Abstract
The estimation of Subjective Visual Vertical (SVV) involves the allocentric, gravitational and egocentric references, which are built by visual, vestibular and somatosensory afferents. Our goals were to assess the influence of plantar cutaneous afferents on the perception of SVV, and to see if there is a difference according to the efficiency of plantar cutaneous afferents. We recruited 48 young and healthy subjects and assessed their SVV and postural performances in quiet stance with a force platform, at 40 or 200 cm, in four ground conditions: on firm ground, on foam, with a bilateral, or with a unilateral 3 mm arch support. We also assessed the efficiency of our subjects’ plantar afferents with the plantar quotient method and divided them in two groups: subjects with a normal use of plantar afferents and subjects with Plantar Exteroceptive Inefficiency (PEI). The results showed significant decreases in the counter clockwise SVV deviation only with the unilateral arch support, at near distance, and among the typically behaving subjects. We conclude that asymmetric foot cutaneous afferents are able to bias the egocentric vertical reference and hence influence the perception of SVV. This influence disappears among subjects with PEI, probably because of a distortion of the plantar signal.
Introduction
Spatial representations require the integration of visual, vestibular and somatosensory cues, which takes place in various areas of the Central Nervous System (CNS). The involved zones which have been identified so far are: the multimodal vestibular areas1, the temporo-occipital, parieto-temporal, parieto-occipital and insular cortex2–4, the supra-marginal gyrus5, the middle temporal gyrus6, the inferior frontal and superior temporal4, and the posterolateral thalamus7,8. These three sensorial modalities enable knowledge of personal and extra-personal space: position of objects in space, positions and displacements of the body in space and spatial configuration of the body segments. Thus, the vestibular system allows to make the distinction between self-motion and movements of the environment. Since motor performance involves an interaction between body and environment9, the availability of spatial reference frames is a prerequisite for the control of posture and movement10–12.
Three inter-dependent spatial references contribute to the representation of Subjective Visual Vertical (SVV): the allocentric reference, in which the objects are localized directly through their spatial configuration; the gravitational reference, which is independent from the position of the body and objects and refers to the orientation of the gravitational vector; and the egocentric reference, in which the position of the objects is determined with respect to the body1. The visual, vestibular and somatosensory afferents contribute respectively to the elaboration of those three references7,13,14, giving rise to the internal models of verticality, with the contribution of top-down influences8.
The influence of the visual information on SVV estimation has been widely studied, as concerns the static15–18 as well as the dynamic cues16,19,20. Vestibular afferents also play a major role in the perception of verticality21–24. The influence of somatosensory cues has been underscored by Anastasopoulos et al.25, who showed that two patients with complete hemisensory loss were insensitive to the deviation of SVV estimate (opposite to the side of the body tilt, i.e. A-effect) when lying on the hypoesthetic side. These authors concluded that the A-effect is primarily somatosensory, not otolithic. More recently, other authors7,26 obtained similar results among larger groups of paraplegics and hemiplegics with unilateral somatosensory loss. They concluded that somatosensory information plays an important role in the construction and update of internal models of verticality. Likewise, it is also known that patients with cerebral lesions show a spontaneous deviation of their SVV estimates. Such deviation (contralateral to the lesion) occurs only in case of hemispatial neglect and is significantly related to the severity of the neglect3,6,27,28. More specifically, the influence of muscular proprioception has been shown by several authors29–32. Visceral afferents also play a role23,33, as well as tactile cues33,34.
As concerns the latter input, it is known that the foot sole behaves like a “dynamometric map” that quantifies the repartition of plantar pressures35. According to those authors, plantar pressure distribution is a precious clue that the Central Nervous System uses to assess the importance of the deviation of the body relative to its vertical reference. Furthermore, Roll et al.36 showed that vibrations applied to the foot soles of restrained and blindfolded subjects give rise to illusory perceptions of whole-body leaning. This result suggests that foot cutaneous afferents contribute to the spatial representation of body posture. However, the existing literature concerning the effects of tactile afferents on SVV only involves the stimulation of body skin during large body tilts (>45°) of seated subjects. To our knowledge, only Faralli et al.37 studied the influence of the podal input on the perception of SVV, but it was among patients with vestibular dysfunction and by stimulating exteroceptive and proprioceptive afferents at the same time. The specific influence of foot cutaneous afferents on the perception of verticality during quiet standing in healthy subjects has never been studied.
Our main hypothesis (hyp.1) was that plantar cutaneous afferents can influence the perception of SVV. In order to manipulate these afferents we resorted to the interposition of a foam pad, which is known to decrease their availability38. Hence, we expected it would increase the deviation in the SVV estimation. We also used bilateral medial arch supports39, which are known to improve the quality of postural control40. We expected that such stimulation would reduce the deviation of the SVV estimate. Finally, we tested a unilateral (right) lateral arch support, which is supposed to induce an opposite (left) shift of the Center of Pressure41 (CoP). Consequently, we expected it would produce an E-effect that is, an error in the SVV estimation opposite to the body tilt42,43.
In a previous publication44, we noticed that among young and healthy people, there was important inter-individual variability as concerns the effects of thin plantar inserts on postural and oculomotor control (see40). We showed that this variability can be explained by the degree to which subjects make use of their plantar afferents. Since plantar cutaneous afferents are normally used by the CNS to ensure postural control45, a decrease of their use (especially among healthy subjects) does not seem to correspond to a simple idiosyncrasy.
Hence, we concluded that this minority of healthy people were unable to properly use their plantar cutaneous afferents both for balance and vergence control. We proposed that it corresponds to a situation revealing inefficiency in plantar cutaneous afferents (Plantar Exteroceptive Inefficiency - PEI). We discussed that PEI could be due to a latent somatosensory dysfunction of the sole’s mechanoreceptors linked to an increase in pressure beneath certain plantar zones (such as the first metatarsal head, as proposed by Janin46). Knowing that an increase in pressure on the mechanoreceptors increases the discharge frequency of these receptors47,48, we argued that such distortion of the plantar signal makes is more difficult to integrate for the CNS49, thereby leading to neglect them. These first results were confirmed by another experiment50: using the same method, we showed that subjects with PEI were also unable to use plantar and visual afferents for postural control at the same time (visual-podal asynergy).
Here and in our prior publications44,50 our view is that, as concerns functional pathology, there is a progressive transition from physiology towards pathology through an in-between dysfunctional state which is not necessarily symptomatic. Our opinion is in line with earlier studies: according to Canguilhem51,52, there is, for each function, a margin which corresponds to functional adaptability. He considers physiology as an ability to adapt to the changes of one’s environment, and disease as a reduction to the margin of tolerance of the variations of the environment. Winter53 shares the same view specifically as concerns postural control, considering that the adaptability of the CNS explains that pathology may not be apparent at first.
Our previous experiments clearly show that all of the healthy subjects do not use their plantar cutaneous afferents in the same way, and this should be taken into consideration when studying subjects in standing posture. Consequently, we further hypothesized (hyp.2) that PEI would also preclude the influence of foot tactile afferents on the perception of SVV.
