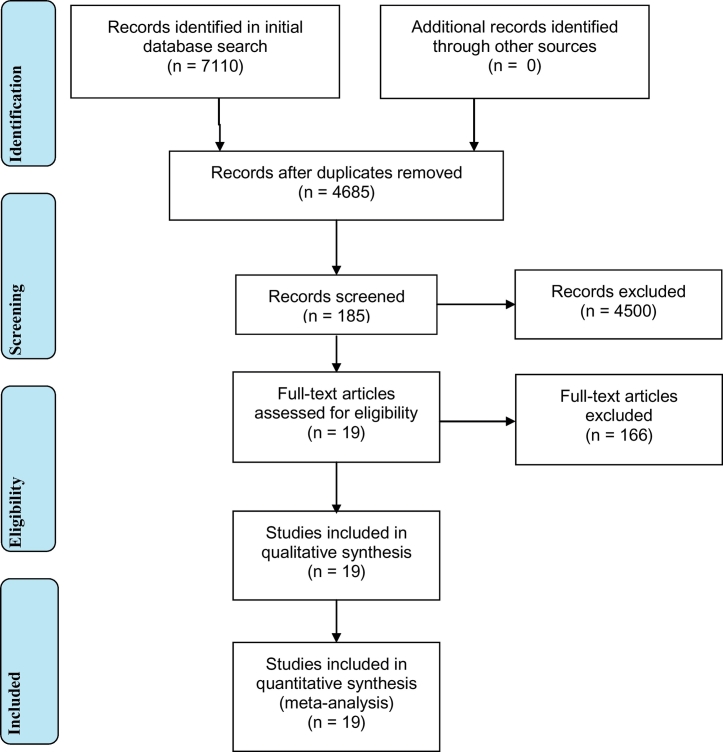
A total of 7110 articles were found from PubMed (2864), MEDLINE - EMBASE (2246) and Scopus (2000) databases. See Table 2, Table 3, supporting Files 1 and 2, and Fig. 2 for detailed search histories and strategies. After removing duplicated articles, 4685 articles were found and further refinement using title and abstract skimming, a total of 185 researches have retained. Finally, a total of 19 studies which fulfil the eligibility criteria were included in the meta-analysis.
One reviewer individually screened all study titles identified through database searches. An initial review of abstracts was performed to identify articles for a more detailed full text review. The final articles were selected if they met the following criteria: 1) if the full text or abstract is available, 2) articles that are published in a peer-reviewed or refereed archival journal in English, 3) articles that are based on original data; i.e., not a review article or meta-analysis, 4) articles that contained reported prevalence estimates from statistical analyses, and 5) articles of studies that were conducted on people working in the meat industry (slaughterhouse and abattoir workers, and butchers). Meta-analysis was done for abattoir and slaughterhouse workers with a total of 19 studies which met the inclusion criteria. Details of the studies' characteristics along with the number of studies included in each are summarized in supporting information Table 1.
Articles with extreme reported seroprevalence rates, that is, 0.0% and 100%, have been included in order to minimize the publication bias especially for positive findings. The prevalence of exposure was selected as the outcome variable in each of the various sub-groups. Odds and risk ratios were also extracted as measures of the strength of association between Q fever and exposures to different risk factors. Initial data extraction was done using Microsoft Excel and compiled data was imported to Stata version 13 [39].
Stratification based on serological test type and whether the study reported findings of an outbreak investigation was performed to determine the presence of heterogeneity in terms of the seroprevalence and potential reporting bias. Random effects meta-analysis was conducted using the metaprop Stata package that pools proportions and presents weighted sub-group and overall pooled estimates with inverse-variance weights obtained from a random-effects model [31] (see supporting File 2). A forest plot with error bars to indicate the 95% confidence interval around each of the [true] prevalence estimates was constructed and Higgin's I2 was used to quantify the amount of heterogeneity in the prevalence estimates, across studies [19,20]. The I2 is calculated using the following formula I2 = 100% × (Q − df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom [20].
Moreover, we used metafor R statistical package [41] for running meta-regression analysis for assessing presence of heterogeneity in the seroprevalences between outbreak and non-outbreak situations.
