Abstract
Study Objectives:
Dravet syndrome is a severe developmental and epileptic encephalopathy, in which 75% of patients have sleep disturbance. Melatonin is often used for sleep problems in childhood; however, there is no quality evidence supporting its use in Dravet syndrome. We hypothesized that melatonin would increase total sleep and quality of life for patients with Dravet syndrome.
Methods:
A double-blind crossover randomized placebo-controlled trial was conducted, comparing 6 mg regular-release melatonin to placebo for patients with Dravet syndrome and sleep disturbance. The primary outcome measure was total sleep measured by actigraphy, with secondary outcomes including wakefulness after sleep onset (WASO), Sleep Disturbance Scale in Children and Quality of Life in Children with Epilepsy 55 questionnaires, caregiver reports of clinical change, seizure diary and serum antiepileptic drug levels. We also compared actigraphy data of patients with Dravet syndrome to an age-matched healthy control group.
Results:
A total of 13 patients completed the study. There was no difference in total sleep or WASO between melatonin and placebo. However, of the 11 patients for whom caregivers reported a clear clinical difference between treatments (blinded), 8 reported improvement on melatonin (P < .05). Interestingly, when compared to patients in the control group, patients with Dravet syndrome had significantly increased total sleep (P = .002).
Conclusions:
Melatonin did not increase total sleep; however, blinded caregiver reports indicate treatment with melatonin provided considerable clinical benefit for some patients with Dravet syndrome and sleep disturbance.
Clinical Trial Registration:
Registry: Australian Government Department of Health, Therapeutic Goods Administration under the Clinical Trials Notification Scheme (protocol number 2241)
Citation:
Myers KA, Davey MJ, Ching M, Ellis C, Grinton BE, Roten A, Lightfoot PA, Scheffer IE. Randomized controlled trial of melatonin for sleep disturbance in dravet syndrome: the DREAMS study. J Clin Sleep Med. 2018;14(10):1697–1704.
Keywords: actigraphy, Dravet syndrome, melatonin, scn1a, sleep
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep disturbance is common in Dravet syndrome and frequently affects quality of life for patients and their families. There are no established therapies for sleep disturbance in Dravet syndrome, and the nature of the sleep disturbance itself remains poorly characterized.
Study Impact: A subset of patients with Dravet syndrome reported dramatic clinical improvement in sleep initiation, maintenance, and quality of life when receiving melatonin. However, melatonin did not result in measurable overall improvements in total sleep or wakefulness after sleep onset. Although definitive evidence of clinical benefit for patients with Dravet syndrome and sleep disturbance was not shown, such individuals should nevertheless consider a trial of melatonin, as some individuals appear to derive dramatic benefits.
INTRODUCTION
Dravet syndrome is a severe infantile-onset developmental and epileptic encephalopathy associated with mutations in SCN1A (OMIM 182389) in more than 80% of cases.1 Usually patients first present with convulsive febrile seizures near 6 months of age, classically prolonged and often hemiclonic.2 Early development is normal, but developmental regression or plateau typically becomes apparent by 2 years.2 Although refractory epilepsy is a major management challenge, people with Dravet syndrome also frequently experience comorbidities including a progressively worsening crouch gait, speech problems, behavioral difficulties, and disturbed sleep.3–7 We recently found that sleep disturbance occurred in 75% of patients with Dravet syndrome;3 however, currently there are no evidence-based therapies whose use is supported in this patient population who have such a challenging and complex range of problems.
One appealing therapeutic candidate is melatonin, a hormone produced endogenously by the pineal gland and involved in sleep-wake cycles.8,9 Synthetically produced melatonin may be given exogenously and there are both immediate and sustained-release formulations available.10 Melatonin is approved by the Food and Drug Administration for treatment of sleep disturbance in older adults but the existing data supporting or refuting use in children with intellectual disability are limited, with no blinded trials in people with Dravet syndrome.10,11 Currently, the best evidence in childhood epilepsy comes from a randomized, double-blind, crossover trial in normally developing children with insomnia and epilepsy, which found that melatonin decreased sleep latency and wakefulness after sleep onset (WASO).12
We conducted a study in people with Dravet syndrome, using a randomized, double-blind, placebo-controlled trial design, to investigate the efficacy and safety of melatonin in treatment of sleep disturbance within this population (the DREAMS study). A secondary aim of the study was to better characterize the nature of sleep disturbance in people with Dravet syndrome by asking caregivers to complete sleep disturbance scale questionnaires, and by comparing 2-week actigraphy results while receiving placebo to an age-matched control group of healthy children with normal sleep.
