Abstract
Study Objectives:
Sleep apnea is often newly diagnosed in patients presenting with ST-segment elevation myocardial infarction (STEMI). We assessed longitudinal changes in apnea-hypopnea index (AHI) and sleep apnea phenotype after STEMI and determined its association with changes in the left ventricular ejection fraction (LVEF).
Methods:
A total of 101 eligible patients with STEMI underwent consecutive sleep studies and echocardiographic studies within 5 days of admission and at 6-month follow-up. Sleep apnea (AHI ≥ 15 events/h) was further divided into obstructive sleep apnea (OSA) or central sleep apnea (CSA).
Results:
Both AHI (mean difference −6.4 events/h, 95% confidence interval [CI] −9.6 to 3.3, P < .001) and LVEF (mean difference 2.6%, 95% CI 1.3 to 4.0, P < .001) improved from baseline to 6 months. The improvement in AHI was associated with an increase in LVEF (β = −.47, 95% CI −.86 to −.07, P = .023) and a decrease in left ventricular end-systolic volume (LVESV) (β = .25, 95% CI .07 to .43, P = .007). Of the patients with OSA at baseline (46%), resolution of OSA was seen in 48% at 6 months. Of those with CSA at baseline (12%), conversion to OSA was seen in 83%. In contrast, among those with no sleep apnea (42%) at baseline, the diagnosis remained the same in 93% at 6 months.
Conclusions:
Concurrent changes in AHI, LVEF, and LVESV were seen after STEMI. Sleep studies performed on admission are reliable in excluding sleep apnea. However, patients with OSA or CSA on admission warrant re-evaluation due to evolution of the sleep apnea phenotype.
Citation:
Tan LL, Ting J, Balakrishnan I, Seneviratna A, Gong L, Chan MY, Tai ES, Richards AM, Tai BC, Ling LH, Lee CH. Sleep apnea evolution and left ventricular recovery after percutaneous coronary intervention for myocardial infarction. J Clin Sleep Med. 2018;14(10):1773–1781.
Keywords: apnea-hypopnea index, echocardiography, left ventricular function, remodeling, screening
BRIEF SUMMARY
Current Knowledge/Study Rationale: Obstructive sleep apnea (OSA) is a highly prevalent and under-diagnosed chronic disorder. Its reported prevalence has risen from below 10% to between 30% and 50% in the general population and to 66% in patients who present with ST-segment elevation myocardial infarction (STEMI). There are limited data on the evolution of apnea-hypopnea index (AHI) during recovery phase of STEMI.
Study Impact: In the 6 months after STEMI, alleviation of sleep apnea is associated with recovery of myocardial function and LV reverse remodeling. A decrease in AHI is correlated with an increase in left ventricular ejection fraction and a decrease in left ventricular end systolic volume. In addition, temporal evolution of sleep apnea phenotype was observed.
INTRODUCTION
Sleep apnea, particularly obstructive sleep apnea (OSA), is increasingly recognized as a cardiovascular risk factor.1,2 In patients presenting with an acute coronary syndrome, those with sleep apnea have a higher incidence of both short- and long-term adverse cardiovascular events than those without sleep apnea.3–7 Most of the current knowledge on the prognostic effect of sleep apnea in acute coronary syndrome is based on sleep studies conducted during the index admission.3,5–7 This approach, compared to postdischarge screening, facilitates timely diagnosis that may translate into better treatment adherence.
However, some small studies have cast doubt on the reliability of sleep studies conducted during acute cardiovascular events.8–10 Among 18 patients admitted to the coronary care unit for various cardiac conditions, the prevalence of sleep apnea decreased from 56% during the acute phase to 28% at 6 weeks.8 Similarly, in 28 patients in whom sleep apnea was diagnosed during admission for an acute coronary syndrome, serial sleep studies showed a progressive reduction in the apnea-hypopnea index (AHI); only six patients still had sleep apnea at the 6-month follow-up.9 In a small study that included 40 patients with acute myocardial infarction who underwent polysomnography and cardiovascular magnetic resonance imaging within 5 days and 12 weeks after the event, apnea and hypopnea events were significantly more reduced in the improved left ventricular ejection fraction (LVEF) group compared with the unchanged LVEF group. This resulted in a significant alleviation of OSA.
In ST-segment elevation myocardial infarction (STEMI), the abrupt cessation of myocardial perfusion leads to contractile dysfunction and a rapid decline in LVEF, which, in survivors, may recover after 3 to 6 months.11,12 The transient myocardial dysfunction during the acute phase often leads to fluid retention that is redistributed rostrally to the neck during sleep, causing upper airway edema and OSA that is reversible.13 In addition, central sleep apnea (CSA), which is closely associated with heart failure,2 may also resolve with recovery of myocardial function. The primary objective of our Sleep Apnea and Myocardial Evolution (SAME) study was to determine the association between the change in AHI and concurrent change in LVEF from baseline to 6-month follow-up in patients who present with STEMI. We hypothesize that as the LVEF improves, there is a concurrent reduction in the AHI. The secondary objectives are to determine (1) the association between the change in AHI and changes in other LV remodeling parameters and (2) the evolution of sleep apnea phenotype (OSA, CSA, and no sleep apnea) from baseline to the 6-month follow-up.
