Abstract
An association between restless legs syndrome (RLS) and obstructive sleep apnea (OSA) has been suggested for decades but has been questioned in recent years given the apparently similar prevalence of RLS among patients with OSA and the general population. Still, marked improvement in symptoms of RLS has been reported in patients with OSA treated with continuous positive airway pressure (CPAP). Whether the effect of OSA treatment on RLS extends to modalities of OSA treatment other than CPAP remains an open question. Here, we report the case of a patient with OSA and comorbid debilitating RLS who underwent upper airway stimulation device implantation and subsequently experienced near-resolution of her severe RLS symptoms.
Upper airway stimulation devices may be an option for patients with OSA and severe RLS intolerant to conventional CPAP modalities.
Citation:
Myc LA, Churnin IT, Jameson MJ, Davis EM. Treatment of comorbid obstructive sleep apnea by upper airway stimulation results in resolution of debilitating symptoms of restless legs syndrome. J Clin Sleep Med. 2018;14(10):1797–1800.
Keywords: obstructive sleep apnea, restless legs syndrome, upper airway stimulator
INTRODUCTION
Restless legs syndrome (RLS), also known as Willis-Ekbom disease, is a sleep-related movement disorder that may significantly and negatively affect quality of life.1 Although the current mainstay of RLS therapies are either dopaminergic or anti-convulsant pharmacologic agents, the absence of a consensus regarding an underlying pathophysiology can be seen as limiting advances in therapeutic modalities for the disease.2
An association between RLS and obstructive sleep apnea (OSA) has been suggested for decades but has been questioned in recent years given the apparently similar prevalence of RLS among patients with OSA and the general population.3,4 Still, marked improvement in symptoms of RLS has been reported in OSA patients treated with continuous positive airway pressure (CPAP).5 Whether the effect of OSA treatment on RLS extends to modalities of OSA treatment other than CPAP remains an open question.
In recent years, an upper airway stimulation (UAS) device received FDA approval and has since been adopted into the therapeutic armamentarium for patients with OSA intolerant to CPAP.6 In addition to objective improvement in both the apnea-hypopnea index (AHI) and oxygen desaturation index (ODI), patients have reported improved outcomes in self-reported measures which were maintained several years out from device implantation.6,7 Here, we report the case of a patient with OSA and comorbid, debilitating RLS who underwent UAS device implantation and subsequently experienced near-resolution of her severe RLS symptoms.
REPORT OF CASE
A 56-year-old woman with a past medical history of RLS presented with severe, chronic, near-nightly leg discomfort with an urge to move and jerk her legs associated with frequent awakening, reduced sleep efficiency, and daytime sleepiness. Her symptoms had been present since her first pregnancy at the age of 23 years. She noted increasingly disruptive and distressing symptoms despite trials of multiple pharmacologic agents including ropinirole, pramipexole, gabapentin, quietiapine, opioids, and iron supplementation. Augmentation was considered a contributing factor to her RLS symptoms, and she was instructed to wean ropinirole and transition to gabapentin. She continued on iron supplementation and healthy weight loss was encouraged. At short-term follow-up she reported worsening RLS symptoms during her ropinirole weaning trial and so this medication was resumed. Severe distress resulting in anxiety and depression persisted as a result of her uncontrolled RLS symptoms and she did require intermittent doses of opiates at night.
During the course of her RLS work-up, she was noted to have snoring and witnessed apneas with an Epworth Sleepiness Scale score of 19/24. She underwent polysomnography which revealed an obstructive AHI of 22.1 events/h, oxygen saturation nadir of 88%, and CPAP requirement of 14 mmH2O. During a 6-month trial, she proved intolerant to CPAP and so was referred for consideration of PAP-alternative modalities.