METHODS
Recruitment, Inclusion/Exclusion Criteria and Trial Design
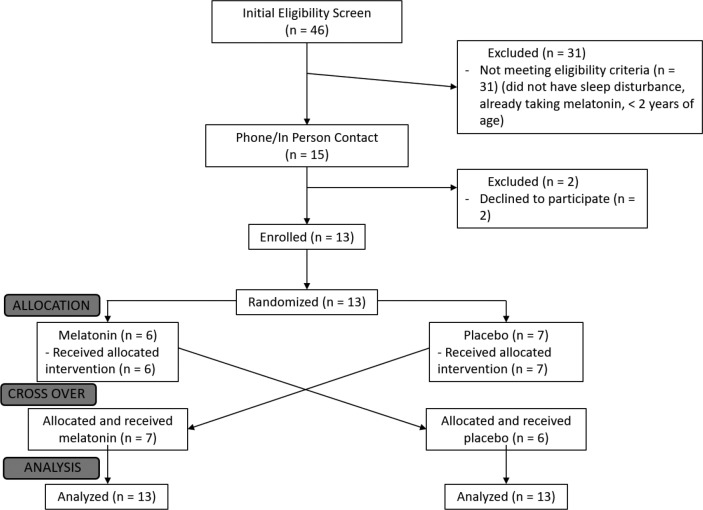
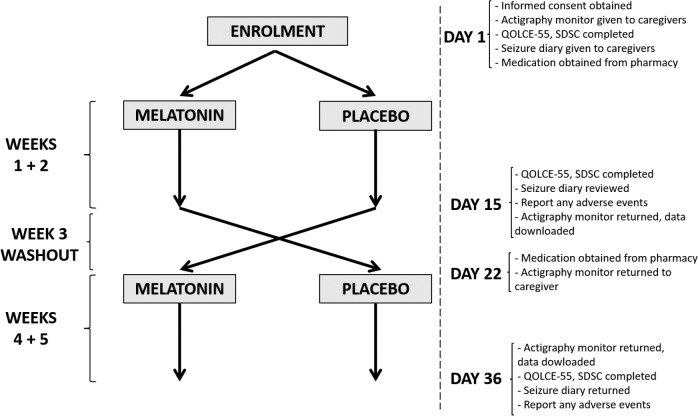
We recruited patients aged 2 to 50 years from the Dravet syndrome clinic at Austin Health (Heidelberg, VIC, Australia). All individuals had Dravet syndrome associated with an SCN1A mutation, diagnosed by a pediatric epileptologist (KAM, IES). Patients were selected if they, or their family, reported disturbed sleep of any kind. Patients were excluded if they had taken melatonin in the past 4 weeks, had a diagnosis of obstructive sleep apnea, had known hypersensitivity to melatonin, had a musculoskeletal abnormality that prevented them from being able to wear the actigraphy wristband, or were pregnant. The study participant flowsheet is shown in Figure 1, and the trial design is summarized in Figure 2.
Figure 1. CONSORT flow diagram.
Figure 2. Trial design.
QOLCE-55 = Quality of Life in Children with Epilepsy 55, SDSC = Sleep Disturbance Scale For Children.
A randomized, double-blind, crossover placebo-controlled trial design was used in which patients received either placebo or 6 mg regular-release melatonin capsules prepared by Melbourne Compounding Centre (Seddon, VIC, Australia). The 6-mg dose was chosen because it is in the range used in prior studies of children with epilepsy.12,13 Caregivers were instructed to give the medication 30 minutes before bedtime for a 2-week period because we were interested in studying melatonin's hypnotic effects. Participants were given an actigraphy bracelet (Actiwatch 2; BMedical Pty Ltd, Milton, Queensland, Australia) to wear for each 2-week treatment period. In a number of cases, the wristband had to be secured with duct tape, and/or under clothing, in order to prevent the participants from spontaneously removing them.
