Abstract
Study Objectives:
Numerous studies have found that obstructive sleep apnea (OSA) causes or exacerbates dementia, including Alzheimer disease and vascular dementia. However, the evidence is often conflicting. Moreover, no study has investigated the effect of surgical treatment for OSA on dementia.
Methods:
This retrospective cohort study analyzed data from the Korea National Health Insurance Corporation. A total of 125,417 participants (age 40 years or older) with a new diagnosis of OSA between 2007 and 2014 were included. The participants were classified into two groups: those who underwent uvulopalatopharyngoplasty (UPPP group, n = 12,664) and those who underwent no surgical treatment (no surgery group, n = 112,753). Propensity score matching by age and sex was used to select the control group of 627,085 participants. Mean follow-up duration was 4.6 ± 2.3 years. The primary endpoint was newly diagnosed Alzheimer dementia, vascular dementia, or other types of dementia.
Results:
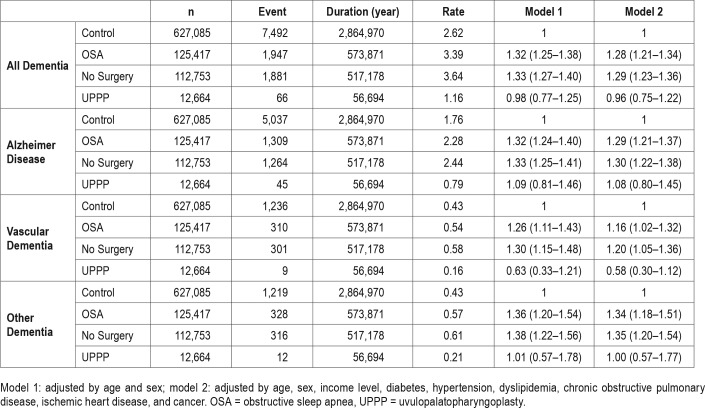
Compared with the control group, the hazard ratio (HR) and 95% confidence interval of dementia was calculated for patients with OSA. In the no-surgery group, the incidence of Alzheimer disease (HR 1.30 [1.22–1.38]), vascular dementia (HR 1.20 [1.05–1.36]), and other types of dementia (HR 1.35 [1.20–1.54]) was significantly higher than those among the control group. In the UPPP group, the incidence of Alzheimer disease (HR 1.08 [0.80–1.45]), vascular dementia (HR 0.58 [0.30–1.12]), and other types of dementia (HR 1.00 [0.57–1.77]) was similar to control levels.
Conclusions:
Uvulopalatopharyngoplasty may have a preventive effect on dementia in patients with OSA.
Citation:
Cho JH, Suh JD, Han KD, Jung JH, Lee HM. Uvulopalatopharyngoplasty may reduce the incidence of dementia caused by obstructive sleep apnea: National Insurance Service Survey 2007–2014. J Clin Sleep Med. 2018;14(10):1749–1755.
Keywords: obstructive sleep apnea, Alzheimer disease, vascular dementia, uvulopalatopharyngoplasty
BRIEF SUMMARY
Current Knowledge/Study Rationale: This study sought to answer the following questions: Does obstructive sleep apnea increase the incidence of dementia? Does uvulopalatopharyngoplasty, the surgical treatment of apnea, reduce the incidence of dementia?
Study Impact: The incidence of Alzheimer disease, vascular dementia, and other types of dementia was higher among patients with obstructive sleep apnea, whereas it was similar to control levels among patients with obstructive sleep apnea who underwent uvulopalatopharyngoplasty. This finding suggests that dementia may be aggravated by obstructive sleep apnea, which could be prevented by uvulopalatopharyngoplasty.