Results
Group constitution
We divided our population into two groups: the Plantar Exteroceptive Inefficient Subjects, who had a plantar quotient below 100 (21 subjects), and the typically behaving subjects, who had a plantar quotient higher than 100 (27 subjects).
Mann-Whitney U tests showed that subjects with PEI and typically behaving subjects did not have significantly different ages (z = 0.12, p = 0.90), heights (z = −0.03, p = 0.98), weights (z = 0.58, p = 0.65), stereoacuity (z = 0.90, p = 0.37), visual acuity (z = −0.22, p = 0.83), amplitude of accommodation (z = 1.83, p = 0.07) and Near Convergence Point (z = −0.81, p = 0.42). Their only significant difference was their plantar quotient (z = −5.89, p < 0.01), the subjects with PEI having a lower plantar quotient than the typically behaving subjects, due to a higher Surface area on firm ground (z = 2.28, p = 0.02, d = 0.82).
Conditions comparisons
Effects of the ground conditions
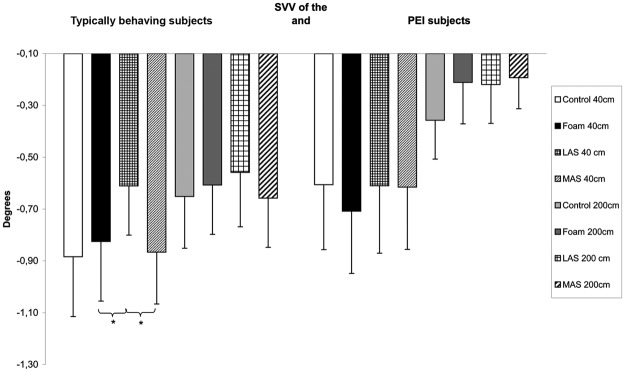
For the typically behaving subjects, the Friedman’s test showed a main effect on the deviation of the SVV (χ²(7,27) = 17.64, p = 0.01). The SVV was significantly less tilted on the left with the lateral arch support compared to foam (z = 2.45, p = 0.04, d = 0.19) and to the medial arch supports (z = 2.55, p = 0.04, d = 0.26). There also was a tendency for the SVV estimate to be less tilted on the left with the lateral arch support compared to Control (z = 1.90, p = 0.06, d = 0.25) at 40 cm (Fig. 1).
Figure 1.
SVV estimation among subjects with Plantar Exteroceptive Inefficiency and typically behaving subjects for each testing condition. Error bars represent the standard errors. Asterisks indicate significant differences, with P with *p < 0.05.
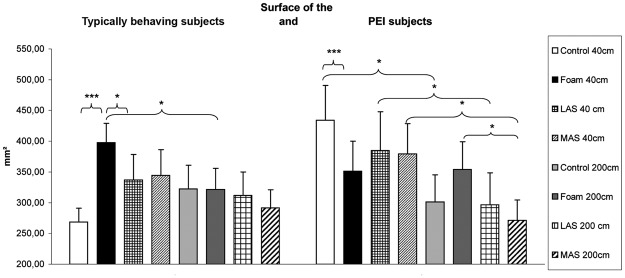
As concerns postural control, there was a main effect of the ground conditions on the Surface area of the CoP (χ²(7,27) = 20.28, p = 0.01). At 40 cm, the Surface area was significantly higher on foam compared to firm ground (z = 4.54, p < 0.01, d = 0.91) and lateral arch support (z = 2.21, p = 0.03, d = 0.32) (Fig. 2).
Figure 2.
Surface area of the Center of Pressure among subjects with Plantar Exteroceptive Inefficiency and typically behaving subjects for each testing condition. Error bars represent the standard errors. Asterisks indicate significant differences, with P with *p < 0.05, ***p < 0.001.
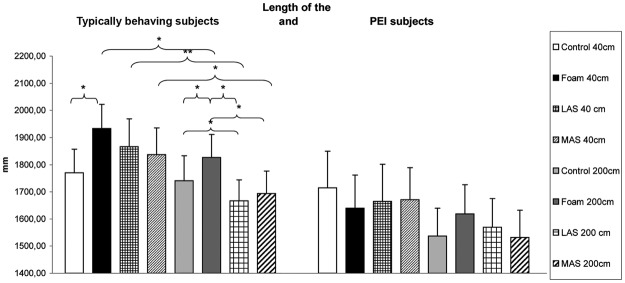
There also was a main effect on the Length of the displacements of the CoP (χ²(7,27) = 27.38, p < 0.01). At 40 cm, the Length of the typically behaving subjects was significantly higher on foam compared to firm ground (z = 3.00, p = 0.04, d = 0.36), and had a tendency to be higher on foam versus lateral arch support (z = 1.87, p = 0.06, d = 0.13). At 200 cm, the Length was higher on foam compared to firm ground (z = 2.11, p = 0.03, d = 0.19), lateral arch support (z = 3.58, p = 0.01, d = 0.38) and medial arch supports (z = 3.17, p = 0.02, d = 0.31). It was lower with lateral arch support compared to firm ground (z = 2.04, p = 0.04, d = 0.17) (Fig. 3).
Figure 3.
Length of the Center of Pressure excursions for the subjects with Plantar Exteroceptive Inefficiency and typically behaving subjects for each testing condition. Error bars represent the standard errors. Asterisks indicate significant differences, with P with *p < 0.05, **p < 0.01.
There was no significant effect on the medio-lateral (X) position of the CoP (χ²(7,27) = 3.61, p = 0.82).
For the subjects with PEI, there was no significant effect of the ground conditions on the estimation of the SVV (χ²(7,21) = 11.47, p = 0.12) (Fig. 1).
As concerns postural control, there was a main effect on the Surface area of the CoP (χ²(7,21) = 25.74, p < 0.01). At 40 cm, the Surface area was lower on foam compared to firm ground (z = 4.01, p < 0.01, d = 0.34), contrary to the typically behaving subjects. At 200 cm, the Surface area was also lower with medial arch supports compared to foam (z = 2.35, p = 0.04, d = 0.46) (Fig. 2).
Finally, there was no significant effect concerning the Length of the CoP displacements (χ²(7,21) = 6.06, p = 0.53 – Fig. 3), and the medio-lateral (X) position of the CoP (χ²(7,21) = 4.76, p = 0.69).
Effects of distance
The results also showed effects of distance. Among the typically behaving subjects, there was a tendency for the SVV estimate to be less tilted on the left at 200 cm than 40 cm on firm ground (z = 1.80, p = 0.07, d = 0.21), and with medial arch supports (z = 1.78, p = 0.08, d = 0.21). The Surface area was significantly lower at 200 cm than 40 cm on foam (z = 2.28, p = 0.04, d = 0.45). The Length was lower at 200 cm than 40 cm on foam (z = 2.39, p = 0.02, d = 0.24), with the lateral arch support (z = 2.91, p < 0.01, d = 0.42) and the medial arch supports (z = 2.14, p = 0.03, d = 0.30).