At baseline, caregivers completed two validated surveys: Sleep Disturbance Scale For Children (SDSC) and Quality of Life in Children with Epilepsy 55 (QOLCE-55).14,15 These questionnaires were chosen because we expected the patient population to be overwhelmingly pediatric, given that our recruitment was from a pediatric neurology clinic. As well, any adult participants would have developmental impairment, so we thought the data from these questionnaires would still be useful, even though they are not designed for this patient population. The same questionnaires were completed at the conclusion of each 2-week period, at which time caregivers were also asked for the number of convulsive seizures that had occurred over the 2 weeks and if any adverse events were noted, and the actigraphy wristbands were returned. There was a washout period of 1 week between the two treatment periods. At the conclusion of the second 2-week treatment period, caregivers were asked if they had noticed any clinical changes during either 2-week period which they suspected were the result of receiving melatonin.
As an optional aspect of the study, participants were invited to provide blood samples for serum antiepileptic drug levels at the conclusion of each treatment period. This was done to study the potential effect of melatonin on hepatic drug metabolism.16
Randomization and Blinding
Randomization was performed by a pharmacist at the Austin Health Clinical Trials Pharmacy. After all patients had completed the study, initial unblinding was partial, so that investigators learned whether individual patients had received “Treatment A” first or second but did not know if “Treatment A” was melatonin or placebo. This allowed statistical analysis to be performed while investigators were still blinded to treatment received. After statistical analysis was complete, final unblinding was performed.
Sample Size Calculation
Based on our initial calculations and estimates, a target sample size of 20 patients was chosen. A rigorous power calculation was not possible because of the lack of knowledge regarding variability of sleep parameters in the Dravet syndrome population. Jain et al.12 found statistically significant differences in a study with a similar design in 10 normally developing children with epilepsy; based on this, 20 was considered a more than adequate sample size, even allowing for increased variability in children and adults with Dravet syndrome.
Comparing Sleep Patterns in Patients With Dravet Syndrome and Patients in the Control Group
As a secondary aim of the study, an age-matched cohort of healthy children and adults without disturbed sleep was recruited. Individuals in the control group were recruited through a call for volunteers to clinic/research staff and their families. These individuals wore the actigraphy monitors for a 2-week period during which time they were asked not to alter their normal routines. Total sleep and WASO were compared between the individuals in the control group and patients with Dravet syndrome when receiving placebo.
Statistical Analysis
The primary outcome measure was average total sleep time over the 2-week period estimated on the basis of actigraphy data. Secondary outcome measures included mean WASO, mean sleep latency, mean sleep efficiency, change in SDSC and QOLCE-55 scores, convulsive seizure frequency, caregiver impression of clinical change, and adverse events.
All continuous data were compared using one-tailed Wilcoxon signed-rank tests, with the exception of Dravet/control comparisons where two-tailed tests were used. Fisher exact test was used to compare the proportion for whom caregivers reported definite improvement for melatonin versus placebo.
Ethics
This study was approved by the Human Research Ethics Committee of Austin Health, Project No. DT16/78. The trial was registered with the Australian Government Department of Health, Therapeutic Goods Administration under the Clinical Trials Notification Scheme (protocol number 2241).