METHODS
Study Design
Patients age 21 years and older who presented to the National University Hospital, Singapore, with STEMI within 12 hours of symptom onset and underwent primary percutaneous coronary intervention were eligible for enrollment. The exclusion criteria included previous myocardial infarction, known OSA on noninvasive positive airway pressure therapy, cardiogenic shock or heart failure exacerbation, the use of mechanical ventilation and/or intra-aortic balloon pump, perceived high risk for malignant arrhythmia, need for oxygen supplementation for medical reasons, active use of sedatives, serum creatinine level of 2.5 mg/dL or higher, life expectancy of less than 1 year, pregnancy, inability to comply with the study protocol, and inability to give informed consent. We did not impose additional criteria (such as body mass index or snoring) to select patients with high probability of having sleep apnea. The SAME study complies with the Declaration of Helsinki, and was approved by the Institutional Review Board (National Healthcare Group, Domain Specific Review Board-C 2012/01047, approved on November 27, 2012, principal investigator: CH-L). All participants provided informed consent. The funding agency and industry sponsor had no role in the study design, data accrual or analysis, or in the generation of this manuscript.
The study flow chart is shown in Figure 1. A total of 178 patients were recruited between April 2013 and January 2016. The recruited patients underwent a sleep study and transthoracic echocardiographic study between days 2 and 5 of admission and again 6 months later using the same devices. The patients and the clinical team were blinded to the results of the sleep study but not to the echocardiographic findings. The clinical management of the recruited patients was left to the discretion of the primary physicians. The 101 patients who completed both the baseline and 6-month sleep studies and echocardiographic studies formed the cohort for this analysis. Table S1 in the supplemental material shows similar baseline characteristics in the analyzed cohort and in the 77 patients who did not complete the study.
Figure 1. Study flow chart.
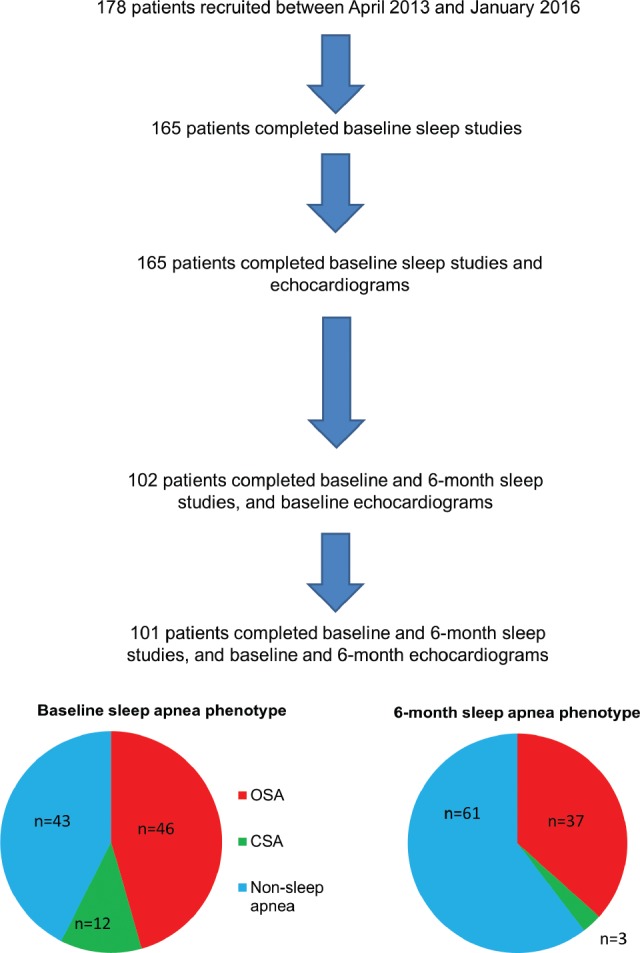
CSA = central sleep apnea, OSA = obstructive sleep apnea.
Screening Questionnaire
All recruited patients completed the Epworth Sleepiness Scale, a validated questionnaire that identifies the perceived likelihood of falling asleep during eight everyday situations.14 Patients were asked to rate their likelihood of falling asleep during each scenario on a scale of 0 to 3, for a total score ranging from 0 to 24. The questionnaire was administered via face-to-face interviews.
Sleep Study
Regardless of the results of the screening questionnaire, all recruited patients were scheduled for an overnight sleep study during the index admission and 6 months after hospital discharge. Sleep studies were performed according to a standard protocol as previously reported7 using a United States Food and Drug Administration–approved level III portable diagnostic device (Embletta Gold, Natus Medical Inc, North York, Ontario, Canada). All recruited patients underwent a sleep study during the index admission and at 6 months after hospital discharge. The Embletta Gold device is validated for use against in-laboratory polysomnography with a sensitivity range of 0.853 to 0.924 and a specificity range of 0.857 to 0.957.15 The measured parameters were nasal airflow (nasal cannula), thoracoabdominal movements (inductive respiratory bands), arterial oxygen saturation (pulse oximetry), snoring episodes (derived from the integrated pressure transducer), limb movements, electrocardiographic readings, and body position (continuous actigraphy).