INTRODUCTION
Obstructive sleep apnea (OSA) is characterized by repetitive upper airway collapse during sleep, which induces frequent arousal and oxygen desaturation.1 It is a common disease worldwide, affecting more than 4% of men and 2% of women, and its prevalence increases with the prevalence of obesity.2 OSA is associated with a range of symptoms, such as fatigue, daytime somnolence, or headaches.1 Moreover, it causes serious complications and increases mortality.1 Dementia is a neurodegenerative disorder characterized by progressive and gradual decline in memory and cognitive functions.3,4 There are different types of dementias according to the etiology. The most frequently encountered type of dementia is Alzheimer disease (AD), accounting for up to 50% to 70% of all cases of dementia. AD is characterized by the loss of neurons and synapses in the cerebral cortex and certain subcortical regions, with accumulation of amyloid plaques and neurofibrillary tangles around or inside neurons.3 The next most common type of dementia is vascular dementia (VD), accounting for approximately 25% of cases of dementia. VD is caused by ischemic or hemorrhagic infarcts affecting multiple brain areas.5 There are also other causes of dementia.4 The most important cause of dementia is aging.3–5 As the elderly population increases, the incidence of dementia is rapidly increasing, resulting in a large burden on society.6 Reducing the incidence of dementia has become an urgent priority.
Recent evidence suggests that OSA exacerbates dementia.3,4 Although the exact mechanism has not been elucidated, it is postulated that different sequelae of OSA exacerbate dementia. OSA causes hypertension, systemic inflammation, intermittent hypoxia, impairment of cerebral perfusion, endothelial dysfunction, alteration of glucose homeostasis, and increase in oxidative stress.3,4 It is possible that the combination of these sequelae cause white matter changes, gray matter atrophy, neurocognitive impairment, and accumulation of amyloid plaques and neurofibrillary tangles, which are typical characteristics of dementia.3,4
Though numerous studies have found that OSA causes or exacerbates dementia, these findings are often conflicting and in most studies the results could not be confirmed because of limited population samples. In addition, the effect of surgical treatment for OSA on dementia has not been previously assessed. The most commonly performed surgical procedure for OSA is uvulopalatopharyngoplasty (UPPP).7,8 The success rate of UPPP based on the apnea-hypopnea index (AHI) reduction is not high.7 However, UPPP is an important therapeutic option in patients refusing or intolerant of continuous positive airway pressure (CPAP).7 Therefore, it is important to evaluate UPPP not only in terms of AHI reduction but also according to its clinical effect, for example, a reduction in the incidence of dementia. However, there are significant difficulties in conducting long-term observational studies of large populations and this explains the limited evidence on patients undergoing UPPP.
The National Health Insurance Service (NHIS) database has recently become available for research purposes in Korea.9 It provides large-scale follow-up data on patients with OSA undergoing UPPP. The purpose of this study is to investigate whether the incidence of dementia increases among patients with OSA and whether UPPP can prevent such an increase, using NHIS data.
METHODS
Data Source
The NHIS is a national insurer managed by the Korean government covering 97% of the Korean population.9 Both outpatient and inpatient claims are reviewed by the NHIS and these include data on diagnoses, procedures, prescription records, demographic information, and direct medical costs. The NHIS also reviews claims from the Medical Assistance Program and the Medical Care for Patriots and Veterans Affairs Scheme, which cover the medical expenses of the Korean population not insured by the NHIS. Therefore, the NHIS database covers the entire Korean population, and contains information regarding all medical claims made in Korea. The NHIS identifies its members by their Korean Resident Registration Number, which removes the risk of duplication or omission when accessing the data. The NHIS database manages claims using the Korean Classification of Disease, sixth edition, a modified version of the International Classification of Diseases, 10th edition (ICD-10), adapted for the Korean health care system. Furthermore, a biennial standardized checkup is recommended for NHIS subscribers. Any researcher can use the NHIS data if the study protocols are approved by the official review committee.