Among the subjects with PEI, the Surface area was significantly lower at 200 cm than 40 cm on firm ground (z = 2.97, p = 0.05, d = 0.57), with lateral arch support (z = 2.07, p = 0.04, d = 0.33), and medial arch supports (z = 2.42, p = 0.05, d = 0.56).
The results are summarized in Figs 2 and 3 and Table 1.
Table 1.
SVV and postural performances.
| SVV (degrees) | Surface area of CoP (mm²) | Length of CoP (mm) | PEI subjects | Typically behaving subjects | PEI subjects | |
|---|---|---|---|---|---|---|
| Typically behaving subjects | PEI subjects | Typically behaving subjects | ||||
| Control | ||||||
| At 40 cm | −0.88 ± 0.23, [−1.35, −0.42] | −0.61 ± 0.25, [−1.13, −0.08] | 269 ± 23, [222, 315] | 434 ± 56, [316, 552] | 1771 ± 86, [1594, 1947] | 1715 ± 136, [1432, 1997] |
| At 200 cm | −0.65 ± 0.20, [−1.06, −0.24] | −0.36 ± 0.15, [−0.68, −0.04] | 322 ± 39, [243, 402] | 301 ± 44, [209, 393] | 1741 ± 92, [1551, 1930] | 1537 ± 102, [1324, 1751] |
| Foam | ||||||
| At 40 cm | −0.82 ± 0.23, [−1.31, −0.34] | −0.71 ± 0.24, [−1.20, −0.22] | 398 ± 32, [333, 463] | 351 ± 49, [249, 453] | 1934 ± 89, [1751, 2117] | 1640 ± 122, [1384, 1895] |
| At 200 cm | −0.61 ± 0.19, [−1.00, −0.22] | −0.21 ± 0.16, [−0.54, 0.12] | 321 ± 34, [251, 392] | 354 ± 45, [260, 448] | 1827 ± 84, [1654, 2000] | 1619 ± 107, [1396, 1842] |
| LAS | ||||||
| At 40 cm | −0.61 ± 0.19, [−1.00, −0.22] | −0.61 ± 0.26, [−1.16, −0.06] | 337 ± 42, [252, 423] | 385 ± 63, [254, 517] | 1867 ± 102, [1656, 2078] | 1665 ± 136, [1381, 1949] |
| At 200 cm | −0.56 ± 0.21, [−0.98, −0.13] | −0.22 ± 0.15, [−0.54, 0.10] | 312 ± 38, [233, 390] | 297 ± 52, [189, 404] | 1667 ± 77, [1508, 1826] | 1569 ± 106, [1348, 1790] |
| MAS | ||||||
| At 40 cm | −0.87 ± 0.20, [−1.27, −0.46] | −0.62 ± 0.24, [−1.12, −0.11] | 344 ± 42, [258, 431] | 379 ± 50, [276, 483] | 1837 ± 98, [1635, 2039] | 1670 ± 119, [1423, 1918] |
| At 200 cm | −0.66 ± 0.19, [−1.05, −0.26] | −0.19 ± 0.12, [−0.44, 0.06] | 292 ± 29, [231, 352] | 271 ± 33, [202, 340] | 1694 ± 82, [1525, 1863] | 1532 ± 101, [1322, 1741] |
Means, standard errors and 95% Confidence Intervals of SVV and postural parameters for each condition among the normal and the Plantar Exteroceptive Inefficient subjects.
Discussion
Effects of the ground conditions
The main result of that experiment is that foot cutaneous afferents influence the perception of SVV, confirming our first hypothesis (hyp.1). Furthermore, this influence disappears among subjects with PEI, confirming our additional hypothesis (hyp.2).
Effects upon SVV
Our subjects had a mean deviation of the SVV (on all of the conditions) −0.60 ± 1.00°, which means they made a counter clockwise error of 0.60 ± 1.00°. These results are in line with the literature: Akin et al.54 found that in young healthy individuals, the deviation in the SVV estimate was <2°.
Among the typically behaving subjects the unilateral lateral arch support decreases the counter clockwise deviation at close distance, showing that this way of manipulating plantar cutaneous afferents influences the perception of verticality. Given that the unilateral lateral arch support is the only tested stimulation to induce a modification of the repartition of plantar pressures between the two feet (as shown by Janin and Dupui41), it seems that this modification biased the egocentric vertical reference of the CNS, resulting in the observed SVV effect. This rationale is in line with the conclusions of Barra et al.55, who showed a correlation between the SVV estimate and an unequal distribution of loading on the feet among stroke survivors. Our results are also in accordance with those of other authors: they give a first confirmation of the proposition of Kavounoudias et al.35 that the CNS uses the information of the foot soles’ pressure distribution in order to build an internal model of verticality. In the same way, Anastasopoulos et al.25 proposed that the egocentric vertical reference (called “idiotropic vector” by Mittelstaedt56) would rely on symmetric somatosensory input, thus explaining the absence of A-effect only among patients with hemisensory loss. It is likely that the same mechanism is involved in healthy subjects, with smaller magnitude. Barra et al.7 and Saeys et al.26 also showed that the deviation of the SVV estimates of paraplegics and hemiplegics only exists in case of unilateral somatosensory loss and is proportional to the availability of somatosensory afferents. They concluded that there exists an internal model of verticality in human, with a subjective vertical constructed by synthesizing available sensory information. Hence, our results suggest that plantar cutaneous afferents, and most especially the comparison of the symmetry/asymmetry of the signals arising from the two feet, is a part of the sensory information which is used in this process.
This finding does not seem to be the result of an E-effect because there was no significant contra-lateral shift of the CoP with such plantar insert. Such absence of postural result in the frontal plane is surprising because Janin and Dupui41 obtained a contra-lateral shift of the CoP with a midfoot 3mm-thick plantar insert. In their experiment, however the subjects were looking straight ahead in a normally lit room, whereas in this one they were in the dark and were instructed to focus on the verticalisation of the laser. Thus, in this double-task paradigm, the Central Nervous System had to manage the SVV estimation and postural control at the same time, without visual afferents. Such challenging situation may explain the prioritization on the adjustment of the SVV and therefore, that absence of postural regulation in the frontal plane.
Contrary to the typically behaving subjects, the subjects with PEI do not show any modification in the SVV deviation with the lateral arch support. Previous work suggested that PEI is due to a non-noxious somatosensory dysfunction which prevents the CNS from correctly processing and using foot cutaneous afferents for both balance and vergence control44. An increase in the pressure beneath the first metatarsal head leading to an increase in the frequency discharge of the sole receptors is thought to be responsible for this dysfunction44,46,50. Thus, the results of this study are in line with the former and show that the presence of PEI also precludes plantar cutaneous afferents from influencing the SVV estimates.
Effects on postural control
The results concerning posture with the thin plantar inserts (lateral arch support and medial arch supports) showed an improvement of the quality of postural control among typically behaving subjects, through a decrease in Surface area and Length compared to the foam or firm ground condition. These findings are in agreement with previous work of our team40,44, even though the postural effects of the medial arch supports were smaller in this experiment. Likewise, it can be due to the double-task here asked for, and the longer recording of postural control (180 seconds), while in the cited experiments the subjects were only looking straight ahead or were performing vergence movements for shorter periods (51 seconds).