3. Results
A total of 7110 articles were identified in the initial searches of PubMed (2864), MEDLINE - EMBASE (2246) and Scopus (2000) databases. After removing duplicate articles, the title and abstracts of 4685 articles were reviewed to identify a total of 185 research articles that met our initial selection criteria. After the final screen 19 seroprevalence studies were included which met the inclusion criteria. Details on the characteristics of included studies are provided in Table 1.
Reported seroprevalence rates ranging from 4.7% to 91.7% among abattoir and slaughterhouse workers have been reported [[1], [2], [3],7,14,17,21,24,27,33]. The magnitude ranged from 4.7% in Trinidad [2] to 43.0% among abattoirs in Australia [1,2,17], and 7.8% in Iran – 91.7% in Spain among slaughterhouse workers [3,7,24,33].
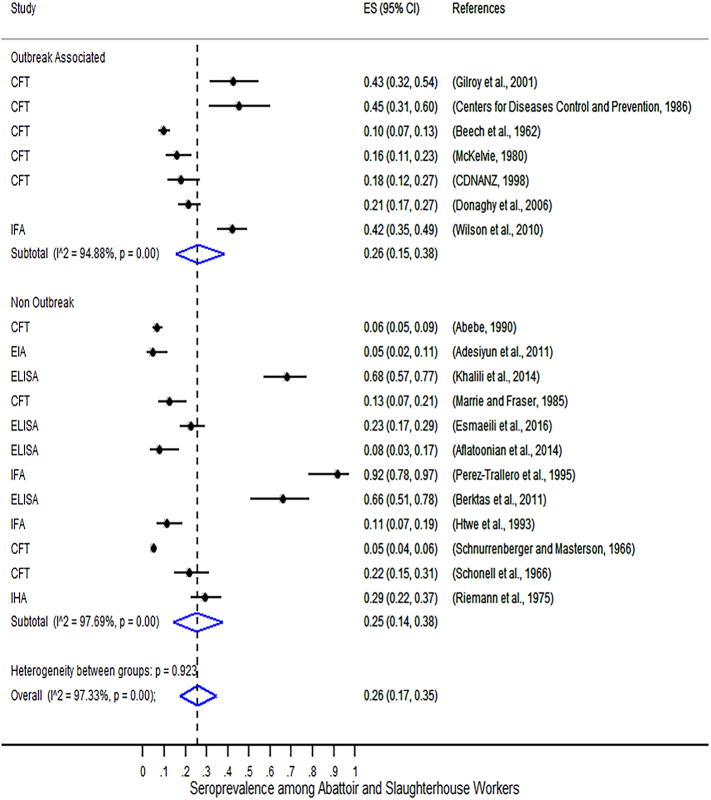
The outputs of the random effects meta-analysis on the seroprevalence of C. burnetii among abattoirs and slaughterhouse workers stratified by outbreak status is shown in Fig. 1. This figure presents the study specific proportions with 95% exact confidence intervals for each study, the sub-group and overall pooled estimate with 95% Wald confidence intervals and the I2 statistic which provides a measure of the total amount of variation (as a percentage) in the prevalence estimates that is due to variation at the individual study level.
Fig. 1.
Forest plot of seroprevalence of C. burnetii among abattoirs and slaughterhouse workers in 19 included studies stratified by outbreak status. Note: CFT = Complement Fixation Test, IFA = Immunofluorescence Assay, and ELISA = Enzyme-Linked Immunosorbent Assay. Also, ES(95% CI) refers to the seroprevalence point estimate (ES) with 95% CI. P = p-value and I2 describes the percentage of total variation across studies that is due to heterogeneity rather than chance.
The result of the meta-analysis shown in Fig. 1 indicates the absence of inter-group heterogeneity on the seroprevalence of C. burnetii (p = 0.932, I2 = 97.33%), both during outbreak and non-outbreak situations, supporting the pooling of all studies into one summary measure: 26% (95%, CI: 17–35%). However, the seroprevalence estimates showed significant intra-group heterogeneity for the three serological tests with I2 exceeding 94% (p < 0.001) for both outbreak and non-outbreak situations.