Study Population and Design
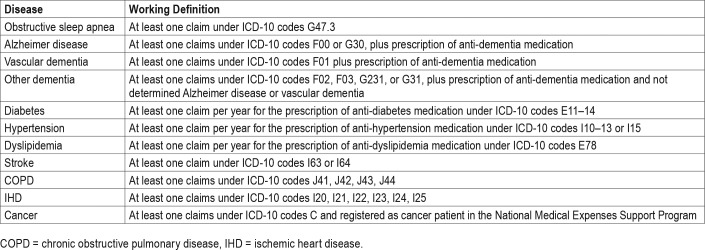
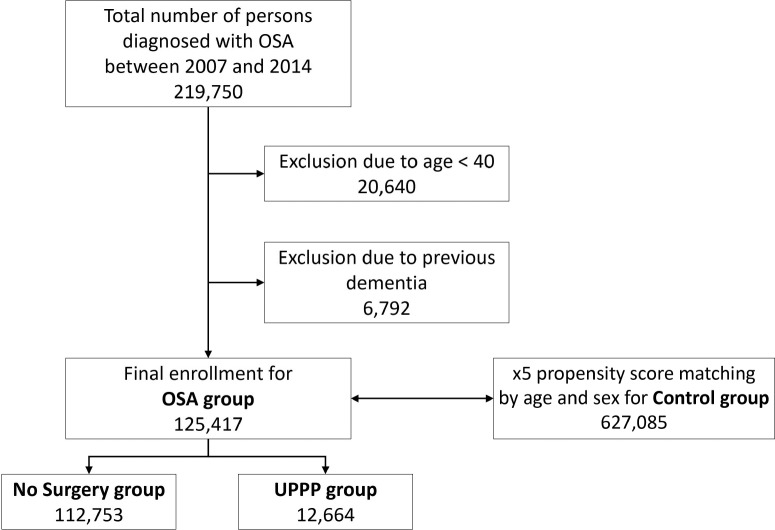
We defined the OSA group as including participants age 40 years or older with newly diagnosed OSA (G47.30) between 2007 and 2014. To select the control group, with a number of participants that was five times that of the OSA group, we used propensity score matching by age and sex with participants in whom OSA was not diagnosed. The OSA group was further divided into two groups: the UPPP group, including patients who underwent UPPP, (Q2196 or Q2197) and the no-surgery group, including participants who did not undergo surgery. The primary endpoint of this study was newly diagnosed dementia (AD, VD, and other types of dementia), which was defined using the insurance claim data (Table 1). Patients in whom dementia was diagnosed prior to enrollment were excluded. The flowchart shows the enrollment process for this study (Figure 1).
Table 1.
Working definitions derived from the insurance claims data.
Figure 1. Enrollment flowchart.
OSA = obstructive sleep apnea, UPPP = uvulopalatopharyngoplasty.
Data Collection
We collected the following baseline data from the NHIS database: age (years), sex, residency (rural or urban), and income level (four quintiles). Data on comorbidities, including diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, ischemic heart disease, and cancer were also collected using the insurance claim data (Table 1).
Statistical Analysis
Data are presented as mean ± standard deviation for age and as proportions for the remaining categorical variables. Comparisons between groups were made using analysis of variance or the chi-square test. The Kaplan-Meier plot without covariance correction was presented to analyze the risk of dementia according to the presence or absence of OSA and whether the patient underwent UPPP. The incidence rate of dementia was calculated by dividing the number of events by the person-time at risk. To determine the hazard ratio of OSA on the incidence of dementia and to compare the incidence of dementia between UPPP and no-surgery groups, the Cox proportional hazards model was used. Two different models were applied: model 1 was adjusted for age and sex and model 2 was based on model 1 and additionally adjusted for income level, diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, ischemic heart disease, and cancer. The proportional hazards assumptions were checked by log-log cumulative survival graphs; the assumptions were met. The results are presented as mean and 95% confidence interval (95% CI). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.2.3 (The R Foundation for Statistical Computing, Vienna, Austria).
Ethical Approval
This study involved routinely collected data, so informed consent was not specifically obtained. The study was exempted by the Institutional Review Board of Konkuk University Hospital due to use of publicly available data (KUH1110066).