RESULTS
Patient Demographics
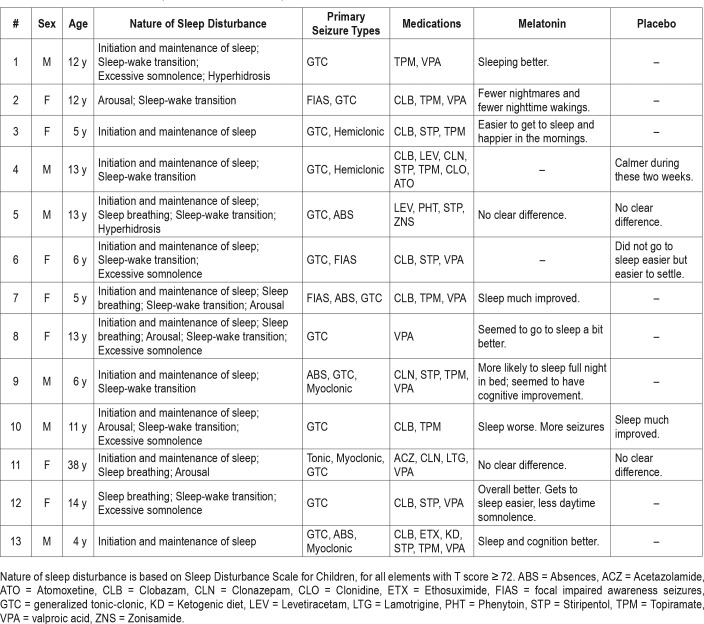
Patient demographics are given in Table 1. Thirteen patients with Dravet syndrome and sleep disturbance were recruited (Table 2), as well as 14 healthy age-matched controls with normal sleep, between June 2016 and July 2017. As shown in Figure 1, 2 eligible patients declined to participate; 31 screened patients were excluded because they did not have sleep disturbance, were already taking melatonin, or were younger than the predetermined age range for the study. All patients with Dravet syndrome completed the study. One participant in the control group, aged 4 years, withdrew from the study because she found the actigraphy wristband annoying; she was replaced and is not included in the Table 1 patient data.
Table 1.
Characteristics of patients with Dravet syndrome and individuals in the control group.
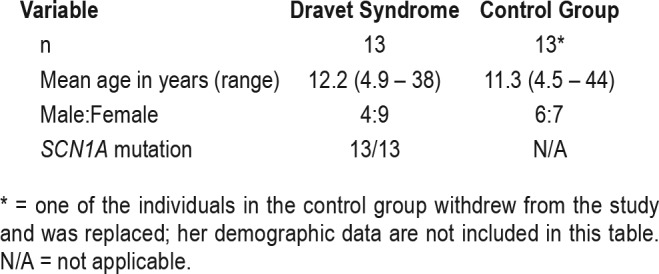
Table 2.
Clinical features of patients with Dravet syndrome.
We had intended to calculate sleep latency and sleep efficiency based on the light sensor on the actigraphy wristband; however, a number of patients had to have the wristband secured with duct tape or under clothing, which prevented collection of the light sensor data and determination of bedtime. Hence, we were unable to conduct sleep latency or sleep efficiency analyses.
Melatonin Versus Placebo in Patients With Dravet Syndrome
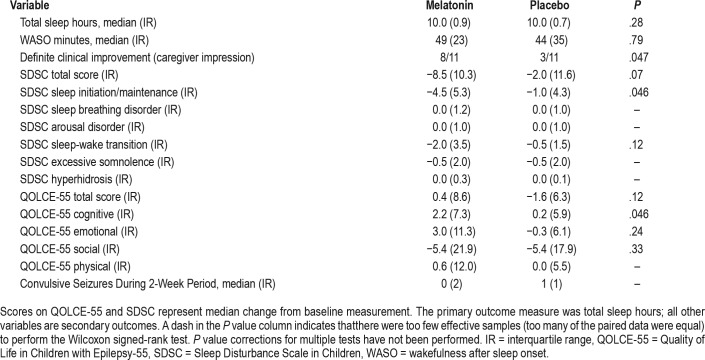
The actigraphy did not demonstrate a significant difference in the primary outcome measure, total sleep, for melatonin compared to placebo in patients with Dravet syndrome, nor was there a significant difference in the secondary measure, WASO (Table 3). Although there was not an overall statistical difference in actigraphy qualitative measures, some patients showed clear objective improvement in sleep patterns while on melatonin (example given in Figure 3).
Table 3.
Melatonin versus placebo in patients with Dravet syndrome.
Figure 3. Actigraphy data from a 5-year-old patient with Dravet syndrome showing a positive response to melatonin.