Meta-regression analysis (summarized in Table 4) indicated absence of statistical significant heterogeneity (p-value >0.05) between outbreak and among serological methods in the seroprevalence of C. burnetii. Hence, we conclude that pooling the seroprevalences for outbreak and non-outbreak situations is supported through a meta-regression.
Table 4.
Table Mixed-effects meta-regression model results on the seroprevalence of Q fever in abattoir and slaughterhouses.
| Source of variation | Category | Estimate | SE | Z | P-Value | 95% CI |
I2 | R2 | |
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Outbreak | Intercept | 0.27 | 0.09 | 3.05 | 0.00 | 0.10 | 0.45 | 99.06% | 0.02% |
| Outbreak | 0.01 | 0.11 | 0.08 | 0.94 | −0.21 | 0.23 | |||
| Diagnosis method | Intercept | 0.18 | 0.12 | 1.57 | 0.12 | −0.05 | 0.41 | 98.90% | 26.86% |
| CFT | 0.01 | 0.14 | 0.06 | 0.96 | −0.26 | 0.27 | |||
| ELISA | 0.22 | 0.16 | 1.40 | 0.16 | −0.09 | 0.52 | |||
| IFA | 0.30 | 0.17 | 1.78 | 0.08 | −0.03 | 0.62 | |||
Mixed-Effects Model (k = 19; tau^2 (estimated amount of residual heterogeneity) estimator: ML (Maximum Likelihood)). I^2 (residual heterogeneity/unaccounted variability). R^2 (amount of heterogeneity accounted for).
4. Discussion
The result of the seroprevalence studies showed that Q fever is endemic in meat processing industries such as abattoirs and slaughterhouses. The result of the meta-analysis indicated that, the seroprevalence of C. burnetii infection remained relatively constant regardless of outbreak situations. A strong association with the meat industry is confirmed [16]. This is in accordance with studies that have shown that abattoir workers are notable at-risk group for Q fever who should be fully protected from this occupational disease [6].
The overall seroprevalence of C. burnetii (26%) determined in this meta-analysis falls within the range of values (30–70%) reported in studies of high risk groups elsewhere [4]. Gilroy et al. [17] indicated that up to sixty-eight (66%) of employees in abattoirs are considered susceptible to primary infection and that unscreened, unvaccinated, non-immune workers developed Q fever after exposure to C. burnetii [17]. Furthermore, Perez-Trallero et al. showed that >86% of slaughterhouse workers in one study had evidence of previous infection by C. burnetii using skin testing and serology? [33].
The detection of higher prevalence of antibodies to C. burnetii in abattoir workers and those who had minimal contact with animals is an indication of presence of pre-existing immunity rather than recent infection [2,17]. Infection with C. burnetii among abattoir and slaughterhouse workers occurs independent of the age, sex, race, years of occupational experience or the types of duties performed in the abattoir or in the offices by the workers [2,14,24]. In addition, the absence of association between seropositivity for C. burnetii and work history, work type, splashing of animal secretions on face or body and occupational injury have also been identified [14]. However, the results of two studies (Wilson et al. [42]; Marrie and Fraser [27]) suggest that being male [OR = 6.4, 95% CI: 1.8–23.4] is independently statistically significantly associated with an increased risk of testing positive for infection with C. burnetii [27,42].
The greatest risk of infection was associated with working in areas where cattle, sheep and swine are slaughtered [11,30]. Marrie and Fraser [27] showed that slaughtering cattle (working on the kill floor) was a significant risk factor for positive antibody titers among slaughterhouse workers [27]. Furthermore, A previous study by Perez-Trallero et al., [33] reported 19.25 higher [OR = 19.25, 95% CI: 5.34, 102.74] odds of Q fever infection for those working in slaughterhouses [33].
In addition, people who work in other occupations in rural settings and that have contact with animals, especially the operators of the livestock industry (veterinarians, tanners, and wool carders) are also at higher risk of seropositivity [24,36].