RESULTS
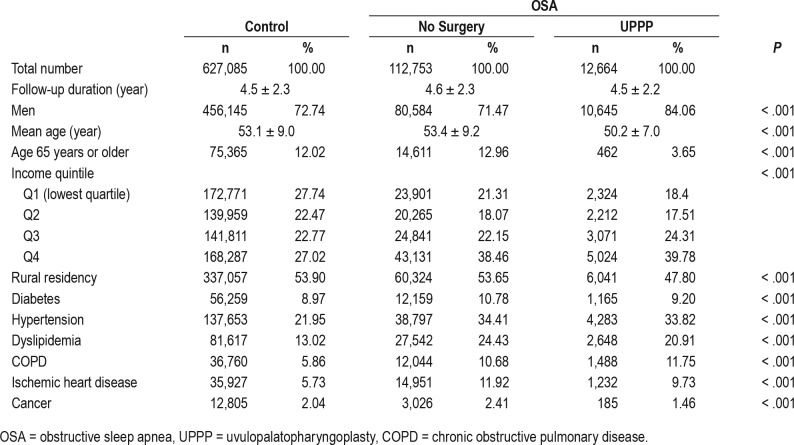
A total of 49,570,064 participants enrolled in the NHIS in 2007, the first year of the research and the numbers were comparable for each year up to 2014. Between 2007 and 2014, there were 125,417 patients in whom OSA was newly diagnosed. Of these, 12,644 underwent UPPP. A total of 627,085 participants were selected as a control group (Figure 1). Demographic data are summarized in Table 2.
Table 2.
Demographics of patients with OSA and controls.
Comparison of Incidence of AD Between OSA and Control Groups
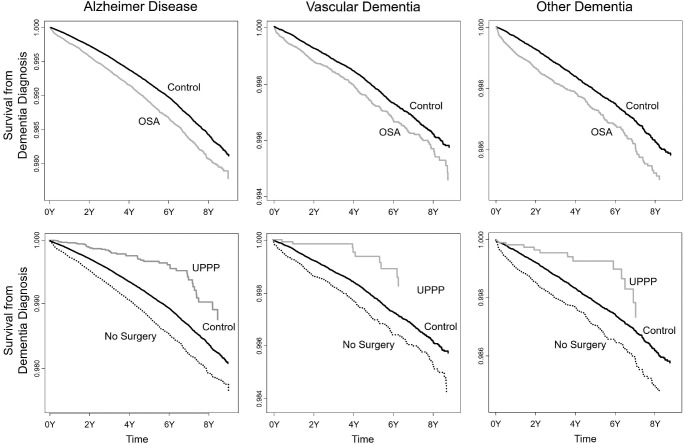
AD occurred more frequently in the OSA group compared to the control group and this finding was significant in both model 1 and model 2. When the OSA group was further analyzed, AD incidence among participants in the UPPP group was similar to that among the control group in both model 1 or model 2, whereas the incidence remained higher in the no-surgery group. The Kaplan-Meier plot showed that compared to the control group, AD occurred more frequently in the OSA group and less frequently in the UPPP group (Figure 2). These results are summarized in Table 3.
Figure 2. Kaplan-Meier plot for incidence of dementia in patients with OSA.
The top row compares the control group with the OSA group, and the bottom row compares the control group with the UPPP group and the no-surgery group. Alzheimer disease, vascular dementia, and other dementia occur more frequently in the OSA group than in the control group, whereas they occur less frequently in the UPPP group. OSA = obstructive sleep apnea, UPPP = uvulopalatopharyngoplasty.
Table 3.
Hazard ratio of OSA for the incidence of dementia.