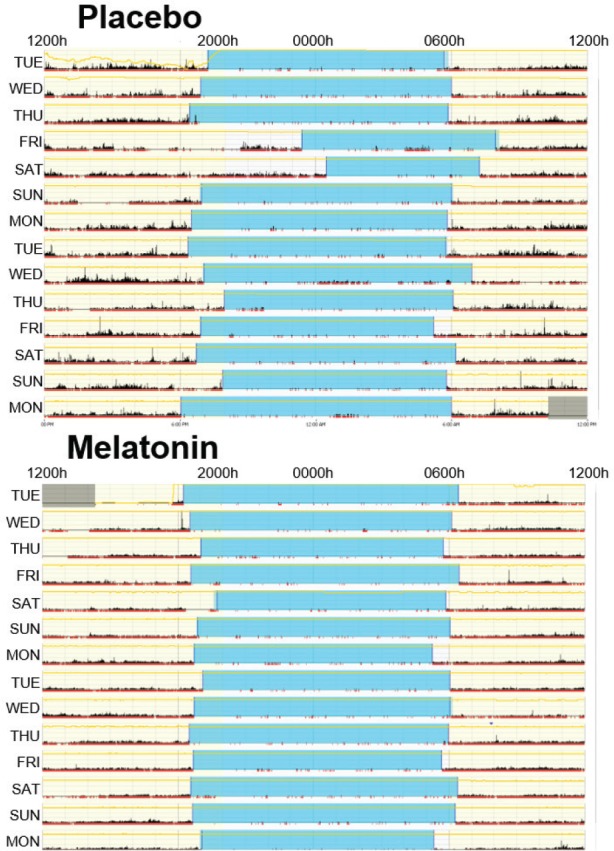
Blue represents the nocturnal sleep period identified and black reflects patient activity. During the 2 weeks on melatonin, the time-to-sleep and time-to-wake is much more consistent, and there is less activity detected during the sleep period.
The total scores of the SDSC and QOLCE-55 did not show statistically significant improvement. However, there was improvement in Sleep Initiation/Maintenance on the SDSC (P = .046 without correction for multiple tests) consistent with the primary effect of melatonin.10 There was also a borderline improvement observed in Cognitive Function on the QOLCE-55 (P = .046 without correction for multiple tests). There was no difference in convulsive seizure frequency, as expected.
The caregivers reported a clear difference in response between the two treatments in 11 of 13 cases. Eight improved with melatonin, whereas three observed improvement with placebo (P < .05). The subjective observations of the caregivers when comparing the two treatments are given for each patient in Table 2. Although the formal study design did not incorporate an open label extension phase, four families were so impressed with the clinical effect that they insisted on continuing therapy despite not yet being unblinded. One family reported that melatonin's effects had “changed their lives” as the patient's sleep disruption had been the major source of stress for the family.
No significant adverse events were reported by caregivers with either treatment. In only one patient were drug levels assessed at the conclusion of both treatment periods; he was on valproate and had an asymptomatic elevation in serum level of 199 μg/mL (normal range 50–125) while receiving melatonin. This level dropped to 126 μg/mL when measured at the conclusion of the placebo period without a change in the patient's valproate dosing. One patient was reported by caregivers to have worse sleep and more seizures while on melatonin; however, caregivers also thought this was within the child's typical week-to-week fluctuations and thus not a significant change from baseline.
Comparison of Actigraphy Among Individuals With Dravet Syndrome and Individuals in the Control Group
When comparing actigraphy from patients with Dravet syndrome on placebo with healthy participants in the control group, mean total sleep was significantly greater in the Dravet group, at 9.99 hours compared to 8.78 hours in the control group (P = .0047, Figure 4). There was no difference in mean WASO, with 56.7 minutes for the Dravet group and 54.2 minutes for controls (P = .76).
Figure 4. Actigraphy comparison of individuals with Dravet syndrome and healthy individuals in the control group.
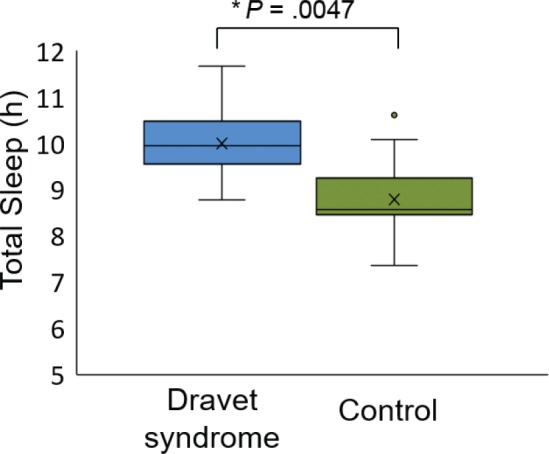
The patients with Dravet syndrome on placebo had greater total sleep than those in the control group.