Sleep study tracings were uploaded to a password-protected electronic system for centralized scoring. The tracings were manually scored by a registered polysomnographic technologist who was blinded to the patients' demographic and clinical characteristics. The primary measure was the AHI, quantified as the total number of apnea and hypopnea episodes per hour of the total recorded time starting from lights-off until lights-on/ awakening as indicated by the patient pressing an event button. An apnea episode was defined as a 90% or greater decrease in airflow from baseline for at least 10 seconds. Apnea episodes were classified as obstructive if paradoxical thoracoabdominal movement was observed and as central if there was no airflow or thoracoabdominal movement. A hypopnea episode was defined as a 30% or greater decrease in airflow from baseline for at least 10 seconds in conjunction with oxygen desaturation of at least 3%. Respiratory event scoring was performed in accordance with the American Academy of Sleep Medicine guidelines.16 Patients were classified into a sleep apnea group (AHI 15 or more events/h) and no sleep apnea group (AHI less than 15 events/h). The sleep apnea group was further divided into an OSA group (> 50% of apnea episodes were obstructive) and a CSA group (> 50% of apnea episodes were central).
Echocardiogram
All patients underwent detailed echocardiographic examinations in the Cardiovascular Imaging Core Laboratory, National University Health System, Singapore. The examinations were performed using Vivid E9 ultrasound system (GE Healthcare, Waukesha, Wisconsin, United States) equipped with an M5s broad-spectrum matrix array transducer. All images were obtained by trained sonographers according to a standardized protocol. The same sonographer (L-G) performed the follow-up scan when possible. Left ventricular end-systolic volume (LVESV), end-diastolic volume (LVEDV), and LVEF were determined by the biplane method of disk summation (modified Simpson rule), and left atrial volume was determined by the biplane area-length method as per American Society of Echocardiography guidelines.17 Measurements of transmitral early (E) and late (A) diastolic velocities and E wave deceleration time were taken from pulsed-wave Doppler recordings at the level of the mitral leaflet tips from the apical imaging window. Tissue Doppler assessment of the mitral annular motion was used to assess the medial and lateral early (e') relaxation diastolic velocities. Echocardiographic measurements were verified by experienced echocardiographers blinded to clinical and polysomnographic data. A single observer (LH-L) analyzed 194 of the 202 (96%) echocardiograms.
Statistical Analysis
The sample size was determined before the SAME study began and was predicated on the primary study objective. We anticipated that the magnitude of association between change in AHI and change in LVEF 6 months from baseline would be 0.35. Assuming a two-sided test at the 5% level of significance and a power of 90%, 80 patients were required to test the hypothesis, so a target enrollment of 100 patients was set, factoring a 20% attrition rate. Because the attrition rate during the study was higher than expected, 178 patients were eventually recruited.
Differences in means of continuous variables between the OSA, CSA, and non-sleep apnea groups were compared using analysis of variance. For categorical attributes, differences in proportions were compared using Fisher exact test. The paired t test was used to compare mean differences in body mass index and in the sleep study and echocardiographic measures at baseline and at 6 months. The strength of association between the change in AHI and the change in LVEF from baseline to the 6-month follow-up was tested with Pearson association coefficient. In addition, the associations between AHI and LVEF, LVESV, LVEDV, left atrial volume index, and E/A ratio were described using simple linear regression analysis and scatter diagrams. Multiple linear regression analysis was implemented to adjust for the baseline sleep apnea phenotype. Logistic regression analysis was used to identify predictors of OSA resolution. All statistical analyses were conducted using STATA v.13 (StataCorp LP, College Station, Texas, United States), assuming a two-sided test with a 5% level of significance.
RESULTS
Baseline Characteristics
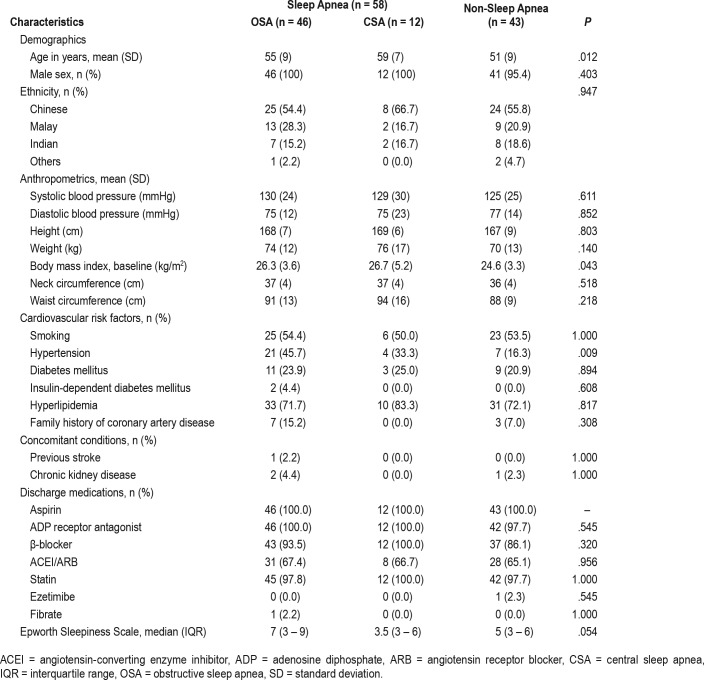
The baseline demographic and clinical characteristics of the 101 patients selected for analysis are shown in Table 1. Based on the baseline sleep study results, sleep apnea was present in 57% of the analyzed cohort. Within the sleep apnea group, 79% had OSA and 21% had CSA. The patients in the OSA and CSA groups were older (P = .012), and had a higher body mass index (P = .043) and a higher prevalence of hypertension (P = .009) than those in the non-sleep apnea group. None of the patients had previous percutaneous coronary intervention or coronary artery bypass surgery.