Comparison of Incidence of VD Between OSA and Control Groups
VD occurred more frequently in the OSA group compared to the control group and this finding was significant in both model 1 and model 2. Further analysis of the OSA group revealed that VD incidence among participants in the UPPP group was similar to that among the control group in both model 1 or model 2, whereas the incidence remained higher in the no-surgery group. The Kaplan-Meier plot showed that compared to the control group, VD occurred more frequently in the OSA group and less frequently in the UPPP group (Figure 2). These results are summarized in Table 3.
Comparison of Incidence of Other Types of Dementia Between OSA and Control Groups
Other types of dementia occurred more frequently in the OSA group compared to the control group and this finding was significant in both model 1 and model 2. When the OSA group was further analyzed, the incidence of other types of dementia increased in the no-surgery group in both models, although it did not increase in the UPPP group. The Kaplan-Meier plot showed that, compared to the control group, other types of dementia occurred more frequently in the OSA group and less frequently in the UPPP group (Figure 2). These results are summarized in Table 3.
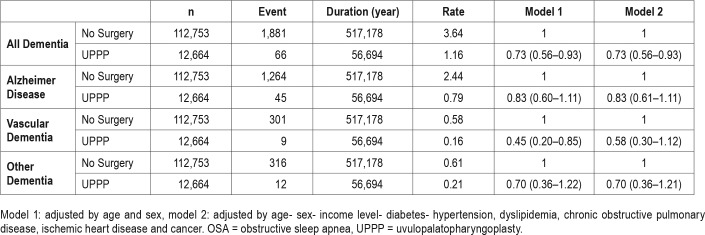
Comparison of Hazard Ratio Between UPPP and No-Surgery Groups for the Incidence of Dementia
All dementia incidence was significantly lower in the UPPP group, compared to the no-surgery group, in both model 1 and model 2. When divided into AD, VD, and other dementia, the incidence of VD was still significantly lower in the UPPP group in model 1, but not in model 2. The incidences of AD and other dementia were not significantly different between the two groups in both model 1 and model 2. The results are summarized in Table 4.
Table 4.
Comparison of hazard ratio between UPPP and no-surgery groups for the incidence of dementia.
DISCUSSION
In this study, we found that the incidence of dementia among patients with OSA was significantly higher than in the control group. However, in patients who underwent UPPP surgery, the incidence of dementia was similar to that of the control group. When comparing UPPP and no-surgery groups, the incidences of all dementia and VD were still lower in the UPPP group, whereas the incidences of AD and other dementia were not significantly different between the two groups. A considerable advantage of this study is that long-term follow-up was conducted for large-scale patient groups using national insurance data. Large-scale studies are essential to determine differences in dementia incidences among patients with OSA and control patients, or among patients who have undergone UPPP and those who have not. A number of cohort studies on OSA have been conducted,10,11 although there are no studies on the long-term effects of UPPP. To date, the effects of UPPP have been determined by the AHI and the success rate is not satisfactory.7,8 However, the clinical significance of AHI-based surgical results is not proven. To the best of our knowledge, this is the first report regarding the clinical feasibility of UPPP to reduce the occurrence of dementia among patients with OSA.
The incidence of dementia in the UPPP group varied according to dementia type. The incidences of AD and other types of dementia were comparable to those observed in the control group (hazard ratio of approximately 1.0); the incidence of VD was even lower, although not statistically significant, than in the control group (hazard ratio of approximately 0.6). When the UPPP and the no-surgery groups were directly compared, VD incidence was the only outcome parameter that was significantly different. This finding may be due to hypoxic preconditioning.12 The brain of patients with OSA is exposed to long-term intermittent hypoxia, which is then normalized after UPPP. Therefore, subsequent hypoxia will be better tolerated in these patients. The main pathogenesis of VD is ischemic brain injury due to infarct, so the hypoxic preconditioning induced by the OSA could reduce brain damage and also the incidence of VD.5
Different covariates that are known to influence the occur-rence of dementia were analyzed to adjust the effect of OSA and UPPP.13 The covariates were age, sex, income level, diabetes, hypertension, dyslipidemia, chronic obstructive pulmonary disease, ischemic heart disease, and cancer. Age is the most important risk factor for the development of dementia.13–17 Dementia incidence increases sharply with age older than 60 years and reaches almost 50% after age 85 years.14 Sex is also an important factor, as dementia is more prevalent among women. Differences according to sex are presumed to be caused by differences in sex hormones, education level, and life expectancy between men and women.14 Income level also plays an important role in the incidence of dementia which increases among populations with lower income levels. There could be many reasons for this trend, such as education level, diet, and lifestyle.15 Diabetes, hypertension, and dyslipidemia are well-known aggravating factors for dementia.16,17 Factors such as chronic obstructive pulmonary disease, ischemic heart disease, and cancer were also included in this study, although their effect has not yet been proven.