One published case control study confirmed the higher incidence of Q fever associated with increased level of exposure to a slaughterhouse (low exposure: OR = 3.0; moderate: OR = 4.7; high: OR = 15.0; chi2 for trend, p = 0.006) and high level of exposure to the slaughterhouse site (OR = 6.8, 95% CI: 1.1–40.3) [5]. A strong association with the meat industry was confirmed [16]. This is in accordance with studies that have shown that abattoir workers are a notable at-risk group for Q fever [6]. It is the study that supports the hypothesis of a sheep lairage being the source of potential exposure, Scottish co-located slaughterhouse and cutting plant. Passing through walkway by the sheep lairage was independently associated with an increased risk of testing positive. Those who passed through the stores were 3.2 (95% CI: 1.7–6.3) times as likely to be a confirmed case, those who passed through walkway by the sheep lairage were 2.1 (95% CI: 1.0–4.3) times as likely [42]. Bell, et al. [6] indicated that because the organism is transmitted in aerosols, it is important that not only abattoir employees but all workers who visit or work on an abattoir site are vaccinated against Q fever. This includes service providers such as electricians, plumbers, telecommunication workers, weights and measures inspectors and transport workers [6].
A randomized, blind, placebo-controlled trial of Q fever and influenza vaccines has been conducted in three Queensland abattoirs, showing occurrence of Q fever cases in unvaccinated workers in all three abattoirs during the follow-up period [38]. However, vaccination administered 10 or more days after the likely period of exposure showed no significant protective effect (RR = 0.57; 95% CI 0.13–2.57; p = 0.60) [17]. The study by Greig et al. [18] showed the average annual risk of infection among abattoir workers to be 45.0 per 1000 (95% CI 42.3–47.6), and 62.6 per 1000 (95% CI 57.5–67.7) over the first 10 years of exposure [18]. Up to 90% of new entrants in high-risk workplaces will be susceptible to Q fever and require vaccination [18].
In summary, the seroprevalence associated with C. burnetii infection ranged from 4.7% to 91.7% among abattoir and slaughterhouse workers was reported in the included studies. From the random effects meta-analysis, seropositivity for C. burnetii in abattoirs and slaughterhouses can be expected in more than a quarter of workers (26%; 95% CI: 17%–35%) regardless of evidence of an outbreak. In addition, seropositivity for C. burnetii is independent of age and years of occupational experience in meat abattoir and slaughterhouses but significantly associated with activities related to slaughtering of cattle, sheep and goats. Vaccination 10 days prior to exposure to environments where C. burnetii may be present was shown to be an effective prevention mechanism. Vaccination programmes for workers in high risk industries is highly recommended for mitigating the incidence of Q fever, and subsequent chronic sufferings and work day offs among abattoir and slaughterhouse workers.
Acknowledgment
We are highly indebted for the University of Queensland for offering this scholarship opportunity and providing us library facility for accessing articles from PubMed, MEDLINE-EMBASE, and Scopus databases through its library.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.onehlt.2018.09.002.
Appendix A. Supplementary data
Scopus Search Strategy [from Health and Life Sciences Subject Areas]. Articles search history and strategy for abattoirs and slaughterhouse workers.