Table 1.
Patient demographic and clinical characteristics.
The angiographic characteristics are shown in Table S2 in the supplemental material. There were no significant differences among the OSA, CSA, and non-sleep apnea groups with regard to infarct-related artery and coronary perfusion before or after percutaneous coronary intervention. The most common infarct-related artery was the left anterior descending artery, followed by the right coronary artery. Drug-eluting stents were placed in all patients.
The body mass indexes at baseline and 6-month follow-up were nearly the same (25.6 ± 3.7 kg/m2 versus 25.6 ± 3.7 kg/m2, mean difference −0.1 kg/m2, 95% confidence interval [CI], −0.3 to 0.2, P = .614).
Baseline and 6-Month Sleep Study
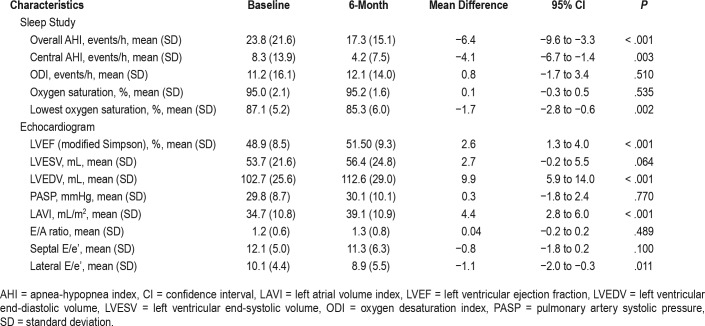
The sleep study results are presented in Table 2. The longitudinal change in AHI from baseline to the 6-month follow-up for individual patients is shown in Figure 2. Overall, the AHI was reduced by 6.4 events per hour (95% CI −9.6 to −3.3, P < .001).
Table 2.
Baseline and 6-month sleep study and echocardiogram.
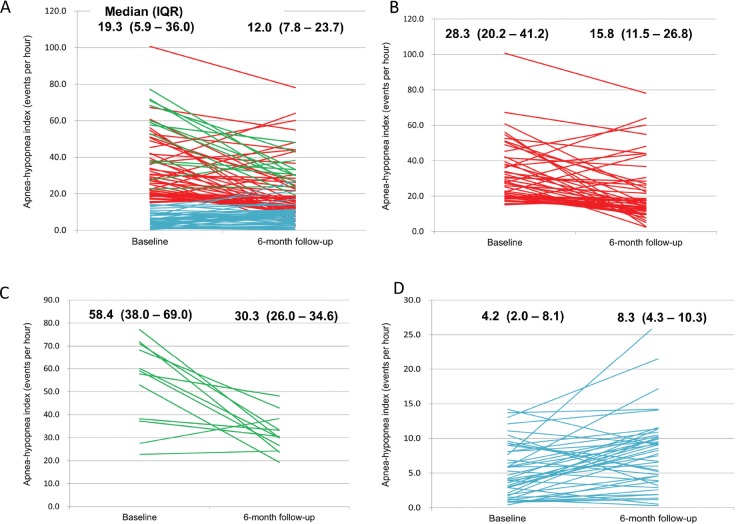
Figure 2. Temporal change in apnea-hypopnea index from baseline to 6-month follow-up for individual patients.
The patients were divided into three groups according to results of their baseline sleep study: obstructive sleep apnea (red line), central sleep apnea (green line), and no sleep apnea (blue line). (A) All patients; (B) patients with obstructive sleep apnea at baseline; (C) patients with central sleep apnea at baseline; (D) patients with no sleep apnea at baseline.
Baseline and 6-Month Echocardiogram
A 6-month echocardiogram was obtained for all patients within 30 days of the 6-month sleep study. The echocardiographic data are shown in Table 2. Overall, the LVEF improved by 2.6% (95% CI 1.3 to 4.0, P < .001) from baseline to the 6-month follow-up. There was also evidence of LV remodeling, with a significant 9.9-mL increase in LVEDV from baseline to the 6-month follow-up (95% CI 5.9 to 14.0, P < .001) and a trend toward an increase in LVESV (P = .064). Among the echo-cardiographic measures of LV diastolic function, the left atrial volume index (P < .001) and lateral E/e' (P = .011) displayed significant changes from baseline.