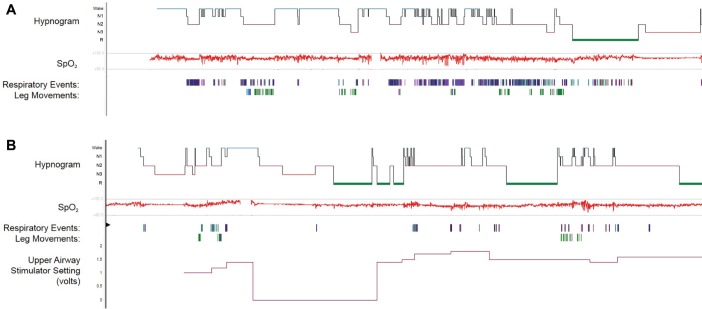
Upon clinical evaluation, her body mass index was 35 kg/m2 with physical examination revealing a midline septum, mild bilateral inferior turbinate hypertrophy, a large scalloped tongue, a 43 cm neck circumference and absence of tonsil-lar hypertrophy or retrognathia. She was noted to be anxious and very restless while sitting. Laboratory data were largely unremarkable, with a serum ferritin of 49 ng/mL. In consideration of UAS therapy, repeat polysomnography was pursued to reassess OSA severity. Polysomnography demonstrated an obstructive AHI of 62 events/h, desaturation nadir of 84%, a periodic limb movement index of 16.2 events/h, and an overall sleep efficiency of 65% (Figure 1A and Table 1). The patient additionally underwent drug-induced sleep endoscopy (DISE) which revealed near complete predominantly anterior-posterior (AP) collapse of the vellum, severe AP collapse of the tongue base, and moderate AP collapse of the epiglottis, with a VOTE (velum, oropharynx, tongue base, and epiglottis) score of 5. Accordingly, she was deemed a candidate for implantation of a hypoglossal nerve stimulator (Inspire IV 3028, Inspire Medical Systems, Inc, Maple Grove, Minnesota, United States) and subsequently underwent surgical implantation.
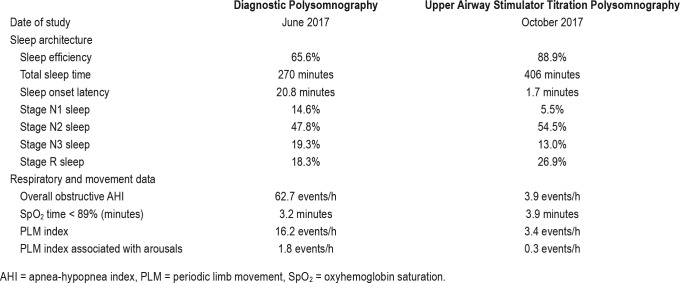
Figure 1. Polysomnography results.
The diagnostic polysomnography (A) shows frequent respiratory events and leg movements which are improved in the subsequent upper airway stimulator titration polysomnography (B). SpO2 = oxyhemoglobin saturation.
Table 1.
Sleep study parameters show interval improvement between the diagnostic polysomnography and upper airway stimulator titration polysomnography.
The device was activated 1 month postoperatively. A titration sleep study performed 2 months postoperatively demonstrated that a setting of 1.6 volts was associated with an obstructive AHI of 4.3 events/h, desaturation nadir of 88%, a periodic limb movement index of 3.4 events/h, and an overall sleep efficiency of 89% (Figure 1B and Table 1). Four months following UAS device activation/titration, the patient was seen in follow-up at which time she reported near complete resolution of her RLS symptoms in the evening hours before bedtime, improvement in sleepiness, and resolution of snoring. Her RLS symptoms started to improve approximately 6 weeks after her UAS device was activated. She was no longer using opiates for RLS control and she had down-titrated her ropinirole dose. She reported good tolerance to UAS and reported an average usage of greater than 5 hours nightly.
DISCUSSION
To our knowledge, ours is the first report of an apparently beneficial effect of an UAS device on RLS. The approximate prevalence of RLS in patients with OSA may be upwards of 8.3%.8 Although the prevalence of the disease may not be increased in patients with OSA, reported improvement in RLS symptoms with OSA-directed therapies at least suggests an augmented severity of RLS unique to this patient population. Most pertinently, Delgado Rodrigues et al. studied 17 patients afflicted with OSA and comorbid RLS who were treated with nasal CPAP. These authors reported improvement in International Restless Legs Syndrome Study Groups Rating Scale (IRLSS) scores (in addition to Epworth Sleepiness Scale and Pichot's questionnaire of fatigue/depression scores) following 3 months of treatment with nasal CPAP.5 More recently, Silva and colleagues reported a retrospective study of 28 patients with both RLS and OSA (mean AHI of 19 events/h) in which over 70% of the patients reported improvement in RLS symptoms following initiation of therapy for OSA.9 Such data highlight the importance of treating OSA in patients with difficult to control RLS.
The observation that a therapy targeting improvement in OSA is associated with improved RLS symptoms may be seen as supporting the hypothesis that the intermittent hypoxia observed in OSA globally activates the putative hypoxiainducible factor pathway in patients with RLS.10 Consistent with this idea, Kaplan et al. have reported that patients with chronic obstructive pulmonary disease (COPD) and RLS were characterized by longer duration of their obstructive disease, more evident obstruction, and worsened hypercapnia and hypoxia than age-matched controls with COPD but without RLS.11 In this scenario, OSA therapies that improve AHI/ ODI, whether conventional CPAP or otherwise, may prove effective in alleviating RLS symptoms in patients with both conditions. Additionally, treatment of OSA with CPAP and other modalities may improve RLS symptoms via reducing wakefulness after sleep onset and improving overall sleep architecture. Such improvement in sleep architecture may limit the overall perception of RLS symptoms thereby positively impacting RLS control.