Metaprop Stata Package for Running the Meta-Analysis
References
- 1.Abebe A. Prevalence of Q fever infection in the Addis Ababa abattoir. Ethiop. Med. J. 1990;28:119–122. [PubMed] [Google Scholar]
- 2.Adesiyun A., Dookeran S., Stewart-Johnson A., Rahaman S., Bissessar S. Frequency of seropositivity for Coxiella burnetii immunoglobulins in livestock and abattoir workers in Trinidad. New Microbiol. 2011;34:219–224. [PubMed] [Google Scholar]
- 3.Aflatoonian M.R., Khalili M., Sami M., Abiri Z. The frequency of IgM anti-Coxiella burnetii (Q fever) antibodies among slaughterhouse workers in Kerman city, 2012. J. Kerman Univ. Med. Sci. 2014;21:368–375. [Google Scholar]
- 4.Aitken I.D., Bögel K., Cračea E., Edlinger E., Houwers D., Krauss H., Rády M., Řeháček J., Schiefer H.G., Kazán J., Schmeer N., Tarasevich I.V., Tringali G. Q fever in Europe: current aspects of aetiology, epidemiology, human infection, diagnosis and therapy. Infection. 1987;15:323–327. doi: 10.1007/BF01647731. [DOI] [PubMed] [Google Scholar]
- 5.Armengaud A., Kessalis N., Desenclos J.C., Maillot E., Brousse P., Brouqui P., Tixier-Dupont H., Raoult D., Provensal P., Obadia Y. Urban outbreak of Q fever, Briancon, France, March to June 1996. Euro Surveill. 1997;2:12–13. doi: 10.2807/esm.02.02.00137-en. [DOI] [PubMed] [Google Scholar]
- 6.Bell M., Patel M., Sheridan J. Q fever vaccination in Queensland abattoirs. Commun. Dis. Intell. 1997;21:29–31. [PubMed] [Google Scholar]
- 7.Berktaş M., Ceylan E., Yaman G., Çiftci I.H. Seroprevalence of Coxiella burnetii antibodies in high risk groups in eastern Turkey. Turkiye Klinikleri J. Med. Sci. 2011;31:45–50. [Google Scholar]
- 8.Bond K.A., Vincent G., Wilks C.R., Franklin L., Sutton B., Stenos J., Cowan R., Lim K., Athan E., Harris O., Macfarlane-Berry L., Segal Y., Firestone S.M. One health approach to controlling a Q fever outbreak on an Australian goat farm. Epidemiol. Infect. 2016;144:1129–1141. doi: 10.1017/S0950268815002368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper A., Hedlefs R., Mcgowan M., Ketheesan N., Govan B. Serological evidence of Coxiella burnetii infection in beef cattle in Queensland. Aust. Vet. J. 2011;89:260–264. doi: 10.1111/j.1751-0813.2011.00794.x. [DOI] [PubMed] [Google Scholar]
- 10.Derrick E.H. "Q" fever, a new fever entity: clinical features, diagnosis and laboratory investigation. Rev. Infect. Dis. 1983;5:790–800. doi: 10.1093/clinids/5.4.790. [DOI] [PubMed] [Google Scholar]
- 11.Donaghy M., Prempeh H., MacDonald N. Outbreak of Q fever in workers at a meat processing plant in Scotland, July 2006. Euro Surveillance. 2006;11 doi: 10.2807/esw.11.34.03031-en. [DOI] [PubMed] [Google Scholar]
- 12.Dupont H.T., Thirion X., Raoult D. Q fever serology: cutoff determination for microimmunofluorescence. Clin. Diagn. Lab. Immunol. 1994;1:189–196. doi: 10.1128/cdli.1.2.189-196.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dyer R.E. Q fever; history and present status. Am. J. Public Health Nation Health. 1949;39:471–477. doi: 10.2105/ajph.39.4.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esmaeili S., Naddaf S.R., Pourhossein B., Shahraki A.H., Amiri F.B., Gouya M.M., Mostafavi E. Seroprevalence of brucellosis, leptospirosis, and q fever among butchers and slaughterhouse workers in South-Eastern Iran. PLoS ONE. 2016;11 doi: 10.1371/journal.pone.0144953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Food Safety, A., European Centre For Disease, P. & Control The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2012. EFSA J. 2014;12 doi: 10.2903/j.efsa.2018.5500. (n/a-n/a) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garner M.G., Longbottom H.M., Cannon R.M., Plant A.J. A review of Q fever in Australia 1991-1994. Aust. N. Z. J. Public Health. 1997;21:722–730. doi: 10.1111/j.1467-842x.1997.tb01787.x. [DOI] [PubMed] [Google Scholar]