DISCUSSION
This randomized, double-blind, placebo-controlled, crossover trial comparing melatonin to placebo for sleep disturbance in Dravet syndrome did not find a significant difference in the predetermined primary outcome variable, total sleep measured by actigraphy. However, the data provide important insights into the nature of sleep disturbance in Dravet syndrome, as well as the possible benefits of melatonin for some patients. The study had a number of limitations including: smaller sample size than planned (though still larger than prior trials with similar designs that found statistically significant results), wide age range of study participants, lack of sleep latency data, and the potential for selection bias and confounding effects of anti-epileptic medications.
When comparing patients with Dravet syndrome to healthy individuals in the control group, somewhat surprisingly, we found that the patients with Dravet syndrome had a greater total sleep time measured by actigraphy. This finding was unexpected as our previous study reported sleep disturbance in 75% of patients with Dravet syndrome based on parental questionnaire.3 Longer total sleep time may reflect a lower quality of sleep in Dravet syndrome; however, this possibility has not been thoroughly investigated. A study of sleep in a mouse model of Dravet syndrome found marked sleep impairment with frequent arousals and electrographic abnormalities in both rapid eye movement and non-rapid eye movement sleep.17 However, a case series of polysomnography studies in human patients with Dravet syndrome failed to show consistent abnormalities, though abnormal non-rapid eye movement sleep microarchitecture was noted.6 Though the nature of sleep disturbance in Dravet syndrome remains unclear, one possibility is that the abnormalities occur because of subtle baseline physiologic abnormalities that may be exacerbated by nocturnal seizures and the effects of antiepileptic polypharmacy. Our data should be considered with caution, as we compared patients over a broad age range, and sleep patterns change considerably through the lifespan.
Given the baseline increased total sleep duration in the group of patients with Dravet syndrome, it is less surprising that melatonin failed to increase this as our primary outcome measure. However, the reports from caregivers indicated that melatonin is highly beneficial for some patients. Of the 11 patients for whom caregivers thought there was a significant difference between treatments, 8 reported improvement when receiving melatonin (P < .05). The parental reports of clinical benefit described subjective improvements in sleep initiation and maintenance, with parents also thinking there were concomitant improvements in cognitive function and quality of life.
The effect of melatonin on sleep latency would have been an interesting and valuable outcome measure, and we had planned to estimate this using the light sensor on the actigraphy bracelet. Unfortunately, this was not possible for practical reasons because many of the patients with Dravet syndrome had to have their bracelets secured with tape or under wristbands or sleeves for behavioral reasons, obscuring the light sensor. On the basis of parental report, sleep latency considerably improved in some patients, but we could not prove this with quantitative data.
From a safety perspective, although no significant adverse effects were reported by caregivers, one patient had a measured serum valproate level in the toxic range while on melatonin. Though this is only a single case, the finding emphasizes that clinicians should consider potential interactions with melatonin and antiepileptic drugs. In vitro data indicate melatonin is metabolized by cytochrome P450 enzymes, primarily CYP1A2, CYP1A1, and CYP2C19, and may inhibit these and other enzymes.18 These are potentially relevant properties for young epilepsy populations, as some patients with Dravet syndrome are on antiepileptic medications partially metabolized by CYP2C19 (eg, phenytoin, phenobarbital, primidone), serum levels of which could be affected with introduction of melatonin. In addition, although melatonin does not affect serum levels of another anti-epileptic drug, carbamazepine, both antiseizure activity and side effects appear to be potentiated when the two medications are combined.19 A final issue related to metabolism is that many people with epilepsy have comorbid mood issues treated with antidepressants, which may be metabolized by a variety of cytochrome P450 enzymes including CYP2C19 and CYP1A1. There is at least one report of an individual on a regimen of antidepressants who experienced severe sedation when melatonin was added.16
Although our double-blind trial did not find a significant difference in total sleep time, the overall impression from parental report was that melatonin is very beneficial in some patients. The negative outcome of our trial likely relates to a few issues. First, total sleep was likely not the optimal parameter choice for our primary outcome measure, given that individuals with Dravet syndrome on placebo had greater total sleep than healthy individuals in the control group. Second, recruitment was challenging because of multiple factors including long travel distances to our clinic, and that many patients were already receiving melatonin. This meant that our findings were underpowered, and a larger cohort size may have delivered more definitive findings.