We are cautious in attributing a therapeutic effect to this new OSA treatment modality in RLS due to factors including (1) multiple potential confounders, (2) a possible placebo effect and (3) the fact that spontaneous remissions in RLS occur with some frequency, but it must be said that RLS diagnostic criteria are subjective by definition. UAS devices may be an option for patients with OSA and severe RLS who are intolerant to conventional PAP modalities. Further studies are needed to validate our observation that treatment of OSA with CPAP alternatives can improve RLS symptomatology. Future use of a prospective study design would allow for use of well-validated tools (such as the IRLSS) to quantify RLS severity and to employ polysomnography to document improvement in periodic limb movements.
DISCLOSURE STATEMENT
Work for this study was performed at the University of Virginia. All authors have seen and approved the manuscript. The use of the Inspire upper airway stimulator is an FDA approved treatment option for moderate to severe obstructive sleep apnea although its impact on the treatment of restless legs syndrome is off-label. The authors report no conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AP
anterior-posterior
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- DISE
drug-induced sleep endoscopy
- IRLSS
International Restless Legs Syndrome Study Groups Rating Scale
- ODI
oxygen desaturation index
- OSA
obstructive sleep apnea
- PLM
periodic limb movement
- RLS
restless legs syndrome
- UAS
upper airway stimulation
REFERENCES
- 1.Happe S, Reese JP, Stiasny-Kolster K, et al. Assessing health-related quality of life in patients with restless legs syndrome. Sleep Med. 2009;10(3):295–305. doi: 10.1016/j.sleep.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Garcia-Borreguero D, Kohnen R, Silber MH, et al. The long-term treatment of restless legs syndrome/willis-ekbom disease: evidence-based guidelines and clinical consensus best practice guidance: a report from the International Restless Legs Syndrome Study Group. Sleep Med. 2013;14(7):675–684. doi: 10.1016/j.sleep.2013.05.016. [DOI] [PubMed] [Google Scholar]
- 3.Chokroverty S, Sachdeo R. Restless limb-myoclonus-sleep apnea syndrome [abstract] Ann Neurol. 1984;16(1):124. [Google Scholar]
- 4.Chokroverty S. Differential diagnoses of restless legs syndrome/Willis-Ekbom Disease: Mimics and comorbidities. Sleep Med Clin. 2015;10(3):249–262. doi: 10.1016/j.jsmc.2015.05.021. [DOI] [PubMed] [Google Scholar]
- 5.Delgado Rodrigues RN, Alvim de Abreu E Silva Rodrigues AA, Pratesi R, Krieger J. Outcome of restless legs severity after continuous positive air pressure (CPAP) treatment in patients affected by the association of RLS and obstructive sleep apneas. Sleep Med. 2006;7(3):235–239. doi: 10.1016/j.sleep.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 6.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. N Engl J Med. 2014;370(2):139–149. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 7.Woodson BT, Strohl KP, Soose RJ, et al. Upper airway stimulation for obstructive sleep apnea: 5-year outcomes. Otolaryngol Head Neck Surg. 2018;159(1):194–202. doi: 10.1177/0194599818762383. [DOI] [PubMed] [Google Scholar]
- 8.Lakshminarayanan S, Paramasivan KD, Walters AS, Wagner ML, Patel S, Passi V. Clinically significant but unsuspected restless legs syndrome in patients with sleep apnea. Mov Disord. 2005;20(4):501–503. doi: 10.1002/mds.20366. [DOI] [PubMed] [Google Scholar]
- 9.Silva C, Peralta AR, Bentes C. The urge to move and breathe - the impact of obstructive sleep apnea syndrome treatment in patients with previously diagnosed, clinically significant restless legs syndrome. Sleep Med. 2017;38:17–20. doi: 10.1016/j.sleep.2017.06.023. [DOI] [PubMed] [Google Scholar]
- 10.Patton SM, Ponnuru P, Snyder AM, Podskalny GD, Connor JR. Hypoxiainducible factor pathway activation in restless legs syndrome patients. Eur J Neurol. 2011;18(11):1329–1335. doi: 10.1111/j.1468-1331.2011.03397.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaplan Y, Inonu H, Yilmaz A, Ocal S. Restless legs syndrome in patients with chronic obstructive pulmonary disease. Can J Neurol Sci. 2008;35(3):352–357. doi: 10.1017/s0317167100008957. [DOI] [PubMed] [Google Scholar]