The observed effects of improvement of stability with the inserts among typically behaving subjects almost entirely disappear among subjects with PEI. They do not show any modification of the Length of their CoP displacements; the only persisting effect is an improvement of stability as indicated by the decrease in the Surface area with the medial arch supports compared to foam at 200 cm. Furthermore, in agreement with previous results44,50, the two groups behave differently on foam: it renders the typically behaving subjects more unstable (increase in Surface area and Length as compared to firm ground), whereas it makes the subjects with PEI more stable in terms of a decrease in their Surface area. Foam smoothes plantar pressure distribution57 and decreases the plantar signal38. Hence, it appears logical that the decrease in the normal foot cutaneous afferents of the typically behaving subjects results in an increase in body sway among them, whereas the decrease in the excessive and disruptive plantar signal of the subjects with PEI makes them more stable. These results are also in line with previous work of our group44,50: they strengthen the hypothesis that, even non noxious, PEI distorts the plantar signal which prevents the affected subjects from properly using these afferents for postural control.
Interestingly, foam interposition produced the expected effects on balance, but no significant effect on SVV. It is known that foam interposition decreases the availability of plantar cues38,44,58 which results in a less stable balance. It is confirmed here by the increase in the Length as compared to the 3 other conditions among the typically behaving subjects. Our results are in line with those of Faralli et al.37 who did not show any effect on the SVV deviation during the perturbation of the podal input of healthy subjects (control group) with a cushion between the feet and the ground. However, these authors did not report the exact characteristics of the cushion (thickness, density, hardness), making impossible to know if it exerts its action rather on plantar exteroception or proprioception44,58.
Thus, the postural and SVV results with all of the stimulations (medial arch supports, lateral arch support, foam) seemed independent, confirming previous work59. It suggests that the influence of plantar afferents on the multimodal vestibular areas involved in SVV estimation1,2 are rather direct than indirect (i.e., following postural changes). Those findings are in line with our previous results concerning vergence and posture40. It is worth noting that we only recorded the displacements of the CoP, but it has been shown that force plates’ measurements reflect the same properties of movements as actual body motions in quiet stance (especially for the hip, shoulder and head60).
Effects of distance
Firstly, it appears that the effects of lateral arch support on SVV appear only at close distance (40 cm), that is, when the proprioceptive extra ocular signals are the most available due to the increase in the vergence angle61. Hence, given that foot cutaneous afferents play a role in the perception of SVV only for that condition (physiologically, for typically behaving subjects), it seems that those cues need to be compared to the eye muscle afferents to produce their effect. These findings suggest that eye muscle proprioception and foot plantar afferents both take part in the elaboration of the egocentric reference and operate in a synergic way in order to estimate verticality, as they do to ensure balance50.
Second, as concerns the control of posture, manipulation of foot tactile afferents produces its effects mainly at far distance (200 cm). It can be explained by the fact that, for postural control, the increase in distance provokes a decrease in the use of visual and oculomotor cues in favour of somatosensory afferents61.
Finally, balance is not significantly improved at close distance and is even of better quality (i.e. lower Surface area and Length) at far distance. At near distance the angular size of the vertical line was larger for geometrical reasons, therefore causing more postural instability than at far distance, in accordance with the original visual interpretation of Paulus et al.62. This result is not in opposition with previous studies of our team showing better stability at near than at far distance50,63. Indeed, these studies did not include such an active task, i.e. exposure to a misaligned vertical line and active effort to judge its verticality. Nevertheless, the present study does confirm that postural control is dependent on distance and interacts with the type of task that the subject is performing. Further research with objective eye movement recording would be of interest to understand better eye movement behavior and postural control at far versus near during the SVV estimation.
Conclusion
This study brings initial evidence of the direct influence of foot cutaneous afferents on the perception of SVV and suggests that they participate in the elaboration of the egocentric reference in synergy with eye muscle proprioception. Moreover, this influence is present only among typically behaving subjects, i.e. who are not affected by PEI.
This experiment is a first step in which we tried different ways of manipulating plantar cutaneous afferents, either attenuating them by foam interposition, or increasing them beneath both feet with the bilateral medial arch supports, or increasing them only beneath one foot with the lateral arch support. The key finding is that only the unilateral stimulation produces an effect on the SVV estimation, suggesting that the symmetry of plantar signals arising from the two feet is information that the CNS uses in order to elaborate an internal model of verticality and assess SVV. In order to confirm those first results, further experiment should focus on protocols aiming at attenuating or stimulating plantar cutaneous afferents in an asymmetrical way.
Those results may have clinical implications. Indeed, it has been showed that elderly fallers64,65, and subjects suffering from scoliosis66, chronic neck pain67, or cerebral lesions inducing visuospatial neglect3,6,27,28 have a more altered perception of SVV than healthy subjects. Insoles with thin plantar inserts could be a cheap and simple mean to help those patients in order to improve their quality of SVV estimation. Further research is required to confirm this hypothesis and assess the potential long term effects.
Methods
Subjects
Forty-height healthy young subjects took part in the study. They were recruited from paramedical schools, 21 males and 27 females, mean age 25 ± 3,3 years, mean height 170.1 ± 8.6 cm, mean body weight 63.7 ± 10.5 kg. Their characteristics are summarized in Table S1 (Supplementary Information).
None of them were taking medication and all of them were asymptomatic. All subjects were emmetropic and wore no glasses. Their visual acuity at close distance was examined by means of Parinaud’s reading test. The results were all normal (2 for 47 subjects, 3 for one of them). Binocular visual function was also assessed with the stereoacuity TNO test and all values were normal, that is 60” of arc or lower. We also measured the Near Convergence Point, which was 5.06 ± 1.82 cm, and the amplitude of accommodation with the push-up method68,69 (we did a mean of 3 measures for both tests). The subjects had a mean of 9.37 dioptres (±1.85), which is within Duane’s normative data (9.5 ± 2 dioptres68). The t-test test did not show any statistical difference with that theoretical physiologic value (p = 0.616).
Experimental device
SVV assessment
The subjects were asked to stand still, barefoot on a force plate. Their perception of the SVV was assessed thanks to the Perspective System (Subjective Vertical and Horizontal v.2.0, produced by FRAMIRAL). It consists of a laser which is projected on the wall in front of the subject, in a dark room in order to avoid visual landmarks15,33,70. The upper end of the laser began tilted left (i.e. counter-clockwise) or right (i.e. clockwise), 15 or 30 degrees, in a random order14,33 and then turned clockwise or counter-clockwise at a speed of 2.5°/s. The examiner held a remote control and stopped the movement of the laser when the subject advised him. The subjects were able to adjust the position of the laser by telling the examiner to turn it “left” or “right” with the remote control, until they felt it corresponded to the vertical position and said “OK”. The examiner recorded the deviation given by the Perspective System device with a precision of 0.01°. The procedure was repeated 10 times for each ground condition and a mean of those 10 measures was calculated7,66,70. The subjects were never informed of the results in order to avoid a corrective feedback33,43.