Association Between Change in AHI and Change in LVEF
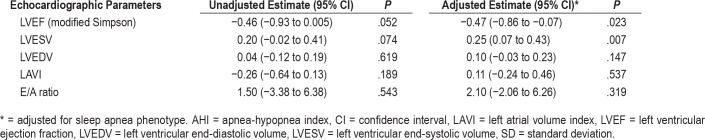
The associations between the change in AHI and the change in the various echocardiographic parameters are shown in Table 3. A negative association was seen between the change in AHI and the change in LVEF from baseline to the 6-month follow-up (P = .023). A positive association was seen between the change in AHI and LVESV (P = .007). Of the 67 patients who showed an increase in LVEF from baseline to the 6-month follow-up, the corresponding decrease in AHI was 8.2 events/h. In the remaining 34 patients who showed no increase or showed a decrease in LVEF, the corresponding decrease in AHI was 3.0 events/h. The mean difference between the group that had increase in LVEF versus the group with no change or with decrease in LVEF was 5.2 events/h (95% CI −1.4 to 11.8, P = .123).
Table 3.
Association between change in AHI and change in echocardiographic parameters.
Evolution of Sleep Apnea Phenotype
The evolution of the sleep apnea phenotype is shown in Figure 3. At baseline, the prevalence of OSA and CSA was 46% and 12%, respectively, and 42% did not have sleep apnea. At 6-month follow-up, the prevalence of OSA and CSA decreased to 37% and 3%, respectively, and 60% did not have sleep apnea. None of the patients underwent OSA therapy during the study period. Of the patients with OSA at baseline, the OSA resolved in 48% at the 6-month follow-up. Of those with CSA at baseline, 83% were converted to a diagnosis of OSA at the 6-month follow-up. In patients with no sleep apnea at baseline, the diagnosis remained the same in 93% at the 6-month follow-up.
Figure 3. Evolution of sleep apnea phenotype from baseline to 6-month follow-up after ST-segment elevation myocardial infarction.
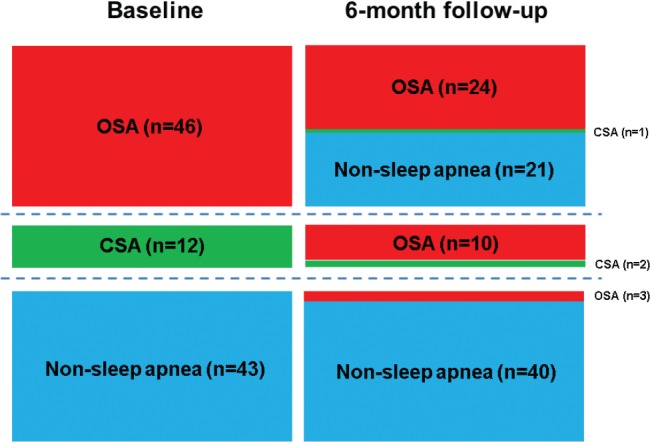
Baseline: obstructive sleep apnea (OSA, n = 46, 46%), central sleep apnea (CSA, n = 12, 12%), no sleep apnea (n = 43, 42%). Six-month follow-up: OSA (n = 37, 37%), CSA (n = 3, 3%), no sleep apnea (n = 61, 60%).
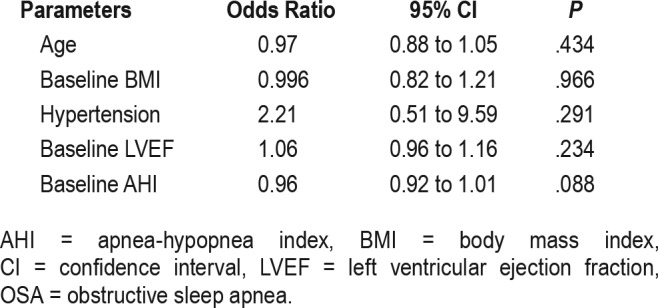
The results of a multivariate logistic regression analysis of the predictors of OSA resolution are shown in Table 4. None of the clinical parameters at baseline reliably predicted the resolution of OSA.
Table 4.
Predictors of OSA resolution in multivariable logistic regression.
Cardiovascular Outcomes
None of the patients received treatment for the OSA. Between 6-month and 18-month follow-up, three patients experienced adverse cardiovascular events. Among the 24 patients in whom OSA persisted, 1 had a nonfatal myocardial infarction. None of the 21 patients in whom OSA resolved at 6-month follow-up had adverse cardiovascular events. Of the 40 patients with no sleep apnea in whom the diagnosis remained unchanged, 1 had a nonfatal myocardial infarction and 1 had a nonfatal stroke. No other adverse cardiovascular events occurred.