Nevertheless, parental reports of improvement were dramatic and more likely on melatonin than placebo. It may be that these improvements relate to both the quality and timing of sleep, which we did not examine in a quantitative manner. The likely benefits include more rapid sleep initiation and enhanced sleep maintenance, leading to improved cognitive function and, importantly for families, improved quality of life for both parents and caregivers. Sleep is a critical modifiable comorbidity for people with epilepsy, especially those with the severe developmental and epileptic encephalopathies. Sleep deserves consideration at each clinical appointment because optimal management can be life-changing for both the patient and his or her family.
CONCLUSIONS
This study did not show significant differences in the primary outcome measure, total sleep, or any of the secondary outcome measures, when patients with Dravet syndrome were receiving melatonin versus placebo. However, the data also indicate that melatonin is a safe therapy for patients with Dravet syndrome and sleep disturbance, and elicits marked clinical improvement in a subset. The underlying nature of sleep disturbance in Dravet syndrome is complex, and may involve the combined effects of nocturnal seizures, antiepileptic drug effects, and inherent cerebral dysfunction related to SCN1A mutation.
DISCLOSURE STATEMENT
Work for this study was performed at Austin Health, University of Melbourne. All authors have seen and approved the manuscript. This study was primarily funded by philanthropic donations to Prof. Scheffer's Dravet syndrome research program, but also supported by funding from the National Health and Medical Research Council (NHMRC) Program Grants (628952, 1091593); Dr. Myers received salary support during the study from a Taking Flight Award from Citizens United for Research in Epilepsy (CURE; grant number 439534) and Prof. Scheffer holds a NHMRC Practitioner Fellowship (1104831). Dr. Myers received a travel grant from Zynerba, and has received research grant funding from Citizens United for Research in Epilepsy and Supporting Families with Koolen-de Vries Syndrome. Dr. Scheffer serves on the editorial boards of Neurology and Epileptic Disorders and has served on the editorial board of Annals of Neurology Epilepsy Currents; has received revenue from Medvet Science and Bionomics for patents; serves on the epilepsy advisory board for Nutricia; may accrue future revenue on a pending patent on therapeutic compound; has received speaker honoraria from Athena Diagnostics, UCB, GSK, Eisai, and Transgenomics; has received funding for travel from Athena Diagnostics, UCB, and GSK; and receives/has received research support from the National Health and Medical Research Council, Australian Research Council, NIH, Health Research Council of New Zealand, March of Dimes, Weizmann Institute, Citizens United for Research in Epilepsy, US Department of Defense, and Perpetual Charitable Trustees. The remaining authors report no conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the participants and their families for supporting this research.