Posture assessment
At the same time the force platform (produced by TechnoConcept, Céreste, France, and using the Standards of the Association Française de Posturologie) assessed the postural performances of our subjects. It consists of two clogs placed side by side; forming a 30° angle with heels separated by 4 cm. Each clog holds 2 strain gauges (one beneath the metatarsal heads, one beneath the heel) which are force - electric tension transducers. The height and weight of the subjects were factored into the calculations of the Center of Pressure’s displacements, which were recorded over a period of 180 s. The equipment contained an Analogue – Digital converter of 16 bits and the sampling frequency of the CoP was 40 Hz. We analyzed the following postural parameters: the medio-lateral (X) mean position of the CoP, the Surface area of CoP excursions and the Length of CoP displacements (total length of the path of the CoP in millimeters). The Surface area of the CoP represents 90% of the instantaneous positions of the CoP included within the confidence ellipse, eliminating the extreme points71.
Testing conditions
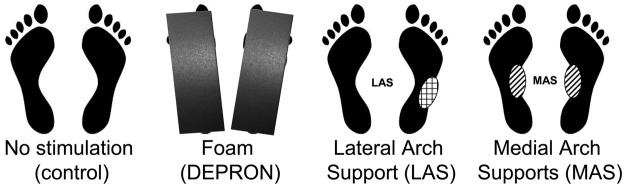
There were 4 plantar stimulation conditions: [1] on firm ground (control condition); [2] on foam (DEPRON, 6 mm-thick, shore rating of 20A, density of 33 kg/m3); [3] with a lateral arch support beneath the right foot; [4] with bilateral medial arch supports. The arch supports were 3 mm-thick plantar inserts, made of polyester resin, shore rating of 60A, density of 250 kg/m3. The lateral arch support was placed beneath the lateral half of the external mid-foot print, whereas the medial arch supports were set beneath the medial half of the external mid-foot print (see Fig. 4).
Figure 4.
Plantar stimulation conditions. DEPRON foam (6 mm-thick, shore rating of 20A, density of 33 kg/m3), lateral arch support, or medial arch supports (3 mm-thick inserts shore rating of 60A, density of 250 kg/m3) were placed beneath the feet.
It has previously been shown that plantar cutaneous afferents can be experimentally manipulated by mechanical means, either attenuating, or stimulating. The attenuation of the information arising from the feet can be obtained by foam interposition between the ground and feet. Among others, Chiang and Wu57, Patel et al.58,72, Yi and Park38 all concluded that foam interposition makes the subjects less stable. In particular, Patel et al.72 showed that such method produced even stronger disrupting effects on stability than hypothermic anesthesia of fast adapting mechanoreceptors of the foot sole. Furthermore, Yi and Park38 directly showed that when healthy subjects are standing on foam, it induces a decrease in plantar cutaneous sensitivity to microfilament touch, similar to the one shown by patients suffering from peripheral sensory neuropathy.
As concerns stimulation of plantar cutaneous afferents, it can be achieved by several means. Plantar exteroceptive signal can be enriched by mechanical vibration of the foot sole, which produces whole-body tilts, opposite to the stimulated foot zone35,45. But postural effects can also be obtained with thin plantar inserts set beneath the feet: clinicians39 proposed that the keen sensibility of plantar mechanoreceptors (10 mN in pressure, 5 microns in deformation47,73 enables them to detect the increase in pressure and deformation of the plantar skin induced by such inserts. Later, Janin and Dupui41 confirmed that they can produce similar effects to those obtained by Kavounoudias et al.35,45. Previous work of our group also showed that such thin plantar inserts significantly improve postural stability40,44.
After a first familiarization trial, the postural and SVV estimation performances of each subject were recorded for each plantar condition at close distance (40 cm) and far distance (200 cm), so that there were in total 8 counterbalanced testing conditions. In order to avoid a phenomenon of habituation of the sole cutaneous mechanoreceptors, a one minute period of seated rest separated each recording74. We tested the effects of the plantar stimulations at close and far distance because it is known that the CNS assigns a different weight to the signals used in postural control according to the distance of the visual target61. At near distance (40 cm) visual and oculomotor signals are particularly used because of the larger retinal slip and vergence angle (respectively); whereas at intermediate and far distances (beyond 90 cm) the CNS mostly uses somatosensory signals61. Thus, testing both distances allowed us to see if the same sensory re-weighting is involved in the estimation of SVV.
Plantar quotient assessment
Thanks to the postural recordings we were able to calculate the plantar quotient75. The plantar quotient is a classical method, used by several authors in order to to assess the weight of plantar input in postural control75–79. It consists in the ratio between the Surface area of the CoP excursions while the subjects stand on foam and the Surface area while they stand on firm ground: plantar quotient = Sfoam/Sfirm ground × 100. Hence, the plantar quotient provides information on the weight of plantar cutaneous afferents used in postural control80,81: the higher it is, the more the subject relies on the information arising from his feet to keep balance. Indeed, foam decreases the information arising from the feet38, normally resulting in a decreased stability38,44,57,58, indicated by a plantar quotient >100. We called the subjects exhibiting such typical behavior on foam “typically behaving ubjects”. In contrast, a plantar quotient <100 identifies a subject whose plantar cutaneous afferents impair postural control instead of being useful to balance, thus revealing a Plantar Exteroceptive Inefficiency (PEI44,50). In the literature, thick (several cm) and compliant foam support surfaces are generally used, leading to both biomechanical and sensorial effects38,58, the latter involving plantar exteroception and proprioception at the same time57,58. Here we used thin and firm foam in order to focus the action upon plantar cutaneous afferents (following44,75,82). Previous work showed that the plantar quotient is a valuable tool to account for interindividual differences in the use of somatosensory cues and to detect PEI, which is defined by a plantar quotient <10044,50.
Statistical analysis
Statistical analysis was performed using non-parametric tests, i.e Mann-Whitney U tests, Friedman’s tests (procedure of Statsoft/Statistica, release 7.1) since the test of Shapiro-Wilk revealed that some of the distributions were not normal and proved impossible to normalize. Post hoc comparisons were done whenever necessary using the test of Wilcoxon, with p < 0.05 considered as significant. The magnitudes of the differences were assessed by the effect size (Cohen’s d).
We applied the Bonferroni-Holm method correction for multiple testing83,84, and the corrected p-values are shown in the text.
Ethics Statement
The investigation adhered to the principles of the Declaration of Helsinki and was approved by the “Conseil d’Evaluation Éthique pour les Recherches en Santé” (CERES) University Paris Descartes, N° IRB: 20153300001072. The subjects gave informed written consent after the nature of the procedure was explained.
Electronic supplementary material
Acknowledgements
The authors wish to thank Chrystal Gaertner and Aurélien Morize for their help in the pre-experimental phase, all the subjects who agreed to take part in the experiment and Scott Lemons for his help in reviewing the English. A. Foisy was financially supported by the COE (College Ostéopathique Européen, 3000€), the API (Association de Posturologie Internationale, 560€) and the Institut d’Assas (375€). None of the sponsors had any involvement in any part of the study.