The most significant weakness of this study is the potential selection bias for the UPPP group. Generally, surgery for OSA is more likely to involve younger, healthier, and more affluent patients because of the risks of anesthesia, complications, and costs of surgery.18 In this study, the UPPP group was younger by approximately 3 years, had a slightly higher income level, and had a lower rate of dyslipidemia, ischemic heart disease, and cancer than the no-surgery group. Therefore, rather than a clear surgical effect, the reason for the low incidence of dementia in the UPPP group may be that patients in that group were younger and healthier than patients in the comparison groups. However, even after adjusting for various factors, the incidence of dementia in the UPPP group was significantly lower than that in the no-surgery group, reaching control levels, which means that the preventive effect of UPPP on the development of dementia is not simply due to confounders. Unfortunately, the analysis of confounding factors did not include body mass index, which was not present in the claim data. Obesity is an important factor because it affects the occurrence of both dementia and OSA; body mass index is a value that most accurately reflects the degree of obesity. Therefore, if body mass index had been included in the analysis, our results might have been quite different.
A more intrinsic limitation of this study is that it does not provide direct evidence that dementia can be exacerbated by OSA and subsequently prevented by UPPP. Although there is a reasonable likelihood of this exacerbation and prevention, our conclusions are solely based on comparison of the incidence of dementia in the no-surgery group, the UPPP group, and the control group. Another limitation is that the adequacy of the diagnosis and the severity of disease could not be demonstrated. We could not determine the AHI values and symptoms for all patients from the medical records; therefore, we identified patients with OSA on the basis of the diagnoses in the medical records.
A further issue to be noted was that we did not know how many of the patients with OSA who did not undergo UPPP (the no-surgery group) were offered alternative treatment, such as CPAP or a mandibular advancing device (MAD). In Korea, UPPP is the treatment of choice for OSA, over CPAP or MAD.18 Although it is not known with certainty, we assumed that the cost of surgery is low (approximately $700) and that many Koreans are reluctant to wear the machine in the long term.18 Approximately 3,000 patients are prescribed CPAP annually and its adherence rate is very low.19 Even fewer patients are prescribed MAD.19 Considering that the number of patients with OSA recruited in this study was 125,417 and their mean follow-up period was approximately 4.5 years, it is reasonable to assume that most patients in the no-surgery group did not receive active treatment for OSA and their condition naturally progressed.
CONCLUSIONS
The incidence of dementia was significantly higher among patients with OSA than among patients in the control group, whereas it was similar to the incidence among patients in the control group after UPPP. This study provides new insight that UPPP may have a preventive effect on dementia in patients with OSA.
DISCLOSURE STATEMENT
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea. (HI15C1512). All the authors have no conflicts of interest. All the authors have seen and approved the manuscript.