- 17.Gilroy N., Formica N., Beers M., Egan A., Conaty S., Marmion B. Abattoir-associated Q fever: a Q fever outbreak during a Q fever vaccination program. Aust. N. Z. J. Public Health. 2001;25:362–367. doi: 10.1111/j.1467-842x.2001.tb00595.x. [DOI] [PubMed] [Google Scholar]
- 18.Greig J.E., Patel M.S., Clements M.S., Taylor N.K. Control strategies for Q fever based on results of pre-vaccination screening in Victoria, 1988 to 2001. Aust. N. Z. J. Public Health. 2005;29:53–57. doi: 10.1111/j.1467-842x.2005.tb00749.x. [DOI] [PubMed] [Google Scholar]
- 19.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 20.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Htwe K.K., Yoshida T., Hayashi S., Miyake T., Amano K., Morita C., Yamaguchi T., Fukushi H., Hirai K. Prevalence of antibodies to Coxiella burnetii in Japan. J. Clin. Microbiol. 1993;31:722–723. doi: 10.1128/jcm.31.3.722-723.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kaplan M.M., Bertagna P. The geographical distribution of Q fever. Bull. World Health Organ. 1955;13:829–860. [PMC free article] [PubMed] [Google Scholar]
- 23.Kersh G.J., Fitzpatrick K.A., Self J.S., Biggerstaff B.J., Massung R.F. Long-term immune responses to Coxiella burnetii after vaccination. Clin. Vaccine Immunol. 2013;20:129–133. doi: 10.1128/CVI.00613-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Khalili M., Mosavi M., Diali H.G., Mirza H.N. Serologic survey for Coxiella burnetii phase II antibodies among slaughterhouse workers in Kerman, southeast of Iran. Asian Pacific J. Trop. Biomed. 2014;4:S209–S212. doi: 10.12980/APJTB.4.2014C1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marmion B.P., Ormsbee R.A., Kyrkou M., Wright J., Worswick D., Cameron S., Esterman A., Feery B., Collins W. Vaccine prophylaxis of abattoir-associated Q fever. Lancet. 1984;2:1411–1414. doi: 10.1016/s0140-6736(84)91617-9. [DOI] [PubMed] [Google Scholar]
- 26.Marmion B.P., Ormsbee R.A., Kyrkou M., Wright J., Worswick D.A., Izzo A.A., Esterman A., Feery B., Shapiro R.A. Vaccine prophylaxis of abattoir-associated Q fever: eight years' experience in Australian abattoirs. Epidemiol. Infect. 1990;104:275–287. doi: 10.1017/s0950268800059458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marrie T.J., Fraser J. Prevalence of antibodies to coxiella burnetii among veterinarians and slaughterhouse workers in Nova Scotia. Can. Vet. J. 1985;26:181–184. [PMC free article] [PubMed] [Google Scholar]
- 28.Maurin M., Raoult D. Q fever. Clin. Microbiol. Rev. 1999;12:518–553. doi: 10.1128/cmr.12.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raoult D., Marrie T., Mege J. Natural history and pathophysiology of Q fever. Lancet Infect. Dis. 2005;5:219–226. doi: 10.1016/S1473-3099(05)70052-9. [DOI] [PubMed] [Google Scholar]
- 30.Mckelvie P. Q fever in a Queensland meatworks. Med. J. Aust. 1980;1:590–593. doi: 10.5694/j.1326-5377.1980.tb135158.x. [DOI] [PubMed] [Google Scholar]
- 31.Nyaga V.N., Arbyn M., Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch. Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'NEILL T.J., Sargeant J.M., Poljak Z. The effectiveness of Coxiella burnetii vaccines in occupationally exposed populations: a systematic review and meta-analysis. Zoonoses Public Health. 2014;61:81–96. doi: 10.1111/zph.12054. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Trallero E., Cilla G., Montes M., Saenz-Dominguez J.R., Alcorta M. Prevalence of Coxiella burnetii infection among slaughterhouse workers in northern Spain. Eur. J. Clin. Microbiol. Infect. Dis. 1995;14:71–73. doi: 10.1007/BF02112627. [DOI] [PubMed] [Google Scholar]
- 34.Reller M.E., Dumler A.J.S., Kliegman R.M., Stanton B.M.D. Q fever (Coxiella burnetii) In: St Geme J., Schor N.F., editors. Nelson Textbook of Pediatrics: Expert Consult. Elsevier; London: 2015. [Google Scholar]