DISCUSSION
Our results demonstrate that in the 6 months after STEMI, alleviation of sleep apnea is associated with recovery of myocardial function and left ventricular reverse remodeling. A decrease in AHI, which is a conventional measure of sleep apnea severity, is correlated with an increase in LVEF and a decrease in LVESV. In addition, temporal evolution of sleep apnea phenotype was observed—48% with OSA had resolution, whereas 83% with CSA evolved to OSA. No change was seen in 93% of patients without sleep apnea, which suggests that sleep studies performed during the index admission for STEMI are reliable in excluding sleep apnea. However, OSA and CSA diagnosed during the index admission may undergo phenotypic evolution.
OSA is a highly prevalent and underdiagnosed chronic disorder. Its reported prevalence has risen from below 10%, to 30% to 50% in the general population and to 66% in patients who present with STEMI.18–21 Affected individuals, most of whom are untreated, experience recurrent cardio-metabolic stress during repeated attempts to breathe against an occluded airway during sleep, leading to hypoxemia, sleep fragmentation, and augmentation of sympathetic activity.1,2 These physiological perturbations often cause blood pressure and heart rate elevation, endothelial dysfunction, and oxidative stress—the converging processes that interact to foster the initiation and progression of coronary artery disease. We recently reported that in a multinational cohort of 1,311 patients (70% presented with acute coronary syndrome) treated with percutaneous coronary intervention, patients with OSA had 1.5 times the risk of experiencing cardiovascular events at a median follow-up of 1.9 years.7 Given the high prevalence and clinical effect of OSA, the National Institutes of Health Sleep Disorders Research Plan in 2011 advocated various goals to improve the diagnosis and treatment of sleep apnea and to enhance the translation of research findings to improve health care.22 In response to that call, the SAME study demonstrated how sleep apnea screening can be incorporated into clinical algorithms for cardiovascular risk stratification after STEMI.
Screening questionnaires for sleep apnea derived from the general population are unreliable in patients with cardiovascular disease.7 A recent attempt to derive a clinically predictive model for OSA in patients with acute coronary syndrome was unsuccessful.23 Given the inability of existing screening tools to accurately identify OSA in patients with cardiovascular disease, a sleep study is the preferred strategy. This is made easier with recent advancements in portable diagnostic devices. Despite the body of evidence linking sleep apnea with adverse cardiovascular events, few data exist on the natural history of sleep apnea after acute myocardial infarction.
Our findings enhance the results of previous studies and provide a deeper understanding of the relationship between sleep apnea and myocardial recovery after an acute myocar-dial infarction. At baseline, the prevalence of OSA (46%) is consistent with findings from earlier reports;4–7,21 however, data on the prevalence of CSA in patients with acute coronary syndrome are limited. The prevalence of CSA in our study (12% in the entire cohort and 21% among those with sleep apnea) is lower than that in a previous study (27.5% in the entire cohort and 50% among those with sleep apnea), presumably because the patients in the previous study had more severe myocardial dysfunction.9 Future studies are warranted to determine the prevalence and effects of CSA in patients with STEMI.
Compared with earlier studies that monitored the change in AHI over a shorter duration (6 to 12 weeks),8,10 the longitudinal follow-up of 6 months in our study provides a more thorough assessment of the relationship between sleep apnea and hemodynamic recovery after an acute myocardial infarction. Notably, almost half of the patients with OSA at baseline had resolution of OSA at the 6-month follow-up. This is in consistent with an earlier study by Buchner and colleagues,10 although we presented a larger sample size and evolution at individual patient level in Figure 2 and Figure 3. Therefore, a re-evaluation at 6 months, especially in patients who have demonstrated LVEF recovery, is necessary before subjecting them to long-term positive airway pressure therapy.10 An alternative strategy is to postpone sleep apnea screening to at least 6 months after an acute myocardial infarction. However, this would mean that patients with OSA post-STEMI would go untreated during the time when their myocardium is recovering from ischemic damage and is most vulnerable to recurrent hypoxemic insults. In our study, 83% of the patients with CSA at baseline converted to OSA at the 6-month follow-up. This finding can be explained by the fact that CSA is closely associated with heart failure,2 and improvement in LVEF over time may lead to the resolution of more central apnea episodes than obstructive apnea episodes. A high index of suspicion is thus needed in this group of patients because most patients with CSA at baseline will eventually warrant positive airway pressure treatment for OSA. Finally, most of the group without sleep apnea at baseline (93%) remained unchanged at the 6-month follow-up. This provides assurance that the sleep study conducted during the index admission is reliable in excluding sleep apnea.