ABBREVIATIONS
- 95% CI
95% confidence interval
- AD
Alzheimer disease
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- NHIS
National Health Insurance Service
- OSA
obstructive sleep apnea
- UPPP
uvulopalatopharyngoplasty
- VD
vascular dementia
REFERENCES
- 1.Eckert DJ, Malhotra A. Pathophysiology of adult obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):144–153. doi: 10.1513/pats.200707-114MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Partinen M. Epidemiology of obstructive sleep apnea syndrome. Curr Opin Pulm Med. 1995;1(6):482–487. doi: 10.1097/00063198-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Pan W, Kastin AJ. Can sleep apnea cause Alzheimer's disease? Neurosci Biobehav Rev. 2014;47:656–669. doi: 10.1016/j.neubiorev.2014.10.019. [DOI] [PubMed] [Google Scholar]
- 4.Daulatzai MA. Evidence of neurodegeneration in obstructive sleep apnea: Relationship between obstructive sleep apnea and cognitive dysfunction in the elderly. J Neurosci Res. 2015;93(12):1778–1794. doi: 10.1002/jnr.23634. [DOI] [PubMed] [Google Scholar]
- 5.Ramos AR, Dib SI, Wright CB. Vascular Dementia. Curr Transl Geriatr Exp Gerontol Rep. 2013;2(3):188–195. doi: 10.1007/s13670-013-0054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clarkson P, Davies L, Jasper R, Loynes N, Challis D. A systematic review of the economic evidence for home support interventions in dementia. Value Health. 2017;20(8):1198–1209. doi: 10.1016/j.jval.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Aurora RN, Casey KR, Kristo D, et al. Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults. Sleep. 2010;33(10):1408–1413. doi: 10.1093/sleep/33.10.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JH, Cho SH, Kim SN, Suh JD, Cho JH. Predicting outcomes after uvulopalatopharyngoplasty for adult obstructive sleep apnea: a meta-analysis. Otolaryngol Head Neck Surg. 2016;155(6):904–913. doi: 10.1177/0194599816661481. [DOI] [PubMed] [Google Scholar]
- 9.Song SJ, Han K, Choi KS, et al. Trends in diabetic retinopathy and related medical practices among type 2 diabetes patients: Results from the National Insurance Service Survey 2006-2013. J Diabetes Investig. 2018;9(1):173–178. doi: 10.1111/jdi.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hla KM, Young T, Hagen EW, et al. Coronary heart disease incidence in sleep disordered breathing: the Wisconsin Sleep Cohort Study. Sleep. 2015;38(5):677–684. doi: 10.5665/sleep.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190(2):218–225. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 12.Shah N, Redline S, Yaggi HK, et al. Obstructive sleep apnea and acute myocardial infarction severity: ischemic preconditioning? Sleep Breath. 2013;17(2):819–826. doi: 10.1007/s11325-012-0770-7. [DOI] [PubMed] [Google Scholar]
- 13.Román GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20(Suppl 2):91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 14.Podcasy JL, Epperson CN. Considering sex and gender in Alzheimer disease and other dementias. Dialogues Clin Neurosci. 2016;18(4):437–446. doi: 10.31887/DCNS.2016.18.4/cepperson. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seifan A1, Schelke M, Obeng-Aduasare Y, Isaacson R. Early life epidemiology of Alzheimer's disease. Neuroepidemiology. 2015;45(4):237–254. doi: 10.1159/000439568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kloppenborg RP, van den Berg E, Kappelle LJ, Biessels GJ. Diabetes and other vascular risk factors for dementia: which factor matters most? A systematic review. Eur J Pharmacol. 2008;585(1):97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 17.Sharp SI, Aarsland D, Day S, Sønnesyn H, Ballard C. Hypertension is a potential risk factor for vascular dementia: systematic review. Int J Geriatr Psychiatry. 2011;26(7):661–669. doi: 10.1002/gps.2572. [DOI] [PubMed] [Google Scholar]
- 18.Kong HW, Kim HA, Han JK, Yun CH. The efficacy of uvulopalatopharyngoplasty in obstructive sleep apnea syndrome. J Korean Sleep Soc. 2004;1(2):32–36. [Google Scholar]
- 19.Choi J, Cho SH. Treatment of obstructive sleep apnea with positive pressure ventilation. Hanyang Med Rev. 2013;33:239–245. [Google Scholar]