- 35.Roest H.I.J., Tilburg J.J.H.C., Van Der Hoek W., Vellema P., Van Zijderveld F.G., Klaassen C.H.W., Raoult D. The Q fever epidemic in the Netherlands: history, onset, response and reflection. Epidemiol. Infect. 2011;139:1–12. doi: 10.1017/S0950268810002268. [DOI] [PubMed] [Google Scholar]
- 36.Schimmer B., Schotten N., Van Engelen E., Hautvast J.L., Schneeberger P.M., van Duijnhoven Y.T. Coxiella burnetii seroprevalence and risk for humans on dairy cattle farms, the Netherlands, 2010-2011. Emerg. Infect. Dis. 2014;20:417–425. doi: 10.3201/eid2003.131111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shapiro A.J., Bosward K.L., Heller J., Norris J.M. Seroprevalence of Coxiella burnetii in domesticated and feral cats in eastern Australia. Vet. Microbiol. 2015;177:154–161. doi: 10.1016/j.vetmic.2015.02.011. [DOI] [PubMed] [Google Scholar]
- 38.Shapiro R.A., Siskind V., Schofield F.D., Stallman N., Worswick D.A., Marmion B.P. A randomized, controlled, double-blind, cross-over, clinical trial of Q fever vaccine in selected Queensland abattoirs. Epidemiol. Infect. 1990;104:267–273. doi: 10.1017/s0950268800059446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Statacorp . 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP. [Google Scholar]
- 40.Tozer S.J., Lambert S.B., Strong C.L., Field H.E., Sloots T.P., Nissen M.D. Potential animal and environmental sources of Q fever infection for humans in Queensland. Zoonoses Public Health. 2014;61:105–112. doi: 10.1111/zph.12051. [DOI] [PubMed] [Google Scholar]
- 41.Viechtbauer W. 2010. Conducting Meta-Analyses in R with the metafor Package. 2010, 36, 48. [Google Scholar]
- 42.Wilson L.E., Couper S., Prempeh H., Young D., Pollock K.G., Stewart W.C., Browning L.M., Donaghy M. Investigation of a Q fever outbreak in a Scottish co-located slaughterhouse and cutting plant. Zoonoses Public Health. 2010;57:493–498. doi: 10.1111/j.1863-2378.2009.01251.x. [DOI] [PubMed] [Google Scholar]
- 43.World Health Assembly . 1950. Third World Health Assembly, Geneva, 8 to 27 May 1950: Resolutions and Decisions: Plenary Meetings Verbatim Records: Committees Minutes and Reports: Annexes. Geneva. [Google Scholar]
- 44.Centers for Disease Control and Prevention (CDC) Q fever among slaughterhouse workers-California. MMWR Morbidity and mortality weekly report. 1986;35(14):223–226. [PubMed] [Google Scholar]
- 45.Beech M.D., Duxbury A.E., Warner P. Q fever in South Australia: an outbreak in a meat-works. J. Hyg. 2009;60:1–20. doi: 10.1017/s0022172400039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schnurrenberger P.R., Masterson R.A. The prevalence of antibodies against selected zoonotic diseases in abattoir workers. Arch. Environ. Health. 1966;13:336–339. doi: 10.1080/00039896.1966.10664566. [DOI] [PubMed] [Google Scholar]
- 47.Schonell M.E., Brotherston J.G., Burnett R.C.S., Campbell J., Coghlan J.D., Moffat M.A.J., Norval J., Sutherland J.A.W. Occupational infections in the Edinburgh Abattoir. Br. Med. J. 1966;2:148–150. doi: 10.1136/bmj.2.5506.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riemann H.P., Brant P.C., Behymer D.E., Franti C.E. Toxoplasma gondii and Coxiella burneti antibodies among Brazilian slaughterhouse employees. Am. J. Epidemiol. 1975;102:386–393. doi: 10.1093/oxfordjournals.aje.a112177. [DOI] [PubMed] [Google Scholar]
- 49.Communicable Diseases Intelligence (CDI) Q fever outbreak in an abattoir in Cooma, NSW. Commun. Dis. Intell. 1998;22:222. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scopus Search Strategy [from Health and Life Sciences Subject Areas]. Articles search history and strategy for abattoirs and slaughterhouse workers.
Metaprop Stata Package for Running the Meta-Analysis