Author Contributions
A.F. and Z.K. conceived and designed the experiment. A.F. conducted the experiment. A.F. analysed the results. A.F. and Z.K. drafted and reviewed the manuscript.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-33268-3.
References
- 1.Lopez, C., Lacour, M. & Borel, L. Perception de la verticalité et représentations spatiales dans les aires corticales vestibulaires in Bipédie, contrôle postural et représentation corticale (eds Lacour, M. & Weber, B.) 35–86 (Solal, 2005).
- 2.Lopez C, Mercier MR, Halje P, Blanke O. Spatiotemporal dynamics of visual vertical judgments: early and late brain mechanisms as revealed by high-density electrical neuroimaging. Neuroscience. 2011;5(181):134–49. doi: 10.1016/j.neuroscience.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 3.Utz KS, et al. Multimodal and multispatial deficits of verticality perception in hemispatial neglect. Neuroscience. 2011;11(188):68–79. doi: 10.1016/j.neuroscience.2011.04.068. [DOI] [PubMed] [Google Scholar]
- 4.Baier B., Suchan J., Karnath H.-O., Dieterich M. Neural correlates of disturbed perception of verticality. Neurology. 2012;78(10):728–735. doi: 10.1212/WNL.0b013e318248e544. [DOI] [PubMed] [Google Scholar]
- 5.Kheradmand A, Lasker A, Zee DS. Transcranial magnetic stimulation (TMS) of the supramarginal gyrus: a window to perception of upright. Cereb Cortex. 2015;25(3):765–71. doi: 10.1093/cercor/bht267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rousseaux M, Braem B, Honoré J, Saj A. An anatomical and psychophysical comparison of subjective verticals in patients with right brain damage. Cortex. 2015;69:60–7. doi: 10.1016/j.cortex.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Barra J, et al. Humans use internal models to construct and update a sense of verticality. Brain. 2010;133(Pt 12):3552–63. doi: 10.1093/brain/awq311. [DOI] [PubMed] [Google Scholar]
- 8.Barra J, Pérennou D. Is the sense of verticality vestibular? Neurophysiol Clin. 2013;43(3):197–204. doi: 10.1016/j.neucli.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Roll J-P, Roll R. Extraocular proprioception as an element of postural reference and spatial coding of retinal information. Agessologie. 1987;28:906–912. [PubMed] [Google Scholar]
- 10.Amblard B, Crémieux J, Marchand AR, Carblanc A. Lateral orientation and stabilization of human stance: static versus dynamic visual cues. Exp Brain Res. 1985;61(1):21–37. doi: 10.1007/BF00235617. [DOI] [PubMed] [Google Scholar]
- 11.Merfeld D, Zupan L, Peterka R. Humans use internal models to estimate gravity and linear acceleration. Nature. 1999;398:615–618. doi: 10.1038/19303. [DOI] [PubMed] [Google Scholar]
- 12.Paillard T. Plasticity of the postural function to sport and/or motor experience. Neurosci Biobehav Rev. 2017;72:129–152. doi: 10.1016/j.neubiorev.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 13.Ohlmann, T. & Luyat, M. La posture référencée et la posture source de références in Nouveautés conceptuelles, instrumentales et cliniques (ed. Lacour, M.) 15–37 (Solal, 2001).
- 14.Clemens I. A. H., De Vrijer M., Selen L. P. J., Van Gisbergen J. A. M., Medendorp W. P. Multisensory Processing in Spatial Orientation: An Inverse Probabilistic Approach. Journal of Neuroscience. 2011;31(14):5365–5377. doi: 10.1523/JNEUROSCI.6472-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mittelstaedt H. The subjective vertical as a function of visual and extraretinal cues. Acta Psychol (Amst). 1986;63(1–3):63–85. doi: 10.1016/0001-6918(86)90043-0. [DOI] [PubMed] [Google Scholar]
- 16.Guerraz M, Poquin D, Ohlmann T. The role of head-centric spatial reference with a static and kinetic visual disturbance. Percept Psychophys. 1998;60:287–295. doi: 10.3758/BF03206037. [DOI] [PubMed] [Google Scholar]
- 17.Isableu B, Ohlmann T, Cremieux J, Amblard B. Selection of spatial frame of reference and postural control variability. Exp Brain Res. 1997;114(3):584–9. doi: 10.1007/PL00005667. [DOI] [PubMed] [Google Scholar]
- 18.Luyat Marion, Mobarek Slimane, Leconte Claire, Gentaz Edouard. The plasticity of gravitational reference frame and the subjective vertical: Peripheral visual information affects the oblique effect. Neuroscience Letters. 2005;385(3):215–219. doi: 10.1016/j.neulet.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 19.Isableu B, Ohlmann T, Cremieux J, Amblard B. How dynamic visual field dependence-independence interacts with the visual contribution to postural control. Hum Mov Sci. 1998;17:367–391. doi: 10.1016/S0167-9457(98)00005-0. [DOI] [Google Scholar]
- 20.Lopez C, Lacour M, Ahmadi AE, Magnan J, Borel L. Changes of visual vertical perception: a long-term sign of unilateral and bilateral vestibular loss. Neuropsychologia. 2007;45:2025–2037. doi: 10.1016/j.neuropsychologia.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Clark B, Graybiel A. Perception of the postural vertical following prolonged bodily tilt in normals and subjects with labyrinthine defects. Acta Otolaryngol. 1964;58:143–148. doi: 10.3109/00016486409121371. [DOI] [PubMed] [Google Scholar]
- 22.Paillard, J. Knowing where and knowing how to get there in Brain and Space (ed. Paillard, J.) 461–481 (Oxford University Press, 1991).