ABBREVIATIONS
- QOLCE-55
Quality of Life in Children with Epilepsy 55
- SDSC
Sleep Disturbance Scale For Children
- WASO
wakefulness after sleep onset
REFERENCES
- 1.De Jonghe P. Molecular genetics of Dravet syndrome. Dev Med Child Neurol. 2011;53(Suppl 2):7–10. doi: 10.1111/j.1469-8749.2011.03965.x. [DOI] [PubMed] [Google Scholar]
- 2.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(Suppl 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 3.Licheni SH, McMahon JM, Schneider AL, Davey MJ, Scheffer IE. Sleep problems in Dravet syndrome: a modifiable comorbidity. Dev Med Child Neurol. 2018;60(2):192–198. doi: 10.1111/dmcn.13601. [DOI] [PubMed] [Google Scholar]
- 4.Turner SJ, Brown A, Arpone M, Anderson V, Morgan AT, Scheffer IE. Dysarthria and broader motor speech deficits in Dravet syndrome. Neurology. 2017;88(8):743–749. doi: 10.1212/WNL.0000000000003635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodda JM, Scheffer IE, McMahon JM, Berkovic SF, Graham HK. Progressive gait deterioration in adolescents with Dravet syndrome. Arch Neurol. 2012;69(7):873–878. doi: 10.1001/archneurol.2011.3275. [DOI] [PubMed] [Google Scholar]
- 6.Dhamija R, Erickson MK, St Louis EK, Wirrell E, Kotagal S. Sleep abnormalities in children with Dravet syndrome. Pediatr Neurol. 2014;50(5):474–478. doi: 10.1016/j.pediatrneurol.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 7.Guzzetta F. Cognitive and behavioral characteristics of children with Dravet syndrome: an overview. Epilepsia. 2011;52(Suppl 2):35–38. doi: 10.1111/j.1528-1167.2011.02999.x. [DOI] [PubMed] [Google Scholar]
- 8.Cavallo A. The pineal gland in human beings: relevance to pediatrics. J Pediatr. 1993;123(6):843–851. doi: 10.1016/s0022-3476(05)80377-4. [DOI] [PubMed] [Google Scholar]
- 9.Srinivasan V, Pandi-Perumal SR, Trahkt I, et al. Melatonin and melatonergic drugs on sleep: possible mechanisms of action. Int J Neurosci. 2009;119(6):821–846. doi: 10.1080/00207450802328607. [DOI] [PubMed] [Google Scholar]
- 10.Bruni O, Alonso-Alconada D, Besag F, et al. Current role of melatonin in pediatric neurology: clinical recommendations. Eur J Paediatr Neurol. 2015;19(2):122–133. doi: 10.1016/j.ejpn.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 11.Gill I, McBrien J. Question 1: Effectiveness of melatonin in treating sleep problems in children with intellectual disability. Arch Dis Child. 2017;102(9):870–873. doi: 10.1136/archdischild-2017-313230. [DOI] [PubMed] [Google Scholar]
- 12.Jain SV, Horn PS, Simakajornboon N, et al. Melatonin improves sleep in children with epilepsy: a randomized, double-blind, crossover study. Sleep Med. 2015;16(5):637–644. doi: 10.1016/j.sleep.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coppola G, Iervolino G, Mastrosimone M, La Torre G, Ruiu F, Pascotto A. Melatonin in wake-sleep disorders in children, adolescents and young adults with mental retardation with or without epilepsy: a double-blind, cross-over, placebo-controlled trial. Brain Dev. 2004;26(6):373–376. doi: 10.1016/S0387-7604(03)00197-9. [DOI] [PubMed] [Google Scholar]
- 14.Goodwin SW, Lambrinos AI, Ferro MA, Sabaz M, Speechley KN. Development and assessment of a shortened Quality of Life in Childhood Epilepsy Questionnaire (QOLCE-55) Epilepsia. 2015;56(6):864–872. doi: 10.1111/epi.13000. [DOI] [PubMed] [Google Scholar]
- 15.Bruni O, Ottaviano S, Guidetti V, et al. The Sleep Disturbance Scale for Children (SDSC). Construction and validation of an instrument to evaluate sleep disturbances in childhood and adolescence. J Sleep Res. 1996;5(4):251–261. doi: 10.1111/j.1365-2869.1996.00251.x. [DOI] [PubMed] [Google Scholar]
- 16.Foster BC, Cvijovic K, Boon HS, et al. Melatonin interaction resulting in severe sedation. J Pharm Pharm Sci. 2015;18(2):124–131. doi: 10.18433/j3ss35. [DOI] [PubMed] [Google Scholar]
- 17.Kalume F, Oakley JC, Westenbroek RE, et al. Sleep impairment and reduced interneuron excitability in a mouse model of Dravet Syndrome. Neurobiol Dis. 2015;77:141–154. doi: 10.1016/j.nbd.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Papagiannidou E, Skene DJ, Ioannides C. Potential drug interactions with melatonin. Physiol Behav. 2014;131:17–24. doi: 10.1016/j.physbeh.2014.04.016. [DOI] [PubMed] [Google Scholar]