Although OSA and CSA may resolve with recovery of LVEF, this does not negate the value of sleep apnea screening during the index admission. Even though a diagnosis of sleep apnea during the index admission may not warrant long-term treatment, it can still serve as a valuable risk marker to identify patients with a high risk of future adverse cardiovascular events. Furthermore, positive airway pressure therapy for OSA has been shown to reduce preload and afterload, which may have a salutary effect on myocardial recovery. Based on the prevailing theory that OSA during the acute phase of STEMI can be attributed to myocardial dysfunction and subclinical fluid retention, it is conceivable that diuretic therapy for patients with STEMI may alleviate OSA and the accompanying nocturnal hypoxic insults. This hypothesis is consistent with the recent finding that early treatment with mineralocorticoid receptor antagonists reduced the mortality rate in patients with STEMI.24
Although we hypothesize that the reduction in AHI is a consequence of LVEF improvement, it can be explained by other mechanisms, notably weight loss and exercise as part of cardiac rehabilitation. Because sleep apnea has been shown to predict a large infarct size and impaired recovery of myocar-dial contractility,25 relief from sleep apnea could have facilitated the recovery of LVEF and reverse remodeling. However, we found that body mass index, an important modifiable risk factor for OSA, remained unchanged between the baseline and 6-month sleep studies. This renders weight reduction leading to alleviation of sleep apnea unlikely. We did not capture data on temporal changes in the duration and intensity of physical activity.
Limitations
First, the 6-month sleep study (but not echocardiogram) had a high default rate. To maintain the power of the study, we extended the recruitment period and recruited 78% more patients than originally planned. Of the 178 patients enrolled in the study, only 101 completed both the sleep study and the echocardiogram at the 6-month follow-up. The second limitation is that we exclusively recruited patients who had undergone primary percutaneous coronary intervention and were clinically stable without oxygen supplementation, which may have selected for patients with less severe myocardial damage. Likewise, our results could not be extrapolated to patients who are not treated with primary percutaneous coronary intervention because the procedure has a known effect of facilitating myocardial recovery. Third, the overnight sleep study was conducted using a portable diagnostic device instead of supervised in-laboratory polysomnography, primarily due to the limited availability of in-laboratory polysomnography and the safety concerns of subjecting patients with acute myocardial infarction to in-laboratory polysomnography. However, a recent multicenter randomized trial showed that clinical management guided by portable diagnostic devices was noninferior to in-laboratory polysomnography in terms of treatment adherence and patient outcomes.26 Fourth, the baseline and 6-month sleep studies were conducted in different environments. Because of the high demand for hospital beds at our institution, we performed a home-based sleep study at the 6-month follow-up. We could not discount the use of alcohol and other sedative medications when the 6-month sleep study was performed. Finally, we used the two-dimensional echocardiographic biplane method of disk summation to obtain the left ventricular volumes. This may be less accurate than volume measurements using cardiac magnetic resonance techniques.
CONCLUSIONS
Sleep apnea is prevalent in patients presenting with STEMI. Improvement in LVEF over 6 months results in a reduction in AHI. Physicians who treat patients with STEMI should be aware of sleep apnea phenotypic evolution when considering sleep apnea screening and treatment. A sleep study performed during the acute phase of a STEMI is reliable in excluding sleep apnea. However, a diagnosis of OSA or CSA may undergo phenotype evolution. Before committing these patients to long-term positive airway pressure therapy, a repeat sleep study at 6 months is necessary, especially if the patient exhibits improvement in LVEF.
DISCLOSURE STATEMENT
This study was supported by Transition Award from the National Medical Research Council of Singapore (Award number: TA/NMRC/012/2012). Easmed Pte, Ltd. provided support in conducting the overnight sleep studies but had no role in study design, data interpretation or manuscript writing. The authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contributions to this study of Miss Venesa Loh, Mr. Glenn Roldan, Professor Judith L. Swain, and Easmed Pte, Ltd.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CSA
central sleep apnea
- LVEDV
left ventricular end diastolic volume
- LVEF
left ventricular ejection fraction
- LVESV
left ventricular end systolic volume
- OSA
obstructive sleep apnea
- STEMI
ST-segment elevation myocardial infarction
REFERENCES