- 23.Mittelstaedt H. Origin and processing of postural information. Neurosci Biobehav Rev. 1998;22:473–478. doi: 10.1016/S0149-7634(97)00032-8. [DOI] [PubMed] [Google Scholar]
- 24.Bronstein AM. The interaction of otolith and proprioceptive information. Ann N Y Acad Sci. 1999;28(871):324–33. doi: 10.1111/j.1749-6632.1999.tb09195.x. [DOI] [PubMed] [Google Scholar]
- 25.Anastasopoulos D, Bronstein A, Haslwanter T, Fetter M, Dichgans J. The role of somatosensory input for the perception of verticality. Ann N Y Acad Sci. 1999;28(871):379–83. doi: 10.1111/j.1749-6632.1999.tb09199.x. [DOI] [PubMed] [Google Scholar]
- 26.Saeys W, et al. Influence of sensory loss on the perception of verticality in stroke patients. Disabil Rehabil. 2012;34(23):1965–70. doi: 10.3109/09638288.2012.671883. [DOI] [PubMed] [Google Scholar]
- 27.Kerkhoff G, Zoelch C. Disorders of visuospatial orientation in the frontal plane in patients with visual neglect following right or left parietal lesions. Exp Brain Res. 1998;122(1):108–20. doi: 10.1007/s002210050497. [DOI] [PubMed] [Google Scholar]
- 28.Funk J, Finke K, Müller HJ, Utz KS, Kerkhoff G. Visual context modulates the subjective vertical in neglect: evidence for an increased rod-and-frame-effect. Neuroscience. 2011;26(173):124–34. doi: 10.1016/j.neuroscience.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 29.Lackner JR, Levine MS. Changes in apparent body orientation and sensory localization induced by vibration of postural muscles: vibratory myesthetic illusions. Aviat Space Environ Med. 1979;50:346–354. [PubMed] [Google Scholar]
- 30.Roll J-P, Vedel J-P, Roll R. Eye, head and skeletal muscle spindle feedback in the elaboration of body references. Prog Brain Res. 1989;80(113–23):57–60. doi: 10.1016/s0079-6123(08)62204-9. [DOI] [PubMed] [Google Scholar]
- 31.McKenna GJ, Peng GC, Zee DS. Neck muscle vibration alters visually perceived roll in normals. J Assoc Res Otolaryngol. 2004;5:25–31. doi: 10.1007/s10162-003-4005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barbieri G, et al. Does proprioception contribute to the sense of verticality? Exp Brain Res. 2008;185(4):545–52. doi: 10.1007/s00221-007-1177-8. [DOI] [PubMed] [Google Scholar]
- 33.Trousselard M, Barraud PA, Nougier V, Raphel C, Cian C. Contribution of tactile and interoceptive cues to the perception of the direction of gravity. Brain Res Cogn Brain Res. 2004;20:355–362. doi: 10.1016/j.cogbrainres.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Nyborg H. Tactile stimulation and perception of the vertical: I. Effect of diffuse vs. specific tactile stimulation. Scand. J. Psychol. 1971;12:1–13. doi: 10.1111/j.1467-9450.1971.tb00599.x. [DOI] [PubMed] [Google Scholar]
- 35.Kavounoudias A, Roll R, Roll JP. Specific whole-body shifts induced by frequency-modulated vibrations of human plantar soles. Neurosci. Lett. 1999;266:181–184. doi: 10.1016/S0304-3940(99)00302-X. [DOI] [PubMed] [Google Scholar]
- 36.Roll R??gine, Kavounoudias Anne, Roll Jean-Pierre. Cutaneous afferents from human plantar sole contribute to body posture awareness. NeuroReport. 2002;13(15):1957–1961. doi: 10.1097/00001756-200210280-00025. [DOI] [PubMed] [Google Scholar]
- 37.Faralli M, Longari F, Ricci G, Ibba MC, Frenguelli A. Influence of extero- and proprioceptive afferents of the plantar surface in determining subjective visual vertical in patients with unilateral vestibular dysfunction. Acta Otorhinolaryngol Ital. 2009;29(5):245–50. [PMC free article] [PubMed] [Google Scholar]
- 38.Yi Y, Park S. Effect of reduced cutaneous cues on motion perception and postural control. Exp Brain Res. 2009;195(3):361–9. doi: 10.1007/s00221-009-1796-3. [DOI] [PubMed] [Google Scholar]
- 39.Villeneuve, P. Cinquième leçon de Posturologie. In Les huit leçons de Posturologie (eds Gagey, P.-M., Bizzo, G., Bonnier, L., Gentaz, R., Guillaume, P. & Marucchi, C.) 35–37. (Association Française de Posturologie, 1990).
- 40.Foisy A., Gaertner C., Matheron E., Kapoula Z. Controlling Posture and Vergence Eye Movements in Quiet Stance: Effects of Thin Plantar Inserts. PLOS ONE. 2015;10(12):e0143693. doi: 10.1371/journal.pone.0143693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janin M, Dupui P. The effects of unilateral medial arch support stimulation on plantar pressure and center of pressure adjustment in young gymnasts. Neurosci. Lett. 2009;461:245–248. doi: 10.1016/j.neulet.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 42.Witkin HA, Asch SE. Studies in space orientation: III. Perception of the upright in the absence of a visual field. J. Exp. Psychol. 1948;38:603–614. doi: 10.1037/h0055372. [DOI] [PubMed] [Google Scholar]
- 43.Jaggi-Schwarz K, Ortega M, Hess BJM. Reciprocal error behavior in estimated body position and subjective visual vertical. Exp Brain Res. 2003;150:122–125. doi: 10.1007/s00221-003-1430-8. [DOI] [PubMed] [Google Scholar]
- 44.Foisy A, Kapoula Z. How Plantar Exteroceptive Efficiency Modulates Postural and Oculomotor Control: Inter-Individual Variability. Front Hum Neurosci. 2016;13(10):228. doi: 10.3389/fnhum.2016.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kavounoudias A, Roll R, Roll JP. Foot sole and ankle muscle inputs contribute jointly to human erect posture regulation. J Physiol. 2001;1,532(Pt 3):869–78. doi: 10.1111/j.1469-7793.2001.0869e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Janin, M. Sensibilité et motricité podales: leur influence sur le contrôle des activités posturo-cinétiques de sujets sains et pathologiques. Thèse de Doctorat. Comportement, Langage, Éducation, Socialisation, Cognition. Toulouse III. (2009).
- 47.Vedel J-P, Roll J-P. Response to pressure and vibration of slowly adapting cutaneous mechanoreceptors in the human foot. Neurosci. Lett. 1982;34:289–294. doi: 10.1016/0304-3940(82)90190-2. [DOI] [PubMed] [Google Scholar]
- 48.Ribot-Ciscar E., Vedel J.P., Roll J.P. Vibration sensitivity of slowly and rapidly adapting cutaneous mechanoreceptors in the human foot and leg. Neuroscience Letters. 1989;104(1-2):130–135. doi: 10.1016/0304-3940(89)90342-X. [DOI] [PubMed] [Google Scholar]
- 49.Weerakkody NS, Percival P, Canny BJ, Morgan DL, Proske U. Force matching at the elbow joint is disturbed by muscle soreness. Somatosens Mot Res. 2003;20(1):27–32. doi: 10.1080/0899022031000083816. [DOI] [PubMed] [Google Scholar]
- 50.Foisy A, Kapoula Z. Plantar Cutaneous Inefficiency, visual-podal asynergy, postural control and best means of remediation. Brain Behav. 2017;00:e00658. doi: 10.1002/brb3.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canguilhem, G. Le normal et le pathologique. PUF. Paris (1966).