- 1.Bradley TD, Floras JS. Obstructive sleep apnea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 2.Javaheri S, Barbe F, Campos-Rodriguez F, et al. Sleep apnea: types, mechanisms, and clinical cardiovascular consequences. J Am Coll Cardiol. 2017;69(7):841–858. doi: 10.1016/j.jacc.2016.11.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee CH, Khoo SM, Chan MY, et al. Severe obstructive sleep apnea and outcomes following myocardial infarction. J Clin Sleep Med. 2011;7(6):616–621. doi: 10.5664/jcsm.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loo G, Tan AY, Koo CY, Tai BC, Richards AM, Lee CH. Prognostic implication of obstructive sleep apnea diagnosed by post-discharge sleep study in patients presenting with acute coronary syndrome. Sleep Med. 2014;15(6):631–636. doi: 10.1016/j.sleep.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Barbé F, Sánchez-de-la-Torre A, Abad J, et al. Spanish Sleep Network. Effect of obstructive sleep apnea on severity and short-term prognosis of acute coronary syndrome. Eur Respir J. 2015;45(2):419–427. doi: 10.1183/09031936.00071714. [DOI] [PubMed] [Google Scholar]
- 6.Mazaki T, Kasai T, Yokoi H, et al. Impact of sleep-disordered breathing on long-term outcomes in patients with acute coronary syndrome who have undergone primary percutaneous coronary intervention. J Am Heart Assoc. 2016;5(6) doi: 10.1161/JAHA.116.003270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee CH, Sethi R, Li R, et al. Obstructive sleep apnea and cardiovascular events after percutaneous coronary intervention. Circulation. 2016;133(21):2008–2017. doi: 10.1161/CIRCULATIONAHA.115.019392. [DOI] [PubMed] [Google Scholar]
- 8.Skinner MA, Choudhury MS, Homan SD, Cowan JO, Wilkins GT, Taylor DR. Accuracy of monitoring for sleep-related breathing disorders in the coronary care unit. Chest. 2005;127(1):66–71. doi: 10.1378/chest.127.1.66. [DOI] [PubMed] [Google Scholar]
- 9.Schiza SE, Simantirakis E, Bouloukaki I, et al. Sleep disordered breathing in patients with acute coronary syndromes. J Clin Sleep Med. 2012;8(1):21–26. doi: 10.5664/jcsm.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buchner S, Greimel T, Hetzenecker A, et al. Natural course of sleep-disordered breathing after acute myocardial infarction. Eur Respir J. 2012;40(5):1173–1179. doi: 10.1183/09031936.00172211. [DOI] [PubMed] [Google Scholar]
- 11.Sheiban I, Fragasso G, Rosano GM, et al. Time course and determinants of left ventricular function recovery after primary angioplasty in patients with acute myocardial infarction. J Am Coll Cardiol. 2001;38(2):464–471. doi: 10.1016/s0735-1097(01)01407-3. [DOI] [PubMed] [Google Scholar]
- 12.Antoni ML, Mollema SA, Atary JZ, et al. Time course of global left ventricular strain after acute myocardial infarction. Eur Heart J. 2010;31(16):2006–2013. doi: 10.1093/eurheartj/ehq198. [DOI] [PubMed] [Google Scholar]
- 13.Carlisle T, Ward NR, Atalla A, Cowie MR, Simonds AK, Morrell MJ. Investigation of the link between fluid shift and airway collapsibility as a mechanism for obstructive sleep apnea in congestive heart failure. Physiol Rep. 2017;5(1) doi: 10.14814/phy2.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 15.Ng SS, Chan TO, To KW, et al. Validation of Embletta portable diagnostic system for identifying patients with suspected obstructive sleep apnea syndrome (OSAS) Respirology. 2010;15(2):336–342. doi: 10.1111/j.1440-1843.2009.01697.x. [DOI] [PubMed] [Google Scholar]
- 16.Thornton AT, Singh P, Ruehland WR, Rochford PD. AASM criteria for scoring respiratory events: Interaction between apnea sensor and hypopnea definition. Sleep. 2012;35(3):425–432. doi: 10.5665/sleep.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1.e14–39.e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 18.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328(17):1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 19.Heinzer R, Vat S, Marques-Vidal P, et al. Prevalence of sleep-disordered breathing in the general population: The HypnoLaus study. Lancet Respir Med. 2015;3(4):310–318. doi: 10.1016/S2213-2600(15)00043-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan A, Cheung YY, Yin J, Lim WY, Tan LW, Lee CH. Prevalence of sleep-disordered breathing in a multiethnic Asian population in Singapore: A community-based study. Respirology. 2016;21(5):943–950. doi: 10.1111/resp.12747. [DOI] [PubMed] [Google Scholar]
- 21.Lee CH, Khoo SM, Tai BC, et al. Obstructive sleep apnea in patients admitted for acute myocardial infarction. Prevalence, predictors, and effect on microvascular perfusion. Chest. 2009;135(6):1488–1495. doi: 10.1378/chest.08-2336. [DOI] [PubMed] [Google Scholar]
- 22. [Accessed June 16, 2017]. https://www.nhlbi.nih.gov/files/docs/resources/sleep/201101011NationalSleepDisordersResearchPlanDHHSPublication11-7820.pdf.
- 23.de Batlle J, Turino C, Sánchez-de-la-Torre A, et al. Spanish Sleep Group. Predictors of obstructive sleep apnea in patients admitted for acute coronary syndrome. Eur Respir J. 2017;49(3) doi: 10.1183/13993003.00550-2016. [DOI] [PubMed] [Google Scholar]
- 24.Beygui F, Cayla G, Roule V, et al. ALBATROSS Investigators. Early Aldosterone Blockade in Acute Myocardial Infarction: The ALBATROSS Randomized Clinical Trial. J Am Coll Cardiol. 2016;67(16):1917–1927. doi: 10.1016/j.jacc.2016.02.033. [DOI] [PubMed] [Google Scholar]
- 25.Buchner S, Satzl A, Debl K, et al. Impact of sleep-disordered breathing on myocardial salvage and infarct size in patients with acute myocardial infarction. Eur Heart J. 2014;35(3):192–199. doi: 10.1093/eurheartj/eht450. [DOI] [PubMed] [Google Scholar]
- 26.Chai-Coetzer CL, Antic NA, Hamilton GS, et al. Physician decision making and clinical outcomes with laboratory polysomnography or limited-channel sleep studies for obstructive sleep apnea: A randomized trial. Ann Intern Med. 2017;166(5):332–340. doi: 10.7326/M16-1301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.