- 52.Canguilhem G. La santé: concept vulgaire et question philosophique. Cah Semin Philos. 1988;8:119–33. [PubMed] [Google Scholar]
- 53.Winter DA. Human balance and posture control during standing and walking. Gait Posture. 1995;3:193–214. doi: 10.1016/0966-6362(96)82849-9. [DOI] [Google Scholar]
- 54.Akin FW, Murnane OD, Pearson A, Byrd S, Kelly KJ. Normative data for the subjective visual vertical test during centrifugation. J Am Acad Audiol. 2011;22(7):460–8. doi: 10.3766/jaaa.22.7.6. [DOI] [PubMed] [Google Scholar]
- 55.Barra J., Oujamaa L., Chauvineau V., Rougier P., Perennou D. Asymmetric standing posture after stroke is related to a biased egocentric coordinate system. Neurology. 2009;72(18):1582–1587. doi: 10.1212/WNL.0b013e3181a4123a. [DOI] [PubMed] [Google Scholar]
- 56.Mittelstaedt H. A new solution to the problem of the subjective vertical. Naturwissenschaften. 1983;70:272–81. doi: 10.1007/BF00404833. [DOI] [PubMed] [Google Scholar]
- 57.Chiang JH, Wu G. The influence of foam surfaces on biomechanical variables contributing to postural control. Gait Posture. 1997;5:239–245. doi: 10.1016/S0966-6362(96)01091-0. [DOI] [Google Scholar]
- 58.Patel M, Fransson PA, Lush D, Gomez S. The effect of foam surface properties on postural stability assessment while standing. Gait Posture. 2008;28(4):649–56. doi: 10.1016/j.gaitpost.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 59.Luyat M, Ohlmann T, Barraud PA. Subjective vertical and postural activity. Acta Psychol (Amst). 1997;95(2):181–93. doi: 10.1016/S0001-6918(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 60.Fransson PA, Hjerpe M, Johansson R. Adaptation of multi-segmented body movements during vibratory proprioceptive and galvanic vestibular stimulation. J Vestib Res. 2007;17(1):47–62. [PubMed] [Google Scholar]
- 61.Le TT, Kapoula Z. Role of ocular convergence in the Romberg quotient. Gait Posture. 2007;27:493–500. doi: 10.1016/j.gaitpost.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 62.Paulus WM, Straube A, Brandt T. Visual stabilization of posture. Physiological stimulus characteristics and clinical aspects. Brain. 1984;107(Pt 4):1143–63. doi: 10.1093/brain/107.4.1143. [DOI] [PubMed] [Google Scholar]
- 63.Kapoula Z, Le TT. Effects of distance and gaze position on postural stability in young and old subjects. Exp Brain Res. 2006;173:438–445. doi: 10.1007/s00221-006-0382-1. [DOI] [PubMed] [Google Scholar]
- 64.Tobis JS, Nayak L, Hoehler F. Visual perception of verticality and horizontality among elderly fallers. Arch Phys Med Rehabil. 1981;62:619–22. [PubMed] [Google Scholar]
- 65.Tobis JS, Reinsch S, Swanson JM, Byrd M, Scharf T. Visual perception dominance of fallers among community-dwelling older adults. J Am Geriatr Soc. 1985;33:330–3. doi: 10.1111/j.1532-5415.1985.tb07132.x. [DOI] [PubMed] [Google Scholar]
- 66.Cakrt O, Slabý K, Viktorinová L, Kolář P, Jeřábek J. Subjective visual vertical in patients with idiopatic scoliosis. J Vestib Res. 2011;21(3):161–5. doi: 10.3233/VES-2011-0414. [DOI] [PubMed] [Google Scholar]
- 67.Docherty S, Schärer R, Bagust J, Humphreys BK. Perception of subjective visual vertical and horizontal in patients with chronic neck pain: a cross-sectional observational study. Man Ther. 2012;17(2):133–8. doi: 10.1016/j.math.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 68.Duane A. Normal values of the amplitude of accommodation at all ages. J Am Med Assoc. 1912;59:1010–1013. doi: 10.1001/jama.1912.04270090254042. [DOI] [Google Scholar]
- 69.Rutstein RP, Fuhr PD, Swiatocha J. Comparing the amplitude of accommodation determined objectively and subjectively. Optom Vis Sci. 1993;70(6):496–500. doi: 10.1097/00006324-199306000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Barnett-Cowan M, Fleming RW, Singh M, Bülthoff HH. Perceived Object Stability Depends on Multisensory Estimates of Gravity. PLoS One. 2011;6:4. doi: 10.1371/journal.pone.0019289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ruhe A, Fejer R, Walker B. On the relationship between pain intensity and postural sway in patients with non-specific neck pain. J Back Musculoskelet Rehabil. 2013;26(4):401–9. doi: 10.3233/BMR-130399. [DOI] [PubMed] [Google Scholar]
- 72.Patel M, Fransson PA, Johansson R, Magnusson M. Foam posturography: standing on foam is not equivalent to standing with decreased rapidly adapting mechanoreceptive sensation. Exp Brain Res. 2011;208(4):519–27. doi: 10.1007/s00221-010-2498-6. [DOI] [PubMed] [Google Scholar]
- 73.Burgess, P., Perl, E., & Iggo, A. Cutaneous mechanoreceptors and nociceptors. In Handbook of Sensory Physiology (ed. Iggo, A.) 29–78 (New-York, Springer-Verlag, 1973).
- 74.Pinsault N, Vuillerme N. Test–retest reliability of centre of foot pressure measures to assess postural control during unperturbed stance. Med Eng Phys. 2009;31:276–286. doi: 10.1016/j.medengphy.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 75.Dujols A. The plantar quotiens and visual-podal conflict. Agressologie. 1991;32:192–4. [PubMed] [Google Scholar]
- 76.Shumway-Cook A, Horak FB. Assessing the influence of sensory interaction of balance. Suggestion from the field. Phys Ther. 1986;66(10):1548–50. doi: 10.1093/ptj/66.10.1548. [DOI] [PubMed] [Google Scholar]
- 77.Fujimoto C, et al. Assessment of diagnostic accuracy of foam posturography for peripheral vestibular disorders: analysis of parameters related to visual and somatosensory dependence. Clin Neurophysiol. 2009;120(7):1408–14. doi: 10.1016/j.clinph.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 78.Di Berardino F, et al. The use of rubber foam pads and “sensory ratios” to reduce variability in static posturography assessment. Gait Posture. 2009;29(1):158–60. doi: 10.1016/j.gaitpost.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 79.Preszner-Domjan A, et al. When does mechanical plantar stimulation promote sensory re-weighing: standing on a firm or compliant surface? Eur J Appl Physiol. 2012;112(8):2979–87. doi: 10.1007/s00421-011-2277-5. [DOI] [PubMed] [Google Scholar]
- 80.Oie KS, Kiemel T, Jeka JJ. Multisensory fusion: simultaneous re-weighting of vision and touch for the control of human posture. Cogn Brain Res. 2002;14(1):164–76. doi: 10.1016/S0926-6410(02)00071-X. [DOI] [PubMed] [Google Scholar]
- 81.Isableu B, Fourre B, Vuillerme N, Giraudet G, Amorim M-A. Differential integration of visual and kinaesthetic signals to upright stance. Exp Brain Res. 2011;212:33–46. doi: 10.1007/s00221-011-2693-0. [DOI] [PubMed] [Google Scholar]
- 82.Leporck, A.-M. & Villeneuve, P. Les épines irritatives d’appui plantaire, objectivation clinique et stabilométrique in Pied, équilibre et posture (ed. Villeneuve, P.) 131–138 (Frison Roche, 1996).
- 83.Holm S. A simple sequentially rejective multiple test procedure. SJOS. 1979;6(2):65–70. [Google Scholar]
- 84.Aickin M, Gensler H. Adjusting for multiple testing when reporting research results: the Bonferroni vs Holm methods. Am J Public Health. 1996;86(5):726–8. doi: 10.2105/AJPH.86.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).